Abstract
Lung volume reduction surgery (LVRS) is a costly procedure that can improve quality and quantity of life. Given the prevalence of emphysema, the costs involved with its management, and resource constraints on all health care delivery systems, evaluating the cost-effectiveness of LVRS is important. In this article, we describe the purposes and principles of cost-effectiveness analysis and how those principles were applied in evaluating LVRS. We present the results of the cost-effectiveness analysis that was conducted alongside the National Emphysema Treatment Trial and other economic studies of LVRS and discuss how these should be interpreted in the context of current reimbursement guidelines.
Keywords: emphysema, lung volume reduction surgery, clinical trial, cost-effectiveness, quality-adjusted life-years
Lung volume reduction surgery (LVRS) has become a treatment option for severe emphysema. After it was introduced in the 1950s, the procedure was abandoned because of high rates of air leaks and other complications. With advances in surgical techniques, there was a resurgence of LVRS in the 1990s. However, methodological limitations of early LVRS evaluations created uncertainties about its efficacy and concerns about the implications of rapid diffusion of an unproven technology (1–6). In response, a collaboration of federal agencies sponsored a large multicenter trial of LVRS: the National Emphysema Treatment Trial (NETT) (7). The results of the NETT have helped to define the role of LVRS in therapy and clarify the risks and potential benefits of treatment.
LVRS is an expensive procedure for a highly prevalent disease. The high cost of the operation, combined with the substantial number of individuals with emphysema in the United States, raised speculation that LVRS could increase national health expenditures by hundreds of millions of dollars annually (8). In a time of rapidly rising health care costs and tightening health care budgets, LVRS was a clear candidate for economic evaluation.
This article reviews the economic data and issues surrounding LVRS, including the background, rationale, and principles of economic evaluation as they apply to LVRS, and summarizes results of the cost-effectiveness analysis that was performed alongside the NETT, the only formal economic evaluation of LVRS that has been conducted to date.
AN ECONOMIC AND POLICY HISTORY OF LVRS
LVRS is an excellent example of a convergence of clinical and economic factors that fosters a rapid diffusion of a technology. For the tens of thousands of patients with severe emphysema, quality of life and long-term survival are typically poor. Comparative studies have shown that quality of life in severe emphysema is often below that of many cancers, coronary artery disease, and AIDS (9–14). Standard medical treatment offers only modest symptom relief, and no intervention except smoking cessation or long-term, domiciliary, supplemental oxygen therapy alters the course of disease. Some medical interventions, such as the use of oral corticosteroids, offer short-term quality of life benefits but have adverse long-term consequences. As is often seen in cancer, this combination of very poor quality of life coupled with the lack of effective therapies creates an intense demand among patients and their families for new technologies that offer the promise of symptom relief, improved survival, or both.
In 1994, results from a small, uncontrolled study in severe emphysema suggested a functional improvement for patients who underwent LVRS (15). This led to a strong movement to use LVRS, and several institutions throughout the country began actively recruiting patients. In less than 18 months from the initial report, the Centers for Medicare and Medicaid Services (CMS; formerly the Health Care Financing Administration [HCFA]) records indicated that over 1,200 LVRS procedures at an average cost of $35,000 per patient had been performed on Medicare beneficiaries nationwide (16). It is likely that a similar or greater number of procedures were performed for individuals in private health plans over the same period.
Noting the sharp increases in claims for lung resection in 1994–1995, the CMS asked the Agency for Healthcare Research and Quality (AHRQ) (at that time called the Agency for Health Care Policy and Research) to conduct an evidence-based review of LVRS. In addition, the CMS learned from an internal claims review that the mortality at 6 months for patients undergoing LVRS approached 17% (17). On the basis of this finding and the AHRQ evidence review, the CMS announced in December of 1995 that Medicare would not pay for LVRS, on the grounds that there was insufficient safety and efficacy evidence to support reimbursement (6).
To what degree did economic considerations contribute to the CMS's decision to suspend reimbursement for LVRS? Agency officials were concerned that hundreds of millions of dollars would be spent on a procedure whose clinical value was unknown and that might be ineffective or even inferior to medical therapy. To plan for a more informed policy, the CMS announced that, in conjunction with the National Heart, Lung, and Blood Institute, they would cosponsor a multicenter, randomized controlled trial to evaluate the efficacy of LVRS. Medicare would cover LVRS and all emphysema-related medical care for patients who agreed to enroll in the trial. In October of 1997, patients began enrolling in what is now known as the NETT.
