Abstract
The National Emphysema Treatment Trial (NETT) has published many articles reporting the various outcomes of lung volume reduction surgery versus medical treatment for patients with severe emphysema. However, long and complex clinical trials like NETT that involve both medical and surgical issues generate multiple manuscripts over a period of years and report an array of various outcomes. As a result, the essential findings of the trial may appear to be fragmented to the clinician or clinical researcher or be lost among the many medical reports published each year. In this review, we summarize in one publication the major medical and surgical outcomes of NETT.
Keywords: emphysema, lung reduction, clinical trial
In 2003, the National Emphysema Treatment Trial (NETT) reported the effects of lung volume reduction surgery (LVRS) versus medical treatment on survival and maximum exercise capacity in 1,218 patients who were randomized to treatment between January 1998 and July 2002 and monitored for a mean of 2.4 years (1). Additional outcomes reported included pulmonary function, oxygen requirement, distance walked in 6 minutes, quality of life, respiratory symptoms, and health care utilization. In 2006, updated analyses of the impact of LVRS on survival and functional measures were reported (2). In these analyses, the median follow-up of the NETT subjects was 4.3 years. The analyses included 40% more patients with functional measures at 2 years after randomization to treatment compared with the original 2003 outcomes manuscript. In addition, separate NETT publications have reported on the prevalence and duration of air leaks after LVRS (3); the predictors of operative morbidity and mortality after LVRS (4); the impact of the surgical approach used to perform LVRS on morbidity, mortality, and functional outcomes (5); as well as the cost-effectiveness of LVRS compared with optimal medical therapy (6, 7).
Large and long clinical trials like NETT necessarily report primary and secondary outcomes in many separate publications as the results become evident. This can be problematic for a therapy like LVRS that involves complexly ill medical patients who require sophisticated physiologic and radiologic assessment and routinely receive care by a multidisciplinary team of thoracic surgeons, pulmonologists, radiologists, nurses, and anesthesiologists. Results are often published in specialty journals outside of the normal readership of many of these disciplines, hampering the clinician's as well as the clinical researcher's ability to quickly and easily understand the essential findings of NETT. Herein, we briefly summarize the essential findings of NETT, based on all of its major publications, on the impact of LVRS in severe emphysema.
IDENTIFICATION OF THE SUBGROUP OF PATIENTS AT HIGH RISK OF DEATH AFTER LVRS
At the initiation of NETT, the investigators provided the data and safety monitoring board (DSMB) with stopping guidelines to be used to identify patient subgroups that benefited from LVRS as well as those whose risk was disproportionately increased. Both the investigators and the DSMB agreed that a 30-day surgical mortality greater than 8% was unacceptable. A stopping guideline was instituted to terminate randomization if the lower 95% confidence limit for 30-day mortality exceeded 8%. The NETT investigators also asked the DSMB to pay special attention to a subgroup of patients who were expected to have substantial benefit from LVRS—with the expectation that this group would have enrollment terminated early if substantial benefit with LVRS was found. The criteria for this group included the following: age ⩽ 70 years; FEV1, 15–25% predicted; PaCO2 ⩽ 50 mm Hg (⩽45 mm Hg in Denver); residual volume > 200% predicted; a low radionuclide perfusion scan ratio of upper to lower lobes (<0.2); a heterogeneous distribution of emphysema on chest computed tomography (CT); and hyperinflation on chest X-ray.
Diffusion capacity of carbon monoxide (DlCO), maximum work capacity, quality of life, race or ethnic group, and sex were also examined to identify patient subgroups that may have had substantial benefit or risk due to LVRS. Analyses for evidence of increased risk or benefit compared with the medically treated group were reviewed by the DSMB every 3 months.
In April 2001, these analyses suggested that patients with a low FEV1, a homogeneous pattern of emphysema on chest CT, and a high perfusion ratio had an increased risk for mortality with LVRS (8). In addition, patients with a low FEV1 and a low diffusion capacity had increased 30-day mortality. The DSMB requested sensitivity analyses varying the cutoff values for FEV1 and DlCO. In May 2001, the DSMB found that the subgroup that was defined by an FEV1 of 20% predicted or less and either a DlCO of 20% predicted or less or a homogeneous pattern of emphysema on chest CT met the criteria for stopping further patient enrollment. The DSMB further concluded that the perfusion ratio added nothing further to the prognostic value of the other three variables.
One hundred forty (13.6%) of the 1,033 patients enrolled into NETT at that time were in this high-risk-for-death subgroup (70 assigned to LVRS and 70 assigned to medical treatment). All 140 patients had an FEV1 of 20% of predicted or less; 94 also had evidence of homogeneous emphysema on chest CT and 87 also had a DlCO of 20% predicted or less. Forty-one patients met all three criteria.
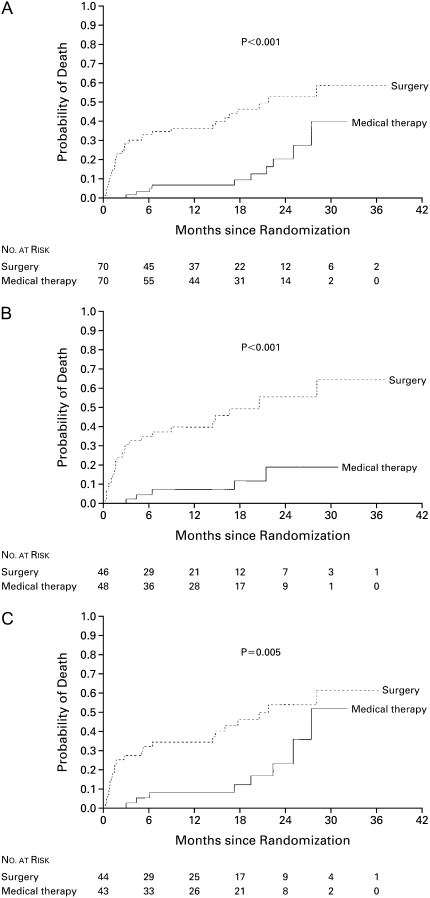
The 30-day mortality in the group receiving LVRS was 16% (95% confidence interval [CI], 8.2–26.7%; P < 0.001) compared with no deaths in the medical group. Patients with all three high-risk characteristics had a 30-day mortality rate of 25% (95% CI, 8.7–49.1%). The type of surgical approach (median sternotomy [MS] or video-assisted thorascopic surgery [VATS]) had no effect. The overall mortality rate was 0.43 deaths per person-year in patients assigned to undergo LVRS as compared with 0.11 deaths per person-year in those assigned to medical treatment. Mortality during 3 years of follow-up is shown in Figure 1 for all high-risk patients (Figure 1A), those with low FEV1 and homogeneous emphysema on CT scan (Figure 1B), and those with low FEV1 and low DlCO (Figure 1C).
Figure 1.
Kaplan-Meier estimates of the probability of death among high-risk patients, according to whether they were randomly assigned to undergo lung volume reduction surgery or receive medical therapy. This intention-to-treat analysis shows the overall results for the high-risk group (A), the subgroup of patients with an FEV1 that was no more than 20% of their predicted value and a homogeneous distribution of emphysema on computed tomography scanning (B), and the subgroup of patients with an FEV1 that was no more than 20% of its predicted value and a carbon monoxide diffusing capacity that was no more than 20% of its predicted value (C). For each analysis, the difference between groups was significant (P < 0.001, P < 0.001, P = 0.005, respectively) by the log-rank test. Reprinted by permission from Reference 8.
The cause of death most frequently reported was respiratory (90% in the LVRS group and 89% in the medically treated group). Sixty percent of the LVRS and 43% of the medically treated patients were receiving mechanical ventilation at the time of death. Pneumonia developed within 30 days of surgery in 30% of the high-risk patients undergoing LVRS.
The physiologic outcomes in patients who survived to the 6-month post–randomization visit were also analyzed. The mean changes from baseline to 6 months for the LVRS compared with medically treated groups were as follows: for exercise capacity, an increase of 4.5 ± 13.0 W versus a decrease of 4.4 ± 14.8 W (P = 0.06); for six-minute-walk distance (6MWD), an increase of 14.9 ± 63.7 m versus a decrease of 21.6 ± 56.7 m (P = 0.03); and for FEV1, an increase of 5.5 ± 6.9% predicted versus a decrease of 0.4 ± 1.9% predicted (P < 0.001). The Quality of Well-Being (QWB) score at 6 months showed a similar decrease (0.01 units) in both groups.
These data demonstrate that patients with severe emphysema as characterized by an FEV1 of 20% predicted or less and either a homogeneous pattern of emphysema on chest CT or a DlCO of 20% predicted or less have high surgical mortality after LVRS with little chance of a clinically meaningful improvement in functional or physiologic benefit. These patients should not be considered for LVRS and they are not approved by Centers for Medicare and Medicaid Services or the Joint Commission for the Accreditation of Healthcare Organization guidelines.
