Abstract
Delineating the extent and distribution of emphysema is an essential component of the evaluation of candidates for lung volume reduction surgery (LVRS). Imaging also may identify contraindications to LVRS, including bronchiectasis and pleural scarring. The chest X-ray is of limited utility in LVRS evaluation. Chest computed tomography (CT) scanning is an essential component of the evaluation, demonstrating the presence of emphysema and its amount and distribution. Clinical experience has shown that a substantial minority of chest CT scans will also demonstrate pulmonary nodules, some of which represent lung cancers. Published series, including the National Emphysema Treatment Trial, consistently demonstrate that patients with upper lobe predominant or heterogeneous emphysema are most likely to benefit from LVRS. Heterogeneity and distribution can also be assessed by radionuclide ventilation perfusion scanning, but this modality adds little additional information to CT scanning.
Keywords: emphysema, lung volume reduction surgery, CT scanning, pulmonary nodule, preoperative evaluation
The hallmarks of advanced chronic obstructive pulmonary disease (COPD) are significant dyspnea on exertion and incompletely reversible airflow obstruction. Although the clinical characterization of the “pink puffer” has been proposed to represent the patient with emphysema, as opposed to chronic bronchitis, clinical experience is that clinical presentation and physical exam are not reliable indicators of the presence or absence of underlying emphysema. In practice, differentiating emphysema from chronic airways disease has been of little importance, as the available therapies for patients with COPD have not been specific to the underlying pathology. This is no longer true now that lung volume reduction surgery (LVRS) has been established as benefiting selected patients with emphysema. Current concepts are that individuals without significant emphysema are unlikely to experience benefit from LVRS (1, 2). Patients with the converse in characteristics, namely significant emphysema without substantial airflow obstruction, are less common and there is little experience with LVRS in such subjects.
Historically, imaging of the chest has had a limited role in the evaluation of patients with obstructive lung disease. Much of the emphasis in the diagnosis and classification of patients with COPD has been on the severity and degree of reversibility of airflow obstruction as assessed by spirometry. Indeed, the commonly used classification systems rely on FEV1 and the FEV1/FVC to classify patients as having airflow obstruction and to assign a severity of impairment (3). Before the reintroduction of LVRS, there was little clinical utility to determining whether the origin of airflow obstruction was due to emphysema or chronic bronchitis, as the therapeutic choices were largely independent of the underlying pathology. One exception to this generalization is establishing the diagnosis of α1-antiprotease deficiency, for which supplementation therapy is often provided when there is evidence of significant airflow obstruction or emphysema.
With recognition that LVRS benefits selected subjects with COPD, establishing the diagnosis of emphysema has assumed new importance. The favorable effects of LVRS are believed to be due to the surgical resection of emphysematous lung tissue and amelioration of its deleterious impact on the mechanical properties of the lung (1, 4–6). Based upon this understanding, the use of techniques to define the presence, severity, and extent of anatomic abnormality assume more importance in selecting appropriate patients for such intervention. Radiographic assessment of patients considering LVRS has three important functions: (1) to establish an anatomic diagnosis of emphysema; (2) to delineate the extent and distribution of emphysema; and (3) to document the presence or absence of other conditions that represent a contraindication to the procedure, or require additional consideration in formulating a treatment recommendation. This radiographic assessment is most often accomplished by using computed tomography (CT) scanning of the chest, although some clinicians also use a radionuclide ventilation–perfusion (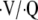 ) scan to provide additional information concerning the heterogeneity and distribution of the functional disruption caused by emphysema.
) scan to provide additional information concerning the heterogeneity and distribution of the functional disruption caused by emphysema.
Arguably, radiographic assessment of the patient considering LVRS using chest CT is the decisive element in determining whether a patient is likely to benefit from the procedure. A body of data from both randomized trials and case report series support the concept that the extent, characteristics, and anatomic distribution of parenchymal destruction are of critical importance in estimating the likelihood of benefit (7–9).
