Abstract
The primary purpose of the National Emphysema Treatment Trial (NETT) was to evaluate the clinical efficacy of lung volume reduction surgery (LVRS) compared with medical therapy as a treatment for advanced emphysema. Transitioning the results of a complex multicenter long-term clinical trial into routine clinical practice is challenging, particularly when the therapy examined is controversial, as was the case in NETT. Aspects of the “clinical art” used by the study investigators to select and treat patients are not always transparent to practitioners reading study publications. At the last NETT Steering Committee meeting, a roundtable discussion was held with investigators, coordinators, Steering Committee leadership, and Data Coordinating Center staff regarding the clinical aspects of patient evaluation and selection and performance of LVRS in advanced emphysema. The questions posed to the meeting participants were ones that are commonly asked by patients and their treating physicians who are considering LVRS and included the following: Why recommend LVRS to a patient? When should LVRS be recommended to a patient? What types of patients are candidates for LVRS? What are the important barriers to performing LVRS? What are the major messages delivered by NETT? It is hoped that answers from NETT investigators to some of these commonly encountered questions will provide clarity and guidance to clinicians faced with the responsibility of considering and discussing LVRS with their patients. NETT investigators were also queried regarding the future directions of research in emphysema and the role that NETT played in shaping that future. The following article is a summary of the highlights of these discussions.
Keywords: emphysema, chronic obstructive pulmonary disease, lung volume reduction surgery, clinical trial, randomized trial
Since the revival of lung volume reduction surgery (LVRS) more than 15 years ago, controversy has surrounded its efficacy as a viable, reproducible form of treatment for severe emphysema. Before conduct of the National Emphysema Treatment Trial (NETT), the literature on LVRS, composed of uncontrolled results reported by single centers, demonstrated marked variability in patient selection criteria, surgical approach, complications, and outcomes in response to LVRS (1–10). These data were further confounded by the fact that most reports described only small numbers of patients with short periods of follow-up. NETT, a multicenter, randomized, controlled long-term trial, was designed to provide definitive answers regarding the independent effects of LVRS in contrast to optimal medical treatment on survival as well as exercise performance, lung function, symptoms, and quality of life (11).
NETT data on the short- and long-term effects of LVRS, as well as the clinical parameters that indicate a preferential response to treatment, have previously been reported (12, 13). However, transitioning a complicated and controversial therapy such as LVRS into routine clinical practice can be problematic. NETT had multiple criteria for inclusion of patients in the trial, some of which are not considered routine for clinical patient assessment. Some of the methodologies used in NETT for conducting radiologic and physiological testing and for characterizing or summarizing those test outcomes were not part of usual daily clinical practice. In addition, the analysis of NETT results turned up additional characterizations of the radiographic, exercise, and lung function data that are important to the identification of patients who benefit from or are harmed by LVRS and that were not standard assessments. Last, some of the data analysis methods used to identify these criteria may be unfamiliar to the practicing physician. As a result, translating the findings of the value of LVRS in patients with severe emphysema from the complex organized confines of NETT into the hands of practicing physicians and institutions not involved with NETT can be daunting. Many of the aspects of the “clinical art” of the selection and performance of LVRS may still remain unclear to the practicing clinician even after reading reports of the primary outcomes of the trial. In fact, the lay press reports that surprisingly few LVRS have been performed after the Centers for Medicare and Medicaid Services (CMS) January 2004 approval for LVRS performance based on NETT results and using NETT methodology to guide patient selection and outcome (14). In 2004, the CMS delegated the LVRS approval and accreditation process to the Joint Commission on Accreditation of Health Care Organizations (JCAHO, Oakbrook Terrace, IL) (15).
To aid clinicians in interpreting the major findings of NETT, and to foster the translation of NETT findings into patient care, a roundtable discussion was held at the last NETT Steering Committee meeting in October 2006 in Baltimore, Maryland. NETT investigators, coordinators, Steering Committee leadership, and Data Coordinating Center staff discussed a series of questions regarding the clinical aspects of the evaluation, selection, and performance of LVRS in patients with severe emphysema. Participants were also queried regarding the future of emphysema research and the role that NETT played in shaping that future. The following is a summary of the highlights of that discussion.
WHY DO YOU RECOMMEND LVRS TO A PATIENT?
Survival, quality of life, dyspnea relief, and improved exercise tolerance were the major reasons cited for recommending LVRS as potential treatment for severe emphysema. Which of these factors, however, were the most important in considering LVRS as treatment for an individual patient sparked discussion and differing opinions among participants.
