Abstract
Increased airway smooth muscle mass is present in fatal and non-fatal asthma. However, little information is available regarding the cellular mechanism (i.e., hyperplasia vs. hypertrophy). Even less information exists regarding the functional consequences of airway smooth muscle remodeling. It would appear that increased airway smooth muscle mass would tend to increase airway narrowing and airflow obstruction. However, the precise effects of increased airway smooth muscle mass on airway narrowing are not known. This review will consider the evidence for airway smooth muscle cell proliferation and hypertrophy in asthma, potential functional effects, and biochemical mechanisms.
Keywords: α-smooth muscle actin, hyperresponsiveness, translational control, migration
The first detailed report of airway structural change in asthma was published over 80 years ago by Huber and Koessler (1), who demonstrated that patients with fatal asthma have substantial thickening of both the airway subepithelial and smooth muscle layers. Since that time, much attention has been focused on the airway structural changes accompanying asthma, and the potential ramifications of these changes for airway function and medical management. Nevertheless, the precise role of these structural changes (“airway remodeling”) in the pathogenesis of airflow obstruction remains unclear. In this review, we will examine the changes in smooth muscle structure that are observed in the airways of patients with asthma, and the potential functional effects of these changes. Finally, avenues for future study will be suggested.
INCREASED AIRWAY SMOOTH MUSCLE MASS IN ASTHMA
Numerous studies have noted increased airway smooth muscle mass in fatal asthma (1–9). Because of the obstacle of obtaining bronchial biopsies that include the full thickness of airway smooth muscle, fewer studies on airway smooth muscle mass in patients with nonfatal asthma exist. However, three reports show increased airway smooth muscle mass in these patients (10–12).
There are several potential pitfalls of this work. First, muscle contraction can exaggerate thickness of the smooth muscle layer. Second, as emphasized by Thomson and colleagues (13), the airway smooth muscle layer may contain varying amounts of connective tissue. Finally, to obtain unbiased estimates, objects must be counted directly in three-dimensional space. Only the most recent studies have used state-of-the-art stereologic techniques.
Ebina and colleagues (9) examined airways of patients with fatal asthma using a dissector probe and serial sections. Two asthmatic subtypes were found: one in which smooth muscle mass was increased only in the central bronchi (type I) and another in which muscle thickness was increased throughout the airway tree (type II). In type I patients, the number of smooth muscle nuclei in the central airways was increased, indicating the presence of airway smooth muscle hyperplasia. In patients with type II asthma, airway smooth muscle cell volume was increased, signifying airway smooth muscle hypertrophy. This report is consistent with other clinical studies suggesting the existence of different asthma phenotypes (14).
Woodruff and colleagues (12) examined biopsies from patients with mild asthma using quantitative morphometry, laser capture microdissection, and real-time polymerase chain reaction. They found that airway smooth muscle cell number was nearly twofold higher in subjects with mild to moderate asthma, whereas there was no increase in cell size between groups. Thus, the cellular mechanism of increased airway smooth muscle mass (i.e., proliferation or hypertrophy) may vary with disease severity.
Benayoun and colleagues (11) also addressed the cellular mechanism of increased airway smooth muscle mass in asthma. They found that the airways of patients with severe asthma had larger smooth muscle cell diameter than control subjects, patients with mild asthma, or patients with chronic obstructive pulmonary disease. Furthermore, there was no evidence of airway smooth muscle cell proliferation, as evidenced by the lack of staining for Ki67, a nuclear marker of cell cycle traversal. Together, these data provide further evidence that smooth muscle hypertrophy contributes to airway remodeling in asthma. However, this study failed to use modern techniques to calculate airway smooth muscle cell size.
Because so few studies have addressed the cellular mechanism of increased airway smooth muscle mass in asthma, it is therefore appropriate to look to animal studies for additional mechanistic information. Ovalbumin sensitization followed by repeated challenge induces features of airway remodeling, including thickening of the peribronchial smooth muscle layer. In guinea pigs, ovalbumin challenge increased the uptake of bromodeoxyuridine, a thymidine analog, in the airway smooth muscle layer (15). Airway smooth muscle proliferation, as evidenced by proliferating cell nuclear antigen staining, has also been found in rats and mice after repeated allergen challenge (16, 17). These data are consistent with the notion that abnormal airway smooth muscle proliferation is present in allergic asthma. On the other hand, Moir and coworkers (18) measured bronchiolar smooth mass content, cell number, and tension development in Brown Norway rats after repeated ovalbumin exposures. Although, as expected, airway smooth muscle mass and maximal tension increased, the authors did not detect increases in the total number of elongated cell nuclei in the muscle bundles, suggesting hypertrophic growth.
Unfortunately, none of the animal studies performed thus far have used advanced stereologic techniques to measure airway smooth muscle volume or cell size, and therefore they add little to the limited human data available. Together with the clinical studies, we can probably conclude that airway smooth muscle mass is increased in asthma, that hyperplasia and hypertrophy may both play a role, and that the balance between cell proliferation and hypertrophy may vary with asthma phenotype, duration, and severity.
AIRWAY SMOOTH MUSCLE CONTRACTILE PROTEIN EXPRESSION IN ASTHMA
Although airway smooth muscle mass appears to be increased in asthma, few studies have assessed the expression of specific contractile proteins. Most reports examining airway smooth muscle mass identified muscle using hematoxylin and eosin or other histologic stains. However, recent studies have used immunostains to detect α-smooth muscle actin and myosin light chain kinase (MLCK) in asthmatic airways. Benayoun and coworkers (11) found that the airways of patients with severe asthma have greater α-smooth muscle actin and MLCK immunoreactivity than control subjects or patients with chronic obstructive pulmonary disease. Woodruff and colleagues (12) found a 50 to 83% increase in α-smooth muscle actin immunoreactivity in patients with mild to moderate asthma. Interestingly, the mRNA expression of contractile protein genes was not increased, consistent with the notion that contractile protein expression may be regulated in a post-transcriptional manner (see below). Finally, bronchial smooth muscle cells from subjects with asthma show higher levels of smooth muscle MLCK mRNA (19).
Moir and colleagues (18) also measured contractile protein expression in their study of ovalbumin-sensitized and -challenged Brown Norway rats. Immunoblots of dissected small bronchioles demonstrated a paradoxical reduction in the expression of contractile proteins, including smooth muscle myosin heavy chain isoform 1, calponin, smoothelin-A, and MLCK, consistent with a switch to a less differentiated growth phenotype. Although these data would appear to contradict findings of increased α-smooth muscle actin and MLCK expression in human asthma, they demonstrate that increased airway smooth muscle mass may not correlate with the expression of contractile proteins, or even contractile function (see below).
FUNCTIONAL EFFECTS OF AIRWAY SMOOTH MUSCLE PROLIFERATION AND HYPERTROPHY
Increased airway smooth muscle mass could generate more shortening, leading to increased airway narrowing and airflow obstruction. Using a mathematical model and actual measurements of airway wall thickness from subjects with asthma, Lambert and colleagues (20) found that increased smooth muscle mass, rather than submucosal or adventitial thickening, was the only structural change likely to substantially increase maximal airways resistance, as is observed in response to bronchoconstrictors in patients with asthma. However, this model assumed no change in the contractile characteristics of the muscle, or of the load on the smooth muscle.
Because airway smooth muscle mass cannot easily be manipulated in humans with asthma, studies examining the issue of functional effects rely on correlations between smooth muscle mass and asthma severity. Numerous studies have examined the relationship between airway subepithelial thickening, another feature of airway remodeling in asthma, and disease severity or duration. However, few studies have correlated airway smooth muscle thickness with function. High-resolution computed tomography scans of patients with stable asthma showed that wall thickness of the right upper lobe apical bronchus, normalized to body surface area, was inversely correlated with airway reactivity to methacholine, suggesting that airway remodeling actually limits airway narrowing (21). However, the airway wall is composed of epithelial, submucosal, smooth muscle, and adventitial layers, and therefore a specific role for airway smooth muscle cannot be determined from this report. In Benayoun and colleagues' report (11), airway smooth muscle mass correlated with asthma severity, suggesting that airway smooth muscle hypertrophy increases airways responsiveness. These data are particularly compelling in light of the fact that subepithelial thickening was inversely correlated with disease severity.