ROLE OF THE CHEST X-RAY IN THE EVALUATION OF THE PATIENT CONSIDERING LVRS
The posteroanterior (PA) and lateral chest radiograph are of limited use in the evaluation for LVRS. Thurlbeck and Simon reported that findings on the chest radiograph have a sensitivity of 40% in detecting emphysema (10). Muller and Coxson state that “limitations of chest radiography in the diagnosis of emphysema include low specificity… considerable interobserver disagreement in the interpretation of findings, and inability to quantify the severity of emphysema” (11). Given these considerations, a chest X-ray is of limited use in LVRS evaluation. If an existing chest radiograph is available for review during the initial evaluation of an LVRS candidate, it should be examined for the presence of significant pleural scarring, pulmonary nodules, and interstitial disease. As described below, however, the likelihood of finding an absolute contraindication to LVRS is small and, given the need for a more precise delineation of the extent and distribution of emphysema, evaluation for LVRS should include a CT scan of the chest.
ROLE OF CT SCANNING IN LVRS EVALUATION
In LVRS evaluation, CT scanning is used to establish the presence of emphysema, quantify the burden of emphysematous destruction, and characterize the distribution of emphysema within the lungs. These factors are used to generate an estimate of the likelihood of improvement after LVRS. The CT scan is also used to identify concomitant conditions that either require evaluation or represent a contraindication to proceeding with LVRS.
DIAGNOSING AND QUANTIFYING EMPHYSEMA ON CHEST CT SCANS
Emphysema is defined as destruction of the lung distal to the terminal bronchiole. Such destruction is recognized radiographically as the absence of normally present lung structure. CT scanning allows the quantitation of the density of the volume of tissue in each voxel, using Hounsfield Units (HU) as the unit of density (−1,000 HU represents air, 0 HU represents water, and +1,000 HU bone). There are two widely used approaches to quantify the amount of emphysema on a CT scan: semiquantitative analysis performed by an observer (12–14) and a quantitative analysis performed by computer software.
In the most commonly used semiquantitative approach, the reader assesses the severity of emphysema at three locations in each lung. For each of these six locations, a numerical score of 0 to 4 is assigned, in which 0 represents no emphysema, 1: 1 to 25%; 2: 26 to 50%; 3: 51 to 75%; and 4: 76 to 100%. In this system, the total score can range from 0 to 24. In addition to this quantitation, a qualitative assessment of the heterogeneity of the emphysema at each location can be made. Published data demonstrate that this assessment can be performed by experienced radiologists or other experienced clinicians, such as pulmonologists, with reasonable agreement (12, 15). In the National Emphysema Treatment Trial (NETT), a training set of CT scans was used to promote consistency in interpretation. Despite this and the use of an interested and trained group of chest radiologists, there was still significant inter- and intra-observer variability in emphysema quantitation.
Automated emphysema quantification can be performed by any of a number of available software programs. The approach used is to identify the lung parenchyma and mathematically “dissect” it away from chest wall, mediastinum, and other anatomic structures. The density of each voxel within the lung field is then assessed. The resulting data can be presented in several different manners, including a histogram of the percentage of lung with densities in specified ranges, pictorially as normal and emphysematous lung, or by a single parameter derived from the histogram.
