Abstract
Chronic obstructive pulmonary disease (COPD) is characterized physiologically by expiratory flow limitation and pathologically by alveolar destruction and enlargement and small and large airway inflammation and remodeling. An imbalance between protease and antiprotease activity in the lung is proposed as the major mechanism resulting in emphysema. The imbalance is mostly due to an increase in the numbers of alveolar macrophages and neutrophils. Emphysema can also develop from increased alveolar wall cell death and/or failure in alveolar wall maintenance. Chronic inflammation and increased oxidative stress contribute to increased destruction and/or impaired lung maintenance and repair. Genetic factors may play an important role in disease susceptibility because only a minority of smokers develops emphysema. Recent literature implicates surfactant instability, malnutrition, and alveolar cell apoptosis as possible etiologies. Identification of cellular and molecular mechanisms of COPD pathogenesis is an area of active, ongoing research that may help to determine therapeutic targets for emphysema.
Keywords: emphysema, apoptosis, protease–antiprotease balance, oxidative stress, hypoxemia
Chronic obstructive pulmonary disease (COPD) is a progressive respiratory condition characterized clinically by dyspnea, cough, and sputum production. Dyspnea is physiologically caused by expiratory flow limitation. Pathologically, COPD lungs show alveolar destruction and enlargement and inflammation of lung parenchyma and airways. The pathogenesis of emphysema is an arena of ongoing, active research, and new developments continue to arise. Emphysema can result from increased alveolar wall cell death and/or failure of alveolar wall maintenance (1). The literature indicates that chronic inflammation and increased oxidative stress contribute to increased destruction and/or impaired lung maintenance and repair in emphysema. Furthermore, because only a minority of smokers develops clinically significant emphysema, genetic factors may play an important role in susceptibility or resistance to cigarette smoke (see Hersh and coworkers, pages 486–493, this symposium [25]). We briefly review a paradigm integrating these mechanisms in generating emphysema. For a detailed review of COPD pathogenesis and a description of related animal studies, readers are referred to recent comprehensive reviews (1–4).
PATHOGENIC MECHANISMS IN EMPHYSEMA
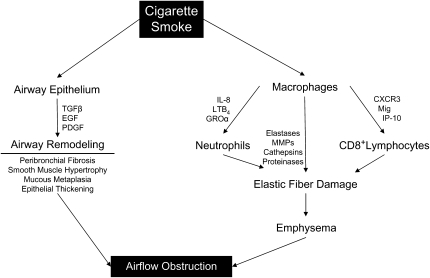
Pathologically, COPD is characterized by diffuse inflammation of lung parenchyma and airways (Figure 1). The inflammatory response in emphysema typically shows evidence of the activation of innate and acquired inflammatory processes. The accumulation of these inflammatory components contributes to the lung injury in these patients and serves as a self-perpetuating stimulus for further immune activation. The mobilization of inflammatory cells to the lung leads to the release of potentially destructive mediators, including tissue proteases and cytokines, which directly contribute to tissue remodeling and destruction. These mediators include chemoattractant factors, most notably chemokines, which serve to attract additional inflammatory cells. The overall inflammatory response serves to trigger structural cells, including vascular endothelial cells and epithelial cells, to produce substantial levels of proinflammatory cytokines, chemokines, and other mediators.
Figure 1.
Macrophages are activated by cigarette smoke and recruit neutrophils and CD8+ lymphocytes to cause elastolysis and emphysema. Similarly, cigarette smoke activates airway epithelium to trigger airway remodeling. Both of these processes result in airflow obstruction. CXCR3 = chemokine CXC receptor 3; EGF = epidermal growth factor; GROα = chemokine growth-regulated protein alpha; IP-10 = IFN-gamma-inducible 10 kD protein; LTB4 = leukotriene B4; Mig = monokine induced by IFN-gamma; MMPs = matrix metalloproteinases; PDGF = platelet-derived growth factor; TGF-β = transforming growth factor-β.
