Abstract
Lung volume reduction surgery (LVRS) produces physiological, symptomatic, and survival benefits in selected patients with advanced emphysema. Because it is associated with significant morbidity, mortality, and cost, nonsurgical alternatives for achieving volume reduction have been developed. Three bronchoscopic lung volume reduction (BLVR) approaches have shown promise and reached later-stage clinical trials. These include the following: (1) placement of endobronchial one-way valves designed to promote atelectasis by blocking inspiratory flow; (2) formation of airway bypass tracts using a radiofrequency catheter designed to facilitate emptying of damaged lung regions with long expiratory times; and (3) instillation of biological adhesives designed to collapse and remodel hyperinflated lung. The limited clinical data currently available suggest that all three techniques are reasonably safe. However, efficacy signals have been substantially smaller and less durable than those observed after LVRS. Studies to optimize patient selection, refine treatment strategies, characterize procedural safety, elucidate mechanisms of action, and characterize short- and longer-term effectiveness of these approaches are ongoing. Results will be available over the next few years and will determine whether BLVR represents a safe and effective alternative to LVRS.
Keywords: bronchoscopic lung volume reduction, endobronchial valves, airway bypass
OVERVIEW, DEFINITIONS, AND SOURCES OF CLINICAL DATA
Bronchoscopic lung volume reduction (BLVR) is a general term that refers to any of several recently developed endobronchial procedures for treating hyperinflation in advanced emphysema (1, 2). Although they share a common objective, these different procedures are based on distinct technologies. None is currently available for general clinical application. None has proven to be consistently effective in generating volume reduction or producing durable clinical benefit in patients. There are no published data summarizing the results of randomized clinical trials involving BLVR. Studies reported to date have all been open label, nonrandomized, and of short duration. Only a fraction of the data that has been collected has been published. Nevertheless, sufficient data are available in manuscripts, abstracts, and scientific presentations to allow a summary of the current state of BLVR and speculation about the future role of BLVR in the treatment of advanced emphysema.
RATIONALE FOR PURSUING BLVR
Enthusiasm for BLVR derives almost entirely from experiences with lung volume reduction surgery (LVRS) (3–7). The early success of LVRS in cohort studies between 1994 and 1997, and subsequent clarification of the mechanistic basis for improvement, provide a physiological rationale for BLVR (8–10). Reducing the overall size of the hyperexpanded emphysematous lung produces space within the less compliant chest cavity for the remaining lung to expand and function during inspiration (Figure 1B). This “resizing” principle, initially proposed by Otto Brantigan in 1956 and defined more precisely by Fessler and Permutt in 1998, explains how resection of lung tissue in emphysema improves overall function of the respiratory system (9, 11). In single-center cohort studies, LVRS improved spirometry, lung volumes, and exercise capacity in appropriately selected patients with emphysema, observations subsequently confirmed in single-center, randomized clinical trials and the multicenter National Emphysema Treatment Trial (NETT).
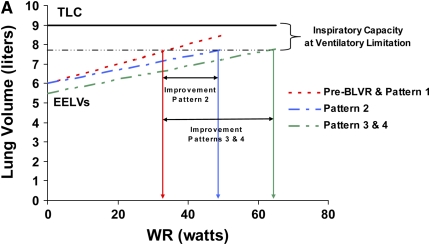
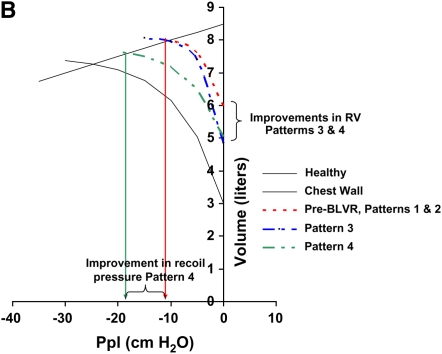
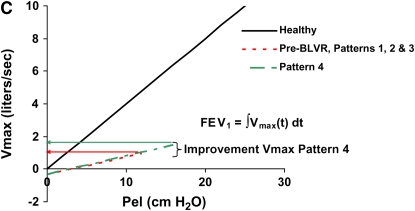
Figure 1.
Patterns of response to bronchoscopic lung volume reduction (BLVR) therapy in terms of exercise capacity, lung volumes and recoil, and expiratory flows. Pattern 1 produces no change in any objective parameters. Pattern 2 produces a beneficial effect in exercise capacity by altering dynamic hyperinflation during exercise (A) through changes in regional lung impedance, without affecting static lung volumes and recoil (B) or expiratory flows (C). Pattern 3 produces larger changes in exercise capacity by altering both static lung volumes (A and B) and dynamic hyperinflation (A), but because lung recoil is not affected, maximal expiratory flows and FEV1 (C) do not substantially change. Pattern 4 produces changes in exercise capacity (A) and residual lung volume (B) similar to pattern 3, but also results in an increase in FEV1 by improving maximal expiratory flows (C) as a result of an increase in recoil pressure.
Enthusiasm for nonsurgical volume reduction has also been fostered by reports showing that LVRS is associated with a high incidence of complications. Procedural (90-d) mortality has ranged from 3 to 19% in cohort studies, and was 5.5% in the NETT. Serious morbidity after LVRS was observed in 59% of patients, with persistent air leak (3%), respiratory failure (22%), pneumonia (18%), cardiac arrhythmias (24%), and myocardial infarction (1%) being most common (12).