As part of the application to become a participating center in the trial, investigators were asked to submit ideas for ancillary studies and several expressed interest in conducting a cost-effectiveness analysis alongside the trial. The AHRQ responded by funding a parallel study to conduct a cost-effectiveness analysis. In the next section, the rationale and methods of cost-effectiveness analysis are reviewed, in particular as they apply to LVRS.
ECONOMIC ANALYSIS OF MEDICAL TECHNOLOGIES: APPLICATION TO LVRS
Given the reality of limited health care budgets, medical care payers are forced to confront the reality that funding new, cost-increasing technologies necessitates spending less in other areas of health care. In this context, some have argued that it is reasonable to consider the outcomes and costs for LVRS relative to outcomes and costs for other medical and surgical procedures for emphysema (18–20). The most widely used approach to compare the relative value of different interventions in creating better health and/or longer life is cost-effectiveness analysis (CEA) (21).
The NETT was one of the first large NIH trials to conduct a parallel analysis of cost-effectiveness alongside the clinical trial. This innovative approach has important advantages. It is an efficient and timely way to obtain data on clinical, economic, and quality of life outcomes simultaneously. Second, performing a CEA alongside a randomized, controlled trial has high internal validity and lower potential for bias (22). The alternative to prospective clinical and economic trials is conducing CEA studies using retrospective data and economic modeling. Such studies can sometimes be performed more quickly but typically rely on weaker evidence and therefore are less well accepted by decision makers and clinicians (23).
Research Question
When comparing a new therapy with an existing therapy for a given population with a given medical condition, cost-effectiveness is measured as the change in costs of care relative to the change in effectiveness of therapy for the new therapy compared with the existing therapy. The difference in costs over the difference in effectiveness of LVRS versus medical therapy (MT), known as the incremental cost effectiveness of LVRS, is derived using the following formula:
 |
(1) |
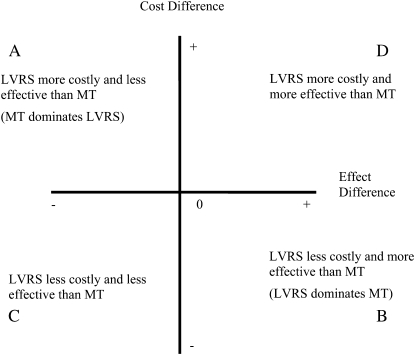
where CLVRS and CMT refer to average total costs, and ELVRS and EMT refer to average total effectiveness for the LVRS and MT arms, respectively. The outcomes of a cost-effectiveness study are typically represented within a four-quadrant CEA plane as shown in Figure 1. The x axis and y axis display the denominator and numerator of Equation 1, respectively. Quadrant A illustrates the scenario where LVRS is less effective (denominator negative) and more costly than MT (numerator positive). In this case, LVRS is said to be dominated by MT; that is, one rejects LVRS and continues with MT. Quadrant B depicts a situation where LVRS improves health outcome and achieves cost savings. In this case, LVRS would be a dominant technology; that is, one would adopt LVRS over MT because LVRS was more effective and less expensive than MT. Quadrant C represents a scenario where LVRS is less expensive than MT but reduces health outcomes compared with standard therapy. Quadrant D shows the cost–outcome relationship for most new medical technologies. Here, health benefits improve but at an additional expense to the health care system. For outcomes in Quadrant D, clinicians, patients, and health care payers must decide whether the improvement in health outcome is worth the additional cost of providing care with the new technology (i.e., what is the maximum acceptable cost-effectiveness ratio from Equation 1). In a health care system with a fixed short-term budget, additional expenditure on a new treatment like LVRS, even if it is considered “cost-effective,” reduces resources that are available to treat other diseases.
Figure 1.
Possible outcomes of lung volume reduction surgery (LVRS) versus medical therapy (MT).
A second important aspect of determining the cost-effectiveness of LVRS versus MT is stating from whose viewpoint or perspective we are evaluating cost-effectiveness. Emphysema involves medical and nonmedical costs and benefits and affects both patients and their families. Accordingly, the societal perspective, which includes all costs and health outcomes that result from LVRS, is the most comprehensive and appropriate measure of cost-effectiveness.