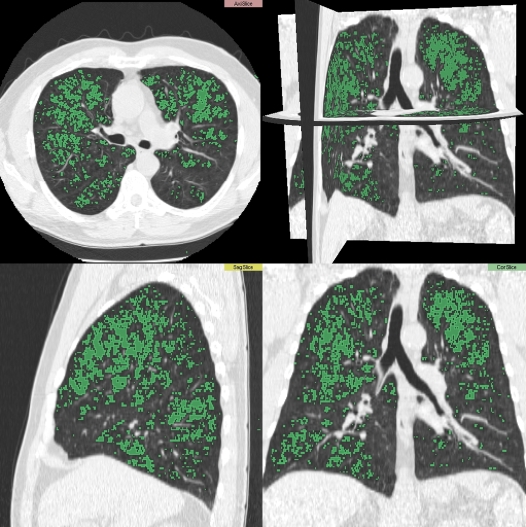
The most common approach to quantitation involves a densitometric analysis, in which a threshold is chosen to differentiate normal lung tissue from emphysema, based on studies relating CT properties to anatomic characteristics of the lung (16, 17). This threshold is typically set at a value ranging from −850 HU to −950 HU, most commonly between −910 HU and −950 HU. Visually, these areas can be identified with a different color than that of “normal” lung tissue (see Figure 1). The amount of emphysema can be reported as the percentage of lung below this threshold, either for the lungs in total or for defined regions. Alternatively, the data are reported as the value from the density histogram that defines the least dense proportion of the lung; the “Perc15” is most commonly used and represents the HU value that divides the least dense 15% of the lung from the densest 85% (with increasing emphysema, the Perc15 is increasingly negative) (18). Of these two approaches, densitometric thresholding to determine the percent of lung that is emphysematous is more commonly used.
Figure 1.
Densitometric analysis showing areas of emphysema in green.
In addition to quantifying the total amount of emphysema present, both the observer and software approaches can also be used to classify the anatomic distribution of emphysema. Of particular importance in the LVRS evaluation is the determination of whether the emphysema is present in an “upper-lobe predominant” distribution or another distribution, such as diffuse or lower-lobe predominant. In the observer-based methodology, this classification is based on subjective assessment and/or scoring the upper location in each chest with a higher number on the semiquantitative scale. In the automated system, the characterization is usually based on the comparison of the percent emphysema in the upper lung (UL) and percent emphysema in lower lung (LL). If % UL > % LL, then the patient is classified as having “upper-lobe predominant” emphysema.
Data from the NETT comparing these two approaches to the classification of emphysema distributions show a modest rate of concordance between the two (kappa = 0.49). In the NETT, there was a high rate of concordance between the automated and radiologist-based approaches in patients that the radiologists classified as “upper-lobe predominant”; of 254 patients with low exercise capacity classified as having upper-lobe predominant disease by the radiologist, 237 (93%) were also classified as upper-lobe predominant by automated quantitative analysis. The automated approach was more likely to classify patients as having upper-lobe predominant disease than was the radiologists' interpretation (758/977 versus 634/977, respectively). These data are consistent with the reports of others that classification of the pattern of emphysema by automated systems differs from that by human observers (19).
Published data show that the automated approach to quantitation of emphysema is highly reproducible. It has not been shown, however, that the automated approach defining the extent and distribution of emphysema offers any substantial advantage in predicting response to LVRS over the characterization supplied by an experienced observer using a semiquantitative scoring approach.
USING CT ANALYSIS TO PREDICT THE RESPONSE TO SURGERY
There are two components of the response to LVRS: short-term risk of morbidity and death and long-term changes in functional status and mortality rates. The CT scan provides important information about each of these components in LVRS patients.
Two closely related characteristics assessed by the CT scan are associated with both risk and benefit of LVRS: “heterogeneity” and the anatomic distribution of emphysema. The latter is often categorized as “upper-lobe predominant” or not and the former as “heterogeneous” or as having “target areas.”
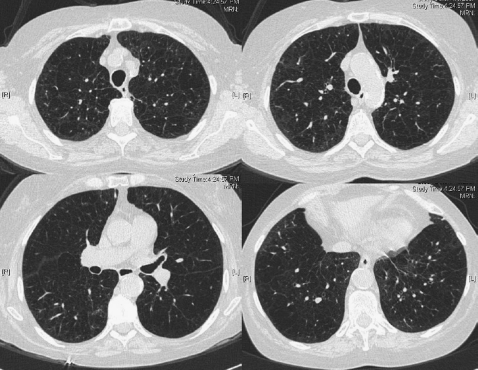
The general clinical experience has been that patients with homogeneous or nonheterogeneous distribution of their emphysema are less likely to benefit from LVRS (7, 8, 20, 21). Examples of this pattern are illustrated in Figures 2 and 3. In addition to the lack of benefit, patients with very severe airflow obstruction, defined as an FEV1 less than or equal to 20% predicted, and a homogeneous distribution of emphysema on CT scan were found to have a high mortality in the NETT, a randomized trial of LVRS compared with medical therapy (18% at 30 d; ∼35% at 5 mo versus 0% and 5%, respectively, in medical control subjects) (22).