THE EFFECT OF LVRS IN SEVERE EMPHYSEMA ON MAJOR PATIENT OUTCOMES IN NETT
NETT Methods
The major outcomes of LVRS in severe emphysema were first published by NETT in 2003 (1). At 17 clinics, patients with severe emphysema underwent comprehensive medical evaluation and optimization of medical care. In eligible patients, baseline measurements were completed after pulmonary rehabilitation but before randomization to LVRS or continued medical treatment. Repeat evaluations were then conducted at 6 and 12 months post–treatment randomization and then yearly thereafter. Primary outcomes were all-cause mortality and maximum exercise capacity (cycle ergometry with a 5- or 10-W incremental load per minute after 3 minutes of unloaded pedaling while breathing 30% oxygen). Secondary outcomes included the following: pulmonary function, 6MWD, and questionnaires probing health-specific quality of life (St. George's Respiratory Questionnaire [SGRQ]), general quality of life (QWB), and dyspnea (University of California at San Diego Shortness of Breath Questionnaire).
The magnitude and distribution of emphysema were characterized on the basis of chest CT by radiologists using a visual scoring scale. Radiologists also classified the craniocaudal pattern of emphysema as predominantly affecting the upper lobes, predominantly affecting the lower lobes, diffuse, or predominantly affecting the superior segments of the lower lobes. The latter three non–upper lobe locations were grouped together for the purposes of analysis and labeled as non–upper lobe predominant emphysema.
Patients randomized to LVRS underwent either bilateral stapled resection via an MS or VATS. The surgical goal was to resect 20 to 30% of the volume of each lung, targeting the most severely affected portions of the lung as indicated by chest CT and visual inspection by the surgeon at the time of operation.
Vital status as of December 2002 was determined by data obtained at the clinical centers and a review of the Social Security Administration's December 2002 Death Master File. Total, 30- and 90-day all-cause mortalities were measured from the day of randomization. The thresholds for improvement in maximum workload (10-W increase) and quality of life (8-point decrease in SGRQ) used for analysis were considered by the NETT investigators to be clinically significant in light of the substantial operative risks posed by LVRS.
All statistical analyses were performed on an intention-to-treat basis. Patients who died or were missing data required for assessment were considered not to have had improvement. The risk ratio (RR) for death was estimated on the basis of the overall mortality in each group after a mean follow-up of 29.2 months. Subgroups of patients with differential risks or benefits were identified using logistic regression analyses that included the following as outcomes: death, improvement in exercise workload, or improvement in SGRQ. Prognostic factors were those identified previously by the NETT investigators or DSMB and stated in the trial protocol as possibly important in affecting patient outcome. These included the following: age, FEV1% predicted, PaCO2, residual volume, radionuclide perfusion scan ratio of upper to lower lobes (<0.2), distribution of emphysema on chest CT, hyperinflation found on chest X-ray, DlCO% predicted, RV/TLC, maximum exercise capacity,  e/Vco2, presence or absence of upper lobe emphysema, degree of dyspnea, quality of life, race/ethnicity, and sex. The prognostic factors were categorized into quartiles, and patients in the worse quartile were compared with patients in the other quartiles.
e/Vco2, presence or absence of upper lobe emphysema, degree of dyspnea, quality of life, race/ethnicity, and sex. The prognostic factors were categorized into quartiles, and patients in the worse quartile were compared with patients in the other quartiles.
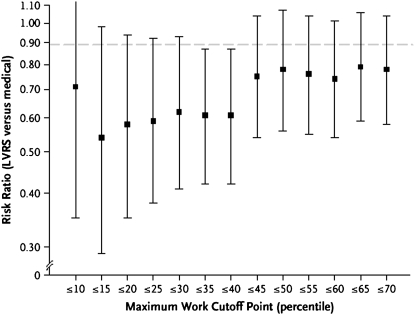
A baseline exercise test in the lowest quartile (⩽25 W) was one of the two prognostic factors that indicated patients at an increased risk for death from LVRS compared with medical therapy. Because of differences in maximum exercise capacity by sex, we found that the sex-specific 40th percentile (25 W for women and 40 W for men) was the best threshold to indicate higher risk for death (Figure 2).
Figure 2.
Sensitivity analysis of sex-specific cutoff points for subgroups defined according to maximal workload. Graph shows the risk ratio (lung volume reduction surgery [LVRS] vs. medical) for percentile cutoff points of baseline maximal workload, ranging from the 10th percentile to the 70th percentile in 5-percentile steps. The cutoff points for a given patient vary according to sex; for example, the 40th percentile for baseline maximal workload for women was 25 W, whereas the 40th percentile for men was 40 W. This graph suggests the 40th percentile of baseline maximal workload as the cutoff point beyond which the risk ratio increases. Reprinted by permission from Reference 1.
RESULTS OF NETT: MAJOR OUTCOMES IN ALL PATIENTS
Between January 1998 and July 2002, 3,777 patients were evaluated for NETT and 1,218 eventually underwent randomization: 608 to LVRS and 610 to continued medical therapy. The study protocol had specified that recruitment would end by July 2002 with an accrual of 2,500 patients and follow-up would end in December 2002. Recruitment was able to end in July 2002 as planned despite a lower subject accrual than expected, because the observed mortality rate was higher than anticipated and the crossover to unassigned therapy rate was much lower than anticipated.
The treatment groups had similar baseline characteristics except that there was a higher proportion assigned to the medical group (Table 1). Of 608 patients assigned to LVRS, 580 (95.4%) underwent surgery (406 [70%] by MS, 174 [30%] by VATS), 21 (3.5%) declined to undergo surgery, and 7 (1.2%) were deemed unsuitable for surgery.
TABLE 1.
CHARACTERISTICS OF ALL 1,218 PATIENTS AT BASELINE
| Characteristic | Surgery Group (n = 608) | Medical Therapy Group (n = 610) |
|---|---|---|
| Age at randomization, yr | 66.5 ± 6.3 | 66.7 ± 5.9 |
| Race or ethnic group, n (%) | ||
| Non-Hispanic white | 581 (96) | 575 (94) |
| Non-Hispanic black | 19 (3) | 23 (4) |
| Other | 8 (1) | 12 (2) |
| Sex, n (%)* | ||
| Female | 253 (42) | 219 (36) |
| Male | 355 (58) | 391 (64) |
| Distribution of emphysema on CT, n (%)† | ||
| Predominantly upper lobe | 385 (63) | 405 (67) |
| Predominantly non-upper lobe | 223 (3J) | 204 (33) |
| Heterogeneous | 330 (54) | 336 (55) |
| Homogeneous | 278 (46) | 274 (45) |
| Perfusion ratios‡ | 0.30 ± 0.21 | 0.28 ± 0.23 |
| Maximal workload, W | 38.7 ± 21.1 | 39.4 ± 22.2 |
| Distance walked in 6 min, ft§ | 1,216.5 ± 312.6 | 1,219.0 ± 316.0 |
| FEV1 after bronchodilator use, % of predicted value | 26.8 ± 7.4 | 26.7 ± 7.0 |
| Total lung capacity after bronchodilator use, % of predicted value | 128.0 ± 15.3 | 128.5 ± 15.0 |
| Residual volume after bronchodilator use, % of predicted value | 220.5 ± 49.9 | 223.4 ± 48.9 |
| DlCO, % of predicted value | 28.3 ± 9.7 | 28.4 ± 9.7 |
| PaO2, mm Hg | 64.5 ± 10.5 | 64.2 ± 10.1 |
| PaCO2, mm Hg | 43.3 ± 5.9 | 43.0 ± 5.8 |
| Total score on St. George's Respiratory Questionnaire‖ | 52.5 ± 12.6 | 53.6 ± 12.7 |
| Average daily Quality of Well-Being score¶ | 0.58 ± 0.12 | 0.56 ± 0.11 |
| Total UCSD Shortness of Breath score** | 61.6 ± 18.1 | 63.4 ± 18.6 |
Definition of abbreviations: CT = computed tomography; DlCO = diffusion capacity of carbon monoxide; UCSD = University of California, San Diego.
Reprinted by permission from Reference 1. Baseline measurements were obtained after rehabilitation but before randomization, except for the DlCO, which was measured before rehabilitation. Plus-minus values are means ± SD.
P for homogeneity = 0.04.
Upper lobe predominance of emphysema was judged subjectively by each center's radiologist, who described the distribution of disease as predominantly upper lobe, predominantly lower lobe, diffuse, or predominantly affecting superior segments of the lower lobes. The latter three choices were grouped as predominantly non-upper lobe. The classification of the emphysema as heterogeneous or homogeneous was based on subjective scores assigned by each center's radiologist to each of three zones in each lung. Data on upper lobe versus non–upper lobe distribution were missing for one patient.
The perfusion ratio is derived from the radionuclide perfusion scan. Each lung is divided into three zones, and a percentage of total perfusion is assigned to each zone. The ratio is calculated as the sum of the percentages assigned to the two upper zones divided by the sum of the percentages assigned to the four middle and lower zones.
To convert values from feet to meters, divide by 3.28.