IDENTIFICATION AND VALUATION OF MEASURES OF EFFECTIVENESS AND COSTS
Measure of Effectiveness
The measure of effectiveness for this cost-effectiveness analysis is quality-adjusted life-years (QALYs), a measure of life expectancy adjusted for patient-derived estimates of quality of life over time. Measures of health-related quality of life, when they reflect individual preferences for particular states of health, are known as utilities. Utilities are measured on a scale ranging from 0.0 (death) to 1.0 (optimal health). The utility values (called preference weights) are obtained by interviewing patients using standardized survey techniques at regular intervals over time and placing them in defined health states. These states are weighted using preferences or utilities derived from representatives of the general population. Average utility weights for the treatment and control groups are multiplied by the percent surviving at each period (those who have died have a utility of 0) to obtain a QALY estimate for each group. The quality of life survey instrument used for the NETT analysis was the Quality of Well-Being (QWB) questionnaire, a validated general health status measure that contains several domains, such as mobility, physical activity, social activity, and symptoms/problems (24). The QWB has been used in studies of patients with COPD for more than 20 years and has been shown to be valid, reliable, and responsive to changes in health status for individuals with this condition (25). Survival estimates for patients in each trial arm for each time period postrandomization are multiplied by utility weights for those time periods and summed to determine total QALYs for LVRS and MT, respectively.
Costs
Costs are derived by identifying resources that are consumed during the course of care and assigning values or prices to each resource. From the perspective of society, relevant costs include the costs of (1) direct medical care (including preoperative evaluation, the operation, and all emphysema-related care after surgery), (2) nonmedical care related to the treatment (such as the cost of traveling to and from the clinic), (3) the value of time that family and friends spend caring for the patient, and (4) the value of the patient's time in treatment. Table 1 itemizes these costs and how they were valued for the NETT.
TABLE 1.
VALUATION OF RESOURCES USED
| Cost Element | Source |
|---|---|
| Medicare-covered services | Medicare reimbursements |
| Emphysema-related drugs | AWP less 15% acquisition + dispensing fee |
| Travel costs | Federal travel reimbursement per mile* |
| Patient time | Wages for persons ≥65† |
| Caregiver time | Wages for persons ≤65† |
Definition of abbreviation: AWP = average wholesale price
U.S. General Services Administration. Privately owned vehicle reimbursement rates. 2003. Available from: http://www.gsa.gov/Portal/content/policies_content.jsp?contentOID=115105&contentType=1006&PMTT=1 (accessed December 3, 2002).
U.S. Department of Labor, Bureau of Labor Statistics. Overview of BLS statistics on wages, earnings, and benefits. 2002. Available from: http://www.bls.gov/bls/wages.htm (accessed December, 2002).
Extrapolating beyond the Trial Period
Cost-effectiveness ratios can be computed for the trial period, but when considering the cost-effectiveness of any treatment, one should consider the duration of time over which costs and benefits accrue to patients. Because LVRS may affect quality of life and survival, it is important to estimate the cost-effectiveness of LVRS projecting costs and outcomes beyond the time horizon of the trial. Such projections require mathematical modeling. It is best to use conservative modeling assumptions that do not inflate the outcomes observed in the trial. The NETT analysis used modeling to project costs, quality of life, and survival beyond the trial. In the case of survival, the relative hazard rate for survival for the surgery group versus the MT group was set at 1 (no survival benefit) beyond observed survival for Year 3. Projected costs and QWB scores were based on trend lines fitted to value observed for each group through Year 3.
Allowance for Uncertainty
Uncertainty in cost-effectiveness can be assessed using one- and multiway sensitivity analysis, where one or more parameters are varied across a range to determine their impact on the incremental analysis. Others advocate using probabilistic methods to better capture combined uncertainty in all model parameters (26, 27). Probabilistic analyses are commonly conducted using Monte Carlo simulations. Output can include confidence intervals and cost-effectiveness acceptability curves, creating a confidence interval around the estimate of incremental cost-effectiveness (28). There is one potential difficulty with confidence intervals for cost-effectiveness ratios: If the interval for the numerator or denominator of the ratio crosses 0, the ratio can range from a negative to positive number. In this case, it is difficult to tell whether the ratio is negative because the new technology is less expensive or less effective than the old technology. To address this potential problem, analysts derive cost-effectiveness acceptability curves (29). The curve represents the probability that the technology in question is associated with a cost per QALY gained that is lower than the corresponding cost-effectiveness ratio that is an “acceptable” threshold to the viewer. The value of this threshold ratio at a probability of 0.5 is the median cost per QALY for the technology.
COST-EFFECTIVENESS OF LVRS: EVIDENCE FROM THE NETT
NETT researchers estimated the cost-effectiveness of LVRS compared with MT during the 3 years after initiation of treatment at $190,000 per QALY gained (Table 2) (30). An important feature of the NETT evaluation was the creation of four post hoc subgroups of patients. These subgroups were defined by combinations of two baseline characteristics: (1) the presence or absence of upper-lobe predominance in emphysema distribution (by computed tomography scan) and (2) low versus high maximal exercise capacity after pulmonary rehabilitation (≤25 watts for female patients, ≤40 watts for male patients). A secondary analysis of the NETT trial enrollees demonstrated differential relative benefits from LVRS in the patient subgroups. There were significant benefits for those with upper lobe emphysema and low initial exercise capacity. For two other groups (upper lobe, high exercise capacity, and non–upper lobe, low exercise capacity), there were significant, but weaker, effects. For the fourth group (non–upper lobe, high exercise capacity), surgery doubled 2-year mortality risk without symptomatic improvement compared with MT.