Several investigators took the viewpoint that the beneficial impact of LVRS on survival was the most important reason to recommend LVRS for treatment. Reasons for this viewpoint extended beyond the obvious benefit of survival increasing longevity, but included the rationale that a therapy that extends survival achieves that through disease modification. For example, many clinical studies can demonstrate that investigational therapies improve the symptoms of a disease, but few have proven that the therapy can modify the underlying disease process. According to this viewpoint, an improvement in survival indicates modification of the underlying emphysema and therefore, without need for further justification, improvements in the patient's lung function, functional status, and quality of life all stem from the beneficial impact of LVRS. Therefore, by improving survival, NETT is a landmark study in the treatment of emphysema by demonstrating that LVRS impacts survival and goes well beyond providing only symptomatic relief.
Some investigators countered that dyspnea reduction, symptom relief, and improvements in exercise tolerance were even more important than survival as reasons to consider LVRS. In fact, some investigators had the opinion that dyspnea, exercise performance, and quality of life are all used synonymously by patients, families, and physicians when making decisions regarding treatment options. In fact, some investigators believed that in a chronic, progressive, and highly symptomatic disease such as emphysema, which profoundly impacts the patient's quality of life, the patient's perception of “feeling better” is the most important treatment goal to achieve, even more than survival.
Several investigators countered that the use of survival or improvements in the symptom complex of patients as clinical yardsticks to decide whether to consider LVRS should not be absolute, and must be customized for the individual patient. For the most part, even if survival is reported to be extended by a treatment in a highly symptomatic chronic disease such as emphysema, if patient quality of life and symptom relief are also not beneficially impacted, the survival benefit may have less value to the patient. Few patients or physicians would consider a treatment successful that extends life in those patients who state that “they would rather die than continue to live like this” but that does not improve their symptoms. However, some patients who are miserable from illness might still want to live longer even under these circumstances.
Other investigators opined that NETT provides data showing that LVRS offers extended survival and symptom reduction, making it a unique treatment option without significant competition in severe emphysema. In fact, some investigators pointed out that the survival benefit of LVRS becomes most important when considering other complex surgical interventions in emphysema such as lung transplantation, which can significantly improve patient symptoms but is accompanied by high morbidity and mortality, as well as costs. In fact, the NETT data support the notion that, in some patients, LVRS may be a superior treatment to lung transplantation, both in terms of patient outcomes and costs.
In summary, most NETT investigators believed that the impacts of LVRS on survival, quality of life, functional performance, and patient symptoms were all important considerations when weighing treatment options. However, the consensus of the NETT group was that the weight assigned to each of the above-cited factors in any particular decision must be individualized for the particular patient under consideration. Detailed discussion regarding the operative and postoperative morbidity and mortality of LVRS must be individualized for each patient on the basis of the results of their radiographic and physiological preoperative assessments. The patient's desires and expectations of LVRS, as well as the interpretation of the patient's lung function, exercise, and radiologic test results and the physician (pulmonologist and surgeon) assessment of the patient, are all key factors to be entered into the decision-making process.
WHEN SHOULD I CONSIDER LVRS FOR A PATIENT?
NETT investigators suggested several factors that should prompt the clinician to consider LVRS for a patient with emphysema. These factors predominantly included the following: the type and severity of the patient's symptoms; the severity and progressive nature of the patient's disease; the particular characteristics of the patient's lung function, chest computed tomography (CT), and exercise testing results; and finally the patient's readiness to undergo comprehensive testing and/or major surgery relative to their desire to attempt to improve their clinical status.
NETT investigators uniformly believed that LVRS should be considered only in severe emphysema after patients have undergone optimal medical treatment, including pulmonary rehabilitation, and fail to improve their clinical status. NETT investigators reported and firmly believed that pulmonary rehabilitation is an important adjunct to medical pharmacotherapy, including oxygen administration, even in patients with severe emphysema (16). Furthermore, several NETT investigators noted that a substantial number of their NETT candidates reported an improvement in their symptoms after pulmonary rehabilitation that was so significant that they withdrew their consent to undergo LVRS if randomized to treatment.
One of the difficulties in determining when patients with emphysema should undergo LVRS is that many of the tests used to determine a patient's suitability are not performed as part of routine clinical practice. NETT demonstrated the value of assessing the severity and distribution of emphysema from chest CT images and measuring maximal workload achieved during symptom-limited cardiopulmonary exercise testing in predicting outcome from LVRS. However, the majority of patients with emphysema have not had chest CT characterization of the severity and distribution of emphysema, or maximal exercise testing performed as part of their usual clinical care evaluation and treatment. For many patients, tests such as chest CT or cardiopulmonary exercise need to be preapproved by the third-party payer before they can be performed. This raises another potential barrier to the broad use of these tools to characterize the patient's suitability for LVRS.