Successful development of BLVR has other potential theoretical benefits as well. Volume reduction procedures that do not cause pleural scarring may be more effective than conventional LVRS for treating lower-lobe emphysema by reducing lung volume without causing formation of peridiaphragmatic scar tissue that can restrict diaphragm motion (13, 14). BLVR procedures that can be performed at the bedside and administered using a gradual, stepwise approach could potentially facilitate weaning of patients with emphysema with chronic respiratory failure from mechanical ventilation (15). Finally, BLVR procedures that are substantially safer than LVRS could make volume reduction therapy available to patients with emphysema with comorbid conditions that preclude surgery.
EXISTING BLVR TECHNOLOGIES
Three different BLVR systems are currently undergoing clinical trials. Two are device-based systems, and one is a biologic drug-based system.
Endobronchial Valves
Endobronchial valve (EV) systems represent a refinement of the predecessor endobronchial blocker or “plug” initially proposed by Sabanathan and colleagues and Watanabe and colleagues (16, 17). EVs are deployed in the proximal airway through a flexible or rigid bronchoscope using a catheter or guidewire. They are designed to block air from entering the target area during inspiration, while allowing gas to exit during exhalation. This is intended to cause collapse and volume reduction by promoting progressive deflation and adsorption atelectasis in damaged regions of lung. EV systems have been designed to accommodate drainage of mucus, reducing the potential for postobstructive pneumonia (2, 18).
Two EV systems are presently under evaluation; both are intended primarily for treatment of heterogeneous upper lobe emphysema. The first is the EV system manufactured by Emphasys Medical (Redwood City, CA) (19–21). It is constructed from biocompatible materials and is simple to deploy and remove. It has an outer cylindrical frame with a circumferential wire mesh, and central lumen that anchors a duck bill–shaped one-way valve. The Emphasys system was the first BLVR device to enter clinical trials, and has been the most extensively studied. Clinical experiences using the Emphasys system in over 100 patients have been published to date. This system is also being evaluated in the multicenter Emphasys Bronchial Valve for Emphysema Palliation Trial (VENT) trial, a randomized, open-label study comparing EV therapy to medical therapy. Enrollment in the VENT trial has been completed, and results are currently pending (22).
Spiration Incorporated (Redmond, WA) manufactures the second EV system. This system has an umbrella design in which an elastomer covering is stretched over a nitinol wire frame that anchors the device in place. Gas under pressure can escape from the lung around the edges of the flexible covering as the umbrella-shaped frame partially collapses, but is prevented from flowing in the forward direction. Similar to the Emphasys system, the Spiration device is deployed into the proximal airway through a flexible or rigid bronchoscope using a catheter, and is easy to deploy and remove. This system has been tested in pilot studies in over 40 patients, and clinical data are available in the form of abstracts and one recently published article (23). The Spiration system is entering clinical testing in a multicenter, randomized, controlled clinical trial.
Airway Bypass System
The second BLVR system currently in clinical trials is the airway bypass system developed by Broncus Incorporated (Mountain View, CA). This system, based on the work of Macklem and colleagues and pioneered by Joel Cooper, is designed to reduce lung volume by altering flow dynamics (regional time constants) and airway closure, rather than by promoting lung collapse (24, 25). A radiofrequency balloon catheter establishes a shunt pathway, referred to as a “fenestration,” between a central airway and a target region of damaged, hyperinflated lung. This newly created passageway facilitates lung emptying, reducing end-expiratory volume without altering lung recoil per se. This approach should theoretically be beneficial for patients with either heterogeneous or homogeneous emphysema, although trials have focused primarily on patients with homogeneous disease.
Treatment using the Broncus system requires three separate devices and three procedures. An initial endobronchial ultrasound is performed to identify vascular structures in the airway, which must be avoided during subsequent steps. Next, a radiofrequency ablation catheter forms the bypass tract through the airway wall into the damaged lung parenchyma. Finally, a drug-eluting stent is placed into the tract to help maintain patency.
This system has been tested in 19 patients, and clinical data are available in the form of an abstract. Initiation of a randomized clinical trial involving the Broncus airway bypass system is expected within the next year.
Biological Remodeling
The third type of BLVR system currently in clinical trials is the biological sealant/remodeling system developed by Aeris Therapeutics (Woburn, MA). Like valve-based systems, it is designed to directly reduce lung volume by collapsing and sealing damaged areas of hyperinflated lung in patients with heterogeneous upper lobe emphysema. The site and mechanism of action are fundamentally different from EV systems, however (26). This system acts at the alveolar rather than the airway level. Furthermore, treatment is intended to produce a permanent change in tissue configuration similar to LVRS, rather than reversible adsorption atelectasis. Treatments are delivered to the alveolar compartment as separate liquid components via a dual lumen catheter passed through the instrument channel of a flexible bronchoscope. The components polymerize distally at the target site to produce collapse and remodeling over several weeks. This system has been tested in 15 patients using single- and repeat-dose regimens, and clinical data are available in the form of abstracts and a single manuscript (34). The Aeris system is currently undergoing evaluation in phase 2 clinical trials.
SUMMARY OF CLINICAL RESPONSES TO BLVR THERAPIES
All BLVR procedures are designed to reduce hyperinflation and achieve lung volume reduction. Available data suggest that this is achieved inconsistently, and to varying degrees.