TABLE 2.
PROJECTED AND OBSERVED COST-EFFECTIVENESS RATIOS FOR LUNG VOLUME REDUCTION SURGERY VERSUS MAXIMAL MEDICAL THERAPY AT OBSERVED AND PROJECTED YEARS OF FOLLOW-UP FROM RANDOMIZATION, USING OBSERVATIONS UP TO 3 AND 5 YEARS POSTRANDOMIZATION
| Incremental Cost Effectiveness Ratio† for Subgroups Defined by:
|
||||
|---|---|---|---|---|
| Time Period | All Patients* | Upper-Lobe Emphysema, Low Exercise Capacity† | Upper-Lobe Emphysema, High Exercise Capacity† | Non–Upper-Lobe Emphysema, Low Exercise Capacity† |
| Observed up to 3 yr | $190,000 | $98,000 | $236,500 | $326,000 |
| Observed up to 5 yr | $140,000 | $77,000 | $170,000 | $225,000 |
| Projected at 10 yr based on 3 yr of follow-up‡ | $58,000 | $21,000 | $54,000 | Dominated§ |
| Projected at 10 yr based on 5 yr of follow-up‡ | $54,000 | $48,000 | $40,000 | $87,000 |
Results exclude 140 patients previously found to be at high risk for death, 3 patients who were not enrolled in Medicare, 8 patients who were enrolled in Medicare+Choice plans, and 1 patient who was missing claims records. Lung volume reduction surgery was not cost-effective for patients in the subgroup with non–upper-lobe predominant emphysema and high exercise capacity who had higher costs and reduced quality-adjusted life-years (QALYs) compared with the medical group.
Costs and QALYs after Year 1 are discounted at 3% per annum.
See text for description of the method of projecting costs and QALYs.
Patients undergoing lung volume reduction surgery in the subgroup with non–upper-lobe predominant emphysema and low exercise capacity had higher total costs and fewer QALYs compared with patients in the medical group.
Because identification of outcomes by patient characteristic may influence clinical practice, it is reasonable to evaluate the cost-effectiveness of subgroups where surgery had more favorable outcomes than MT. In the NETT, three subgroups—upper lobe, low exercise; upper lobe, high exercise; lower lobe, low exercise—had improvements in either quality of life, survival, or both compared with MT patients. The cost-effectiveness analysis of the LVRS at 3-year follow-up was most favorable for patients with upper-lobe emphysema and low exercise capacity ($98,000 per additional QALY; Table 2). Although total health-related costs were higher for this subgroup compared with all patients, the relative improvement in quality-adjusted survival for the surgery versus medical groups was greater, resulting in improved cost-effectiveness. The cost-effectiveness over 3 years of follow-up was much less favorable in the two remaining subgroups of patients: upper-lobe predominant emphysema with high exercise capacity ($236,500 per QALY gained) and non–upper-lobe predominant emphysema with low exercise capacity ($326,000 per QALY gained).
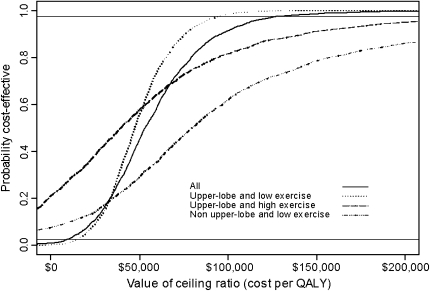
Two years after completion of the trial, we reevaluated the cost-effectiveness of LVRS using data available from the longer follow-up period. Using up to 5 years of follow-up data, the cost-effectiveness of LVRS versus MT was $140,000 per QALY gained (95% confidence interval, $40,155 to $239,359) at 5 years and was projected to be $54,000 per QALY gained at 10-years. In subgroup analysis, the cost-effectiveness of LVRS in patients with upper lobe emphysema and low exercise capacity was $77,000 per QALY gained at 5 years and was projected to be $48,000 per QALY at 10 years. The cost-effectiveness of LVRS for the other subgroups improved in the reanalysis compared with the original report (Table 2) (31). Uncertainty analysis of the 10-year findings suggested a moderate degree of uncertainty, particularly for the subgroups (Figure 2).
Figure 2.