Finally, NETT algorithms for characterizing emphysema severity and distribution on CT scan, or for characterizing workload during cardiopulmonary exercise testing as high or low, are not typically employed by non-NETT centers and physicians. NETT demonstrated that these assessments could be successfully conducted in a large number of patients with severe emphysema, at many different centers with reproducible results and acceptable patient comfort and safety. In fact, one publication has shown that CT assessment of emphysema severity and distribution can be conducted by nonradiologists with acceptable results (17). Transitioning the techniques of NETT assessment and test interpretation into clinical practice was considered feasible by the NETT investigators. However, the NETT investigators believed that a range of educational materials and venues would be required to adequately disseminate this information to the clinical community.
TO WHOM DO YOU OFFER LVRS?
NETT demonstrated that pulmonary function data, maximal workload attained on cardiopulmonary exercise testing, and the severity and pattern of emphysema on chest CT analysis are helpful in differentiating patient response to LVRS (12). Patients with an FEV1 not greater than 20% predicted and either a nonheterogeneous pattern of emphysema on chest CT or a carbon monoxide diffusion capacity not greater than 20% predicted were found to have unacceptably high postoperative mortality and are not considered candidates for LVRS (18). Similarly, patients with a high maximal workload on cardiopulmonary exercise testing and a non–upper lobe–predominant pattern of emphysema on chest CT had unacceptably high mortality after LVRS with questionable benefit, and are also not considered LVRS candidates. Neither of the above categories of patients has CMS approval for LVRS (19).
NETT demonstrated that patients with upper lobe–predominant emphysema on chest CT benefited from LVRS in terms of improvements in lung function, exercise performance, quality of life, and a reduction in patient symptoms (12). Patients with upper lobe–predominant emphysema and a low maximal workload on cardiopulmonary exercise testing also had a significant survival advantage with LVRS compared with optimal medical management, a finding that remains preserved in long-term follow-up (13).
NETT reported that patients with non–upper lobe–predominant emphysema by chest CT analysis and low maximal workload on exercise testing who underwent LVRS had an improvement in lung function and exercise performance, and an improvement in quality of life and patient symptom score compared with medical management. However, the beneficial effects in this patient group were of lesser magnitude and were less durable over time compared with patients with upper lobe–predominant disease (13).
Overall, NETT investigators uniformly offer LVRS to patients with upper lobe–predominant disease because of its demonstrated benefits in improving lung function, exercise performance, quality of life, and patient symptoms. In patients with upper lobe–predominant disease and low exercise performance, NETT investigators believe the significant survival benefit of LVRS to be an important factor to consider when discussing the potential benefits of LVRS.
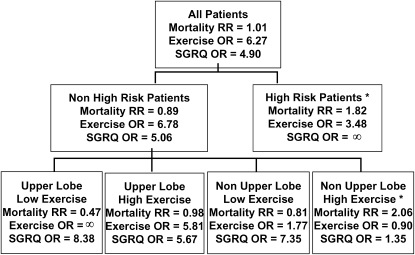
In patients with non–upper lobe–predominant emphysema on chest CT and low exercise performance on cardiopulmonary exercise testing, NETT investigators are selective in considering LVRS as a potential treatment option. In fact, most NETT investigators infrequently offer LVRS to this patient group. Non–upper lobe–predominant emphysema also includes patients with isolated emphysema of the superior segments of the lower lobes, and some NETT investigators believe that this patient group would have better response to LVRS than other categories of patients with non–upper lobe disease (e.g., diffuse emphysema) and should be more favorably considered for surgery. NETT investigators believe that the desires and personal considerations of the patient, as well as the availability of other medical alternatives, need to be carefully factored when considering LVRS in this patient group. An overview of the likelihood of benefit with LVRS on survival, maximal workload attained on exercise testing, and patient symptoms for each of the NETT subgroups is shown in Figure 1.
Figure 1.
National Emphysema Treatment Trial (NETT) subgroup treatment effects. Effect of lung volume reduction surgery (LVRS) versus medical treatment on mortality, maximal workload achieved on cardiopulmonary exercise testing (improvement of more than 10 W vs. not improved), and disease-specific quality of life as measured by the St. George's Respiratory Questionnaire (SGRQ; improvement of more than 8 units in total score vs. not improved). OR = odds ratio; RR = relative risk; *Patients not considered candidates for LVRS by the NETT or approved for LVRS by the Centers for Medicare and Medicaid Services or the Joint Commission on Accreditation of Healthcare Organizations.
WHO SHOULD DO THE PREOPERATIVE TESTING TO ASSESS LVRS CANDIDACY?
Patients and their physicians will not know if they are appropriate candidates for LVRS if they have not had lung function and exercise testing or a chest CT. To transition the testing found useful by NETT to evaluate patients for LVRS into routine clinical practice, the practicality of who performs the tests and where the tests are performed needs to be addressed.