The St. George's Respiratory Questionnaire is a 51-item questionnaire on the health-related quality of life with regard to respiratory symptoms that is completed by the patient; the total score ranges from 0 to 100, with lower scores indicating better health-related quality of life.
The Quality of Well-Being scale is a 77-item quality-of-life questionnaire completed by the patient. The average daily score ranges from 0 to 1, with higher scores indicating better quality of life.
The UCSD Shortness of Breath Questionnaire is a 24-item questionnaire about dyspnea that is completed by the patient; the total score ranges from 0 to 120, with lower scores indicating less shortness of breath.
The 90-day mortality rate in the LVRS group was 7.9% (95% CI, 5.9–10.3%) and higher compared with the medically treated group (1.3%; 95% CI, 0.6–2.6%; P < 0.001). All clinics reported similar rates of patient mortality and incidence and types of morbidity, including intraoperative and postoperative complications.
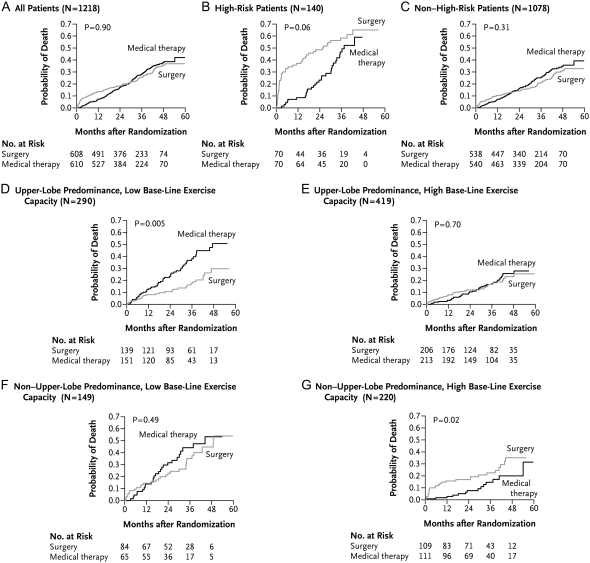
During the mean 29.2 months of follow-up post–randomization to treatment, 157 patients assigned to LVRS and 160 patients assigned to medical therapy died. There was no significant difference in overall mortality rates between the two groups, although there was a higher initial mortality rate in the LVRS group after performance of the surgery (Figure 3A).
Figure 3.
Kaplan-Meier estimates of the probability of death as a function of the number of months after randomization. P values were derived by Fisher's exact test for the comparison between groups over a mean follow-up period of 29.2 months. High-risk patients were defined as those with an FEV1 ⩽ 20% predicted and either homogeneous emphysema or DlCO ⩽ 20% predicted. A low baseline exercise capacity was defined as a maximal workload at or below the sex-specific 40th percentile (25 W for women and 40 W for men); a high exercise capacity was defined as a workload above this threshold. This was an intention-to-treat analysis. Reprinted by permission from Reference 1.
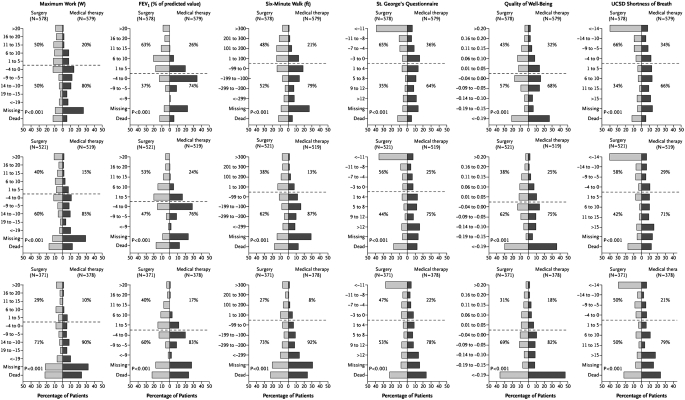
Exercise capacity improved by more than 10 W in 28, 22, and 15% of LVRS patients after 6, 12, and 24 months, respectively, as compared with 4, 5, and 3% of the medically treated patients (P < 0.001 for each time point comparison; Table 2 and Figure 4). As shown in Table 2 and Figure 4, LVRS patients were more likely than the medical group to have improvements in 6MWD, FEV1% predicted, level of dyspnea, and disease-specific as well as general quality of life assessments.
TABLE 2.
IMPROVEMENT IN EXERCISE CAPACITY AND HEALTH-RELATED QUALITY OF LIFE AT 24 MONTHS
| Improvement in Exercise Capacity, n/total n (%)*
|
Improvement in Health-related Quality of Life, n/total n (%)*
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Patients | Surgery Group | Medical Therapy Group | Odds Ratio | P Value | Surgery Group | Medical Therapy Group | Odds Ratio | P Value |
| All patients | 54/371 (15) | 10/378 (3) | 6.27 | <0.001 | 121/371 (33) | 34/378 (9) | 4.90 | <0.001 |
| High risk† | 4/58 (7) | 1/48 (2) | 3.48 | 0.37 | 6/58 (10) | 0/48 | — | 0.03 |
| Other | 50/313 (16) | 9/330 (3) | 6.78 | <0.001 | 115/313 (37) | 34/330 (10) | 5.06 | <0.001 |
| Subgroups‡ | ||||||||
| Predominantly upper lobe emphysema | ||||||||
| Low exercise capacity | 25/84 (30) | 0/92 | — | <0.001 | 40/84 (48) | 9/92 (10) | 8.38 | <0.001 |
| High exercise capacity | 17/115 (15) | 4/138 (3) | 5.81 | 0.001 | 47/115 (41) | 15/138 (11) | 5.67 | <0.001 |
| Predominantly non–upper lobe emphysema | ||||||||
| Low exercise capacity | 6/49 (12) | 3/41 (7) | 1.77 | 0.50 | 18/49 (37) | 3/41 (7) | 7.35 | 0.001 |
| High exercise capacity | 2/65 (3) | 2/59 (3) | 0.90 | 1.00 | 10/65 (15) | 7/59 (12) | 1.35 | 0.61 |
Reprinted by permission from Reference 1.
Improvement in exercise capacity in patients followed for 24 months after randomization was defined as an increase in the maximal workload of more than 10 W from the patient's post–rehabilitation baseline value. Improvement in the health-related quality of life in patients followed for 24 months after randomization was defined as a decrease in the score on the St. George's Respiratory Questionnaire of more than 5 points (on a 100-point scale) from the patient's post–rehabilitation baseline score. For both analyses, patients who died or who missed the 24-month assessment were considered not to have improvement. Odds ratios are for improvement in the surgery group as compared with the medical therapy group. P values were calculated by Fisher's exact test. A low baseline exercise capacity was defined as a post–rehabilitation baseline maximal workload at or below the sex-specific 40th percentile (25 W for women and 40 W for men); a high exercise capacity was defined as a workload above this threshold.
High-risk patients were defined as those with an FEV1 that was 20% or less of the predicted value and either homogeneous emphysema on computed tomography or a DlCO that was 20% or less of the predicted value.
High-risk patients were excluded from the subgroup analyses. For improvement in exercise capacity, P for interaction = 0.005; for improvement in health-related quality of life, P for interaction = 0.03. These P values were derived from binary logistic-regression models with terms for treatment, subgroup, and the interaction between the two, with the use of an exact-score test with 3 degrees of freedom. Other factors that were considered as potential variables for the definition of subgroups included the baseline FEV1, DlCO, partial pressure of arterial CO2, residual volume, ratio of residual volume to total lung capacity, ratio of expired ventilation in 1 minute to CO2 excretion in 1 minute, distribution of emphysema (heterogeneous vs. homogeneous), perfusion ratio, score for health-related quality of life and Quality of Well-Being score, age, race or ethnic group, and sex.
Figure 4.
Histograms of changes from post–rehabilitation baseline in exercise capacity (maximal workload), percentage of predicted value for FEV1, distance walked in 6 minutes, health-related quality of life (St. George's Respiratory Questionnaire), quality of life (Quality of Well-Being Scale), and dyspnea (University of California, San Diego [UCSD], Shortness of Breath Questionnaire) after 6, 12, and 24 months of follow-up for all patients shown in the first, second, and third rows of data, respectively. The category “missing” includes patients who were too ill to complete the procedure or who declined to complete the procedure but did not explain why. For the Quality of Well-Being Scale, patients who died were assigned a score of 0 on the questionnaire for the visit, and patients who did not complete the questionnaire were assigned a score equal to half the lowest score observed for the visit. P values were determined by the Wilcoxon rank-sum test. The degree to which the bars are shifted to the upper left of the chart indicates the degree of relative benefit of lung volume reduction surgery over medical treatment. The percentage shown in each quadrant is the percentage of patients in the specified treatment group with a change in the outcome falling in that quadrant. This is an intention-to-treat analysis. Reprinted by permission from Reference 1.
A subgroup of 140 patients had been identified to be at high risk for death with LVRS as previously outlined, and longer follow-up confirmed that initial report.