Figure 2.
Diffuse distribution of emphysema.
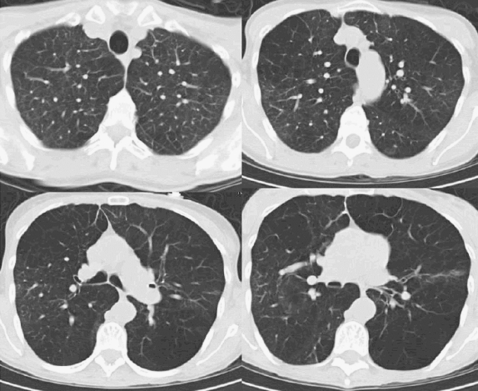
Figure 3.
Non–upper-lobe distribution of emphysema.
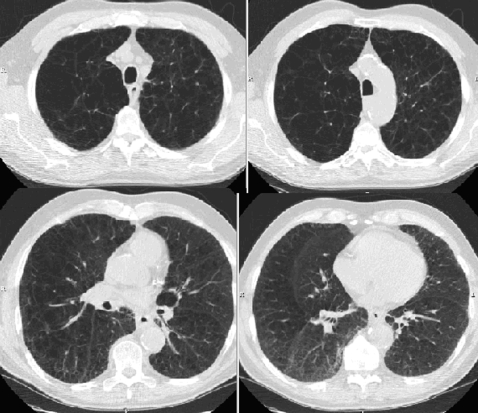
The original reports of the reintroduction of LVRS suggested that patients with upper-lobe predominant or heterogeneous distribution of emphysema on CT scan experience significant benefit from LVRS. An example of this pattern is shown in Figure 4. On the basis of this, the NETT was designed in part to test the hypothesis that patients with upper-lobe predominant or heterogeneous emphysema are more likely to benefit from LVRS. The results of the trial were consistent with this hypothesis. In NETT, two preoperative characteristics were retrospectively identified as having important prognostic value for functional, health-related quality of life and mortality benefit: upper-lobe distribution of emphysema and post-rehabilitation exercise capacity. Patients with upper-lobe predominant emphysema and low exercise capacity (⩽25 watts for women, ⩽40 watts for men) had a substantial reduction in mortality (risk ratio of 0.47, P = 0.005). Mortality was not affected in patients with upper-lobe predominant disease and high exercise capacity. Table 1 summarizes the relationship of other measures of response to LVRS and the anatomic distribution of emphysema on CT scans.
Figure 4.
Upper-lobe predominant distribution of emphysema.
TABLE 1.
DISTRIBUTION OF EMPHYSEMA AND RESPONSE TO LVRS IN NETT
| Improvement in Exercise Capacity
|
Improvement in Health-related Quality of Life
|
|||
|---|---|---|---|---|
| LVRS | Medical | LVRS | Medical | |
| Upper-lobe predominant emphysema on CT | ||||
| Low Exercise | 30% | 0%* | 48% | 10%* |
| High Exercise | 15% | 3%* | 41% | 11%* |
| Non–upper-lobe predominant emphysema on CT | ||||
| Low Exercise | 12% | 7% | 37% | 7%* |
| High Exercise | 3% | 3% | 15% | 12% |
Definition of abbreviations: CT = computed tomography; LVRS = lung volume reduction surgery; NETT = National Emphysema Treatment Trial.
Response of patient to LVRS in the NETT. Improvement in exercise capacity is measured by cardiopulmonary exercise testing; the percentages represent the proportion of patients with a 10-watt improvement over prerandomization baseline in maximal workload attained on cycle ergometry. Improvement in health-related quality of life is measured with the St. George's Respiratory Questionnaire (SGRQ); the percentages represent the proportion of patients with an 8-point decrease (twice the MCID for improvement) in SGRQ score. Table adapted from Reference 7.