Ten-year cost-effectiveness acceptability curves for lung volume reduction surgery (LVRS) versus medical therapy for all patients and for three subgroups with significantly improved clinical outcomes in the LVRS arm (reduced mortality, improved quality of life, or both). The curve represents the probability that LVRS is associated with a cost per quality-adjusted life-year (QALY) gained that is lower than the corresponding cost-effectiveness ratios displayed on the x axis. The value of the ceiling ratio at a probability of 0.5 is the median cost per QALY for LVRS. Solid horizontal lines denote 95% confidence limits for the projections.
Other Studies of the Cost-Effectiveness of LVRS
Five Canadian centers conducted a randomized, controlled trial of LVRS compared with optimal MT (best medical care [BMC]). After pulmonary rehabilitation, 32 patients were randomized to the LVRS arm and 30 patients to the BMC arm. Unlike the NETT, the study was designed with a general measure of quality of life, the Health Utility Index, as the primary outcome. Patients were followed for 2 years. The overall 2-year survival was similar in each arm of the study. The Health Utility Index showed a clinically important improvement in quality of life for the LVRS group at the 2-year assessment (0.104) but was underpowered to detect modest improvements, and the difference failed to achieve statistical significance (0.104; 95% confidence interval, −0.06 to 0.27; P = 0.19). There was a nonsignificant gain in QALYs of 0.21 (P = 0.19) in the LVRS arm over the BMC arm. The incremental cost-effectiveness of LVRS compared with BMC was $133,900 per QALY gained (2006 Canadian dollars) (32).
Implications of Cost-Effectiveness for Reimbursement and Use of LVRS
Many experts propose that the cost-effectiveness of LVRS should be considered relative to outcomes and costs for other medical and surgical procedures (6, 33, 34). Over the observation period, the cost-effectiveness of LVRS compared with MT was relatively unfavorable compared with other common thoracic surgical procedures (Table 3), but the projected results suggested that LVRS could have a level of cost-effectiveness that is considered reasonable. It does not seem that the results of the cost-effectiveness analysis played a central role in the national coverage decision by CMS to cover LVRS for selected patients.
TABLE 3.
COST-EFFECTIVENESS RATIOS FOR OTHER PROCEDURES IN THORACIC SURGERY
| Procedure | Cost-Effectiveness Ratios (in 2002 dollars per quality-adjusted life-year gained) |
|---|---|
| Coronary artery bypass surgery | 8,300–64,000* |
| Lung transplantation | 133,000–216,000† |
| Heart transplantation compared with medical therapy | 65,000‡ |
| Implantable cardioverter defibrillator for survivors of cardiac arrest with a low cardiac ejection fraction | 47,000§ |
Pliskin JS, Stason WB, Weinstein MC, Cohn PF, McEnany MT, Braun P. Coronary artery bypass graft surgery: clinical decision making and cost-effectiveness analysis. Med Decis Making 1981;1:10–28. Weinstein MC, Stason WB. Cost-effectiveness of coronary artery bypass surgery. Circulation 1982;66:III56–66. Harvard Center for Risk Analysis. The CEA registry: standardizing the methods and practices of cost-effectiveness analysis. Available from: http://www.hsph.harvard.edu/cearegistry/ (accessed February 13, 2002).
Ramsey SD, Patrick DL, Albert RK, Larson EB, Wood DE, Raghu G. The cost-effectiveness of lung transplantation: a pilot study. University of Washington Medical Center Lung Transplant Study Group. Chest 1995;108:1594–1601. Al MJ, Koopmanschap MA, van Enckevort PJ, Geertsma A, van der Bij W, de Boer WJ, TenVergert EM. Cost-effectiveness of lung transplantation in The Netherlands: a scenario analysis. Chest 1998;113:124–130.
van Hout B, Bonsel G, Habbema D, van der Maas P, de Charro F. Heart transplantation in the Netherlands; costs, effects and scenarios. J Health Econ 1993;12:73–93.
Harvard Center for Risk Analysis. The CEA registry: standardizing the methods and practices of cost-effectiveness analysis. Available from: http://www.hsph.harvard.edu/cearegistry/ (accessed February 13, 2002). Owens DK, Sanders GD, Harris RA, McDonald KM, Heidenreich PA, Dembitzer AD, Hlatky MA. Cost-effectiveness of implantable cardioverter defibrillators relative to amiodarone for prevention of sudden cardiac death. Ann Intern Med 1997;126:1–12.
Assuming public and private health plans agree to pay for LVRS, how much will these procedures add to the nation's health budget? Before the trial, many predicted that LVRS would be a significant financial burden for health insurers (35). The CMS has specified that LVRS would be performed at NETT facilities and sites that have been approved by Medicare as lung transplant facilities (36) and has delegated to the Joint Commission on Accreditation of Healthcare Organizations a process for certifying additional LVRS centers (37). The CMS implemented its final coverage policy on January 5, 2004 (38). Some private health insurance plans have agreed to cover LVRS for persons under age 65 since the CMS coverage policy was implemented (39, 40). The private health plans that cover LVRS do not seem to have facility restrictions similar to the CMS policy.