There are few studies comparing the force-generating capacity of asthmatic airway smooth muscle with normal muscle (22–27). At least one (25) showed increased constrictor sensitivity, but others showed normal or reduced contractile function. However, these early studies focused on isometric rather than isotonic contractile function, and did not normalize force for muscle cross-sectional area.
Mitchell and colleagues showed that passive sensitization of human bronchi augments smooth muscle shortening velocity and capacity (28). These data are consistent with the notion that airway smooth muscle shortening velocity is increased in patients with asthma. However, this study did not specifically address the function of hyperplastic or hypertrophic muscle.
Again, because of the paucity of human data, it is appropriate to look to animal studies for additional mechanistic information. Many animal models of asthma also show a correlation between airway smooth muscle thickness and responsiveness, for example in a study by Henderson and colleagues (29). In this study, airway smooth muscle thickness and methacholine responsiveness increased with ovalbumin sensitization and challenge and decreased with montelukast treatment. However, other studies have shown an uncoupling of airway smooth muscle proliferation and responsiveness. After repeated allergen exposures of rats and mice, airway hyperresponsiveness and allergic inflammation resolved but airway smooth muscle proliferation persisted (16, 17). Furthermore, treatment of allergen-exposed rats with an endothelin receptor antagonist attenuated airway smooth muscle bromodeoxyuridine incorporation but had no effect on cholinergic bronchial reactivity (30). Consistent with human airways, canine and murine airways show increased smooth muscle shortening velocity and capacity after passive sensitization (31, 32). However, only one animal study has directly examined the effects of smooth muscle hypertrophy or hyperplasia on ex vivo airway function. Zheng and colleagues (33) measured the mechanical properties of guinea pig airway explants treated with cardiotrophin, a member of the IL-6 family. Cardiotrophin increases the size and protein synthesis of cultured human bronchial smooth muscle cells (34). Cardiotrophin also increased the airway smooth muscle content of guinea pig airway explants (33). However, maximal isometric stress and perhaps shortening were decreased, suggesting that the contractile apparatus of hypertrophic airway smooth muscle cells may not be completely functional. On the other hand, as reflected in Moir and coworkers' study (18), cell hypertrophy is a complicated phenotype consisting not only of changes in protein synthesis or cell size but also expression of contractile proteins and incorporation of these proteins into filaments. Thus, other conditions or stimuli leading to airway smooth muscle hyperplasia or hypertrophy may yield different results.
The paucity of information examining the contractile function of airways with increased smooth muscle mass led us to consider models of vascular smooth muscle hyperplasia/hypertrophy. In hypoxia-induced pulmonary hypertension, smooth muscle and connective tissue content are both increased (35). Hypoxia increased passive tissue stiffness and reduced active stress, again suggesting that the contractile apparatus of hypertrophic airway smooth muscle may not be completely functional. These data are also consistent with a reduction in contractile force due to a change in load. On the other hand, active shortening was increased and velocity of shortening was unchanged. Similar results were found in hyperoxia and monocrotaline-induced hypertension.
With regard to studies in cells, stimulation of postconfluent cultures with either serum or growth factors has been shown to markedly repress the expression of contractile proteins such as α-smooth muscle actin and MLCK (36–39). Conversely, serum-deprived cells demonstrate increased contractile protein expression. These data are consistent with the notion that proliferating airway smooth muscle cells may express lesser amounts of contractile proteins (“proliferative phenotype”), and generate less contractile force than nonproliferating cells.
On the other hand, bronchial smooth muscle cells from subjects with asthma show increased MLCK expression as well as maximum shortening capacity and velocity compared with normal cells (19). Furthermore, treatment of human airway smooth muscle cells with transforming growth factor (TGF)-β induced cellular hypertrophy (as evidenced by changes in protein synthesis, cell size, contractile protein expression, and incorporation of contractile proteins into filaments), while increasing cell shortening in response to contractile agonists (40). Interestingly, our preliminary studies have shown that, whereas both cardiotrophin-1 and TGF-β increase cell size, protein synthesis, and α-smooth muscle actin expression, TGF-β induces greater changes in cell size and incorporation of α-actin into contractile filaments (Figure 1). Recently, it has been shown that endothelin increases human bronchial smooth muscle cell size to a significantly greater extent than cardiotrophin-1 (41). These data are consistent with the notion that the contractile function of hypertrophic muscle may vary with the treatment or stimulus inducing the hypertrophy.
Figure 1.
Confocal micrographs showing primary human bronchial smooth muscle cells stained for α-smooth muscle actin (red) and phalloidin (green). Left panel: Control cells are thin and express minimal α-actin. Middle panel: Transforming growth factor-β increases cell size, α-actin expression, and incorporation into filaments (colocalization appears yellow-orange). Right panel: cardiotrophin (CT)-1 increases cell size and α-actin but there is less incorporation into filaments.
BIOCHEMICAL MECHANISMS OF AIRWAY SMOOTH MUSCLE PROLIFERATION
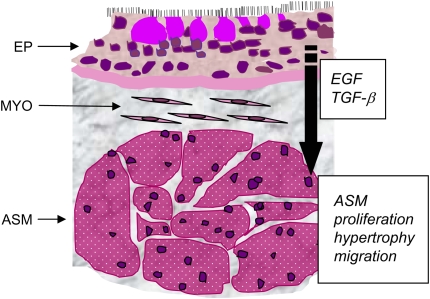
Cultured airway smooth muscle cells proliferate in response to a considerable number of stimuli, including peptide growth factors ligating receptor tyrosine or serine/threonine kinases and bronchoconstrictor substances associated with G protein–coupled seven-transmembrane receptors. The former include platelet-derived growth factor (PDGF), epidermal growth factor (EGF), and TGF-β (42–45), whereas the latter include histamine, thrombin, endothelin, and tryptase (46–56). EGF and TGF-β are synthesized by airway epithelial cells (57, 58), consistent with the notion that epithelial damage induces production of paracrine growth factors, which stimulate airway smooth muscle growth (Figure 2).
Figure 2.
Schematic demonstrating the close proximity of airway epithelium and airway smooth muscle (ASM). Paracrine growth factors produced by the airway epithelium, such as epidermal growth factor (EGF) and transforming growth factor (TGF)-β, may induce ASM proliferation, hypertrophy or migration. EP = epithelium; MYO = myofibroblasts; ASM = airway smooth muscle bundles in cross-section.
TGF-β may play a unique role in the airway remodeling process. TGF-β stimulates fibroblasts to synthesize and secrete proteins of the extracellular matrix; thus, in subjects with asthma, the degree of subepithelial fibrosis correlates with the amount of eosinophil-associated TGF-β mRNA (59). TGF-β is increased in the airways of patients with severe asthma compared with patients with less severe disease (59–62). As noted above, TGF-β induces human airway smooth muscle hypertrophy (40, 56), and has been noted to either enhance (55, 56) or inhibit airway smooth muscle proliferation in vitro (63, 64). Although it may be difficult to reconcile these disparate effects, increased cell growth (i.e., size) and contractile protein expression occur in both hyperplasia and hypertrophy (see below). Differential effects may also be explained by the presence or absence of other factors (serum, EGF).