P ⩽ 0.001 in comparison of LVRS with Medical.
IDENTIFICATION OF OTHER CONDITIONS REQUIRING ATTENTION: PULMONARY NODULES
A number of studies have suggested that both chronic airflow obstruction (23, 24) and emphysema (25) are independent risk factors for the development of lung cancer in addition to cigarette smoking. CT scans of middle-aged current and ex-smokers reveals nodules (defined in various ways) in 13 to 50% of exams, the large majority of which are benign (26–30). It is not surprising, therefore, that a number of investigators have noted that nodules are detected in a sizable minority of patients undergoing CT scanning for consideration of LVRS. Hazelrigg and colleagues reported that 39.5% of 281 patients had a nodule identified on CT scan; of these, 52 had benign characteristics, 78 were resected, and 20 were followed. Seventeen of the resected nodules were malignant, 13 of which were felt to be primary lung cancers (31). Pigula and coworkers reported that 10 of 128 patients undergoing LVRS had neoplasm, six of which were primary lung cancers (32). McKenna and colleagues reported 51 of 325 patients (16%) who underwent LVRS had nodules; approximately 20% of these were non–small cell lung cancers (33). Rozenshtein and colleagues reported detecting nodules “suspicious for lung cancer” in 17 of 148 (11%) patients; 16 were resected, of which 9 were non–small cell carcinomas (34). In the NETT, 174 patients were excluded from randomization (of >3,700 patients screened) due to findings of “other disease” on the chest CT scan. Of these patients, 104 had a nodule that required further evaluation or surgical excision. Thus, depending on the definitions used, published experience suggests that 10 to 30% of LVRS candidates will have nodules visualized on CT scans and that approximately 3 to 5% will have previously undiagnosed non–small cell lung cancer.
By accepted criteria for resectability of non–small cell lung cancer, LVRS candidates are at high risk and/or have lung function impairment considered prohibitive for surgical therapy. There is a limited experience of combining LVRS procedures and surgical resection, based on the premise that the LVRS component of the operation should result in an improvement in lung function (33, 35). Early clinical results suggest that highly selected patients tolerate the combined procedure well and have an early course that is not different than that of patients undergoing either procedure alone.
The clinician evaluating patients for LVRS should be prepared to deal with the identification of pulmonary nodules and have an approach in mind for their subsequent evaluation and management. The Fleischner Society has outlined one such set of recommendations (36).
IDENTIFICATION OF OTHER CONDITIONS: EXCLUSIONS FROM LVRS
Using criteria adopted from common clinical practice in the field, the NETT excluded candidates with clinically significant bronchiectasis, pleural or interstitial disease, or a bulla occupying greater than one third of the volume of the lung. CT scanning identified 35 patients with clinically significant bronchiectasis, 21 with pleural or interstitial lung disease, and 14 with a giant bulla. Extrapolating from these data, between 2 and 5% of potential LVRS candidates will manifest one of these exclusionary criteria.
THE ROLE OF RADIONUCLIDE VENTILATION/PERFUSION SCANNING
An alternate method of assessing the regional distribution of functional derangement of the lung is radionuclide ventilation perfusion scanning. Wang and colleagues concluded that upper-lobe predominance and heterogeneity identified from perfusion scanning were “modestly” predictive of response to LVRS (37). Others have concluded, however, that it adds little to CT scanning in LVRS evaluation (5, 38, 39).
CONCLUSIONS
Radiological assessment with a chest CT scan is a key tool for identifying patients likely to benefit from LVRS. Patients with upper-lobe predominant emphysema on CT scan are most likely to benefit from LVRS. A minority of patients will be identified by CT scanning as having contraindications to LVRS, such as clinically significant bronchiectasis, pleural disease, or unsuspected interstitial disease. Detection of pulmonary nodules is a common occurrence in evaluation; carefully selected patients may benefit from concomitant LVRS and nodule resection.