In NETT, the clinical centers used standardized procedures to perform and interpret pulmonary function tests, exercise tests, six-minute-walk tests, and chest CT with a particular focus on the patient's candidacy for LVRS (11). To optimize the quality of data collection, investigators and their staff were instructed during several group training sessions to use similar protocols to perform the techniques. In addition, onsite visits were conducted at each center to evaluate test performance during the course of NETT. Although it was feasible during NETT for all subjects to have all of their LVRS testing, both screening and follow-up testing, performed only at each NETT center, this is impractical for routine clinical care. Patient travel expenses and inconveniences, third-party payer practices, and physician–patient referral patterns are some of the important issues that make it unrealistic for every potential patient candidate to have all of their testing for LVRS conducted only at LVRS-approved centers.
The NETT investigators made the following recommendations to overcome some barriers in performing patient evaluations to determine LVRS candidacy. The NETT investigators believe that it is important for JCAHO-approved LVRS centers to develop a collaborative regional network with health providers who care for patients with chronic obstructive pulmonary disease (COPD). Continuing medical education programs, written materials, close contact (e.g., close communication, physician-to-physician contacts, regular updates of patient and physician LVRS informational written materials) and other types of educational contacts that are coordinated by the regional LVRS centers are needed to educate referral primary care physicians, pulmonologists, thoracic surgeons, and other health care providers about the role of LVRS in the management of patients with severe emphysema.
The NETT investigators recommended that referral physicians obtain pulmonary function and six-minute-walk tests, an arterial blood gas, chest X-ray, and a high-resolution chest CT in their local community after optimal medical treatment including pulmonary rehabilitation. These tests should be performed only when patients are in stable condition, using the procedural format and predicted pulmonary function standards as outlined in NETT and endorsed by the JCAHO. The data can then be used to screen patients with COPD who are refractory to optimized medical treatment for potential candidacy for LVRS by their regional JCAHO-approved LVRS center.
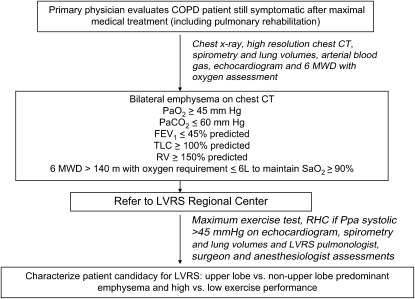
At the LVRS center, patients who are potential LVRS candidates can undergo post–pulmonary rehabilitation maximal cardiopulmonary exercise testing to better characterize their likelihood of benefit. An echocardiogram should be performed to evaluate left ventricular function and to screen for obvious secondary pulmonary hypertension. In those patients with a suspected pulmonary artery systolic pressure of at least 45 mm Hg, the presence of right ventricular dysfunction, or the inability to measure pulmonary arterial pressure on echocardiography, right heart catheterization should be performed to exclude occult severe secondary pulmonary hypertension. Pulmonary function and six-minute-walk tests; preoperative blood work; preoperative evaluations by the LVRS center pulmonologist, surgeon, and anesthesiologist; and other testing deemed necessary to assess the individual patient's condition and suitability for surgery can be done before LVRS. Figure 2 shows an outline of the LVRS testing that is suggested to be coordinated between the community referral physician and the regional LVRS center.
Figure 2.
Suggested schema for the coordination of lung volume reduction surgery (LVRS) testing between community physicians and LVRS regional centers. 6-MWD = six-minute-walk distance; COPD = chronic obstructive pulmonary disease; CT = computed tomography; RHC = right heart catheterization; RV = residual volume.
Postsurgical testing can then be coordinated between the LVRS center and the patient's local clinical care provider to maximize the collection of data. This information is used to assess the patient's response to LVRS and the need for additional medical interventions.
The NETT investigators believe that this form of coordination of testing and care between the regional LVRS centers and the patient's primary clinical care team will overcome some of the important barriers that now exist for patients to receive LVRS.
WHAT ARE THE MOST IMPORTANT BARRIERS TO PERFORMING LVRS?
NETT investigators remarked that it is their perception after talking to many practicing physicians that restricting the performance of LVRS to prior NETT or lung transplantation centers or centers that receive JCAHO approval limits the number of patients who receive LVRS. However, as NETT demonstrated, patients who received LVRS are fragile, and their postsurgical care can be complicated. NETT investigators believe that LVRS should be done only by those with the skill and facilities to manage these types of compromised patients with advanced pulmonary disease undergoing pulmonary resection. NETT investigators also believe it is the perception of many clinicians that the testing required for LVRS assessment is complicated and exhaustive. Hopefully, the measures enumerated in the prior section may alter these perceptions held by non-NETT clinicians and make the coordination of pre- and postoperative LVRS testing more efficient for patients as well as their primary care providers.