The NETT cost-effectiveness research team originally estimated that 10,000 persons may meet eligibility criteria for LVRS annually, with a resulting impact on national health expenditures of $100 million to $300 million per year, depending on patients' interest in the procedure and their suitability after pulmonary rehabilitation. An analysis of Medicare claims for LVRS since the coverage was implemented shows that the actual budget impact has been substantially less. Between January 2004 and September 2005, the CMS paid only 258 claims for LVRS (31). This modest use suggests that, given the evidence regarding clinical and economic benefit, there is relatively limited demand for the LVRS, despite the relatively open CMS coverage policy. LVRS-related expenditures by CMS are likely less than $21 million annually.
Future Research on the Cost-Effectiveness of LVRS
There is little likelihood that other large-scale, controlled studies of LVRS will be conducted in the future. New technologies that do not involve sternotomy have been developed to reduce nonfunctional lung volume and are being studied in large-scale clinical trials. If these technologies are shown to be effective in clinical testing, it would be reasonable to compare economic outcomes to current LVRS. Clinical trials necessarily involve more intensive patient monitoring than typical clinical practice, and the depth of experience of the participating clinical centers also influences economic outcomes (41). A reasonable question is then whether the cost-effectiveness of LVRS as performed in clinical practice compares with the result calculated from the clinical trial. As with clinical outcomes, cost-effectiveness in typical practice may be less favorable than what was seen in the trial. The Joint Commission on Accreditation of Healthcare Organizations standards may help reduce the discrepancy between outcomes and costs observed in the NETT and those in clinical practice. These considerations should encourage further research into the clinical and economic effects of LVRS after sufficient patient experience in clinical practice has accumulated.
CONCLUSIONS
The NETT was a uniquely funded study that included clinical and economic endpoints for evaluating LVRS. The economic evaluation reported alongside the primary outcomes of the trial suggested that LVRS had a relatively unfavorable cost-effectiveness overall but had more favorable economic outcomes for patients with low exercise capacity and upper lobe emphysema on post hoc analysis. Reevaluation using longer follow-up data and projecting beyond the trial horizon suggested the LVRS may have favorable cost-effectiveness, particularly for the aforementioned subgroup. Since the report of the trial, use of LVRS among Medicare patients nationally has been modest, limiting the financial impact of the procedure.
The National Emphysema Treatment Trial (NETT) is supported by contracts with the National Heart, Lung, and Blood Institute (N01HR76101, N01HR76102, N01HR76103, N01HR76104, N01HR76105, N01HR76106, N01HR76107, N01HR76108, N01HR76109, N01HR76110, N01HR76111, N01HR76112, N01HR76113, N01HR76114, N01HR76115, N01HR76116, N01HR76118, and N01HR76119), the Centers for Medicare and Medicaid Services (CMS), and the Agency for Healthcare Research and Quality (AHRQ).
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Flaherty KR, Kazerooni EA, Curtis JL, Iannettoni M, Lange L, Schork MA, Martinez FJ. Short-term and long-term outcomes after bilateral lung volume reduction surgery: prediction by quantitative CT. Chest 2001;119:1337–1346. [DOI] [PubMed] [Google Scholar]
- 2.Geddes D, Davies M, Koyama H, Hansell D, Pastorino U, Pepper J, Agent P, Cullinan P, MacNeill SJ, Goldstraw P. Effect of lung-volume-reduction surgery in patients with severe emphysema. N Engl J Med 2000;343:239–245. [DOI] [PubMed] [Google Scholar]
- 3.Sciurba FC, Rogers RM, Keenan RJ, Slivka WA, Gorcsan J 3rd, Ferson PF, Holbert JM, Brown ML, Landreneau RJ. Improvement in pulmonary function and elastic recoil after lung-reduction surgery for diffuse emphysema. N Engl J Med 1996;334:1095–1099. [DOI] [PubMed] [Google Scholar]
- 4.Gelb AF, McKenna RJ Jr, Brenner M, Schein MJ, Zamel N, Fischel R. Lung function 4 years after lung volume reduction surgery for emphysema. Chest 1999;116:1608–1615. [DOI] [PubMed] [Google Scholar]
- 5.Pompeo E, Marino M, Nofroni I, Matteucci G, Mineo TC. Reduction pneumoplasty versus respiratory rehabilitation in severe emphysema: a randomized study. Pulmonary Emphysema Research Group. Ann Thorac Surg 2000;70:948–953. [Discussion, 954.] [DOI] [PubMed] [Google Scholar]
- 6.Agency for Health Care Policy and Research. Lung volume reduction surgery for end-stage chronic obstructive pulmonary disease. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service; 1996. Publication No. 96-0062.