RESULTS OF NETT: MAJOR OUTCOMES IN NON–HIGH-RISK PATIENTS
Among the 1,078 NETT patients who were not high risk, the 30-day mortality was 2.2% with LVRS and 0.2% with medical therapy (P < 0.001). The 90-day mortality rate was 5.2% with LVRS and 1.5% with medical treatment (P = 0.001; Table 3). One month after randomization, 28.1% of the LVRS patients as compared with 2.2% of the medically treated patients were either hospitalized, living in a nursing or rehabilitation facility, or unavailable to interview but not known to be dead (P < 0.001; Table 4). At 8 months post–randomization to treatment, these differences became nonsignificant (Table 4), except for the subgroup of upper lobe–predominant, low-exercise patients who continued to demonstrate more independent living after LVRS compared with medically treated patients at 18- and 27-month assessments (P = 0.04 and P = 0.002, respectively).
TABLE 3.
MORTALITY AMONG ALL PATIENTS AND IN SUBGROUPS
| 90-Day Mortality*†
|
Total Mortality*†
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Patients | Surgery Group | Medical Therapy Group | P Value | Surgery Group | Medical Therapy Group | Risk Ratio | P Value | ||
| All patients | 48/608 (7.9 [5.9–10.3]) | 8/610 (1.3 [0.6–2.6]) | <0.001 | 157/608 | 0.11 | 160/610 | 0.11 | 1.01 | 0.90 |
| High risk‡ | 20/70 (28.6 [18.4–40.6]) | 0/70 (0 [0–5.1]) | <0.001 | 42/70 | 0.33 | 30/70 | 0.18 | 1.82 | 0.06 |
| Other | 28/538 (5.2 [3.5–7.4]) | 8/540 (1.5 [0.6–2.9]) | 0.001 | 115/538 | 0.09 | 130/540 | 0.10 | 0.89 | 0.31 |
| Subgroups§ | |||||||||
| Patients with predominantly upper lobe emphysema | |||||||||
| Low exercise capacity | 4/139 (2.9 [0.8–7.2]) | 5/151 (3.3 [1.1–7.6]) | 1.00 | 26/139 | 0.07 | 51/151 | 0.15 | 0.47 | 0.005 |
| High exercise capacity | 6/206 (2.9 [1.1–6.2]) | 2/213 (0.9 [0.1–3.4]) | 0.17 | 34/206 | 0.07 | 39/213 | 0.07 | 0.98 | 0.70 |
| Patients with predominantly non–upper lobe emphysema | |||||||||
| Low exercise capacity | 7/84 (8.3 [3.4–16.4]) | 0/65 (0 [0–5.5]) | 0.02 | 28/84 | 0.15 | 26/65 | 0.18 | 0.81 | 0.49 |
| High exercise capacity | 11/109 (10.1 [5.1–17.3]) | 1/111 (0.9 [0.02–4.9]) | 0.003 | 27/109 | 0.10 | 14/111 | 0.05 | 2.06 | 0.02 |
Reprinted by permission from Reference 1.
Mortality was measured from the date of randomization in both treatment groups. Total mortality rates are based on a mean follow-up of 29.2 months. P values were calculated by Fisher's exact test. Risk ratios are for the risk in the surgery group as compared with the risk in the medical therapy group. A low baseline exercise capacity was defined as a post–rehabilitation baseline maximal workload at or below the sex-specific 40th percentile (25 W for women and 40 W for men); a high-exercise capacity was defined as a workload above this threshold.
Values are numbers of death/total number with percentages in parentheses and 95% confidence intervals in brackets.
High-risk patients were defined as those with an FEV1 that was 20% or less of the predicted value and either homogeneous emphysema on computed tomography or a DlCO that was 20% or less of the predicted value.
High-risk patients were excluded from the subgroup analyses. For total mortality, P for interaction = 0.004; this P value was derived from binary logistic-regression models with terms for treatment, subgroup, and the interaction between the two, with the use of an exact-score test with 3 degrees of freedom. Other factors that were considered as potential variables for the definition of subgroups included the baseline FEV1, DlCO, partial pressure of arterial CO2, residual volume, ratio of residual volume to total lung capacity, ratio of expired ventilation in 1 minute to CO2 excretion in 1 minute, distribution of emphysema (heterogeneous vs. homogeneous), perfusion ratio, score for health-related quality of life and Quality of Well-Being score, age, race or ethnic group, and sex.
TABLE 4.
PATIENT'S PLACE OF RESIDENCE ACCORDING TO THE TIME SINCE RANDOMIZATION
| All Patients
|
Non–High-Risk Patients
|
Upper Lobe Predominance, Low Exercise Capacity
|
Upper Lobe Predominance, High Exercise Capacity
|
Non–Upper Lobe Predominance, High Exercise Capacity
|
Non–Upper Lobe Predominance, High Exercise Capacity
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Surgery | Medical Therapy | Surgery | Medical Therapy | Surgery | Medical Therapy | Surgery | Medical Therapy | Surgery | Medical Therapy | Surgery | Medical Therapy |
| 1 Month | ||||||||||||
| Private home, % | 66.8 | 97.5 | 69.7 | 97.6 | 65.5 | 97.4 | 74.3 | 96.7 | 66.7 | 100 | 68.8 | 98.2 |
| Nursing home or rehabilitation facility, % | 0.7 | 0 | 0.6 | 0 | 0.7 | 0 | 0.5 | 0 | 1.2 | 0 | 0 | 0 |
| Acute care hospital, % | 24.0 | 0.3 | 23.1 | 0.4 | 29.5 | 0.7 | 19.4 | 0.5 | 21.4 | 0 | 22.9 | 0 |
| Living, no data, % | 4.9 | 2.0 | 4.5 | 1.9 | 2.9 | 2.0 | 3.9 | 2.8 | 7.1 | 0 | 5.5 | 0.9 |
| Dead, % | 3.6 | 0.2 | 2.2 | 0.2 | 1.4 | 0 | 1.9 | 0 | 3.6 | 0 | 2.8 | 0.9 |
| No. of patients | 6.8 | 610 | 538 | 540 | 139 | 151 | 206 | 213 | 84 | 65 | 109 | 111 |
| P value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||||||
| Median time since surgery, mo | 0.7 | 0.7 | 0.8 | 0.7 | 0.8 | 0.7 | ||||||
| 2 Months | ||||||||||||
| Private home, % | 78.3 | 96.1 | 80.9 | 95.9 | 77.0 | 93.4 | 83.0 | 96.2 | 78.6 | 98.5 | 83.5 | 97.3 |
| Nursing home or rehabilitation facility, % | 0.7 | 0.2 | 0.7 | 0.2 | 2.2 | 0 | 0 | 0.5 | 1.2 | 0 | 0 | 0 |
| Acute care hospital, % | 8.4 | 0.3 | 8.6 | 0.2 | 13.0 | 0 | 8.7 | 0 | 7.1 | 1.5 | 3.7 | 0 |
| Living, no data, % | 5.8 | 2.8 | 5.0 | 3.0 | 5.0 | 4.6 | 5.8 | 3.3 | 6.0 | 0 | 2.8 | 1.8 |
| Dead, % | 6.9 | 0.7 | 4.8 | 0.7 | 2.9 | 2.0 | 2.4 | 0 | 7.1 | 0 | 10.1 | 0.9 |
| No. of patients | 608 | 610 | 538 | 540 | 139 | 151 | 206 | 213 | 84 | 65 | 109 | 111 |
| P value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.001 | ||||||
| Median time since surgery, mo | 1.7 | 1.7 | 1.8 | 1.7 | 1.7 | 1.7 | ||||||
| 4 Months | ||||||||||||
| Private home, % | 84.5 | 94.3 | 87.7 | 94.6 | 90.7 | 91.4 | 90.3 | 95.3 | 79.8 | 98.5 | 85.3 | 95.5 |
| Nursing home or rehabilitation facility, % | 0.8 | 0.2 | 0.9 | 0.2 | 1.4 | 0.7 | 0.5 | 0 | 2.4 | 0 | 0 | 0 |
| Acute care hospital, % | 1.8 | 0.5 | 2.0 | 0.6 | 2.9 | 0 | 1.5 | 0.5 | 3.6 | 0 | 0.9 | 1.8 |
| Living, no data, % | 4.4 | 2.8 | 3.7 | 2.4 | 2.2 | 4.0 | 3.9 | 2.4 | 6.0 | 0 | 3.7 | 1.8 |
| Dead, % | 8.4 | 2.3 | 5.6 | 2.2 | 2.9 | 4.0 | 3.9 | 1.9 | 8.3 | 1.5 | 10.1 | 0.9 |
| No. of patients | 608 | 610 | 538 | 540 | 139 | 151 | 206 | 213 | 84 | 65 | 109 | 111 |
| P value | <0.001 | <0.001 | 0.89 | 0.05 | 0.001 | 0.008 | ||||||
| Median time since surgery, mo | 3.7 | 3.7 | 3.7 | 3.6 | 3.6 | 3.7 | ||||||
| 8 Months | ||||||||||||
| Private home, % | 85.3 | 90.9 | 88.6 | 91.2 | 89.5 | 84.8 | 91.2 | 94.0 | 86.4 | 89.2 | 84.5 | 96.0 |
| Nursing home or rehabilitation facility, % | 0.5 | 0.2 | 0.6 | 0.2 | 1.5 | 0.7 | 0 | 0 | 1.2 | 0 | 0 | 0 |