NETT investigators also believe that the limited availability of outpatient pulmonary rehabilitation programs in some regions of the country is also a potential barrier to the broader performance of LVRS. Compounding the lack of available programs is the absence of a Medicare national coverage policy for outpatient pulmonary rehabilitation, outside of that in preparation for LVRS. In fact, NETT investigators believe that the current payment scheme for preoperative pulmonary rehabilitation before LVRS, whereby the reimbursement flows via the LVRS center to the rehabilitation center, is problematic. The lack of direct reimbursement to the rehabilitation centers performing pulmonary rehabilitation hinders the willingness of those centers to accept patients. The investigators believe that the favorable effects of outpatient pulmonary rehabilitation on patient symptoms, quality of life, and exercise tolerance in severe emphysema demonstrated in NETT substantiate its role as a standard of care for this patient group (16). It is the strong opinion of the investigators that NETT has proven outpatient pulmonary rehabilitation is effective in severe emphysema. They believe that appropriate reimbursement strategies should be developed by CMS to consider pulmonary rehabilitation as a covered benefit.
Despite NETT publications demonstrating an improvement in survival as well as improvements in lung function, exercise performance, and quality of life, NETT investigators also believe that many practicing physicians still remain unaware of the benefits of LVRS and what constitutes an appropriate patient candidate (12, 13). Many NETT investigators believe that this stems from the publication describing the patient group that was identified to be at high risk for death with LVRS (e.g., FEV1 ⩽ 20% predicted and either nonheterogeneous emphysema on chest CT or diffusion capacity ⩽ 20% predicted) early in NETT and censored from further enrollment. Many believe that the title of that publication, “Patients at High Risk of Death after Lung-Volume–Reduction Surgery,” has been erroneously interpreted as all patients are at high risk for death with LVRS (18). Many believe that the stigma that LVRS increases the risk of death in all subjects, both in the lay and medical press, has continued to this date (14, 20). Correcting this misperception will require a variety of educational formats geared toward patients as well as non-NETT clinicians.
NETT investigators also believe that LVRS is perceived by many in the medical community to be too costly. In part, they believe this perception is fueled by the cost-effectiveness analysis of LVRS that was published simultaneously with the main outcomes paper (21). However, as noted in the initial and follow-up cost-effectiveness analysis reports (22), LVRS is on par with costs of other palliative procedures such as joint replacement surgery and coronary artery revascularization, and is significantly less expensive than lung transplantation. Furthermore, the cost-effectiveness of LVRS is significantly enhanced when only considering patients with the clinical characteristics that predict the most favorable response to surgery. NETT investigators believe that the performance of a well-designed cost-effectiveness analysis done in parallel with the main trial was a strength of NETT, which makes it unique in the investigation of the effectiveness of new surgical therapies.
WHAT DO YOU BELIEVE ARE THE MOST IMPORTANT QUESTIONS REGARDING LVRS THAT REMAIN UNANSWERED AFTER NETT?
The meeting participants identified several important questions that remain unanswered regarding LVRS after completion of NETT. One of the most important questions is the mechanism responsible for improved survival with LVRS, especially in the upper lobe–predominant emphysema, low-exercise subgroup of patients. Whether patients with heterogeneous, but not upper lobe–predominant, emphysema can also have a beneficial response to LVRS remains an outstanding question. In addition, whether patients with predominantly unilateral emphysema respond favorably to LVRS is unknown. Additional questions are as follows: the status of patient outcomes with LVRS post-CMS approval, the impact of the JCAHO certification process on the number of LVRS programs, and patient access to LVRS on a national level.
NETT investigators also believe that choosing between lung transplantation and LVRS can be problematic, especially in patients with non–upper lobe–predominant emphysema and low exercise performance. NETT did not address this issue nor what obstacles are posed to the surgeon or the patient when lung transplantation is performed after LVRS. Non-NETT single-center data suggest that lung transplantation may produce more substantial improvements in lung function, exercise performance, and quality of life, but is associated with higher morbidity and mortality and health care costs compared with LVRS (23). The new Lung Allocation Scoring system for lung transplantation listing results in lower scores for potential emphysema lung transplantation candidates compared with other lung diseases, potentially limiting their access to transplantation. The NETT investigators suggested that a properly conducted multicenter trial of LVRS versus transplantation involving adequate numbers of patients with emphysema who are similar in terms of baseline physiology, age, and CT pattern of emphysema would be required to definitely answer the risks and rewards of lung transplantation versus LVRS.
WHAT DO YOU BELIEVE ARE THE MAJOR MESSAGES DELIVERED BY NETT?