The National Emphysema Treatment Trial (NETT) is supported by contracts with the National Heart, Lung, and Blood Institute (N01HR76101, N01HR76102, N01HR76103, N01HR76104, N01HR76105, N01HR76106, N01HR76107, N01HR76108, N01HR76109, N01HR76110, N01HR76111, N01HR76112, N01HR76113, N01HR76114, N01HR76115, N01HR76116, N01HR76118, and N01HR76119), the Centers for Medicare and Medicaid Services (CMS), and the Agency for Healthcare Research and Quality (AHRQ).
Conflict of Interest Statement: G.R.W. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. E.H. is a shareholder of VIDA Diagnostics, which is commercializing software used in the NETT trials. He is also an advisor to Siemens Medical System's Chest CT group and he serves on numerous advisory groups helping with the application of medical imaging methods as a safety or outcomes measure. J.J.R. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Ingenito EP, Loring SH, Moy ML, Mentzer SJ, Swanson SJ, Reilly JJ. Physiological characterization of variability in response to lung volume reduction surgery (LVRS). J Appl Physiol 2003;94:20–30. [DOI] [PubMed] [Google Scholar]
- 2.Fessler HE, Permutt S. Lung volume reduction surgery and airflow limitation. Am J Respir Crit Care Med 1998;157:715–722. [DOI] [PubMed] [Google Scholar]
- 3.Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Fukuchi Y, Jenkins C, Rodriguez-Roisin R, van Weel C, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD Executive Summary. Am J Respir Crit Care Med 2007;176:532–555. [DOI] [PubMed] [Google Scholar]
- 4.Rogers RM, Coxson HO, Sciurba FC, Keenan RJ, Whittall KP, Hogg JC. Preoperative severity of emphysema predictive of improvement after lung volume reduction surgery: use of CT morphometry. Chest 2000;118:1240–1247. [DOI] [PubMed] [Google Scholar]
-
5.Hunsaker AR, Ingenito EP, Reilly JJ, Costello P. Lung volume reduction surgery for emphysema: correlation of CT and
 imaging with physiologic mechanisms of improvement in lung function. Radiology 2002;222:491–498. [DOI] [PubMed] [Google Scholar]
imaging with physiologic mechanisms of improvement in lung function. Radiology 2002;222:491–498. [DOI] [PubMed] [Google Scholar] - 6.Ingenito EP, Hunsaker A, Pugatch R, Evans RB, Loring SL, Reilly JJ Jr, Mentzer SJ, Swanson SJ, DeCamp MM, Sugarbaker DJ. CT assessment of emphysema, lung physiology, and response to volume reduction surgery [abstract]. Am J Respir Crit Care Med 1997;155:A794. [Google Scholar]
- 7.Fishman A, Martinez F, Naunheim K, Piantadosi S, Wise R, Ries A, Weinmann G, Wood DE. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med 2003;348:2059–2073. [DOI] [PubMed] [Google Scholar]
- 8.Slone RM, Pilgram TK, Gierada DS, Sagel SS, Glazer HS, Yusen R, Cooper JD. Lung volume reduction surgery: comparison of preoperative radiologic features and clinical outcome. Radiology 1997;204:685–693. [DOI] [PubMed] [Google Scholar]
- 9.Gierada DS, Slone RM, Bae KT, Yusen RD, Lefrak SS, Cooper JD. Pulmonary emphysema: comparison of preoperative quantitative CT and physiologic index values with clinical outcome after lung- volume reduction surgery. Radiology 1997;205:235–242. [DOI] [PubMed] [Google Scholar]
- 10.Thurlbeck WM, Simon G. Radiographic appearance of the chest in emphysema. AJR Am J Roentgenol 1978;130:429–440. [DOI] [PubMed] [Google Scholar]