In addition to inflammation, oxidative stress caused by cigarette smoke inhalation plays a significant role in generating emphysema. The major consequence of the oxidative stress is the activation of the transcription factor nuclear factor-κB, which activates proinflammatory cytokine transcription (5, 6). Recent evidence suggests that cigarette smoke inhibits histone deacetylase, further promoting the release of proinflammatory cytokines (7). Therefore, oxidant injury and lung inflammation act in concert to increase alveolar destruction or compromise maintenance and repair of alveolar structure. Table 1 outlines the established and some proposed mechanisms behind COPD pathogenesis.
TABLE 1.
ESTABLISHED AND PROPOSED MECHANISMS OF CHRONIC OBSTRUCTIVE PULMONARY DISEASE PATHOGENESIS
| Established mechanisms |
| Protease–antiprotease imbalance |
| Histone deacetylase inhibition |
| Airway inflammation with macrophages, neutrophils, and CD8+ lymphocytes |
| Oxidant injury |
| Proposed mechanisms |
| Malnutrition |
| Surfactant instability |
| Alveolar cell apoptosis |
Protease–Antiprotease Balance
A prevailing hypothesis in the generation of emphysema is protease–antiprotease imbalance (4). A delicate balance between protease and antiprotease activity is required for proper lung maintenance (1). Derangements of this balance may result in increased destruction and inappropriate repair of lungs, ultimately causing emphysema. For example, α1-antitrypsin deficiency is a well-described genetic risk factor for emphysema (8). Furthermore, several animal models show development of emphysema with intratracheal instillation of elastolytic enzymes (1). An animal model of matrix metalloproteinase (MMP)-12 knockout mice showed resistance to development of emphysema in mice exposed to cigarette smoke (9). Macrophages and neutrophils are main sources of proteases in lungs, and many studies have shown correlations between the degree of macrophage and neutrophil inflammation and severity of airflow obstruction (10). In addition to neutrophil elastase and MMP-12, there are other proteases that may play an important role, including MMP-8 (a collagenase), MMP-9 (a gelatinase), and cathapsins S, L (in macrophages), and G, and proteinase-3 (in neutrophils) (4).
In addition to destruction of the matrix, elastin fragments generated by proteinases have a chemotactic effect on monocytes and thus increase the inflammatory and protease burden in the lung, creating a positive feedback loop that results in continuous destruction of lung parenchyma (9, 11, 12). In short, protease–antiprotease imbalance can degrade the lung matrix and affect alveolar structure maintenance by altering the matrix and cell signaling (1).
Maintenance of Alveolar Structure and Apoptosis
The neonatal lung develops from interactions between foregut endoderm and mesenchyma. This development is completed by alveolarization that continues into the neonatal period. Development and maintenance of alveolar structure depends on the concerted interaction among various elements of the alveolar wall. Massaro and Massaro suggested that this remarkable phenomenon of lung parenchymal plasticity is related to amount of oxygen consumption and nutritional status (13).
Many studies have suggested that malnutrition and starvation may contribute to the development of emphysema. Studies by Sahebjami and colleagues suggest that caloric restriction may result in loss of alveoli and lung cells and that increasing nutrition restores normal structure (14). Autopsies undertaken on starved patients during World War II revealed signs of emphysema in relatively young individuals (15). Finally, patients with anorexia nervosa have decreased diffusing capacity and increased emphysema via computed tomography (CT) imaging compared with a control group in one trial (16). In an editorial review, Massaro and Massaro differentiated the regulated alveolar loss of malnutrition from tobacco-induced emphysema (17). Massaro and Massaro suggest that malnutrition results in regulated and spontaneously reversible alveolar loss in response to an internal stimulus (calorie restriction), whereas tobacco-induced emphysema is an unregulated spontaneously irreversible process due to exposure exogenous agents (17).
The literature suggests that surfactant may play an important role in the maintenance of alveolar structure. Blockage of neutral lipid metabolism has been shown to cause emphysema, increased lung neutrophils and macrophages, increased expression of proinflammatory cytokines and MMPs, and Clara cell hypertrophy and hyperplasia in a lysosomal acid lipase knockout mouse model (18). Emphysema- and inflammation-related remodeling is also seen in the absence of surfactant proteins A, C, and D with findings similar to lysosomal acid lipase knockout mice (19). In short, the plasticity of alveoli allows constant adjustment of lung parenchyma, and interference with this process leads to enlargement and destruction of the alveolar space.