The signaling pathways regulating airway smooth muscle proliferation have been reviewed elsewhere (65). Briefly, airway smooth muscle mitogenesis depends chiefly on stimulation of the extracellular signal regulated kinase (ERK) and phosphatidylinositol (PI) 3–kinase pathways. Activation of ERK is required for DNA synthesis in bovine, rat, and human airway smooth muscle (51, 66–68). ERK is an upstream activator of cyclin D1 promoter activity (69). Bronchoalveolar lavage fluid from asthmatic airways increases ERK activation, cyclin D1 protein abundance, DNA synthesis, and cell number of cultured human airway smooth muscle cells (70), consistent with the notion that ERK signaling constitutes an important regulator of cell cycle entry and progression in airway smooth muscle.
Chemical inhibitors of PI 3-kinase inhibit airway smooth muscle cyclin D1 protein expression (71) and DNA synthesis (68, 71, 72). Constitutive activation of PI 3-kinase in bovine tracheal myocytes is sufficient for transcription from the cyclin D1 promoter but does not induce ERK activation (71), implying that PI 3-kinase signaling occurs independently of ERK. Likewise, inhibitors of PI 3-kinase had no effect on ERK activation (68).
An important downstream target of PI 3-kinase appears to be the GTPase Rac1. Rac1 is required for cyclin D1 expression in bovine tracheal myocytes (73). Overexpression of active Rac1 does not activate ERK in bovine tracheal myocytes, and Rac1-induced transcription from the cyclin D1 promoter is insensitive to a chemical mitogen-activated protein kinase/ERK (MEK) inhibitor (73), suggesting that Rac1-mediated cell cycle progression, like that induced by PI 3-kinase, is independent of ERK activity. Finally, active PI 3-kinase and Rac1 each activate the cyclin D1 promoter via the cAMP response element binding protein (CREB)/activating transcription factor (ATF)-2 binding site, suggesting that, in the context of cyclin D1 expression, PI 3-kinase and Rac1 lie on the same signaling pathway. Rac1 constitutes part of the NADPH oxidase complex that generates superoxide and H2O2 (74, 75). Intracellular H2O2 is increased after mitogen treatment of rat tracheal myocytes (76), bovine tracheal myocytes (73), and human bronchial smooth muscle cells (77). Accordingly, treatment with antioxidants attenuates both mitogen-activated cyclin D1 expression and DNA synthesis in these cells (73, 76, 77). Finally, in bovine cells, Rac1 induces transactivation of the cyclin D1 promoter CREB/ATF2 binding site, which is attenuated by antioxidants (71). Taken together, these data suggest that growth factor–induced airway smooth muscle proliferation is regulated by a PI 3-kinase/Rac1/NADPH oxidase pathway.
It has been reported that cultured airway myocytes from subjects with asthma proliferate faster than do those from nonasthmatic individuals (78). Although corticosteroids inhibit proliferation of normal airway myocytes by reducing cyclin D1 expression and retinoblastoma protein phosphorylation (79), they apparently fail to inhibit proliferation of airway myocytes from subjects with asthma, an effect attributed to dysfunctional interaction between C/EBPα and the glucocorticoid receptor (80). However, these results have not been verified by others, and whether this mechanism contributes importantly to smooth muscle accumulation in the asthmatic airway wall remains unknown.
BIOCHEMICAL MECHANISMS REGULATING AIRWAY SMOOTH MUSCLE HYPERTROPHY
The study of biochemical pathways regulating airway smooth muscle hypertrophy has been limited due to the difficulty of establishing cell models. Two models using cell cycle arrest suggest that cell size and contractile protein expression are regulated in a post-transcriptional manner. In the first model, canine tracheal myocytes demonstrated high levels of SM22 and smooth muscle myosin heavy chain (smMHC) mRNA expression during rapid cell proliferation but only accumulated contractile protein during long-term serum deprivation (81). In the second model, airway smooth muscle cells conditionally immortalized with a thermolabile simian virus 40 (SV40) large tumor (T) antigen demonstrated increased α-smooth muscle actin and MLCK protein abundance but not mRNA expression upon shift to the nonpermissive temperature, which inactivates large T antigen and induces expression of p57 and p21Waf1/Cip1 (82). More recent studies have used physiologic stimuli, such as cardiotrophin (34) and TGF-β (40). In the case of TGF-β, α-smooth muscle actin protein expression is regulated at both the transcriptional and translational levels.
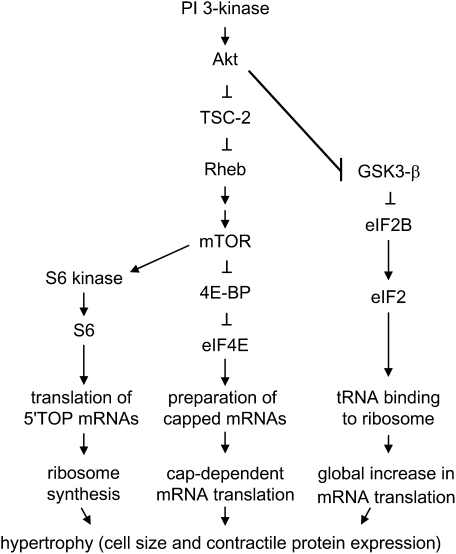
Regulation of protein synthesis is achieved primarily by the phosphorylation of various eukaryotic translation initiation factors (eIFs) (Figure 3). There are two highly regulated steps in the translation initiation pathway (Figure 4). First, the mRNA is prepared for ribosome binding by the eIF4 group of initiation factors. Translation of the majority of eukaryotic mRNAs is initiated through a 7-methylguanosine cap structure at the 5′ end of mRNA. The cap is recognized and “clamped” by the 24-kD eIF4E. Recognition of the cap structure allows unwinding of secondary mRNA structure by another initiation factor, the RNA helicase eIF4A. However, eiF4E is normally bound to inhibitory proteins termed “4E-binding proteins” (4E-BPs). Release of eIF4E typically requires 4E-BP1 phosphorylation by mammalian target of rapamycin (mTOR) (83, 84), although other 4E-BP kinases exist. mTOR phosphorylation, in turn, is regulated by a complex signaling pathway, including PI 3-kinase, the serine/threonine kinase Akt, the GTPase activating protein tuberous sclerosis complex (TSC)-2, and the GTPase Rheb (85–87). More recently, it has been discovered that mTOR exists in two distinct multiprotein complexes, one rapamycin-sensitive (mTORC1) and one rapamycin-insensitive (mTORC2) (88).
Figure 3.
Signaling intermediates and eukaryotic translation initiation factors regulating cell size and contractile protein expression. The eIF4E and eIF2 pathways regulate the efficiency of translation, whereas the p70 ribosomal S6 kinase pathway regulates translational capacity by increasing the synthesis of ribosomes.
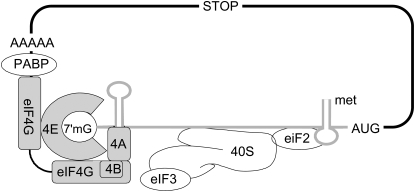
Figure 4.
Translation initiation. eIF2 recruits the initiator tRNA to the 40 S ribosomal subunit to form the 43S preinitiation complex. eIF4E binds to the 7-methylguanosine cap structure at the 5′ end of mRNA. Binding of the scaffold protein eiF4G to the convex dorsal surface of eIF4E allows recruitment of several other factors to the mRNA, including eIF4A and polyA binding protein (PABP). The helicase activity of eIF4A, which is enhanced by eIF4B, is believed to be required for unwinding of secondary structures in the 5′ untranslated region, allowing subsequent movement of the whole complex until the initiation codon (AUG) is recognized by the anticodon of the Met-tRNA.
Second, concurrent with the preparation of mRNA, the 43S preinitiation complex, composed of initiator methionyl-tRNA and the 40S ribosomal subunit, must be formed. eIF2, a multimer consisting of α, β, and γ subunits, functions to recruit methionyl tRNA and conduct it as a tRNA–eIF2–GTP ternary complex to the 40S ribosomal subunit. eIF2 GTP loading is determined by the activity of eIF2B, a guanine nucleotide exchange factor. eIF2Bɛ phosphorylation by glycogen synthase kinase (GSK)-3β inhibits its GDP/GTP exchange activity, thereby limiting binding of methionyl tRNA to the 40S ribosomal subunit. However, phosphorylation of GSK3β by the serine threonine kinase Akt inactivates it, leading to eIF2B dephosphorylation and activation, and a general enhancement of translation initiation, which is independent of the 7-methylguanosine mRNA cap (89).