| Acute care hospital, % | 0.7 | 0.5 | 0.8 | 0.4 | 0.8 | 0 | 0.5 | 1.0 | 1.2 | 0 | 1.0 | 0 |
| Living, no data, % | 2.2 | 3.1 | 2.0 | 3.1 | 3.0 | 4.8 | 2.6 | 2.5 | 0 | 3.1 | 1.0 | 0 |
| Dead, % | 11.2 | 5.3 | 8.0 | 5.1 | 5.3 | 9.7 | 5.7 | 2.5 | 11.1 | 7.7 | 13.6 | 2.0 |
| No. of patients | 580 | 582 | 510 | 512 | 133 | 145 | 193 | 201 | 81 | 65 | 103 | 101 |
| P value | 0.002 | 0.16 | 0.22 | 0.26 | 0.60 | 0.005 | ||||||
| Median time since surgery, mo | 7.7 | 7.8 | 7.7 | 7.8 | 7.7 | 7.7 | ||||||
| 18 Months | ||||||||||||
| Private home, % | 78.4 | 83.8 | 83.0 | 84.3 | 87.3 | 77.2 | 84.4 | 90.9 | 76.7 | 74.1 | 80.0 | 87.5 |
| Nursing home or rehabilitation facility, % | 0.2 | 0.6 | 0.2 | 0.7 | 0.9 | 1.6 | 0 | 0.5 | 0 | 0 | 0 | 0 |
| Acute care hospital, % | 0.6 | 0.8 | 0.7 | 0.7 | 0 | 0.8 | 1.2 | 0.5 | 1.4 | 0 | 0 | 1.3 |
| Living, no data, % | 3.3 | 2.1 | 2.9 | 2.0 | 2.5 | 0.8 | 3.0 | 1.6 | 2.7 | 1.7 | 3.3 | 5.0 |
| Dead, % | 17.6 | 12.8 | 13.2 | 12.3 | 9.3 | 19.5 | 11.4 | 6.5 | 19.2 | 24.1 | 16.7 | 6.3 |
| No. of patients | 518 | 517 | 448 | 447 | 118 | 123 | 167 | 186 | 73 | 58 | 90 | 80 |
| P value | 0.02 | 0.60 | 0.04 | 0.06 | 0.67 | 0.14 | ||||||
| Median time since surgery, mo | 17.7 | 17.7 | 17.7 | 17.7 | 17.7 | 17.8 | ||||||
| 27 Months | ||||||||||||
| Private home, % | 73.8 | 72.8 | 79.1 | 74.9 | 81.1 | 61.2 | 79.0 | 83.1 | 75.9 | 61.9 | 78.9 | 85.5 |
| Nursing home or rehabilitation facility, % | 0.3 | 0.8 | 0.3 | 0.6 | 1.1 | 1.0 | 0 | 0.7 | 0 | 0 | 0 | 0 |
| Acute care hospital, % | 0.8 | 0.5 | 0.6 | 0.6 | 1.1 | 1.0 | 0 | 0.7 | 1.9 | 0 | 0 | 0 |
| Living, no data, % | 4.2 | 4.0 | 4.4 | 3.7 | 4.4 | 4.1 | 7.3 | 3.4 | 1.9 | 0 | 1.4 | 6.5 |
| Dead, % | 21.0 | 22.0 | 15.6 | 20.3 | 12.2 | 32.7 | 13.7 | 12.2 | 20.4 | 38.1 | 19.7 | 8.1 |
| No. of patients | 401 | 401 | 339 | 350 | 90 | 98 | 124 | 148 | 54 | 42 | 71 | 62 |
| P value | 0.74 | 0.16 | 0.002 | 0.41 | 0.11 | 0.25 | ||||||
| Median time since surgery, mo | 26.7 | 26.8 | 26.7 | 26.8 | 26.6 | 26.9 | ||||||
Reprinted by permission from Reference 1.
High-risk patients had with an FEV1 that was 20% or less of the predicted value and either homogeneous emphysema or a DlCO that was 20% or less of the predicted value. Upper lobe predominance of emphysema was judged subjectively by each center's radiologist; choices were upper lobe predominance, lower lobe predominance, diffuse, or superior segments of lower lobes predominantly involved. The latter three choices were grouped as non–upper lobe predominance. A low baseline exercise capacity was defined as a post–rehabilitation baseline maximal workload at or below the sex-specific 40th percentile (25 W for women, 40 W for men); a high exercise capacity was defined as a workload above this threshold. All subgroup analyses excluded high-risk patients. P values are for homogeneity.
Total mortality in the non–high-risk NETT patients was 0.09 deaths per person-year in the LVRS group and 0.10 deaths per person-year in the medically treated group (RR, 0.89; P = 0.31; Table 3 and Figure 3C). NETT patients who underwent LVRS were more likely to have improvements in 6MWD, maximum exercise capacity, FEV1% predicted, and general as well as disease-specific quality of life compared with the medically treated group (P < 0.001 for each comparison; Figure 4).
PREOPERATIVE PREDICTORS OF OUTCOMES WITH LVRS IN NON–HIGH-RISK NETT PATIENTS
The only baseline factors associated with differences in mortality between the treatment groups were the craniocaudal distribution of emphysema on chest CT (presence or absence of upper lobe–predominant emphysema, P for interaction = 0.02) and postrehabilitation maximum exercise test (low or high exercise, P for interaction = 0.01).
Patients were divided into four subgroups based on combinations of high and low exercise with upper lobe– or non-upper lobe–predominant emphysema by chest CT analysis. In 290 patients with upper lobe–predominant emphysema and low exercise capacity, LVRS patients had a lower risk of death than medical patients (RR, 0.47; P = 0.005; Figure 3D and Table 3). LVRS patients were also more likely to have a more than 10-W improvement in maximum exercise at 24 months (30 vs. 0%, P < 0.001; Table 2) and more than an 8-point improvement in the SGRQ at 24 months (48 vs. 10%, P < 0.001; Table 2).
In the 419 patients with upper lobe–predominant emphysema and high exercise, there was no impact of LVRS on survival (RR, 0.98; P = 0.70). But LVRS patients were more likely than medically treated patients to have a more than 10-W improvement in maximum exercise capacity at 24 months (15 vs. 3%, P = 0.001; Table 2) and to have more than an 8-point improvement in SGRQ (41 vs. 11%, P < 0.001; Table 2).
The 149 patients with non-upper lobe–predominant disease and low exercise capacity had no impact of LVRS on their risk of death (RR, 0.81; P = 0.49), and similar chance of improvement in maximum exercise capacity at 24 months (12 vs. 7%, P = 0.50). However, patients had a greater likelihood of improvement in SGRQ at 24 months (37 vs. 7%, P = 0.001; Table 2).
In 220 patients with non-upper lobe–predominant emphysema and high exercise at baseline, LVRS-treated patients had a greater risk for death than the medical group (RR, 2.06, P = 0.02) and similar chances for improvements in maximum exercise capacity at 24 months (3% both groups, P = 1.0) and SGRQ (15 vs. 12%, P = 0.61; Table 2).
These NETT data at a mean follow-up of 29.2 months after randomization to treatment showed that LVRS provided no survival benefit over medical treatment, even with exclusion of the subgroup that was at high risk for death with LVRS. LVRS did produce significant and sustained improvements in exercise capacity, 6MWD, relief of dyspnea, and general and disease-specific quality of life assessments. However, the NETT investigators noted that the mean follow-up at the time analysis was only 2.4 years and that longer follow-up would not only establish the durability of benefits from LVRS on functional and physiologic performance but also assess the effect of LVRS on long-term survival.
NETT: LONG-TERM FOLLOW-UP OF THE EFFECTS OF LVRS VERSUS MEDICAL TREATMENT IN SEVERE EMPHYSEMA
Through December 2002, patients were reexamined at 6 and 12 months postrandomization and then yearly thereafter (2). From January 2003 through December 2003, patients returned for examinations at 6 months and then 2, 3, and 5 years after randomization. Patients who were 1 and 4 years postrandomization between January 2003 and December 2003 completed a telephone interview and mailed quality of life questionnaires. From January through June 2004, patients completed quality of life questionnaires a final time by mail. Survival was determined as of September 2005 by clinical center reports and by review of the most recent Social Security Master Death file.