Recent studies point to the role of alveolar structure maintenance failure and apoptosis as a contributing mechanism to emphysema development (20). This failed maintenance can target one or more cell types and produce changes similar to emphysema. For example, vascular endothelial growth factor, an endothelial cell survival factor, is abundant in the lung, and its blockade results in apoptosis-dependent airspace enlargement (21–23). In contrast, apoptosis of type II pneumocytes may compromise the production of surfactant, which produces changes similar to emphysema in animal models (18, 19). Furthermore, the maintenance of the alveolar structure depends upon the interaction between different cell types and the lung's matrix. Thus, failure of one cell type may result in damage to the other cell types and failure of the overall maintenance program. Tsao and colleagues, in a mouse model of lung over expression of placental-like growth factor, reported pulmonary emphysema starting at 6 months of age, which became prominent at 12 months of age (24). The mice showed increased apoptosis of type II pneumocytes and reduced mRNA of vascular endothelial growth factor and platelet–endothelial cell adhesion molecule-1, indicating a reduced number of endothelial cells. The authors speculated that the apoptosis of type II pneumocytes may have resulted in a reduction of endothelial cells (24).
CONCLUSIONS
Ongoing inflammation and oxidative stress results in damage to airspace structure and disturbance of the normal maintenance of alveolar structure. Data strongly support a role for protease–antiprotease imbalance in the development of emphysema. Furthermore, recent studies support the additional role of apoptosis in emphysema. Identification of the cellular and molecular mechanisms involved in the development of emphysema may have important implications for the development of new targets for therapeutic intervention.
The National Emphysema Treatment Trial (NETT) is supported by contracts with the National Heart, Lung, and Blood Institute (N01HR76101, N01HR76102, N01HR76103, N01HR76104, N01HR76105, N01HR76106, N01HR76107, N01HR76108, N01HR76109, N01HR76110, N01HR76111, N01HR76112, N01HR76113, N01HR76114, N01HR76115, N01HR76116, N01HR76118, and N01HR76119), the Centers for Medicare and Medicaid Services (CMS), and the Agency for Healthcare Research and Quality (AHRQ).
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Tuder RM, McGrath S, Neptune E. The pathobiological mechanisms of emphysema models: what do they have in common? Pulm Pharmacol Ther 2003;16:67–78. [DOI] [PubMed] [Google Scholar]
- 2.Tuder RM, Yoshida T, Arap W, Pasqualini R, Petrache I. State of the art: cellular and molecular mechanisms of alveolar destruction in emphysema: an evolutionary perspective. Proc Am Thorac Soc 2006;3:503–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mahadeva R, Shapiro SD. Animal models of pulmonary emphysema. Curr Drug Targets Inflamm Allergy 2005;4:665–673. [DOI] [PubMed] [Google Scholar]
- 4.Shapiro SD. Proteolysis in the lung. Eur Respir J Suppl 2003;44:30s–32s. [DOI] [PubMed] [Google Scholar]
- 5.Rahman I, Kilty I. Antioxidant therapeutic targets in COPD. Curr Drug Targets 2006;7:707–720. [DOI] [PubMed] [Google Scholar]
- 6.Yang SR, Chida AS, Bauter MR, Shafiq N, Seweryniak K, Maggirwar SB, Kilty I, Rahman I. Cigarette smoke induces proinflammatory cytokine release by activation of NF-kappaB and posttranslational modifications of histone deacetylase in macrophages. Am J Physiol Lung Cell Mol Physiol 2006;291:L46–L57. [DOI] [PubMed] [Google Scholar]