Finally, the translation of mRNAs with 5′ terminal oligopyrimidine (TOP) tracts, many of which encode elongation factors and ribosomal proteins involved in mRNA translation, is up-regulated by phosphorylation of the S6 ribosomal protein. S6 ribosomal protein is in turn phosphorylated by p70 S6 kinase. In contrast to the above pathways that regulate the efficiency of translation, this pathway regulates the translational capacity by increasing the synthesis of ribosomes.
Despite human studies indicating the presence of airway smooth muscle hypertrophy and increased contractile protein expression in asthma, little information is available concerning the signaling intermediates and translation initiation factors involved. In confluent serum-deprived canine tracheal myocyte cultures, PI 3-kinase and mTOR are required for smooth muscle elongation and smMHC protein accumulation (81). In conditionally immortalized human bronchial smooth muscle cell lines, inhibition of PI 3-kinase and mTOR each attenuated temperature shift–induced cell enlargement (90). Chemical inhibitors of PI 3-kinase and mTOR also decreased 4E-BP phosphorylation and increased the binding of 4E-BP to eIF4E. Furthermore, cells transduced with a 4E-BP-1 double phosphorylation mutant failed to undergo a change in cell size after temperature shift. Together, these data show that PI 3-kinase and mTOR promote cap-dependent protein synthesis and cell size change via phosphorylation of 4E-BP, and that eIF4E is required for airway smooth muscle hypertrophy. However, in TGF-β–stimulated primary bronchial smooth muscle cells, rapamycin, which effectively blocked p70 ribosomal S6 kinase phosphorylation, had no effect on cell size and α-smooth muscle actin expression (40), suggesting that 4E-BP phosphorylation may occur independently of mTORC1. Furthermore, because TGF-β–induced hypertrophy occurred in the presence of p70 S6 kinase dephosphorylation, these data suggest that the up-regulation of 5′ TOP mRNAs was not required for the observed hypertrophic response.
THE CELLULAR OUTCOME OF PROTEIN SYNTHESIS, PROLIFERATION, OR HYPERTROPHY MAY BE REGULATED BY CELL CYCLE EVENTS
Although the cellular mechanism of airway smooth muscle accumulation, hypertrophy, or hyperplasia may vary in individual patients, increased cell growth (i.e., size) and contractile protein expression occur in both cases. (Mammalian cells must undergo cell enlargement before mitosis.) In the case of hypertrophy, cells do not traverse the cell cycle, whereas in proliferation, mitosis occurs. Thus, the biochemical mechanisms underlying hypertrophy and hyperplasia may overlap. Furthermore, in the context of increasing protein synthesis, the cellular outcome (i.e., hyperplasia or hypertrophy) is likely to be highly influenced by cell cycle regulation. For example, in cardiomyocytes, which cannot reenter the cell cycle, activation of mitogenic signaling pathways leads to cell hypertrophy. Similarly, expression of p57 and p21Waf1/Cip1 induces cell cycle arrest and hypertrophy of cultured human airway smooth muscle cells (34). Nevertheless, the contribution of cell cycle arrest to airway smooth muscle hypertrophy has not been well studied.
AIRWAY SMOOTH MUSCLE MIGRATION
Cell migration could be in part responsible for the pathogenesis of airway smooth muscle hyperplasia and remodeling in asthma. It is also possible that migration of smooth muscle cells toward the lumen of the airway underlies the appearance of myofibroblasts in the subepithelium of the asthmatic airway (91). In support of this hypothesis, segmental challenge of asthmatic airways increases the number of myofibroblasts in the airway subepithelium (92). Because myofibroblasts are a rich source of extracellular matrix proteins, such as collagen, fibronectin, and tenascin, each of which have been found in the subepithelium of patients with asthma (91), myofibroblast migration to the subepithelium may be a key factor in the development of subepithelial fibrosis.
Airway smooth muscle cells migrate in response to a number of physiologic stimuli in vitro, including PDGF, basic fibroblast growth factor, TGF-β, and IL-1β (93, 94). Recent studies suggest that chemokines, small (8–12 kD) proteins involved in the regulation of leukocyte trafficking, may also regulate airway smooth muscle migration. For example, the CCR3 ligand eotaxin induces migration of airway smooth muscle cells in vitro, and airway smooth muscle CCR3 expression is increased in asthma (95). Airway smooth muscle cells also express CXCR1, CXCR2, and CCR7, and migrate in response to the CXCR1/2 ligand IL-8 and the CCR7 ligand CCL19 (96, 97). Moreover, β-agonists and corticosteroids inhibit migration (94).
The signaling pathways regulating airway smooth muscle migration have been well studied. Activation of a p21-activating kinase/p38 mitogen–activated protein kinase heat shock protein 27 signaling pathway is required for the migration of canine tracheal smooth muscle cells (93, 98). Activation of the tyrosine kinase Src is necessary and sufficient for the migration of cultured human airway smooth muscle cells (99). In contrast, activation of protein kinase A inhibits migration (94).
The source of the migrating airway smooth muscle cells in vivo (i.e., the original airway smooth muscle layer, circulating fibrocytes, or bone marrow– or lung-derived mesenchymal stem cells) remains unknown. Recent studies have demonstrated the presence of circulating fibrocytes and mesenchymal stem cells in the airways, suggesting that airway myofibroblasts may originate from distant sites. Allergen exposure induces the accumulation of CD34-, collagen I–, and α-smooth muscle actin–positive cells to areas of collagen deposition below the epithelium in patients with allergic asthma (100). (CD34, like CD45, is an antigen found on hematopoietic progenitor cells.) PKH-26–labeled mouse fibrocytes purified from peripheral blood and injected into the tail vein of BALB/c mice home to the airway subepithelium of animals sensitized and challenged with ovalbumin but not those exposed to phosphate-buffered saline. Furthermore, human adult bronchial fibroblast–like cells isolated from the lung tissue of patients undergoing lobectomy show a pattern of surface markers characteristic of bone marrow–derived mesenchymal stem cells (101). In contrast to fibrocytes, these cells are negative for CD34 and CD45 and positive for CD73 (SH3/SH4), CD105 (SH2/endoglin), and CD166 (SB10 or leukocyte cell adhesion molecule). STRO-1 antigen, another human mesenchymal stem cell marker, was only weakly expressed in these cells. Similar to marrow fibroblasts, the bronchial fibroblast–like cells underwent adipogenic, osteogenic, and chondrogenic differentiation when cultured under appropriate inducible conditions. Finally, we have found that tracheal aspirates from premature infants undergoing mechanical ventilation for respiratory distress express the mesenchymal stem cell markers STRO-1, CD73, CD90, CD105, and CD166, but not hematopoietic and endothelial cell markers. These cells are capable of adipocytic and osteogenic differentiation, and express high levels of α-smooth muscle actin upon TGF-β stimulation (102). Taken together, these results indicate that circulating fibrocytes or mesenchymal stem cells may function as myofibroblast precursors and contribute to the genesis of subepithelial fibrosis in asthma. On the other hand, pilot studies in dogs showing an absence of airway smooth muscle cells 6 months after thermoplasty are inconsistent with the notion that mesenchymal cells repopulate the airway smooth muscle layer with disease, injury, or aging.