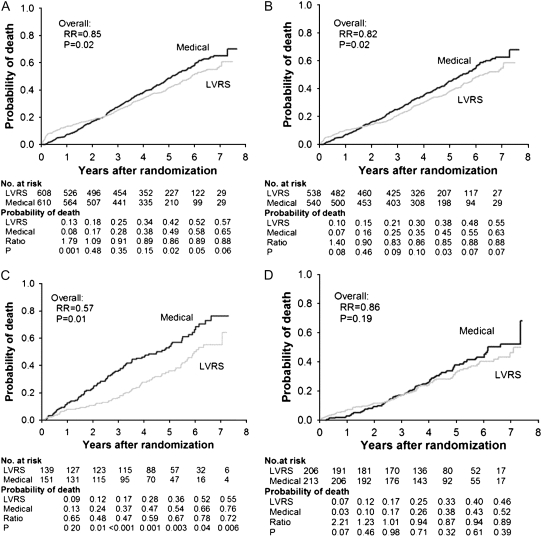
Figure 5A shows the probability of death as a function of years after randomization to LVRS or medical therapy for all 1,218 patients (median follow-up, 4.3 yr). Total mortality rate was 0.11 deaths per person-year in the LVRS and 0.13 in the medical groups, respectively (RR, 0.85; P = 0.02). This improvement in survival in the LVRS group versus the medically treated group occurred despite the expected higher earlier postoperative mortality in the LVRS group.
Figure 5.
Kaplan-Meier estimates of the cumulative probability of death as a function of years after randomization to lung volume reduction surgery (LVRS) (gray line) or medical treatment (black line) for (A) all patients and (B–D) non–high-risk and upper lobe–predominant subgroups of patients. The P value is from the Fisher's exact test for difference in the proportions of patients who died during the 4.3 years (median) of follow-up. Shown below each graph are the numbers of patients at risk, the Kaplan-Meier probabilities, the ratio of the probabilities (LVRS:medical), and P value for the difference in these probabilities. This is an intention-to-treat analysis. (A) All patients (n = 1,218). (B) Non–high-risk patients (n = 1,078). (C) Upper lobe predominant and low baseline exercise capacity (n = 290). (D) Upper lobe predominant and high exercise capacity (n = 419). RR = relative risk. Reprinted by permission from Reference 2.
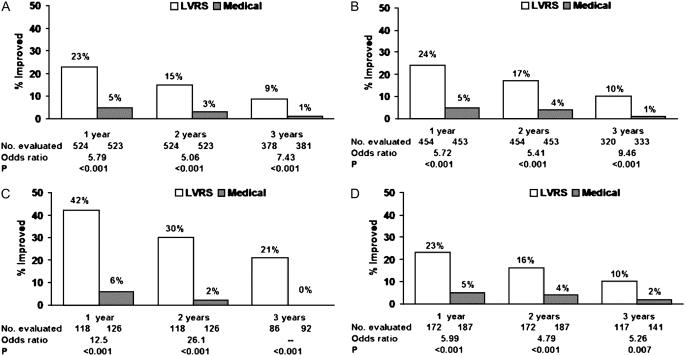
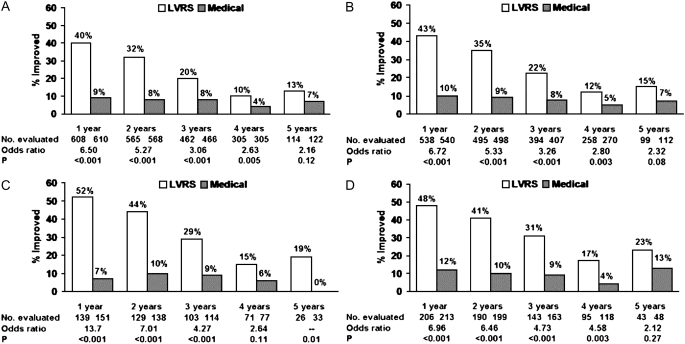
Exercise capacity improved more than 10 W in 23, 15, and 9% of patients post-LVRS compared with 5, 3, and 1% of medically treated patients at 1, 2, and 3 years of follow-up (P < 0.001 at each time point). SGRQ decreased more than 8 units in 40, 32, 20, 10, and 13% of LVRS patients compared with 9, 8, 8, 4, and 7% of medically treated patients at 1–5 years of follow-up (P < 0.001, Years 1–3; P = 0.005, Year 4; P = 0.12, Year 5).
EFFECT OF SUBGROUP CLASSIFICATION ON LVRS PATIENTS' LONG-TERM SURVIVAL AND FUNCTIONAL OUTCOME
The evidence for differential risk and benefit on the basis of subgroup classification using the craniocaudal pattern of emphysema on chest CT and baseline exercise capacity was further substantiated in the updated analysis.
In the 290 patients with upper lobe–predominant emphysema and low exercise capacity, the LVRS patients had a marked survival advantage compared with the medically treated group (RR, 0.57; P = 0.01; Figure 5C). These LVRS patients were also more likely to have significant improvements in exercise capacity and quality of life (Figures 6C and 7C, respectively).
Figure 6.
Improvement in exercise capacity (increase in maximum work of >10 W above the patient's post–rehabilitation baseline) at 1, 2, and 3 years after randomization to lung volume reduction surgery (LVRS) (open bars) or medical treatment (solid bars) for (A) all patients and (B–D) non–high-risk and upper lobe–predominant subgroups of patients. Shown below each graph are the numbers of patients evaluated, the odds ratio for improvement (LVRS:medical), and the Fisher's exact P value for difference in proportion improved. Patients who died or who did not complete the assessment were considered not improved. This is an intention-to-treat analysis. (A) All patients (n = 1,218). (B) Non–high-risk patients (n = 1,078). (C) Upper lobe predominant and low baseline exercise capacity (n = 290). (D) Upper lobe predominant and high exercise capacity (n = 419). Reprinted by permission from Reference 2.
Figure 7.
Improvement in health-related quality of life (decrease in St. George's Respiratory Questionnaire total score of >8 units below the patient's post–rehabilitation baseline) at 1, 2, 3, 4, and 5 years after randomization to lung volume reduction surgery (LVRS) (open bars) or medical therapy (solid bars) for (A) all patients and (B–D) non–high-risk and upper lobe–predominant subgroups of patients. Shown below each graph are the numbers of patients evaluated, the odds ratio for improvement (LVRS:medical), and the Fisher's exact P value for difference in proportion improved. Patients who died or who did not complete the assessment were considered not improved. This is an intention-to-treat analysis. (A) All patients (n = 1,218). (B) Non–high-risk patients (n = 1,078). (C) Upper lobe predominant and low baseline exercise capacity (n = 290). (D) Upper lobe predominant and high exercise capacity (n = 419). Reprinted by permission from Reference 2.
In the 419 patients with upper lobe–predominant emphysema and baseline high exercise capacity, mortality was similar regardless of treatment (RR, 0.86; P = 0.19). However, LVRS patients were more likely to have significant and sustained improvements in exercise capacity (P < 0.01) and disease-specific quality of life (P < 0.001) (Figures 6D and 7D, respectively).
In the 149 patients with non–upper lobe emphysema and low exercise capacity, the risk of death was similar (RR, 0.80; P = 0.31) for patients treated with LVRS or medical management, as well as the chance for an improvement in exercise capacity (P > 0.99 at 3 yr). Although LVRS patients had a greater chance for more than an 8-point improvement in SGRQ compared with medical patients at Years 1 and 2 postrandomization, this effect disappeared at Year 3.
In the non–upper lobe emphysema and high-exercise patient group, LVRS-treated patients had a similar risk of death (RR, 1.10; P = 0.79) and low chances of improvement in maximum exercise (3 vs. 4%, P > 0.99) or SGRQ (19 vs. 9%, P = 0.07) compared with the medically treated group.
The long-term follow-up data demonstrate that LVRS has a survival advantage compared with medical treatment alone in patients with severe emphysema. This effect is primarily driven by patients who have upper lobe–predominant emphysema on chest CT and low exercise performance on maximum exercise testing. The magnitude and durability of the physiologic and functional improvements previously identified in follow-up at 29.2 months were confirmed with long-term follow-up.
PREDICTORS OF OPERATIVE MORTALITY AND CARDIOPULMONARY MORBIDITY AFTER LVRS IN NETT
NETT sought to identify the predictors of operative mortality and major pulmonary and cardiovascular morbidity after LVRS (4). A host of predictors of mortality and morbidity were analyzed in the 511 non–high-risk patients who underwent LVRS. The predictors included demographic characteristics, pulmonary function, the extent and pattern of emphysema characterized by chest CT, exercise capacity measured on maximum exercise testing, dyspnea, and quality of life. Operative mortality was defined as death within 90 days of surgery. Major pulmonary morbidity was defined as tracheostomy, failure to wean from mechanical ventilation, pneumonia, reintubation, or mechanical ventilation of more than 3 days within 30 days of surgery. Major cardiovascular morbidity was defined as myocardial infarction, pulmonary embolus, or cardiac arrhythmia requiring treatment within 30 days of surgery.
The incidence of operative mortality within 90 days of LVRS was 5.5%; major pulmonary and cardiovascular morbidity occurred in 29.8 and 20% of patients, respectively. Intraoperatively, 91% of patients had no complications, 2.2% had transient hypoxemia, and 1.2% developed an arrhythmia.