- 7.Barnes PJ, Adcock IM, Ito K. Histone acetylation and deacetylation: importance in inflammatory lung diseases. Eur Respir J 2005;25:552–563. [DOI] [PubMed] [Google Scholar]
- 8.Stoller JK, Aboussouan LS. Alpha1-antitrypsin deficiency. Lancet 2005;365:2225–2236. [DOI] [PubMed] [Google Scholar]
- 9.Hautamaki RD, Kobayashi DK, Senior RM, Shapiro SD. Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science 1997;277:2002–2004. [DOI] [PubMed] [Google Scholar]
- 10.Saetta M, Turato G, Maestrelli P, Mapp CE, Fabbri LM. Cellular and structural bases of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001;163:1304–1309. [DOI] [PubMed] [Google Scholar]
- 11.Hunninghake GW, Davidson JM, Rennard S, Szapiel S, Gadek JE, Crystal RG. Elastin fragments attract macrophage precursors to diseased sites in pulmonary emphysema. Science 1981;212:925–927. [DOI] [PubMed] [Google Scholar]
- 12.Senior RM, Griffin GL, Mecham RP. Chemotactic activity of elastin-derived peptides. J Clin Invest 1980;66:859–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Massaro D, Massaro GD. Pulmonary alveolus formation: critical period, retinoid regulation and plasticity. Novartis Found Symp 2001;234:229–236. [DOI] [PubMed] [Google Scholar]
- 14.Sahebjami H. Nutrition and the pulmonary parenchyma. Clin Chest Med 1986;7:111–126. [PubMed] [Google Scholar]
- 15.Fliederbaum J. Clinical aspects of hunger disease in adults. In: M. Winick, editor. Hunger disease: studies by the Jewish Physicians in the Warsaw Ghetto. New York: John Wiley & Sons; 1979. pp. 11–36.
- 16.Coxson HO, Chan IH, Mayo JR, Hlynsky J, Nakano Y, Birmingham CL. Early emphysema in patients with anorexia nervosa. Am J Respir Crit Care Med 2004;170:748–752. [DOI] [PubMed] [Google Scholar]
- 17.Massaro D, Massaro GD. Hunger disease and pulmonary alveoli. Am J Respir Crit Care Med 2004;170:723–724. [DOI] [PubMed] [Google Scholar]
- 18.Lian X, Yan C, Yang L, Xu Y, Du H. Lysosomal acid lipase deficiency causes respiratory inflammation and destruction in the lung. Am J Physiol Lung Cell Mol Physiol 2004;286:L801–L807. [DOI] [PubMed] [Google Scholar]
- 19.Yan C, Du H. Alveolus formation: what have we learned from genetic studies? J Appl Physiol 2004;97:1543–1548. [DOI] [PubMed] [Google Scholar]
- 20.Yoshida T, Tuder RM. Pathobiology of cigarette smoke-induced chronic obstructive pulmonary disease. Physiol Rev 2007;87:1047–1082. [DOI] [PubMed] [Google Scholar]
- 21.Papaioannou AI, Kostikas K, Kollia P, Gourgoulianis KI. Clinical implications for vascular endothelial growth factor in the lung: friend or foe? Respir Res 2006;7:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kasahara Y, Tuder RM, Taraseviciene-Stewart L, Le Cras TD, Abman S, Hirth PK, Waltenberger J, Voelkel NF. Inhibition of VEGF receptors causes lung cell apoptosis and emphysema. J Clin Invest 2000;106:1311–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang K, Rossiter HB, Wagner PD, Breen EC. Lung-targeted VEGF inactivation leads to an emphysema phenotype in mice. J Appl Physiol 2004;97:1559–1566. [DOI] [PubMed] [Google Scholar]
- 24.Tsao PN, Su YN, Li H, Huang PH, Chien CT, Lai YL, Lee CN, Chen CA, Cheng WF, Wei SC, et al. Overexpression of placenta growth factor contributes to the pathogenesis of pulmonary emphysema. Am J Respir Crit Care Med 2004;169:505–511. [DOI] [PubMed] [Google Scholar]
- 25.Hersh CP, DeMeo DL, Silverman EK. National Emphysema Treatment Trial state of the art: genetics of emphysema. Proc Am Thorac Soc 2008;5:486–493. [DOI] [PMC free article] [PubMed] [Google Scholar]