- 11.Muller NL, Coxson H. Chronic obstructive pulmonary disease. 4: imaging the lungs in patients with chronic obstructive pulmonary disease. Thorax 2002;57:982–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bankier AA, De Maertelaer V, Keyzer C, Gevenois PA. Pulmonary emphysema: subjective visual grading versus objective quantification with macroscopic morphometry and thin-section CT densitometry. Radiology 1999;211:851–858. [DOI] [PubMed] [Google Scholar]
- 13.Bergin C, Muller N, Nichols DM, Lillington G, Hogg JC, Mullen B, Grymaloski MR, Osborne S, Pare PD. The diagnosis of emphysema: a computed tomographic-pathologic correlation. Am Rev Respir Dis 1986;133:541–546. [DOI] [PubMed] [Google Scholar]
- 14.Goddard PR, Nicholson EM, Laszlo G, Watt I. Computed tomography in pulmonary emphysema. Clin Radiol 1982;33:379–387. [DOI] [PubMed] [Google Scholar]
- 15.Hersh CP, Washko GR, Jacobson FL, Gill R, Estepar RS, Reilly JJ, Silverman EK. Interobserver variability in the determination of upper lobe-predominant emphysema. Chest 2007;131:424–431. [DOI] [PubMed] [Google Scholar]
- 16.Coxson HO, Rogers RM, Whittall KP, D'Yachkova Y, Pare PD, Sciurba FC, Hogg JC. A quantification of the lung surface area in emphysema using computed tomography. Am J Respir Crit Care Med 1999;159:851–856. [DOI] [PubMed] [Google Scholar]
- 17.Muller NL, Staples CA, Miller RR, Abboud RT. “Density mask”: an objective method to quantitate emphysema using computed tomography. Chest 1988;94:782–787. [DOI] [PubMed] [Google Scholar]
- 18.Newell JD Jr, Hogg JC, Snider GL. Report of a workshop: quantitative computed tomography scanning in longitudinal studies of emphysema. Eur Respir J 2004;23:769–775. [DOI] [PubMed] [Google Scholar]
- 19.Stavngaard T, Shaker SB, Bach KS, Stoel BC, Dirksen A. Quantitative assessment of regional emphysema distribution in patients with chronic obstructive pulmonary disease (COPD). Acta Radiol 2006;47:914–921. [DOI] [PubMed] [Google Scholar]
- 20.Berger RL, Wood KA, Cabral HJ, Goodnight-White S, Ingenito EP, Gray A, Miller J, Springmeyer SC. Lung volume reduction surgery: a meta-analysis of randomized clinical trials. Treat Respir Med 2005;4:201–209. [DOI] [PubMed] [Google Scholar]
- 21.Teschler H, Thompson AB, Stamatis G. Short- and long-term functional results after lung volume reduction surgery for severe emphysema. Eur Respir J 1999;13:1170–1176. [DOI] [PubMed] [Google Scholar]
- 22.National Emphysema Treatment Trial Research Group. Patients at high risk of death after lung-volume-reduction surgery. N Engl J Med 2001;345:1075–1083. [DOI] [PubMed] [Google Scholar]
- 23.Samet JM, Humble CG, Pathak DR. Personal and family history of respiratory disease and lung cancer risk. Am Rev Respir Dis 1986;134:466–470. [DOI] [PubMed] [Google Scholar]
- 24.Schabath MB, Delclos GL, Martynowicz MM, Greisinger AJ, Lu C, Wu X, Spitz MR. Opposing effects of emphysema, hay fever, and select genetic variants on lung cancer risk. Am J Epidemiol 2005;161:412–422. [DOI] [PubMed] [Google Scholar]
- 25.de Torres JP, Bastarrika G, Wisnivesky JP, Alcaide AB, Campo A, Seijo LM, Pueyo JC, Villanueva A, Lozano MD, Montes U, et al. Assessing the relationship between lung cancer risk and emphysema detected on low-dose ct of the chest. Chest 2007;132:1932–1938. [DOI] [PubMed] [Google Scholar]