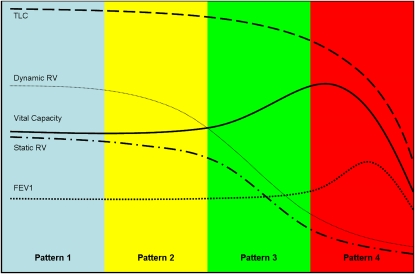
Results indicate that response patterns to BLVR have been of four general types (Figures 1 and 2), defined here as patterns 1–4. A pattern 1 response is one in which patients have reported feeling “better” and subjective functional (dyspnea scores) and composite (health-related quality of life [HRQOL]) outcomes have improved without any meaningful change in objective physiological outcome measures (i.e., changes ⩽ minimal clinically important difference [MCID] standards). Although this pattern of response may truly benefit patients, it is difficult to distinguish from a placebo effect. Thus, attributing improvement to the intervention based upon this pattern of response in the absence of a randomized, blinded, placebo-controlled comparison is not possible.
Figure 2.
Patterns of response to bronchoscopic lung volume reduction (BLVR) pattern 1: treatment produces no change in lung physiology. Pattern 2: treatment reduces dynamic gas trapping (dynamic RV), which improves exercise capacity. Static volumes and spirometry are unaffected. Pattern 3: treatment reduces static and dynamic gas trapping (static RV and dynamic RV), but has minimal effect on total lung capacity (TLC). FVC increases, but FEV1 does not. Pattern 4: treatment reduces static and dynamic gas trapping (static RV and dynamic RV) and TLC. FVC and FEV1 improve. Beyond a critical threshold, further reductions in TLC have a negative impact on VC and then FEV1, and the benefits of treatment diminish.
A pattern 2 response is one in which subjective outcomes as well as objective measures of exercise capacity have improved. However, spirometry and lung volumes are not substantially improved (Figures 1 and 2). Although small changes in exercise capacity measured by six-minute-walk distance (6MWD) may reflect the combined effects of training and placebo, MCID improvements are likely due to the effects of BLVR treatment on dynamic hyperinflation. Such benefits can occur in the absence of changes in static residual volume (RV) or VC.
A pattern 3 response is one in which subjective (dyspnea) and objective (exercise capacity) functional measures improve, one or more composite measures (HRQOL, exacerbations) improve, and measures of lung volume (RV, inspiratory capacity, and VC) improve. However, FEV1, long considered the standard for assessing the effectiveness of treatments for obstructive lung disease, does not improve (Figures 1 and 2). This pattern of response is likely due to beneficial effects of BLVR on both static and dynamic hyperinflation. FEV1, which is determined primarily by lung recoil and airway resistance upstream of the site of flow limitation, is unaffected, as neither recoil nor airway resistance is affected by therapy with this kind of response.
A pattern 4 response is one in which subjective and objective functional measures, composite measures, and physiological measures of both lung volumes (RV, inspiratory capacity, and VC) and expiratory flows (FEV1) improve in a meaningful way (Figures 1 and 2). This is the pattern of response observed with successful LVRS and explained mechanistically by the Fessler-Permutt analysis (9).
In studies published to date, declines in lung function within the first 90 days of BLVR have been uncommon. When observed, these have been attributed to procedural complications or chronic obstructive pulmonary disease (COPD) exacerbations rather than unexpected device failures or design faults. Whether there are specific groups of patients with emphysema at high risk for BLVR, analogous to those identified in the NETT as high risk for LVRS, remains to be determined (27).
EVs
Data from abstracts and seven manuscripts using the Emphasys valve, and from a single manuscript using the Spiration valve, suggest that EVs are capable of producing initial responses that span this entire spectrum in patients with upper lobe emphysema (19–21, 27, 29–31). Experience to date suggests that the most effective therapeutic approach has involved placement of valves into subsegmental and segmental airways to occlude airways to an entire lobe.
The initial experience with EV in eight patients treated with the Emphasys valve demonstrated pattern 4 responses in three, with improvements in FEV1 (Δ = +34%), RV (Δ = −11%), and DlCO (Δ = +29%) at 30 days. Several of these patients had radiographic confirmation of partial lobar collapse at treatment sites. Similar beneficial effects have been described by Yim and colleagues and by Venuta and colleagues (20, 28). Other studies have documented less impressive results, however. Cohort studies from Australia, England, Belgium, and Brazil have demonstrated pattern 1 and 2 responses (21, 29–32). The Spiration valve has also generated pattern 1 responses, without post-treatment functional or physiological benefit at 6 months (23).
Hopkinson and colleagues has shown that EV therapy reduces dynamic hyperinflation during exercise (30). In a cohort of 19 patients demonstrating a pattern 2 response, EV therapy reduced gas trapping and ventilatory limitation during cycle ergometry, confirming that despite the absence of reductions in static lung volumes, EBV therapy can have beneficial physiological effects.
A recent publication summarizing the broad experience using EV therapy in 98 patients confirms wide variability in therapeutic response (33). Overall, the 98-patient cohort showed small improvements in physiological parameters that failed to meet MCID criteria for FEV1 (ΔFEV1 = 60 ± 210 ml, 10.7 ± 26.2%), VC (120 ± 470 ml, 9 ± 23.9%), or 6MWD (36.9 ± 90 m) at 90-day follow-up, most consistent with a pattern 1 long-term response. Subjective outcomes were not reported in this study.