CONCLUSIONS
The airways of patients with asthma demonstrate both airway smooth muscle hyperplasia and hypertrophy, the balance of which may vary with asthma phenotype, duration, severity, and airway generation. Data examining the effects of smooth muscle remodeling on airway function are extremely limited. Although asthma severity may correlate with smooth muscle hypertrophy, other studies suggest that the contractile apparatus of hyperplastic or hypertrophic airway smooth muscle cells may be dysfunctional. The effect of increased smooth muscle mass on airway function may vary with cellular mechanism, airway stiffness, or the stimulus involved. Although the signaling pathways regulating airway smooth muscle proliferation have been well studied, the biochemical mechanisms regulating hypertrophy and contractile apparatus mRNA translation are less well known. On the basis of the above, important areas of future research include the following: (1) additional analyses of airway smooth muscle stereology and contractile apparatus mRNA expression in severe asthma and animal models of asthma; (2) studies examining the contractile function of hyperplastic/hypertrophic airway smooth muscle, using either airways from humans with asthma, animals exposed to chronic allergen challenge, airway explants treated with relevant stimuli (e.g., TGF-β), or airway smooth muscle cells in three-dimensional culture; (3) further studies examining the phenotype of asthmatic airway smooth muscle; and (4) studies examining the translational control pathways regulating airway smooth muscle hypertrophy.
Supported by National Institutes of Health grant HL79339.
Conflict of Interest Statement: J.K.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.B.H. received a $100,000 research grant from GlaxoSmithKline in 2004–2006.
References
- 1.Huber HL, Koessler KK. The pathology of bronchial asthma. Arch Intern Med 1922;30:689–760. [Google Scholar]
- 2.Dunhill MS, Massarella GR, Anderson JA. A comparison of the quantitative anatomy of the bronchi in normal subjects, in status asthmaticus, in chronic bronchitis and in emphysema. Thorax 1971;24:176–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takizawa T, Thurlbeck WM. Muscle and mucous gland size in the major bronchi of patients with chronic bronchitis, asthma and asthmatic bronchitis. Am Rev Respir Dis 1971;104:331–336. [DOI] [PubMed] [Google Scholar]
- 4.Heard BE, Hossain S. Hyperplasia of bronchial smooth muscle in asthma. J Pathol 1973;110:319–332. [Google Scholar]
- 5.Sobonya RE. Quantitative structural alterations in long-standing allergic asthma. Am Rev Respir Dis 1984;130:289–292. [DOI] [PubMed] [Google Scholar]
- 6.James AL, Pare PD, Hogg JC. The mechanics of airway narrowing in asthma. Am Rev Respir Dis 1989;139:242–246. [DOI] [PubMed] [Google Scholar]
- 7.Ebina M, Yaegashi H, Chiba R, Takahashi T, Motomiya M, Tanemura M. Hyperreactive site in the airway tree of asthmatic patients revealed by thickening of bronchial muscles: a morphometric study. Am Rev Respir Dis 1990;141:1327–1332. [DOI] [PubMed] [Google Scholar]
- 8.Saetta M, Di Stefano A, Rosina C, Thiene G, Fabbri LM. Quantitative structural analysis of peripheral airways and arteries in sudden fatal asthma. Am Rev Respir Dis 1991;143:138–143. [DOI] [PubMed] [Google Scholar]
- 9.Ebina M, Takahashi T, Chiba T, Motomiya M. Cellular hypertrophy and hyperplasia of airway smooth muscles underlying bronchial asthma: a 3-D morphometric study. Am Rev Respir Dis 1993;148:720–726. [DOI] [PubMed] [Google Scholar]
- 10.Carroll N, Elliot J, Morton A, James A. The structure of large and small airways in nonfatal and fatal asthma. Am Rev Respir Dis 1993;147:405–410. [DOI] [PubMed] [Google Scholar]
- 11.Benayoun L, Druilhe A, Dombret MC, Aubier M, Pretolani M. Airway structural alterations selectively associated with severe asthma. Am J Respir Crit Care Med 2003;167:1360–1368. [DOI] [PubMed] [Google Scholar]
- 12.Woodruff PG, Dolganov GM, Ferrando RE, Donnelly S, Hays SR, Solberg OD, Carter R, Wong HH, Cadbury PS, Fahy JV. Hyperplasia of smooth muscle in mild to moderate asthma without changes in cell size or gene expression. Am J Respir Crit Care Med 2004;169:1001–1006. [DOI] [PubMed] [Google Scholar]
- 13.Thomson RJ, Bramley AM, Schellenberg RR. Airway muscle stereology: implications for increased shortening in asthma. Am J Respir Crit Care Med 1996;154:749–757. [DOI] [PubMed] [Google Scholar]
- 14.Wenzel SE, Schwartz LB, Langmack EL, Halliday JL, Trudeau JB, Gibbs RL, Chu HW. Evidence that severe asthma can be divided pathologically into two inflammatory subtypes with distinct physiologic and clinical characteristics. Am J Respir Crit Care Med 1999;160:1001–1008. [DOI] [PubMed] [Google Scholar]
- 15.Panettieri RA, Murray RK, Eszterhas AJ, Bilgen G, Martin JG. Repeated allergen inhalations induce DNA synthesis in airway smooth muscle and epithelial cells in vivo. Am J Physiol Lung Cell Mol Physiol 1998;274:L417–L424. [DOI] [PubMed] [Google Scholar]
- 16.Leung S-Y, Eynott P, Noble A, Nath P, Chung KF. Resolution of allergic airways inflammation but persistence of airway smooth muscle proliferation after repeated allergen exposures. Clin Exp Allergy 2004;34:213–220. [DOI] [PubMed] [Google Scholar]
- 17.McMillan SJ, Lloyd CM. Prolonged allergen challenge in mice leads to persistent airway remodelling. Clin Exp Allergy 2004;34:497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moir LM, Leung S-Y, Eynott PR, McVicker CG, Ward JPT, Chung KF, Hirst SJ. Repeated allergen inhalation induces phenotypic modulation of smooth muscle in bronchioles of sensitized rats. Am J Physiol Lung Cell Mol Physiol 2003;284:L148–L159. [DOI] [PubMed] [Google Scholar]
- 19.Ma X, Wang Y, Stephens NL. Serum deprivation induces a unique hypercontractile phenotype of cultured smooth muscle cells. Am J Physiol Cell Physiol 1998;274:C1206–C1214. [DOI] [PubMed] [Google Scholar]
- 20.Lambert RK, Wiggs BR, Kuwano K, Hogg JC, Pare PD. Functional significance of increased airway smooth muscle in asthma and COPD. J Appl Physiol 1993;74:2771–2781. [DOI] [PubMed] [Google Scholar]
- 21.Niimi A, Matsumoto H, Takemura M, Ueda T, Chin K, Mishima M. Relationship of airway wall thickness to airway sensitivity and airway reactivity in asthma. Am J Respir Crit Care Med 2003;168:983–988. [DOI] [PubMed] [Google Scholar]