Within 30 days of surgery, 58.7% of the patients had at least one postoperative complication. Arrhythmia was the most common complication; it occurred in 23.5% of the patients and required treatment in 18.6%. Pneumonia developed in 18.2% of patients, 21.8% required at least one reintubation, 11.7% were readmitted to the intensive care unit, 8.2% underwent tracheostomy, and 5.1% of patients failed to wean from mechanical ventilation within 3 days of LVRS.
The sole predictor of operative mortality was the presence of non-upper lobe–predominant emphysema as assessed by a radiologist (relative odds, 2.99; P = 0.009). Pulmonary morbidity was greater in patients who were older (relative odds, 1.05; P = 0.02), had lower FEV1 (relative odds, 0.97; P = 0.05), or had lower DlCO (relative odds, 0.97; P = 0.01). Cardiovascular morbidity was higher in those patients who were older (relative odds, 1.07; P = 0.004), used oral steroids (relative odds, 1.72; P = 0.04), or had non-upper lobe–predominant emphysema on chest CT on quantitative image analysis (relative odds, 2.67; P < 0.001).
These data show that LVRS can be performed in compromised patients with emphysema with acceptable mortality; the incidence of major cardiopulmonary morbidity is high.
AIR LEAKS AFTER LVRS
Air leaks after LVRS are common and often prolonged. NETT investigated the effectiveness of a number of different buttressing products and techniques on the occurrence of postoperative air leaks after LVRS (3). Of the 608 patients assigned to LVRS as part of NETT, 580 (95%) actually underwent surgery. Of these, 552 had detailed 30-day postoperative air leak data for analysis. NETT investigators recorded the materials used for staple line buttressing, including bovine pericardium and polytetrafluoroethylene and fibrin glue (combined with bovine pericardium in all but one case in which it was combined with polytetrafluoroethylene). In less than 5% of operative cases, no buttressing materials were used (Table 5). Staplers were predominantly open and endoscopic and manufactured by U.S. Surgical Corporation (Norwalk, CT) or Ethicon (Cincinnati, OH). A few 3M devices (St. Paul, MN) were used.
TABLE 5.
SURGICAL VARIABLES IN RELATION TO AIR LEAK OCCURRING AFTER CHEST CLOSURE WITHIN 30 DAYS OF LUNG VOLUME REDUCTION SURGERY
| Air Leak
|
|||||
|---|---|---|---|---|---|
| Variable | n | % of 552 | n | % of n | P |
| Surgical approach* | 0.9 | ||||
| Median sternotomy | 382 | 69 | 344 | 90 | |
| VATS | 165 | 30 | 148 | 90 | |
| Staple line buttressing | |||||
| Pericardium | 399 | 73 | 363 | 91 | 0.14 |
| PTFE | 166 | 30 | 146 | 88 | 0.3 |
| Fibrin glue | 32 | 5.8 | 27 | 84 | 0.3 |
| None | 26 | 4.7 | 25 | 96 | 0.4 |
| Stapler | |||||
| U.S. Surgical | 384 | 69 | 309 | 80 | 0.2 |
| Ethicon | 174 | 31 | 142 | 82 | 0.19 |
| 3M | 20 | 3.6 | 18 | 90 | 0.9 |
| Intraoperative adjunct procedures | |||||
| Tenting | 60 | 11 | 55 | 92 | 0.6 |
| Pleurodesis | 53 | 9.7 | 48 | 91 | 0.8 |
Definition of abbreviations: PTFE = polytetrafluoroethylene; VATS = video-assisted thorascopic surgery.
Reproduced by permission from Reference 3. Categories are generally not mutually exclusive, and not all missing values have been identified, although they were uncommon and are not included in computations. For surgical approach, the P value represents a statistical comparison of air leak occurrence between the two techniques. For the choice of stapler, buttressing material, or adjunct procedures, the P value represents a comparison between the reference product or technique and all alternatives.
In two patients, a clamshell incision was used, and in three patients, a thoracotomy was performed on one side.
Any air leak after the patient left the operating room and within 30 days of LVRS was recorded. The postoperative day when the air leak ceased was also recorded, except in three patients in whom the data were not available. Air leak duration was calculated as the interval from the day of LVRS to the day of the clinically observed cessation of air leak.
Risk factors for post-LVRS air leak prevalence were identified using multivariable logistic regression analysis and included the following: demographic factors (age, sex, and race/ethnicity), preoperative emphysema treatment (oral or inhaled corticosteroids), pulmonary function and gas exchange, maximum workload attained on post–pulmonary rehabilitation maximum exercise test, distribution of emphysema by chest CT analysis, and the type of surgical LVRS procedure performed.
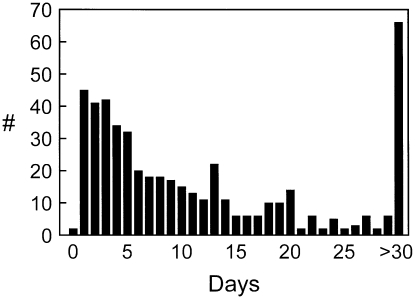
After LVRS, 496 of the 552 patients for whom detailed data on air leak occurrence were available had air leaks at some point 30 days after thoracotomy. The median duration of the air leak was 7 days (Figure 8), but 66 patients had air leaks 30 days or longer postoperatively. Air leak duration was longer in whites (P < 0.0001), in those with lower FEV1 (P = 0.0003), in those with lower diffusion capacity (P = 0.06), in those taking inhaled steroids (P = 0.004), in those with upper lobe–predominant emphysema (P = 0.04), and in patients with moderate or marked pleural adhesions (P = 0.007).
Figure 8.
The number of patients (in 496/552 lung volume reduction surgery [LVRS] patients who had detailed data on air leak duration) who stopped leaking air via chest tubes (#, y axis) per postoperative day for the first 30 days post-LVRS. The number of patients with air leak persisting at least 30 days is shown in the extreme right-hand bar. Reprinted by permission from Reference 3.
Surgical approach (MS vs. VATS), use of buttressing materials, stapler brand, and intraoperative adjunctive procedures were not associated with fewer or a shorter duration of air leaks (P ⩾ 0.2). Postoperative complications occurred more frequently in matched patients having air leaks (57 vs. 30%, P = 0.0004) and postoperative stay was longer (11.8 ± 6.5 vs. 7.6 ± 4.4 d, P = 0.0005).
These data show that air leak complicates LVRS in 90% of patients, is prolonged in half of the patients, and contributes to a more prolonged and complicated hospital course. The prevalence and duration of air leak are increased by patient factors such as more severe airflow obstruction, a lower diffusion capacity, upper lobe–predominate disease, the use of inhaled corticosteroids, and the presence of significant pleural adhesions. In contrast, neither the surgical approach used to perform LVRS (MS vs. VATS) nor surgical techniques, such as choice of suture line buttressing material, nor use of pleurodesis or pleural tents had any significant effect on the prevalence or duration of postoperative air leaks.
IMPACT OF SURGICAL APPROACH USED TO PERFORM LVRS
A secondary goal of NETT was to compare the effects of MS or VATS approaches to LVRS on patient mortality, morbidity, and functional outcomes (5). Before NETT, the optimal surgical approach to perform LVRS was unknown. Case series had supported the use of either MS or VATS approaches. However, randomized, prospective, and controlled data that compared the functional outcomes or long-term effects of using either of the two approaches were lacking.
In NETT, LVRS was performed by MS only at eight centers and VATS only at three centers. Six centers randomized the approach to LVRS. Two types of comparisons were performed after excluding the high-risk patients (those with FEV1 ⩽ 20% predicted and either DlCO ⩽ 20% predicted or homogeneous emphysema on CT scan): the nonrandomized comparison, an analysis of all MS patients (n = 359) versus all VATS patients (n = 152), and an analysis of those randomized to VATS.
NONRANDOMIZED COMPARISON
The 90-day mortality was 5.9% for MS and 4.6% for VATS (P = 0.67). Overall mortality was 0.08 deaths per person-year for MS and 0.10 deaths per person-year for VATS (VATS:MS RR, 1.18; P = 0.42).
Complication rates were low in both groups. There was no difference between MS and VATS in terms of mean intraoperative blood loss (P = 0.55) or need for transfusion (P = 0.99). The mean operating time was 21.7 minutes shorter for MS than VATS (P < 0.001), hypoxemia occurred less frequently with MS than VATS (0.8 vs. 5.3%, P = 0.004), and there were fewer reports of intraoperative complications in MS compared with VATS (93 vs. 86.2% with no intraoperative complications, P = 0.02).
Median hospital length of stay was longer for MS than for VATS (10 vs. 9 d, P = 0.1) and, by 30 days postsurgery, 70.5% of MS patients were living independently compared with 80.9% of the VATS cohort (P = 0.02). Functional outcomes were similar between the two groups at 12 and 24 months of follow-up. Costs associated with the LVRS and associated hospitalization were less for VATS compared with MS (P = 0.03), as were total costs (medical and nonmedical) during the 6 months after surgery (P = 0.005).