- 26.Swensen SJ, Jett JR, Sloan JA, Midthun DE, Hartman TE, Sykes AM, Aughenbaugh GL, Zink FE, Hillman SL, Noetzel GR, et al. Screening for lung cancer with low-dose spiral computed tomography. Am J Respir Crit Care Med 2002;165:508–513. [DOI] [PubMed] [Google Scholar]
- 27.Henschke CI, McCauley DI, Yankelevitz DF, Naidich DP, McGuinness G, Miettinen OS, Libby DM, Pasmantier MW, Koizumi J, Altorki NK, et al. Early Lung Cancer Action Project: overall design and findings from baseline screening. Lancet 1999;354:99–105. [DOI] [PubMed] [Google Scholar]
- 28.Henschke CI, Yankelevitz DF, Libby DM, Pasmantier MW, Smith JP, Miettinen OS. Survival of patients with stage I lung cancer detected on CT screening. N Engl J Med 2006;355:1763–1771. [DOI] [PubMed] [Google Scholar]
- 29.Gohagan J, Marcus P, Fagerstrom R, Pinsky P, Kramer B, Prorok P. Baseline findings of a randomized feasibility trial of lung cancer screening with spiral CT scan vs chest radiograph: the Lung Screening Study of the National Cancer Institute. Chest 2004;126:114–121. [DOI] [PubMed] [Google Scholar]
- 30.Diederich S, Wormanns D, Semik M, Thomas M, Lenzen H, Roos N, Heindel W. Screening for early lung cancer with low-dose spiral CT: prevalence in 817 asymptomatic smokers. Radiology 2002;222:773–781. [DOI] [PubMed] [Google Scholar]
- 31.Hazelrigg SR, Boley TM, Weber D, Magee MJ, Naunheim KS. Incidence of lung nodules found in patients undergoing lung volume reduction. Ann Thorac Surg 1997;64:303–306. [DOI] [PubMed] [Google Scholar]
- 32.Pigula FA, Keenan RJ, Ferson PF, Landreneau RJ. Unsuspected lung cancer found in work-up for lung reduction operation. Ann Thorac Surg 1996;61:174–176. [DOI] [PubMed] [Google Scholar]
- 33.McKenna RJ Jr, Fischel RJ, Brenner M, Gelb AF. Combined operations for lung volume reduction surgery and lung cancer. Chest 1996;110:885–888. [DOI] [PubMed] [Google Scholar]
- 34.Rozenshtein A, White CS, Austin JH, Romney BM, Protopapas Z, Krasna MJ. Incidental lung carcinoma detected at CT in patients selected for lung volume reduction surgery to treat severe pulmonary emphysema. Radiology 1998;207:487–490. [DOI] [PubMed] [Google Scholar]
- 35.Pompeo E, De Dominicis E, Ambrogi V, Mineo D, Elia S, Mineo TC. Quality of life after tailored combined surgery for stage I non-small-cell lung cancer and severe emphysema. Ann Thorac Surg 2003;76:1821–1827. [DOI] [PubMed] [Google Scholar]
- 36.MacMahon H, Austin JH, Gamsu G, Herold CJ, Jett JR, Naidich DP, Patz EF Jr, Swensen SJ. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology 2005;237:395–400. [DOI] [PubMed] [Google Scholar]
- 37.Wang SC, Fischer KC, Slone RM, Gierada DS, Yusen RD, Lefrak SS, Pilgram TK, Cooper JD. Perfusion scintigraphy in the evaluation for lung volume reduction surgery: correlation with clinical outcome. Radiology 1997;205:243–248. [DOI] [PubMed] [Google Scholar]
- 38.Cleverley JR, Desai SR, Wells AU, Koyama H, Eastick S, Schmidt MA, Charrier CL, Gatehouse PD, Goldstraw P, Pepper JR, et al. Evaluation of patients undergoing lung volume reduction surgery: ancillary information available from computed tomography. Clin Radiol 2000;55:45–50. [DOI] [PubMed] [Google Scholar]
- 39.Thurnheer R, Engel H, Weder W, Stammberger U, Laube I, Russi EW, Bloch KE. Role of lung perfusion scintigraphy in relation to chest computed tomography and pulmonary function in the evaluation of candidates for lung volume reduction surgery. Am J Respir Crit Care Med 1999;159:301–310. [DOI] [PubMed] [Google Scholar]