- 22.Goldie RG, Spina D, Henry PJ, Lulich KM, Paterson JW. In vitro responsiveness of human asthmatic bronchus to carbachol, histamine, beta-adrenoceptor agonists and theophylline. Br J Clin Pharmacol 1986;22:669–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Jongste JC, Mons H, Van Strik R, Bonta IL, Kerrebijn KF. Comparison of human bronchiolar smooth muscle responsiveness in vitro with histological signs of inflammation. Thorax 1987;42:870–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whicker SD, Armour CL, Black JL. Responsiveness of bronchial smooth muscle from asthmatic patients to relaxant and contractile agonists. Pulm Pharmacol 1988;1:25–31. [DOI] [PubMed] [Google Scholar]
- 25.Bai TR. Abnormalities in airway smooth muscle in fatal asthma: a comparison between trachea and bronchus. Am Rev Respir Dis 1991;143:441–443. [DOI] [PubMed] [Google Scholar]
- 26.Black JL. Pharmacology of airway smooth muscle in chronic obstructive pulmonary disease and in asthma. Am Rev Respir Dis 1991;143:1177–1181. [DOI] [PubMed] [Google Scholar]
- 27.Bjorck T, Gustafsson LE, Dahlen SE. Isolated bronchi from asthmatics are hyperresponsive to adenosine, which apparently acts indirectly by liberation of leukotrienes and histamine. Am Rev Respir Dis 1992;145:1087–1091. [DOI] [PubMed] [Google Scholar]
- 28.Mitchell RW, Ruhlmann E, Magnussen H, Leff AR, Rabe KF. Passive sensitization of human bronchi augments smooth muscle shortening velocity and capacity. Am J Physiol 1994;267:L218–L222. [DOI] [PubMed] [Google Scholar]
- 29.Henderson WR Jr, Tang L-O, Chu S-J, Tsao S-M, Chiang GKS, Jones F, Jonas M, Pae C, Wang H, Chi EY. A role for cysteinyl leukotrienes in airway remodeling in a mouse asthma model. Am J Respir Crit Care Med 2002;165:108–116. [DOI] [PubMed] [Google Scholar]
- 30.Salmon M, Liu Y-C, Mak JCW, Rousell J, Huang T-J, Hisada T, Nicklin PL, Fan Chung K. Contribution of upregulated airway endothelin-1 expression to airway smooth muscle and epithelial cell DNA synthesis after repeated allergen exposure of sensitized Brown-Norway rats. Am J Respir Cell Mol Biol 2000;23:618–625. [DOI] [PubMed] [Google Scholar]
- 31.Jiang H, Rao K, Halayko AJ, Kepron W, Stephens NL. Bronchial smooth muscle mechanics of a canine model of allergic airway hyperresponsiveness. J Appl Physiol 1992;72:39–45. [DOI] [PubMed] [Google Scholar]
- 32.Fan T, Yang M, Halayko A, Mohapatra SS, Stephens NL. Airway responsiveness in two inbred strains of mouse disparate in IgE and IL-4 production. Am J Respir Cell Mol Biol 1997;17:156–163. [DOI] [PubMed] [Google Scholar]
- 33.Zheng X, Zhou D, Seow CY, Bai TR. Cardiotrophin-1 alters airway smooth muscle structure and mechanical properties in airway explants. Am J Physiol Lung Cell Mol Physiol 2004;287:L1165–L1171. [DOI] [PubMed] [Google Scholar]
- 34.Zhou D, Zheng X, Wang L, Stelmack G, Halayko AJ, Dorscheid D, Bai TR. Expression and effects of cardiotrophin-1 in human airway smooth muscle cells. Br J Pharmacol 2003;140:1237–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Griffith SL, Rhoades RA, Packer CS. Pulmonary arterial smooth muscle contractility in hypoxia-induced pulmonary hypertension. J Appl Physiol 1994;77:406–414. [DOI] [PubMed] [Google Scholar]
- 36.Blank RS, Thompson MM, Owens GK. Cell cycle versus density dependence of smooth muscle alpha actin expression in cultured rat aortic smooth muscle cells. J Cell Biol 1988;107:299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Panettieri RA, Murray RK, DePalo LR, Yadvish PA, Kotlikoff MI. A human airway smooth muscle cell line that retains physiological responsiveness. Am J Physiol Cell Physiol 1989;256:C329–C335. [DOI] [PubMed] [Google Scholar]
- 38.Halayko AJ, Salari H, Ma X, Stephens NL. Markers of airway smooth muscle cell phenotype. Am J Physiol Lung Cell Mol Physiol 1996;270:L1040–L1051. [DOI] [PubMed] [Google Scholar]
- 39.Mitchell RW, Halayko AJ, Kahraman S, Solway J, Wylam ME. Selective restoration of calcuim coupling to muscarinic M(3) receptors in contractile cultured airway myocytes. Am J Physiol Lung Cell Mol Physiol 2000;278:L1091–L1100. [DOI] [PubMed] [Google Scholar]
- 40.Goldsmith AM, Bentley JK, Zhou L, Jia Y, Bitar KN, Fingar DC, Hershenson MB. Transforming growth factor-beta induces airway smooth muscle hypertrophy. Am J Respir Cell Mol Biol 2006;34:247–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McWhinnie R, Pechkovsky DV, Zhou D, Lane D, Halayko AJ, Knight DA, Bai TR. Endothelin-1 induces hypertrophy and inhibits apoptosis in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 2007;292:L278–L286. [DOI] [PubMed] [Google Scholar]
- 42.Kelleher MD, Abe MK, Chao TS, Jain M, Green JM, Solway J, Rosner MR, Hershenson MB. Role of MAP kinase activation in bovine tracheal smooth muscle mitogenesis. Am J Physiol Lung Cell Mol Physiol 1995;268:L894–L901. [DOI] [PubMed] [Google Scholar]
- 43.Hirst SJ, Barnes PJ, Twort CHC. PDGF isoform-induced proliferation and receptor expression in human cultured airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 1996;270:L415–L428. [DOI] [PubMed] [Google Scholar]
- 44.Hirst SJ, Barnes PJ, Twort CH. Quantifying proliferation of cultured human and rabbit airway smooth muscle cells in response to serum and platelet-derived growth factor. Am J Respir Cell Mol Biol 1992;7:574–581. [DOI] [PubMed] [Google Scholar]
- 45.Krymskaya VP, Hoffman R, Eszterhas A, Kane S, Ciocca V, Panettieri RA Jr. EGF activates ErbB-2 and stimulates phosphatidylinositol 3-kinase in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 1999;276:L246–L255. [DOI] [PubMed] [Google Scholar]
- 46.Panettieri RA, Yadvish PA, Kelly AM, Rubinstein NA, Kotlikoff MI. Histamine stimulates proliferation of airway smooth muscle and induces c-fos expression. Am J Physiol Lung Cell Mol Physiol 1990;259:L365–L371. [DOI] [PubMed] [Google Scholar]
- 47.Stewart AG, Grigoriadis G, Harris T. Mitogenic actions of endothelin-1 and epidermal growth factor in cultured airway smooth muscle. Clin Exp Pharmacol Physiol 1994;21:277–285. [DOI] [PubMed] [Google Scholar]
- 48.Panettieri RA Jr, Hall IP, Maki CS, Murray RK. α-Thrombin increases cytosolic calcium and induces human airway smooth muscle cell proliferation. Am J Respir Cell Mol Biol 1995;13:205–216. [DOI] [PubMed] [Google Scholar]
- 49.Malarkey K, Chilvers ER, Lawson MF, Plevin R. Stimulation by endothelin-1 of mitogen-activated protein kinases and DNA synthesis in bovine tracheal smooth muscle cells. Br J Pharmacol 1995;116:2267–2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shapiro PS, Evans JN, Davis RJ, Posada JA. The seven-transmembrane-spanning receptors for endothelin and thrombin cause proliferation of airway smooth muscle cells and activation of the extracellular regulated kinase and c-Jun NH2-terminal kinase groups of mitogen-activated protein kinases. J Biol Chem 1996;271:5750–5754. [DOI] [PubMed] [Google Scholar]