The fact that LVRS is a disease-modifying therapy for emphysema, and that clinical phenotyping of the patient with emphysema is important in determining the response to treatment, are what NETT investigators believe to be the major messages of NETT. LVRS now joins long-term oxygen therapy (24) and smoking cessation (25) as the only therapies that improve survival, physiological, and/or functional status in advanced emphysema. Work is needed to determine the mechanisms that improve survival with LVRS to enhance these treatment effects and produce even more potent and durable responses to therapy.
That the patterns of maximal workload attained on maximal exercise testing (high vs. low workload) and the radiographic presentation of emphysema (upper lobe vs. non–upper lobe predominant) can help predict differential patient responses to LVRS heralds a new concept in designing clinical trials and assessing patient responses to treatment. Similar to the staging that occurs in medical oncology, NETT demonstrated that emphysema is a diverse disease with protean pulmonary and nonpulmonary manifestations that can affect outcome. Careful characterization of the patient's extent and severity of disease by multimodality testing may help define the different clinical phenotypes of COPD or emphysema as well as their requirements for different treatments. Support for this notion is provided by other studies that demonstrate the value of multidimensional indices composed of pulmonary and nonpulmonary factors in predicting survival in COPD (26).
NETT demonstrated the value of the qualitative interpretation of emphysema by chest CT analysis in selecting candidates for LVRS and determining their response to surgery. This raises the question as to whether the pattern of emphysema on chest CT should be assessed more commonly in clinical trials. If so, should the assessment be qualitative or quantitative? Should the determination of the severity and pattern of emphysema be used as part of routine clinical assessment? Finally, what are the technical obstacles for the use of the qualitative or quantitative assessment of emphysema by chest CT in clinical investigation or by the clinician? Although NETT demonstrated the value of chest CT analysis in predicting patient outcome and response to therapy, much work needs to be done to answer the above questions and others to make this an effective and easily used tool.
The experience of NETT suggests that future studies should also be designed to be large enough and broad enough to be capable of determining patient characteristics that predict favorable responses to treatment. Clinicians and patients can then use the information to make choices based on a more individualized assessment of their potential risks and benefits.
The inclusion criteria of NETT were purposely broad and the study was designed with survival as the primary outcome, thus ensuring the ability to use test results to characterize not only the type of response to LVRS but the magnitude and durability of the response. The NETT investigators encourage future investigators to consider such factors when designing their trials.
NETT also demonstrated that large, long-term clinical trials in a complex and ill patient population can be done, but require careful integration and care with multiple health care professionals. The integral role of the clinical coordinator to successfully complete the study as well as serve as the patient's advocate throughout a prolonged period was well demonstrated in NETT. NETT coordinators not only paced the patient though the course of the study protocol, but frequently became the patient's advocate and primary conduit for a range of medical advice. Similar roles were played at times by rehabilitation and other ancillary staff who bonded to the patient over the course of their prolonged participation in the study. Such patient–coordinator bonding and aid in a range of medical and nonmedical issues are paramount to consider in designing trials, such as NETT, that examine intensive therapies in a complex and severely ill patient group.
CONCLUSIONS
Table 1 summarizes the major points on each of the issues discussed at the conference. The NETT investigators hope that the results of this discussion will improve the clinician's awareness of the benefits of LVRS and stimulate further communication between patients and physicians regarding the role of LVRS as treatment in advanced emphysema.
TABLE 1.
CLINICIAN'S GUIDE TO LUNG VOLUME REDUCTION SURGERY: SUMMARY
| • Why do you recommend LVRS to a patient? |
| Survival |
| Quality of life |
| Dyspnea relief |
| Improved exercise tolerance |
| • When should I consider LVRS for a patient? |
| Type and severity of patient's symptoms |
| Progressive nature of disease |
| Results of patient's lung function, exercise, and chest CT |
| Patient willingness to undergo comprehensive testing, rehabilitation and major surgery |
| • To whom do you offer LVRS? |
| Upper lobe–predominant emphysema by chest CT |
| Non–upper lobe–predominant emphysema and postrehabilitation low exercise performance |
| • Who should do the preoperative testing to assess LVRS candidacy? |
| Referral physicians obtain lung function, six-minute walk, arterial blood gases, and high-resolution chest CT after pulmonary rehabilitation |
| LVRS center performs maximal exercise test post rehabilitation, echocardiogram, right heart catheterization if Ppa exceeds 45 mm Hg on echocardiogram, preoperative anesthesiology, pulmonary and thoracic surgery evaluations and lung function, six-minute walk, and other preoperative blood work |
| • What are the most important barriers to performing LVRS? |
| Limited awareness by clinicians of the benefits of LVRS or profile of optimal candidates |
| Relegation of LVRS to select centers |
| Limited availability of pulmonary rehabilitation |
| Misperceptions that LVRS is too expensive or screening testing is too exhaustive and complicated |
| • What are the most important questions regarding LVRS that remain unanswered after NETT? |
| Mechanisms of improvement with LVRS |
| Whether patients with heterogeneous but not upper lobe–predominant disease benefit from LVRS |
| If patients with unilateral heterogeneous emphysema benefit from LVRS |
| • What are the major messages delivered by NETT? |
| LVRS is a disease-modifying therapy |
| Clinical phenotype of the patient with COPD is important to assess the natural history of disease and response to therapy |
Definition of abbreviations: COPD = chronic obstructive pulmonary disease; CT = computed tomography; LVRS = lung volume reduction surgery; NETT = National Emphysema Treatment Trial.