These data suggest that EV therapy is capable of producing initial responses similar to that to LVRS (pattern 4 responses) in selected cases, but responses are variable, criteria for identifying responders are poorly defined, and benefits are often short lived. However, even in the absence of changes in FEV1, FVC, and RV, these devices can produce physiological benefit by altering regional airflow impedance and reducing dynamic hyperinflation.
EV systems have proven quite safe in clinical trials. Mortality has been on the order of 1%. Serious procedure-related morbidity, manifest as pneumothorax, pneumonia, or COPD exacerbations, has been reported to be 3–17%. Average length of stay after EV therapy has been 2–4 days; length of stay as long as 90 days has been reported in patients with serious complications.
Airway Bypass Formation
The published experience with the Broncus Technologies bypass tract system is quite limited. A report summarizing the initial clinical results is available in abstract form (25).
Data from 19 patients showed an immediate improvement in dyspnea score and lung physiology (FEV1, FVC, and RV), which was not sustained at 30 days (25). In a subset of patients (8 of 19) with homogeneous disease and marked baseline hyperinflation (total lung capacity > 133% predicted), reductions in RV (−1.1 L), improvements VC (+189 ml), and improvements in dyspnea (Medical Research Council Dyspnea Score) and HRQOL were observed out to 6 months.
Morbidity and mortality data associated with bypass tract therapy have not yet been published.
Biological Remodeling
The published experience with the Aeris biological remodeling system is also limited. Clinical data are available in several abstracts and a single peer-reviewed manuscript (34, 35). Initial studies have focused on demonstrating system safety; subtherapeutic dosing at two subsegmental sites and low therapeutic dosing at four subsegmental sites have been evaluated in patients with heterogeneous upper lobe emphysema.
The initial experience in six patients with upper lobe disease demonstrated that unilateral treatment at two sites produced no symptomatic or physiological benefit. At 6-month follow-up, treatment at four sites unilaterally produced small improvements in symptom scores (change in Medical Research Council dyspnea = −1.3 ± 1.2 U) and HRQOL (change in St. George's Respiratory Questionnaire total score = −11.0 ± 11.6 U), and small improvements in physiological parameters (ΔFEV1 = +20 ± 67 ml, +3.0 ± 10.2%; ΔFVC = +250 ± 151 ml, +9.2 ± 6.5%; ΔRV = −630 ± 360 ml, −11.8 ± 5.8%) and exercise capacity (Δ6MWD = +71.9 ± 61.8 m) that generally failed to meet MCID criteria (34). Two of three patients receiving four-site treatment experienced pattern 3 responses; the overall response for all six patients was a pattern 1 response with failure to meet MCID criteria for exercise capacity or physiological parameters.
These data suggest that, at low doses in patients with heterogeneous emphysema, the Aeris biological system produces mild volume reduction and small changes in physiology and exercise capacity. It is likely that the benefits of therapy are due to reductions in both static and dynamic hyperinflation. However, because only limited data are available for this system at a very limited number of clinical sites, additional information is required to assess the effectiveness of this approach.
Preliminary safety data using the Aeris biological remodeling system have been favorable. There have been no deaths in the 15 patients treated to date. COPD exacerbations have occurred in two patients. All patients have been discharged to home on the day after treatment.
BLVR VERSUS LVRS
Results from clinical trials suggest that subsets of patients treated with EV, bypass tract therapy, and biological remodeling have achieved meaningful physiological and clinical benefit. In general, however, these treatments have produced pattern 1 responses, with at best small overall objective improvements. By contrast, pattern 3 and 4 responses have consistently been observed in randomized clinical trials involving bilateral LVRS in patients with upper lobe–predominant disease (Table 1) (3, 5, 36–46). BLVR responses also appear to be less durable than those of LVRS (29, 32). Although the therapeutic benefits of LVRS diminish over time due to disease progression and possibly treatment-related acceleration of lung function loss, objective benefit has been observed for years after treatment. This is particularly true for patients with upper lobe–predominant emphysema (47). By contrast, physiological benefits after BLVR often diminish substantially within months.
TABLE 1.
PUBLISHED RESPONSES TO BLVR AND LVRS
| Volume Reduction Therapy | Exercise Capacity (6MWD meters) | Spirometry | Lung Volumes | Overall Response Pattern |
|---|---|---|---|---|
| Emphasys endobronchial valve* | Range of group mean responses across 6 studies (+0 to +152m) | Range of group mean responses 6 studies (−4% to +46%) Meta analysis | Range of group mean responses 6 studies (0% to −11%) | Pattern 1 |
| Meta analysis | FEV1 = 11 ± 26% | Meta analysis | ||
| +37 ± 90 m | FVC = 9 ± 24% | −5 ± 17% | ||
| Biological sealant/remodeling system† | Combined mean response of 2 and 4 site treatments | Combined mean response of 2 and 4 site treatments (n=15 patients) | Range of group mean responses (n=15 patients) | Pattern 1 |
| + 30 ± 34 m | FEV1 = −1 ± 13% | −3 ± 7% | ||
| FVC = 10 ± 14% | ||||
| Bypass tract system | Sufficient published data not currently available | |||
| Unilateral LVRS‡ (includes responses for heterogeneous and homogeneous patients) | Summary 3 month responses from 3 studies | Summary 3 month responses from 3 studies | Summary 3 month responses from 3 studies | Pattern 4 |
| 56 m | FEV1 = +24% | −16% | ||
| FVC = +18% | ||||
| Bilateral LVRS for homogeneous disease§ (includes results from patients with alpha-1 trypsin deficiency) | Summary 3 month responses from 3 studies | Summary 3 month responses from 3 studies | Summary 3 month responses from 3 studies | Pattern 4 |
| 75 m | FEV1 = +35% | −22% | ||
| FVC = +23% | ||||
| Bilateral LVRS for heterogeneous disease‖ | Summary 3-6 month responses from 4 small randomized studies | Summary 3-6 month responses from 4 small randomized studies | Summary 3-6 month responses from 4 small randomized studies | |
| 47 m | FEV1 = +34% | RV = −23% | Type 4 | |
| FVC = +16% | ||||
Results summarized from references 18, 20, 21, 29, 30, 31.