- 7.The National Emphysema Treatment Trial Research Group. Rationale and design of the National Emphysema Treatment Trial: a prospective randomized trial of lung volume reduction surgery. Chest 1999;116:1750–1761. [DOI] [PubMed] [Google Scholar]
- 8.Gentry C. Second opinion: why Medicare covers a new lung surgery for just a few patients. Wall Street Journal June 29, 1998:A1.
- 9.Kaplan RM, Atkins CJ, Timms R. Validity of a quality of well-being scale as an outcome measure in chronic obstructive pulmonary disease. J Chronic Dis 1984;37:85–95. [DOI] [PubMed] [Google Scholar]
- 10.Kaplan RM, Anderson JP, Patterson TL, McCutchan JA, Weinrich JD, Heaton RK, Atkinson JH, Thal L, Chandler J, Grant I. Validity of the Quality of Well-Being Scale for persons with human immunodeficiency virus infection. HNRC Group. HIV Neurobehavioral Research Center. Psychosom Med 1995;57:138–147. [DOI] [PubMed] [Google Scholar]
- 11.Kaplan RM, Hartwell SL, Wilson DK, Wallace JP. Effects of diet and exercise interventions on control and quality of life in non-insulin-dependent diabetes mellitus. J Gen Intern Med 1987;2:220–228. [DOI] [PubMed] [Google Scholar]
- 12.Ganiats TG, Palinkas LA, Kaplan RM. Comparison of Quality of Well-Being scale and Functional Status Index in patients with atrial fibrillation. Med Care 1992;30:958–964. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan RM. Quality of life assessment for cost/utility studies in cancer. Cancer Treat Rev 1993;19(Suppl A):85–96. [DOI] [PubMed] [Google Scholar]
- 14.Kaplan RM. Decisions about prostate cancer screening in managed care. Curr Opin Oncol 1997;9:480–486. [DOI] [PubMed] [Google Scholar]
- 15.Cooper JD, Trulock EP, Triantafillou AN, Patterson GA, Pohl MS, Deloney PA, Sundaresan RS, Roper CL. Bilateral pneumectomy (volume reduction) for chronic obstructive pulmonary disease. J Thorac Cardiovasc Surg 1995;109:106–116. [Discussion, 116] [DOI] [PubMed] [Google Scholar]
- 16.Huizenga HF, Ramsey SD, Albert RK. Estimated growth of lung volume reduction surgery among Medicare enrollees: 1994 to 1996. Chest 1998;114:1583–1587. [DOI] [PubMed] [Google Scholar]
- 17.Holohan TV, Handelsman H. Lung-volume reduction surgery for end-stage chronic obstructive pulmonary disease. Health Technol Assess (Rockv) 1996;:1–30. [PubMed]
- 18.Snider GL. Health-care technology assessment of surgical procedures: the case of reduction pneumoplasty for emphysema. Am J Respir Crit Care Med 1996;153:1208–1213. [DOI] [PubMed] [Google Scholar]
- 19.Make BJ, Fein AM. Is volume reduction surgery appropriate in the treatment of emphysema? No. Am J Respir Crit Care Med 1996;153:1205–1207. [DOI] [PubMed] [Google Scholar]
- 20.Utz JP, Hubmayr RD, Deschamps C. Lung volume reduction surgery for emphysema: out on a limb without a NETT. Mayo Clin Proc 1998;73:552–566. [DOI] [PubMed] [Google Scholar]
- 21.Gold MR, Siegal JE, Russel LB, Weinstein MC, editors. Cost-effectiveness in health and medicine. New York: Oxford University Press; 1996.
- 22.Ramsey S, Willke R, Briggs A, Brown R, Buxton M, Chawla A, Cook J, Glick H, Liljas B, Petitti D, et al. Good research practices for cost-effectiveness analysis alongside clinical trials: the ISPOR RCT-CEA Task Force report. Value Health 2005;8:521–533. [DOI] [PubMed] [Google Scholar]
- 23.Neumann PJ. Why don't Americans use cost-effectiveness analysis? Am J Manag Care 2004;10:308–312. [PubMed] [Google Scholar]
- 24.Kaplan RM, Anderson JP. The General Health Policy Model: an integrated approach. In: Spilker B, editor. Quality of life and pharmacoeconomics in clinical trials, 2nd ed. Philadelphia: Lippincott-Raven Publishers; 1996. pp. 309–322.