The National Emphysema Treatment Trial (NETT) is supported by contracts with the National Heart, Lung, and Blood Institute (N01HR76101, N01HR76102, N01HR76103, N01HR76104, N01HR76105, N01HR76106, N01HR76107, N01HR76108, N01HR76109, N01HR76110, N01HR76111, N01HR76112, N01HR76113, N01HR76114, N01HR76115, N01HR76116, N01HR76118, and N01HR76119), the Centers For Medicare and Medicaid Services (CMS), and the Agency for Healthcare Research and Quality (AHRQ).
Conflict of Interest Statement: Neither author has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
Attendees at the NETT Steering Committee meeting included the following: Amanda Blackford, Neil Brister, Gerard Criner, Malcolm DeCamp, Philip Diaz, Jeremy Falk, Alfred Fishman, Patricia Jellen, Steven Kadiev, Robert Kaplan, Samuel Krachman, Mark Krasna, Anne Marie Kuzma, Imogene Lambie, Charlene Levine, David Lipson, Barry Make, Fernando Martinez, Catherine Meldrum, Yvonne Meli, Omar Minai, Zab Mosenifar, Iris Moskowitz, Keith Naunheim, Namrata Patel, Steven Piantadosi, John Reilly, Steven Scharf, Frank Sciurba, Alice Sternberg, Byron Thomashow, James Tonascia, Susan Tonascia, James Utz, George Washko, and Gail Weinmann.
References
- 1.Cooper JD, Trulock EP, Triantafillou AN, Patterson GA, Pohl MS, Deloney PA, Sundaresan RS, Roper CL. Bilateral pneumectomy (volume reduction) for chronic obstructive pulmonary disease. J Thorac Cardiovasc Surg 1995;109:106–116. [DOI] [PubMed] [Google Scholar]
- 2.McKenna RJ Jr, Brenner M, Gelb AF, Mullin M, Singh N, Peters H, Panzera J, Calmese J, Schein MJ. A randomized, prospective trial of stapled lung reduction versus laser bullectomy for diffuse emphysema. J Thorac Cardiovasc Surg 1996;111:317–321. [Discussion, p. 322.] [DOI] [PubMed] [Google Scholar]
- 3.McKenna RJ Jr, Brenner M, Fischel RJ, Gelb AF. Should lung volume reduction surgery be unilateral or bilateral? J Thorac Cardiovasc Surg 1996;112:1331–1339. [Discussion, pp. 1338–1339.] [DOI] [PubMed] [Google Scholar]
- 4.Bingisser R, Zollinger A, Hauser M, Bloch KE, Russi EW, Weder W. Bilateral volume reduction surgery for diffuse pulmonary emphysema by video-assisted thoracoscopy. J Thorac Cardiovasc Surg 1996;112:875–882. [DOI] [PubMed] [Google Scholar]
- 5.Cooper JD, Patterson GA, Sundaresean RS, Trulock EP, Yusen RD, Pohl MS, Lefrak SS. Results of 150 consecutive bilateral lung volume reduction procedures in patients with severe emphysema. J Thorac Cardiovasc Surg 1996;112:1319–1330. [Discussion, pp. 1329–1330.] [DOI] [PubMed] [Google Scholar]
- 6.Miller JI Jr, Lee RB, Mansour KA. Lung volume reduction surgery: lessons learned. Ann Thorac Surg 1996;61:1464–1469. [Discussion, pp. 1468–1469.] [DOI] [PubMed] [Google Scholar]
- 7.Daniel TM, Chan BK, Bhaskar V, Parekh JS, Walters PE, Reeder J, Truwit JD. Lung volume reduction surgery: case selection, operative technique, and clinical results. Ann Surg 1996;223:526–533. [Discussion, pp. 532–533.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Argenziano M, Moazami N, Thomashow B, Jellen PA, Gorenstein LA, Rose EA, Weinberg AD, Steinglass KM, Ginsburg ME. Extended indications for volume reduction pneumoplasty in advanced emphysema. Ann Thorac Surg 1996;62:1588–1597. [DOI] [PubMed] [Google Scholar]
- 9.Wisser W, Tschernko E, Senbaklavaci O, Kontrus M, Wanke T, Wolner E, Klepetko W. Functional improvement after volume reduction: sternotomy versus videoendoscopic approach. Ann Thorac Surg 1997;63:822–828. [Discussion, pp. 827–828.] [DOI] [PubMed] [Google Scholar]
- 10.Healthcare Finance Administration. Report to Congress. Lung volume reduction surgery and Medicare coverage policy: implications of recently published evidence. Washington, DC: Department of Health and Human Services; 1998.