Results summarized from references 34 and 40.
Results summarized from references 36, 38, 39.
Results summarized from references 40, 41, 42.
Results summarized from references 3, 6, 7, 43.
The reasons for limited effectiveness of BLVR therapy in early trials are modality dependent. Variability in response to EV therapy has been attributed to extensive collateral ventilation pathways distal to sites of valve placement in patients with advanced emphysema (48). These pathways allow continued access of inhaled gas to the target zone, preventing effective collapse. Even with “lobar targeting,” substantial, lasting volume reduction is rarely achieved with EV therapy (29, 32).
The effectiveness of airway bypass therapy has been limited by premature closure of newly formed fenestrations. To address this, placement of a drug-eluting stent into each tract has become a standard part of treatment. These stents are intended to maintain airway bypass tract patency. Whether this approach will prove successful is yet to be determined.
Biological remodeling therapy, which is designed to block collateral ventilation and promote volume reduction at the alveolar level, has shown early signs of promise, but insufficient data are currently available to determine whether this strategy will ultimately prove effective. The subtherapeutic dosing employed in early safety trials has not allowed a thorough assessment of potential efficacy. Early benefit observed with four-site treatments has diminished with time, possibly due to metabolism and partial dissolution of the biological hydrogel (49). A more durable hydrogel formulation is currently entering clinical trials.
The lack of effectiveness of BLVR therapy cited above is not meant to imply that it does not or cannot work. Some of the reasons for the apparent clinical and physiological limitations of BLVR are obvious. Testing to date has primarily involved unilateral treatment. EV results suggest that responses to unilateral treatment may be superior to bilateral treatment for reasons that are unclear (33). With biological remodeling, bilateral therapy is expected to be more effective than unilateral therapy. Currently, it is reasonable to conclude that, for all BLVR approaches, “full dose” treatment (i.e., number of sites to treat) and strategies for delivering this dose (single session vs. sequential treatment) are still being determined.
In addition, differences in patient selection undoubtedly account for some of the differences in clinical outcomes between BLVR therapy and LVRS as well. Some patients included in initial BLVR trials were individuals excluded from consideration for LVRS, and were therefore likely suboptimal candidates for BLVR. Selection criteria for identifying “optimal candidates” for BLVR have not yet been established. Although most studies have relied heavily upon NETT patient selection criteria, refinements will likely be required to optimize outcomes to BLVR therapy (5, 50).
CURRENT STATUS AND FUTURE DIRECTIONS OF BLVR
Presently, BLVR remains an experimental procedure of unproven benefit. More objective data are needed to determine the clinical utility of each BLVR procedure. As these treatments undergo evaluation, it will be important for physicians to interpret responses in the context of the trial design and study endpoints. In an open-label (unblinded) study, where every patient knows that he/she is receiving a theoretically beneficial treatment, even statistically significant improvements in subjective functional (dyspnea scores) and composite outcomes (HRQOL), with little or no change in physiological parameters, must be viewed with skepticism. Dr. Carl Simonton, in his book Getting Well Again, has convincingly argued that the placebo effect can be quite real in patients with chronic debilitating diseases (51). The ability of the human body to heal when an individual believes he or she is healed can be substantial, albeit poorly understood. Thus, improvements in subjective outcomes should be accompanied by improvements in physiological parameters that can rationally explain them.
CONCLUSIONS AND RECOMMENDATIONS
Beyond smoking cessation and oxygen therapy, LVRS is the only treatment shown to alter the natural history of emphysema. Patients with upper lobe–predominant disease, low exercise capacity, and both FEV1 and DlCO > 20% of predicted receive the most benefit, demonstrating improvements in symptoms and physiology, and reduced mortality (5, 50). LVRS should be recommended for these individuals.
Is there currently a role for BLVR in this subpopulation? Proponents of BLVR could argue that its relative safety, and the option for subsequent LVRS in the event of a suboptimal BLVR response, make trial participation a reasonable first option. However, it is difficult to justify recommending an unproven experimental treatment when an established, effective, reasonably safe alternative exists.
What is the role of BLVR in the treatment of other forms of advanced emphysema? That will ultimately be determined by the results of ongoing clinical trials. It is reasonable to consider BLVR for the patients who refuse LVRS, or who are not candidates for LVRS but still qualify for trial participation. Only through completion of these studies will the pulmonary community learn whether BLVR therapy is in fact a clinically useful therapeutic modality.