RANDOMIZED COMPARISON
When the above analyses were restricted to the patients who were randomized to surgical approach, the findings were comparable to those generated by the nonrandomized comparison.
These data show that morbidity and mortality are comparable using either MS or VATS approaches to perform LVRS. The VATS approach to perform LVRS, however, allowed earlier patient recovery at reduced costs.
COST-EFFECTIVENESS OF LVRS
Because of the potential profound economic impact of LVRS if broadly applied to the emphysema patient population, NETT conducted a parallel prospective cost-effectiveness study of LVRS (6). For the cost analysis, the value of medical goods and services, transportation to and from health care facilities, and time spent by family and friends in caring for the patients and time spent by the patient in receiving treatment were estimated.
The number of quality-adjusted life-years was derived from the adjustment of survival data for health care preferences; the latter are also referred as “utilities.” Weights for the utilities were obtained from data from the QWB assessment, a self-administered tool that attempts to measure the patient's quality of life. Scores for each of the QWB domains (acute and chronic symptoms, self-care, mobility, physical activity and functioning, and social activity) were converted to utility weights using a scale that ranges from 0 (death) to 1.0 (optimal quality of life).
Measurement of the utilization of medical care was based on Medicare claims for study participants. Medicare reimbursed providers for trial-related medical care throughout all phases of NETT from screening to care post–randomization to treatment. These services included inpatient care, outpatient care provided by physicians, ambulatory laboratory and radiologic services, supplemental oxygen costs, 100 days of skilled care hospitalization costs, and hospice care. Medications for emphysema were recorded on follow-up visit questionnaires. Travel distances to facilities were estimated for each subject. Each subject also gave estimates of the weekly average number of hours of care provided by family or friends.
Cost-effectiveness was calculated as the ratio of the difference in costs between the LVRS and medically treated groups divided by the difference in the quality-adjusted life-years gained between the two groups. The cost-effectiveness ratio was then computed for the 3-year trial period and projections were computed for 5 and 10 years postrandomization.
The subgroup of NETT patients identified to be at greater risk of death with little chance of any substantial benefit after LVRS (FEV1 ⩽ 20% predicted and either homogeneous emphysema on chest CT or DlCO ⩽ 20% predicted) was excluded from the cost-effectiveness analysis because LVRS is not considered to be a viable treatment option in this patient group.
In the first 6 months after randomization to treatment, mean number of inpatient hospital days was higher in the LVRS group compared with the medical group (23.3 vs. 3 d, P < 0.001), as well as the percentage of patients hospitalized more than 25 days (26 vs. 3%, P < 0.001). During the first year after treatment, the mean number of hospital days (24.9 vs. 4.9 d, P < 0.001), days of ambulatory care (10.3 vs. 8.6 d, P = 0.005), and nursing home admissions (0.1 vs. 0.0, P = 0.005) were greater in the LVRS than the medical treatment group. However, in the second year post-treatment, the mean number of hospital days (3.2 vs. 6.1 d, P = 0.005) and emergency room visits (0.5 vs. 0.7, P = 0.04) were lower in the LVRS group than in the medical group. During the third year post-treatment, there were no significant differences between the groups in measures of health care utilization.
The mean total cost per patient was higher in the LVRS group than in the medical group in the first 12 months after randomization to treatment ($71,515 vs. $23,371, P < 0.001), mainly because of costs incurred within the first 6 months due to the operation and hospitalization postoperatively ($62,753 vs. $12,932, P < 0.001). In contrast, the mean total cost of care was lower in the LVRS compared with the medical patients in the second year post-treatment ($13,222 vs. $21,319, P < 0.001) and trended to be lower in the third year post–randomization to treatment ($14,215 vs. $17,870, P = 0.08). Mean total medical cost per patient during Months 7–36 was almost $10,000 lower in the LVRS compared with the medically treated group ($36,199 vs. $49,628, P < 0.001), predominantly due to fewer hospital days in the LVRS group.
The mean total cost per person at 3 years was $98,952 in the LVRS group and $62,560 in the medically treated group (P < 0.001). The per-person direct medical costs in the LVRS group were $80,818 compared with $43,689 in the medical group over the 3-year period after randomization to treatment. The nonmedical costs were not different between the two groups.
After 3 years of follow-up, the mean number of quality-adjusted life-years gained was greater in the LVRS compared with the medical group (1.46 vs. 1.27, P < 0.001). The mean number of quality-adjusted life-years gained was also significantly greater at 1 and 2 years of follow-up.
The cost-effectiveness ratio for LVRS versus medical treatment at 3 years was $190,000 per quality-adjusted life-year gained and was projected to be $53,000 per quality-adjusted life-year gained at 10 years.
Cost-effectiveness analyses were also performed for three subgroups of patients determined to have differential response to LVRS versus medical therapy based on chest CT analysis and workload attained on maximum exercise testing. The cost-effectiveness of LVRS compared with medical treatment in the subgroup with upper lobe–predominant disease by chest CT and low exercise on maximum exercise testing was $98,000 per quality-adjusted life-year gained at 3 years and projected to be $21,000 at 10 years.
The cost-effectiveness analysis was updated using data obtained for patients during the extension of NETT follow-up performed in 2003 (7). Updated data showed that the cost-effectiveness ratio fell for the LVRS group compared with medical group from $190,000 to $140,000 per patient, but the projected 10-year costs per subgroup deviated substantially from the prior projections. These data show that medical care for the patient with severe emphysema is costly, and that the cost-effectiveness of LVRS depends on patient characteristics determined by chest CT and performance on maximum exercise testing.
CONCLUSIONS
Over its 10 years of operation, NETT has published over 42 articles examining the evaluation, optimal patient characterization, surgical approach, and outcomes of LVRS in severe emphysema. It is hoped that, by succinctly summarizing the major findings of NETT in one comprehensive review, clinicians and researchers will be more aware of the most important factors relevant to the performance of LVRS.
The National Emphysema Treatment Trial (NETT) is supported by contracts with the National Heart, Lung, and Blood Institute (N01HR76101, N01HR76102, N01HR76103, N01HR76104, N01HR76105, N01HR76106, N01HR76107, N01HR76108, N01HR76109, N01HR76110, N01HR76111, N01HR76112, N01HR76113, N01HR76114, N01HR76115, N01HR76116, N01HR76118, and N01HR76119), the Centers for Medicare and Medicaid Services (CMS), and the Agency for Healthcare Research and Quality (AHRQ).
Conflict of Interest Statement: G.J.C. has had research grants from Aeris Therapeutics and Emphysas Medical Incorporated over the past 5 years. A.L.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.National Emphysema Treatment Trial Research Group. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med 2003;348:2059–2073. [DOI] [PubMed] [Google Scholar]
- 2.Naunheim KS, Wood DE, Mohsenifar Z, Sternberg AL, Criner GJ, DeCamp MM, Deschamps CC, Martinez FJ, Sciurba FC, Tonascia J, et al. Long-term follow-up of patients receiving lung-volume-reduction surgery versus medical therapy for severe emphysema. Ann Thorac Surg 2006;82:431–443. [DOI] [PubMed] [Google Scholar]
- 3.DeCamp MM, Blackstone EH, Naunheim KS, Krasna MJ, Wood DE, Meli YM, McKenna RJ; for the NETT Research Group. Patient and surgical factors influencing air leak after lung volume reduction surgery: lessons learned from the National Emphysema Treatment Trial. Ann Thorac Surg 2006;82:197–207. [DOI] [PubMed] [Google Scholar]
- 4.Naunheim KS, Wood, DE, Krasna MJ, Decamp MM, Ginsburg ME, McKenna RJ, Criner GJ, Hoffman EA, Sternberg AL, Deschamps CC; for the National Emphysema Treatment Trial Research Group. Predictors of operative mortality and cardiopulmonary morbidity in the National Emphysema Treatment Trial. J Thorac Cardiovasc Surg 2006;131:43–53. [DOI] [PubMed] [Google Scholar]
- 5.National Emphysema Treatment Trial Research Group. Safety and efficacy of median sternotomy versus video-assisted thoracic surgery for lung volume reduction surgery. J Thorac Cardiovasc Surg 2004;127:1350–1360. [DOI] [PubMed] [Google Scholar]
- 6.National Emphysema Treatment Trial Research Group. Cost effectiveness of lung-volume-reduction surgery for patients with severe emphysema. N Engl J Med 2003;348:2092–2102. [DOI] [PubMed] [Google Scholar]
- 7.Ramsey SD, Shroyer AL, Sullivan SD, Wood DE. Updated evaluation of the cost-effectiveness of lung volume reduction surgery. Chest 2007;131:823–832. [DOI] [PubMed] [Google Scholar]
- 8.National Emphysema Treatment Trial Research Group. Patients at high risk of death after lung volume reduction surgery. N Engl J Med 2001;345:1075–1083. [DOI] [PubMed] [Google Scholar]