- 25.Ries AL, Kaplan RM, Limberg TM, Prewitt LM. Effects of pulmonary rehabilitation on physiologic and psychosocial outcomes in patients with chronic obstructive pulmonary disease. Ann Intern Med 1995;122:823–832. [DOI] [PubMed] [Google Scholar]
- 26.Briggs AH, Goeree R, Blackhouse G, O'Brien BJ. Probabilistic analysis of cost-effectiveness models: choosing between treatment strategies for gastroesophageal reflux disease. Med Decis Making 2002;22:290–308. [DOI] [PubMed] [Google Scholar]
- 27.Griffin S, Claxton K, Hawkins N, Sculpher M. Probabilistic analysis and computationally expensive models: necessary and required? Value Health 2006;9:244–252. [DOI] [PubMed] [Google Scholar]
- 28.Chaudhary MA, Stearns SC. Estimating confidence intervals for cost-effectiveness ratios: an example from a randomized trial. Stat Med 1996;15:1447–1458. [DOI] [PubMed] [Google Scholar]
- 29.Briggs AH, O'Brien BJ, Blackhouse G. Thinking outside the box: recent advances in the analysis and presentation of uncertainty in cost-effectiveness studies. Annu Rev Public Health 2002;23:377–401. [DOI] [PubMed] [Google Scholar]
- 30.National Emphysema Treatment Trial Research Group. Cost effectiveness of lung-volume-reduction surgery for patients with severe emphysema. N Engl J Med 2003;348:2092–2102. [DOI] [PubMed] [Google Scholar]
- 31.Ramsey SD, Sullivan SD, Shroyer AL, Wood DE. Updated evaluation of the cost-effectiveness of lung volume reduction surgery. Chest 2007;131:823–832. [DOI] [PubMed] [Google Scholar]
- 32.Miller JD, Malthaner RA, Goldsmith CH, Goeree R, Higgins D, Cox PG, Tan L, Road JD. A randomized clinical trial of lung volume reduction surgery versus best medical care for patients with advanced emphysema: a two-year study from Canada. Ann Thorac Surg 2006;81:314–320. [Discussion, 320–311.] [DOI] [PubMed] [Google Scholar]
- 33.Drazen JM. Surgery for emphysema: not for everyone. N Engl J Med 2001;345:1126–1127. [DOI] [PubMed] [Google Scholar]
- 34.Fein AM. Lung volume reduction surgery: answering the crucial questions. Chest 1998;113(Suppl):277S–282S. [DOI] [PubMed] [Google Scholar]
- 35.Grady D. Medicare to pay for major lung operation. New York Times August 21, 2003;A22.
- 36.Centers for Medicare and Medicaid Services. Facilities eligible for reimbursement for lung volume reduction surgery [Internet] [accessed December 7, 2006]. U.S. Department of Health and Human Services. Available from: http://www.cms.hhs.gov/MedicareApprovedFacilitie/LVRS/list.asp?filterType=none&filterByDID=0&sortByDID=1&sortOrder=ascending&intNumPerPage=10
- 37.Joint Commission on Accreditation of Healthcare Organizations. Lung volume reduction surgery certification requirements, 6/19/06 [Internet] [accessed December 7, 2006]. Available from: http://www.jointcommission.org/NR/rdonlyres/12EFD2D8–6FC9–498C–9AD8–944D8794CF45/0/LVRS_final_addendum.pdf
- 38.Centers for Medicare and Medicaid Services. Medicare national coverage determinations—lung volume reduction surgery: Pub 100–03, transmittal 44. [Internet] 2005 December 2 [cited 2007 December 4]. Available from: www.cms.hhs.gov/transmittals/downloads/R44NCD.pdf
- 39.Cigna Healthcare Coverage Position. Lung volume reduction surgery [Internet] [accessed December 7, 2006]. Coverage position number: 0218. Effective date: November 15, 2006. Available from: http://www.cigna.com/health/provider/medical/procedural/coverage_positions/medical/mm_0218_coveragepositioncriteria_lung_volume_reduction_surgery.pdf
- 40.The Regence Group. Medical policy on lung volume reduction surgery [Internet] [accessed December 4, 2007]. Available from: http://regence.com/trgmedpol/surgery/sur31.html
- 41.Wagner JL, Alberts SR, Sloan JA, Cha S, Killian J, O'Connell MJ, Van Grevenhof P, Lindman J, Chute CG. Incremental costs of enrolling cancer patients in clinical trials: a population-based study. J Natl Cancer Inst 1999;91:847–853. [DOI] [PubMed] [Google Scholar]