The National Emphysema Treatment Trial (NETT) is supported by contracts with the National Heart, Lung, and Blood Institute (N01HR76101, N01HR76102, N01HR76103, N01HR76104, N01HR76105, N01HR76106, N01HR76107, N01HR76108, N01HR76109, N01HR76110, N01HR76111, N01HR76112, N01HR76113, N01HR76114, N01HR76115, N01HR76116, N01HR76118, and N01HR76119), the Centers for Medicare and Medicaid Services (CMS), and the Agency for Healthcare Research and Quality (AHRQ).
Conflict of Interest Statement: E.P.I. is founder, chief scientific officer, and a stock owner of Aeris Therapeutics, one of the companies that is developing a method and commercial product for performing bronchoscopic lung volume reduction. D.E.W. has received research funding as part of the clinical trial of the Spiratin IBV device. J.P.U. may be the Mayo principal investigator (PI) in the Aeris multicenter trial. The budget has not been determined, and the final participation decision has not been made. He is a co-PI for the Mayo site in the VENT trial (Eric Edell is PI at the Mayo site).
References
- 1.Maxfield RA. New and emerging minimally invasive techniques for lung volume reduction. Chest 2004;125:777–783. [DOI] [PubMed] [Google Scholar]
- 2.Toma TP. The flexible bronchoscopic approach to lung volume reduction. Pneumologia 2001;50:97–100. [PubMed] [Google Scholar]
- 3.Criner GJ, Cordova FC, Furukawa S, Kuzma AM, Travaline JM, Leyenson V, O'Brien GM. Prospective randomized trial comparing bilateral lung volume reduction surgery to pulmonary rehabilitation in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999;160:2018–2027. [DOI] [PubMed] [Google Scholar]
- 4.Cooper JD, Patterson GA. Lung-volume reduction surgery for severe emphysema. Chest Surg Clin N Am 1995;5:815–831. [PubMed] [Google Scholar]
- 5.Fishman A, Martinez F, Naunheim K, Piantadosi S, Wise R, Ries A, Weinmann G, Wood DE. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med 2003;348:2059–2073. [DOI] [PubMed] [Google Scholar]
- 6.Geddes D, Davies M, Koyama H, Hansell D, Pastorino U, Pepper J, Agent P, Cullinan P, MacNeill SJ, Goldstraw P. Effect of lung-volume-reduction surgery in patients with severe emphysema. N Engl J Med 2000;343:239–245. [DOI] [PubMed] [Google Scholar]
- 7.Pompeo E, Marino M, Nofroni I, Matteucci G, Mineo TC. Reduction pneumoplasty versus respiratory rehabilitation in severe emphysema: a randomized study. Pulmonary Emphysema Research Group. Ann Thorac Surg 2000;70:948–953. [Discussion, p. 954.] [DOI] [PubMed] [Google Scholar]
- 8.Ingenito EP, Loring SH, Moy ML, Mentzer SJ, Swanson SJ, and Reilly JJ. Interpreting improvement in expiratory flows after lung volume reduction surgery in terms of flow limitation theory. Am J Respir Crit Care Med 2001;163:1074–1080. [DOI] [PubMed] [Google Scholar]
- 9.Fessler HE, Permutt S. Lung volume reduction surgery and airflow limitation. Am J Respir Crit Care Med 1998;157:715–722. [DOI] [PubMed] [Google Scholar]
- 10.Fessler HE, Scharf SM, Permutt S. Improvement in spirometry following lung volume reduction surgery: application of a physiologic model. Am J Respir Crit Care Med 2002;165:34–40. [DOI] [PubMed] [Google Scholar]
- 11.Brantigan OC. Surgical treatment of pulmonary emphysema. Md State Med J 1957;6:409–414. [PubMed] [Google Scholar]
- 12.Naunheim KS, Wood DE, Krasna MJ, DeCamp MM, Jr, Ginsburg ME, McKenna RJ, Jr, Criner GJ, Hoffman EA, Sternberg AL, and Deschamps C. Predictors of operative mortality and cardiopulmonary morbidity in the National Emphysema Treatment Trial. J Thorac Cardiovasc Surg 2006;131:43–53. [DOI] [PubMed] [Google Scholar]
- 13.Cassina PC, Teschler H, Konietzko N, Theegarten D, Stamatis G. Two-year results after lung volume reduction surgery in α1-antitrypsin deficiency versus smoker's emphysema. Eur Respir J 1998;12:1028–1032. [DOI] [PubMed] [Google Scholar]
- 14.Gelb AF, McKenna RJ, Brenner M, Fischel R, and Zamel N. Lung function after bilateral lower lobe lung volume reduction surgery for α1-antitrypsin emphysema. Eur Respir J 1999;14:928–933. [DOI] [PubMed] [Google Scholar]
- 15.Criner GJ, O'Brien G, Furukawa S, Cordova F, Swartz M, Fallahnejad M, D'Alonzo G. Lung volume reduction surgery in ventilator-dependent COPD patients. Chest 1996;110:877–884. [DOI] [PubMed] [Google Scholar]