- 51.Whelchel A, Evans J, Posada J. Inhibition of ERK activation attenuates endothelin-stimulated airway smooth muscle prolifeation. Am J Respir Cell Mol Biol 1997;16:589–596. [DOI] [PubMed] [Google Scholar]
- 52.Walker TR, Moore SM, Lawson MF, Panettieri RAJ, Chilvers ER. Platelet-derived growth factor-BB and thrombin activate phosphoinositide 3-kinase and protein kinase B: role in mediating airway smooth muscle proliferation. Mol Pharmacol 1998;54:1007–1015. [DOI] [PubMed] [Google Scholar]
- 53.Vichi P, Whelchel A, Knot H, Nelson M, Kolch W, Posada J. Endothelin-stimulated ERK activation in airway smooth-muscle cells requires calcium influx and Raf activation. Am J Respir Cell Mol Biol 1999;20:99–105. [DOI] [PubMed] [Google Scholar]
- 54.Brown JK, Jones CA, Rooney LA, Caughey GH, Hall IP. Tryptase's potent mitogenic effects in human airway smooth muscle cells are via nonproteolytic actions. Am J Physiol Lung Cell Mol Physiol 2002;282:L197–L206. [DOI] [PubMed] [Google Scholar]
- 55.Cohen P, Rajah R, Rosenbloom J, Herrick DJ. IGFBP-3 mediates TGF-beta1-induced cell growth in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 2000;278:L545–L551. [DOI] [PubMed] [Google Scholar]
- 56.Sturrock A, Huecksteadt TP, Norman K, Sanders KA, Murphy TM, Chitano P, Wilson K, Hoidal JR, Kennedy TP. Nox4 mediates TGF-β1-induced retinoblastoma protein phosphorylation, proliferation and hypertrophy in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 2007;292:L1543–L1555. [DOI] [PubMed] [Google Scholar]
- 57.Amishima M, Munakata M, Nasuhara Y, Sato A, Takahashi T, Homma Y, Kawakami Y. Expression of epidermal growth factor and epidermal growth factor receptor immunoreactivity in the asthmatic human airway. Am J Respir Crit Care Med 1998;157:1907–1912. [DOI] [PubMed] [Google Scholar]
- 58.Vignola AM, Chanez P, Chiappara G, Merendino A, Pace E, Rizzo A, laRocca AM, Bellia V, Bonsignore G, Bousquet J. Transforming growth factor-β expression in mucosa biopsies in asthma and chronic bronchitis. Am J Respir Crit Care Med 1997;156:591–599. [DOI] [PubMed] [Google Scholar]
- 59.Minshall EM, Leung DYM, Matin RJ, Song YL, Cameron L, Ernst P, Hamid Q. Eosinophil-associated TGF-β1 mRNA expression and airways fibrosis in bronchial asthma. Am J Respir Cell Mol Biol 1997;17:326–333. [DOI] [PubMed] [Google Scholar]
- 60.Ohno I, Nitta Y, Yamauchi K, Hoshi H, Honma M, Woolley K, O'Byrne P, Tamura G, Jordana M, Shirato K. Transforming growth factor beta 1 gene expression by eosinophils in asthmatic airway inflammation. Am J Respir Cell Mol Biol 1996;15:404–409. [DOI] [PubMed] [Google Scholar]
- 61.Tillie-Leblond I, Pugin J, Marquette C-H, Lamblin C, Saulnier F, Brichet A, Wallaert B, Tonnel A-B, Gosset P. Balance between proinflammatory cytokines and their inhibitors in bronchial lavage from patients with status asthmaticus. Am J Respir Crit Care Med 1999;159:487–494. [DOI] [PubMed] [Google Scholar]
- 62.Nomura A, Uchida Y, Sakamoto T, Ishii Y, Masuyama K, Morishima Y, Hirano K, Sekizawa K. Increases in collagen type I collagen synthesis in asthma: the role of eosinophils and transforming growth factor-beta. Clin Exp Allergy 2002;32:860–865. [DOI] [PubMed] [Google Scholar]
- 63.Krymskaya VP, Hoffman R, Eszterhas A, Ciocca V, Panettieri PA. TGF-1 modulates EGF-stimulated phosphatidylinositol 3-kinase activity in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 1997;273:L1220–L1227. [DOI] [PubMed] [Google Scholar]
- 64.Cohen MD, Ciocca V, Panettieri RA Jr. TGF-beta 1 modulates human airway smooth-muscle cell proliferation induced by mitogens. Am J Respir Cell Mol Biol 1997;16:85–90. [DOI] [PubMed] [Google Scholar]
- 65.Zhou L, Hershenson MB. Mitogenic signaling pathways in airway smooth muscle. Respir Physiolo Neurobiol 2003;137:295–308. [DOI] [PubMed] [Google Scholar]
- 66.Karpova AY, Abe MK, Li J, Liu P, Rhee JM, Kuo WL, Hershenson MB. MEK1 is required for PDGF-induced ERK activation and DNA synthesis in tracheal myocytes. Am J Physiol 1997;272:L558–L565. [DOI] [PubMed] [Google Scholar]
- 67.Lew DB, Dempsey BK, Zhao Y, Muthalif M, Fatima S, Malik KU. beta-hexosaminidase-induced activation of p44/42 mitogen-activated protein kinase is dependent on p21Ras and protein kinase C and mediates bovine airway smooth-muscle proliferation. Am J Respir Cell Mol Biol 1999;21:111–118. [DOI] [PubMed] [Google Scholar]
- 68.Krymskaya VP, Penn RB, Orsini MJ, Scott PH, Plevin RJ, Walker TR, Eszterhas AJ, Amrani Y, Chilvers ER, Panettieri RA Jr. Phosphatidylinositol 3-kinase mediates mitogen-induced human airway smooth muscle cell proliferation. Am J Physiol Lung Cell Mol Physiol 1999;277:L65–L78. [DOI] [PubMed] [Google Scholar]
- 69.Ramakrishnan M, Musa NL, Li J, Liu P, Pestell RG, Hershenson MB. Catalytic activation of extracellular signal-regulated kinases induces cyclin D1 expression in airway smooth muscle. Am J Respir Cell Mol Biol 1998;18:736–740. [DOI] [PubMed] [Google Scholar]
- 70.Naureckas ET, Ndukwu IM, Halayko AJ, Maxwell C, Hershenson MB, Solway J. Bronchoalveolar lavage fluid from asthmatic subjects is mitogenic for human airway smooth muscle. Am J Respir Crit Care Med 1999;160:2062–2066. [DOI] [PubMed] [Google Scholar]
- 71.Page K, Li J, Wang Y, Kartha S, Pestell RG, Hershenson MB. Regulation of cyclin D1 expression and DNA synthesis by phosphatidylinositol 3-kinase in airway smooth muscle cells. Am J Respir Cell Mol Biol 2000;23:436–443. [DOI] [PubMed] [Google Scholar]
- 72.Scott PH, Belham CM, al-Hafidh J, Chilvers ER, Peacock AJ, Gould GW, Plevin R. A regulatory role for cAMP in phosphatidylinositol 3-kinase/p70 ribosomal S6 kinase-mediated DNA synthesis in platelet-derived-growth-factor-stimulated bovine airway smooth-muscle cells. Biochem J 1996;318:965–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Page K, Li J, Hodge JA, Liu PT, Vanden Hoek TL, Becker LB, Pestell RG, Rosner MR, Hershenson MB. Characterization of a Rac1 signaling pathway to cyclin D1 expression in airway smooth muscle cells. J Biol Chem 1999;274:22065–22071. [DOI] [PubMed] [Google Scholar]
- 74.Abo A, Pick E, Hall A, Totty N, Teahan CG, Segal AW. Activation of the NADPH oxidase involves the small GTP-binding protein p21rac1. Nature 1991;353:668–670. [DOI] [PubMed] [Google Scholar]
- 75.Abo A, Boyhan A, West I, Thrasher AJ, Segal AW. Reconstitution of neutrophil NADPH oxidase activity in the cell-free system by four components: p67-phox, p47-phox, p21rac1, and cytochrome b-245. J Biol Chem 1992;267:16767–16770. [PubMed] [Google Scholar]
- 76.Brar SS, Kennedy TP, Whorton AR, Murphy TM, Chitano P, Hoidal JR. Requirement for reactive oxygen species in serum-induced and platelet-derived growth factor-induced growth of airway smooth muscle. J Biol Chem 1999;274:20017–20026. [DOI] [PubMed] [Google Scholar]