- 11.National Emphysema Treatment Trial Research Group. Rationale and design of the National Emphysema Treatment Trial: a prospective randomized trial of lung volume reduction surgery. Chest 1999;116:1750–1761. [DOI] [PubMed] [Google Scholar]
- 12.National Emphysema Treatment Trial Research Group. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med 2003;348:2059–2073. [DOI] [PubMed] [Google Scholar]
- 13.Naunheim KS, Wood DE, Mohsenifar Z, Sternberg AL, Criner GJ, DeCamp MM, Deschamps CC, Martinez FJ, Sciurba FC, Tonascia J, et al.; National Emphysema Treatment Trial Research Group. Long-term follow-up of patients receiving lung-volume-reduction surgery versus medical therapy for severe emphysema. Ann Thorac Surg 2006;82:431–443. [DOI] [PubMed] [Google Scholar]
- 14.Kolata G. Medicare says it will pay, but patients say “no thanks.” New York Times March 3, 2006. [PubMed]
- 15.Joint Commission on Accreditation of Healthcare Organizations. Lung volume reduction surgery certification requirements [Internet] [accessed December 2007]. Available from: http://www.jointcommission.org/NR/rdonlyres/12EFD2D8-6FC9-498C-9AD8-944D8794CF45/0/LVRS_final_addendum.pdf
- 16.Ries A, Make B, Lee S, Krasna M, Bartels M, Crouch R, Fishman A; National Emphysema Treatment Trial Research Group. The effects of pulmonary rehabilitation in the National Emphysema Treatment Trial. Chest 2005;128:3799–3809. [DOI] [PubMed] [Google Scholar]
- 17.Hersh CP, Washko GR, Jacobson FL, Gill R, Estepar RS, Reilly JJ, Silverman EK. Interobserver variability in the determination of upper lobe–predominant emphysema. Chest 2007;131:424–431. [DOI] [PubMed] [Google Scholar]
- 18.National Emphysema Treatment Trial Research Group. Patients at high risk of death after lung-volume-reduction surgery. N Engl J Med 2001;345:1075–1083. [DOI] [PubMed] [Google Scholar]
- 19.Centers for Medicare and Medicaid Services (CMS), Department of Health and Human Services (DHHS). Revision to §240.1 of Publication 100-03: Lung volume reduction surgery (reduction pneumoplasty) [CMS Manual System, Publication 100-03, Medicare National Coverage; November 4, 2003] [accessed December 2007]. Available from: http://www.cms.hhs.gov/Transmittals/Downloads/R3NCD.pdf
- 20.Grady D. Results of costly emphysema operation are mixed, study finds. New York Times May 21, 2003.
- 21.NETT Research Group. Cost effectiveness of lung-volume-reduction surgery for patients with severe emphysema. N Engl J Med 2003;348:2092–2102. [DOI] [PubMed] [Google Scholar]
- 22.Ramsey SD, Shroyer AL, Sullivan SD, Wood DE. Updated evaluation of the cost-effectiveness of lung volume reduction surgery. Chest 2007;131:823–832. [DOI] [PubMed] [Google Scholar]
- 23.Weinstein M, Martin U, Crookshank A, Chatila W, Vance G, Gaughan J, Criner GJ. Mortality and functional performance in severe emphysema after lung volume reduction or transplant. COPD 2007;4:15–22. [DOI] [PubMed] [Google Scholar]
- 24.Nocturnal Oxygen Therapy Trial Group. Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: a clinical trial. Ann Intern Med 1980;93:391–398. [DOI] [PubMed] [Google Scholar]
- 25.Anthonisen NR, Skeans MA, Wise RA, Manfreda J, Kanner RE, Connett JE; Lung Health Study Research Group. The effects of a smoking cessation intervention on 14.5-year mortality: a randomized clinical trial. Ann Intern Med 2005;142:233–239. [DOI] [PubMed] [Google Scholar]
- 26.Celli BR, Cote CG, Marin JM, Casanova C, Montes de Oca M. Mendez RA. Pinto Plata V, Cabral HJ. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 2004;350:1005–1012. [DOI] [PubMed] [Google Scholar]