- 16.Watanabe S, Shimokawa S, Yotsumoto G, Sakasegawa K. The use of a Dumon stent for the treatment of a bronchopleural fistula. Ann Thorac Surg 2001;72:276–278. [DOI] [PubMed] [Google Scholar]
- 17.Sabanathan S, Richardson J, Pieri-Davies S. Bronchoscopic lung volume reduction. J Cardiovasc Surg (Torino) 2003;44:101–108. [PubMed] [Google Scholar]
- 18.Toma TP, Hopkinson NS, Polkey MI, Geddes DM. Endobronchial volume reduction: a myth or a marvel? Semin Respir Crit Care Med 2004;25:399–404. [DOI] [PubMed] [Google Scholar]
- 19.Toma TP, Hopkinson NS, Hillier J, Hansell DM, Morgan C, Goldstraw PG, Polkey MI, Geddes DM. Bronchoscopic volume reduction with valve implants in patients with severe emphysema. Lancet 2003;361:931–933. [DOI] [PubMed] [Google Scholar]
- 20.Venuta F, de Giacomo T, Rendina EA, Ciccone AM, Diso D, Perrone A, Parola D, Anile M, Coloni GF. Bronchoscopic lung-volume reduction with one-way valves in patients with heterogenous emphysema. Ann Thorac Surg 2005;79:411–416. [Discussion, pp. 416–417.] [DOI] [PubMed] [Google Scholar]
- 21.Snell GI, Holsworth L, Borrill ZL, Thomson KR, Kalff V, Smith JA, Williams TJ. The potential for bronchoscopic lung volume reduction using bronchial prostheses: a pilot study. Chest 2003;124:1073–1080. [DOI] [PubMed] [Google Scholar]
- 22.Leroy S, Marquette CH. VENT: International study of bronchoscopic lung volume reduction as a palliative treatment for emphysema [in French]. Rev Mal Respir 2004;21(6 Pt 1):1144–1152. [DOI] [PubMed] [Google Scholar]
- 23.Wood DE, McKenna RJ, Jr, Yusen RD, Sterman DH, Ost DE, Springmeyer SC, Gonzalez HX, Mulligan MS, Gildea T, Houck WV, et al. A multicenter trial of an intrabronchial valve for treatment of severe emphysema. J Thorac Cardiovasc Surg 2007;133:65–73. [DOI] [PubMed] [Google Scholar]
- 24.Lausberg HF, Chino K, Patterson GA, Meyers BF, Toeniskoetter PD, Cooper JD. Bronchial fenestration improves expiratory flow in emphysematous human lungs. Ann Thorac Surg 2003;75:393–397. [Discussion, p. 398.] [DOI] [PubMed] [Google Scholar]
- 25.Macklem PT, Cardosa P, Snell G, Hopkins P, Sybrecht GW, Pierce J, Cooper JD. Airway bypass: a new treatment for emphysema [abstract]. Proc Am Thorac Soc 2006;167:A726. [Google Scholar]
- 26.Ingenito EP, Berger RL, Henderson AC, Reilly JJ, Tsai L, Hoffman A. Bronchoscopic lung volume reduction using tissue engineering principles. Am J Respir Crit Care Med 2003;167:771–778. [DOI] [PubMed] [Google Scholar]
- 27.National Emphysema Treatment Trial Research Group. Patients at high risk of death after lung-volume-reduction surgery. N Engl J Med 2001;345:1075–1083. [DOI] [PubMed] [Google Scholar]
- 28.Yim AP, Hwong TM, Lee TW, Li WW, Lam S, Yeung TK, Hui DS, Ko FW, Sihoe AD, Thung KH, et al. Early results of endoscopic lung volume reduction for emphysema. J Thorac Cardiovasc Surg 2004;127:1564–1573. [DOI] [PubMed] [Google Scholar]
- 29.Toma TP, Hopkinson J, Hillier J, Ujita M, Dusmet M, Goldstraw P, Morgan C, Hansell DM, Polkey MI, Geddes D. Effect of unilateral total lobar occlusion with bronchoscopic valve implants in patients with severe heterogeneous emphysema. Am J Respir Crit Care Med 2004;165:A576. [Google Scholar]
- 30.Hopkinson NS, Toma TP, Hansell DM, Goldstraw P, Moxham J, Geddes DM, Polkey MI. Effect of bronchoscopic lung volume reduction on dynamic hyperinflation and exercise in emphysema. Am J Respir Crit Care Med 2005;171:453–460. [DOI] [PubMed] [Google Scholar]
- 31.Germonpre PR, Verbraeken J, Vints AM, Van Ranst D, De Backer WA. Effect of endobronchialo valves in patients with severe emphysema. Am J Respir Crit Care Med 2004;165:A576. [Google Scholar]
- 32.de Oliveira HG, Macedo-Neto AV, John AB, Jungblut S, Prolla JC, Menna-Barreto SS, Fortis EA. Transbronchoscopic pulmonary emphysema treatment: 1-month to 24-month endoscopic follow-up. Chest 2006;130:190–199. [DOI] [PubMed] [Google Scholar]
- 33.Wan IY, Toma TP, Geddes DM, Snell G, Williams T, Venuta F, Yim AP. Bronchoscopic lung volume reduction for end-stage emphysema: report on the first 98 patients. Chest 2006;129:518–526. [DOI] [PubMed] [Google Scholar]
- 34.Washko G, Kenney L, Pinto-Plata V, Celli B, Reilly JJ. Initial experience with a tissue engineering approach to bronchoscopic lung voume reduction in humans with emphysema [abstract]. Chest 2005;180:A230. [Google Scholar]