- 77.Brar SS, Kennedy TP, Sturrock AB, Huecksteadt TP, Quinn MT, Murphy TM, Chitano P, Hoidal JR. NADPH oxidase promotes NF-kappa B activation and proliferation in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 2002;282:L782–L795. [DOI] [PubMed] [Google Scholar]
- 78.Johnson PRA, Roth M, Tamm M, Hughes M, Ge Q, King G, Burgess JK, Black JL. Airway smooth muscle cell proliferation is increased in asthma. Am J Respir Crit Care Med 2001;164:474–477. [DOI] [PubMed] [Google Scholar]
- 79.Fernandes D, Guida E, Koutsoubos V, Harris T, Vadiveloo P, Wilson JW, Stewart AG. Glucocorticoids inhibit proliferation, cyclin D1 expression, and retinoblastoma protein phosphorylation, but not activity of the extracellular-regulated kinases in human cultured airway smooth muscle. Am J Respir Cell Mol Biol 1999;21:77–88. [DOI] [PubMed] [Google Scholar]
- 80.Roth M, Johnson PRA, Rudiger JJ, King GG, Ge Q, Burgess JK, Anderson G, Tamm M, Black JL. Interaction between glucocorticoids and β2 agonists on bronchial airway smooth muscle cells through synchronised cellular signalling. Lancet 2002;360:1293–1299. [DOI] [PubMed] [Google Scholar]
- 81.Halayko AJ, Kartha S, Stelmack GL, McConville J, Tam J, Camoretti-Mercado B, Forsythe SM, Hershenson MB, Solway J. Phophatidylinositol-3 kinase/mammalian target of rapamycin/p70S6K regulates contractile protein accumulation in airway myocyte differentiation. Am J Respir Cell Mol Biol 2004;31:266–275. [DOI] [PubMed] [Google Scholar]
- 82.Zhou L, Li J, Goldsmith AM, Newcomb DC, Giannola DM, Vosk RG, Eves EM, Rosner MR, Solway J, Hershenson MB. Human bronchial smooth muscle cell lines show a hypertrophic phenotype typical of severe asthma. Am J Respir Crit Care Med 2004;169:703–711. [DOI] [PubMed] [Google Scholar]
- 83.Beretta L, Gingras AC, Svitkin YV, Hall MN, Sonenberg N. Rapamycin blocks the phosphorylation of 4E–BP1 and inhibits cap-dependent initiation of translation. EMBO J 1996;15:658–664. [PMC free article] [PubMed] [Google Scholar]
- 84.Brunn GJ, Hudson CC, Sekuli A, Williams JM, Hosoi H, Houghton PJ, Lawrence JC Jr, Abraham RT. Phosphorylation of the translational repressor PHAS-I by the mammalian target of rapamycin. Science 1997;277:99–101. [DOI] [PubMed] [Google Scholar]
- 85.Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol 2002;4:648–657. [DOI] [PubMed] [Google Scholar]
- 86.Tee AR, Fingar DC, Manning BD, Kwiatkowski DJ, Cantley LC, Blenis J. Tuberous sclerosis complex-1 and -2 gene products function together to inhibit mammalian target of rapamycin (mTOR)-mediated downstream signaling. Proc Natl Acad Sci USA 2002;99:13571–13576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang Y, Gao X, Saucedo LJ, Ru B, Edgar BA, Pan D. Rheb is a direct target of the tuberous sclerosis tumour suppressor proteins. Nat Cell Biol 2003;5:578–581. [DOI] [PubMed] [Google Scholar]
- 88.Jacinto E, Loewith R, Schmidt A, Lin S, Ruegg MA, Hall A, Hall MN. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat Cell Biol 2004;6:1122–1128. [DOI] [PubMed] [Google Scholar]
- 89.Welsh GI, Miller CM, Loughlin AJ, Price NT, Proud CG. Regulation of eukaryotic initiation factor eIF2B: glycogen synthase kinase-3 phosphorylates a conserved serine which undergoes dephosphorylation in response to insulin. FEBS Lett 1998;421:125–130. [DOI] [PubMed] [Google Scholar]
- 90.Zhou L, Goldsmith AM, Bentley JK, Jia Y, Rodriguez ML, Abe MK, Fingar DC, Hershenson MB. 4E-binding protein phosphorylation and eukaryotic initiation factor-4E release are required for airway smooth muscle hypertrophy. Am J Respir Cell Mol Biol 2005;33:195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Brewster CE, Howarth PH, Djukanovic R, Wilson J, Holgate ST, Roche WR. Myofibroblasts and subepithelial fibrosis in bronchial asthma. Am J Respir Cell Mol Biol 1990;3:507–511. [DOI] [PubMed] [Google Scholar]
- 92.Gizycki MJ, Adelroth E, Rogers AV, O'Byrne PM, Jeffery PK. Myofibroblast involvement in the allergen-induced late response in mild atopic asthma. Am J Respir Cell Mol Biol 1997;16:664–673. [DOI] [PubMed] [Google Scholar]
- 93.Hedges JC, Dechert MA, Yamboliev IA, Martin JL, Hickey E, Weber LA, Gerthoffer WT. A role for p38(MAPK)/HSP27 pathway in smooth muscle cell migration. J Biol Chem 1999;274:24211–24219. [DOI] [PubMed] [Google Scholar]
- 94.Goncharova EA, Billington CK, Irani C, Vorotnikov AV, Tkachuk VA, Penn RB, Krymskaya VP, Panettieri RA Jr. Cyclic AMP-mobilizing agents and glucocorticoids modulate human smooth muscle cell migration. Am J Respir Cell Mol Biol 2003;29:19–27. [DOI] [PubMed] [Google Scholar]
- 95.Joubert P, Lajoie-Kadoch S, Labonte I, Gounni AS, Maghni K, Wellemans V, Chakir J, Laviolette M, Hamid Q, Lamkhioued B. CCR3 expression and function in asthmatic airway smooth muscle cells. J Immunol 2005;175:2702–2708. [DOI] [PubMed] [Google Scholar]
- 96.Govindaraju V, Michoud M-C, Al-Chalabi M, Ferraro P, Powell WS, Martin JG. Interleukin-8: novel roles in human airway smooth muscle cell contraction and migration. Am J Physiol Cell Physiol 2006;291:C957–C965. [DOI] [PubMed] [Google Scholar]
- 97.Kaur D, Saunders R, Berger P, Siddiqui S, Woodman L, Wardlaw A, Bradding P, Brightling CE. Airway smooth muscle and mast cell-derived CC chemokine ligand 19 mediate airway smooth muscle migration in asthma. Am J Respir Crit Care Med 2006;174:1179–1188. [DOI] [PubMed] [Google Scholar]
- 98.Dechert MA, Holder JM, Gerthoffer WT. p21-activated kinase 1 participates in tracheal smooth muscle cell migration by signaling to p38 MAPK. Am J Physiol Cell Physiol 2001;281:C123–C132. [DOI] [PubMed] [Google Scholar]
- 99.Krymskaya VP, Goncharova EA, Ammit AJ, Lim PN, Goncharov DA, Eszterhas A, Panettieri RA Jr. Src is necessary and sufficient for human airway smooth muscle cell proliferation and migration. FASEB J 2005;19:428–430. [DOI] [PubMed] [Google Scholar]
- 100.Schmidt M, Sun G, Stacey MA, Mori L, Mattoli S. Identification of circulating fibrocytes as precursors of bronchial myofibroblasts in asthma. J Immunol 2003;171:380–389. [DOI] [PubMed] [Google Scholar]
- 101.Sabatini F, Petecchia L, Tavian M, Jodon de Villeroche V, Rossi GA, Brouty-Boye D. Human bronchial fibroblasts exhibit a mesenchymal stem cell phenotype and multilineage differentiating potentialities. Lab Invest 2005;85:962–971. [DOI] [PubMed] [Google Scholar]
- 102.Hennrick KT, Keeton AG, Nanua S, Kijek TG, Goldsmith AM, Sajjan US, Bentley JK, Lama VN, Moore BB, Schumacher RE, et al. Lung cells from neonates show a mesenchymal stem cell phenotype. Am J Respir Crit Care Med 2007;175:1158–1164. [DOI] [PubMed] [Google Scholar]