- 35.Reilly J, Washko G, Pinto-Plata V, Velez E, Kenney L, Berger R, Celli B. Biological lung volume reduction: a new bronchoscopic therapy for advanced emphysema. Chest 2007;131:1108–1113. [DOI] [PubMed] [Google Scholar]
- 36.Cooper JD, Patterson GA, Sundaresan RS, Trulock EP, Yusen RD, Pohl MS, Lefrak SS. Results of 150 consecutive bilateral lung volume reduction procedures in patients with severe emphysema. J Thorac Cardiovasc Surg 1996;112:1319–1329. [Discussion, pp. 1329–30.] [DOI] [PubMed] [Google Scholar]
- 37.Ingenito EP, Evans RB, Loring SH, Kaczka DW, Rodenhouse JD, Body SC, Sugarbaker DJ, Mentzer SJ, DeCamp MM, Reilly JJ, Jr. Relation between preoperative inspiratory lung resistance and the outcome of lung-volume-reduction surgery for emphysema. N Engl J Med 1998;338:1181–1185. [DOI] [PubMed] [Google Scholar]
- 38.Meyers BF, Yusen RD, Guthrie TJ, Davis G, Pohl MS, Lefrak SS, Patterson GA, Cooper JD. Outcome of bilateral lung volume reduction in patients with emphysema potentially eligible for lung transplantation. J Thorac Cardiovasc Surg 2001;122:10–17. [DOI] [PubMed] [Google Scholar]
- 39.Mineo TC, Pompeo E, Mineo D, Rogliani P, Leonardis C, Nofroni I. Results of unilateral lung volume reduction surgery in patients with distinct heterogeneity of emphysema between lungs. J Thorac Cardiovasc Surg 2005;129:73–79. [DOI] [PubMed] [Google Scholar]
- 40.Sciurba FC, Rogers RM, Keenan RJ, Slivka WA, Gorcsan J, 3rd, Ferson PF, Holbert JM, Brown ML, Landreneau RJ. Improvement in pulmonary function and elastic recoil after lung-reduction surgery for diffuse emphysema. N Engl J Med 1996;334:1095–1099. [DOI] [PubMed] [Google Scholar]
- 41.Kotloff RM, Tino G, Palevsky HI, Hansen-Flaschen J, Wahl PM, Kaiser LR, Bavaria JE. Comparison of short-term functional outcomes following unilateral and bilateral lung volume reduction surgery. Chest 1998;113:890–895. [DOI] [PubMed] [Google Scholar]
- 42.Geiser T, Schwizer B, Krueger T, Gugger M, Hof VI, Dusmet M, Fitting JW, Ris HB. Outcome after unilateral lung volume reduction surgery in patients with severe emphysema. Eur J Cardiothorac Surg 2001;20:674–678. [DOI] [PubMed] [Google Scholar]
- 43.Hamacher J, Bloch KE, Stammberger U, Schmid RA, Laube I, Russi EW, Weder W. Two years' outcome of lung volume reduction surgery in different morphologic emphysema types. Ann Thorac Surg 1999;68:1792–1798. [DOI] [PubMed] [Google Scholar]
- 44.Tutic M, Bloch KE, Lardinois D, Brack T, Russi EW, Weder W. Long-term results after lung volume reduction surgery in patients with α1-antitrypsin deficiency. J Thorac Cardiovasc Surg 2004;128:408–413. [DOI] [PubMed] [Google Scholar]
- 45.Wisser W, Tschernko E, Wanke T, Senbaclavaci O, Kontrus M, Wolner E, Klepetko W. Functional improvements in ventilatory mechanics after lung volume reduction surgery for homogeneous emphysema. Eur J Cardiothorac Surg 1997;12:525–530. [DOI] [PubMed] [Google Scholar]
- 46.Goldstein RS, Todd TR, Guyatt G, Keshavjee S, Dolmage TE, van Rooy S, Krip B, Maltais F, LeBlanc P, Pakhale S, et al. Influence of lung volume reduction surgery (LVRS) on health related quality of life in patients with chronic obstructive pulmonary disease. Thorax 2003;58:405–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Naunheim KS, Wood DE, Mohsenifar Z, Sternberg AL, Criner GJ, DeCamp MM, Deschamps CC, Martinez FJ, Sciurba FC, Tonascia J, et al. Long-term follow-up of patients receiving lung-volume-reduction surgery versus medical therapy for severe emphysema by the National Emphysema Treatment Trial Research Group. Ann Thorac Surg 2006;82:431–443. [DOI] [PubMed] [Google Scholar]
- 48.Fessler HE. Collateral ventilation, the bane of bronchoscopic volume reduction. Am J Respir Crit Care Med 2005;171:423–424. [DOI] [PubMed] [Google Scholar]
- 49.Pinto-Plata V, Reilly JJ, Refaely Y, Duurkens VAM, Brooks J, Celli B, Berger RL. Biologic lung volume reduction (BLVR) for advanced emphysema. Chest 2006;190:A100. [Google Scholar]
- 50.Gelb AF, McKenna RJ, Jr, Brenner M, Epstein JD, Zamel N. Lung function 5 years after lung volume reduction surgery for emphysema. Am J Respir Crit Care Med 2001;163:1562–1566. [DOI] [PubMed] [Google Scholar]
- 51.Simonton C, Mathews-Simonton S, Creighton JL. Getting well again, 1st ed. New York: Bantam Books; 1992.