Abstract
Chronic obstructive pulmonary disease (COPD) is characterized by an abnormal persistent inflammatory response to cigarette smoke. This noxious insult leads to emphysema and airway remodeling, manifested by squamous and mucous metaplasia of the epithelium, smooth muscle hypertrophy, and airway wall fibrosis. These pathologic abnormalities interact synergistically to cause progressive airflow obstruction. Although it has been accepted that the spectrum of COPD is vast, the reasons for the development of different phenotypes from the same exposure to cigarette smoke have not been determined. Furthermore, it is becoming increasingly clear that airways disease and emphysema often coexist in many patients, even with a clear clinical phenotype of either emphysema or chronic bronchitis. Recent studies have focused on the nature of the inflammatory response to cigarette smoke, the inflammatory cell lines responsible for COPD pathogenesis, and new biomarkers for disease activity and progression. New cytokines are being discovered, and the complex interactions among them are being unraveled. The inflammatory biomarker that has received the most attention is C-reactive protein, but new ones that have caught our attention are interleukin (IL)-6, tumor necrosis factor-α, IL-8, and IL-10. Further research should focus on how these new concepts in lung inflammation interact to cause the various aspects of COPD pathology.
Keywords: chronic obstructive pulmonary disease, pathology, airway inflammation, emphysema, inflammatory biomarkers
Chronic obstructive pulmonary disease (COPD) is a progressive and debilitating disease that affects between 10 and 24 million adults in the United States (1), and is the fourth leading cause of death worldwide (2). Cigarette smoking has been firmly established as the most important risk factor for the development of COPD. Studies have found that smokers have a two- to threefold increase in the rate of decline in forced expiratory volume in one second (FEV1) compared with nonsmokers (3, 4). Interestingly, however, only about 15 to 20% of smokers develop clinically significant COPD (5), suggesting that genetic predisposition and environmental factors play a role in the pathogenesis of the disease. Cigarette smoking elicits airway inflammation in all of those who smoke, but in those that develop airflow obstruction there is persistent inflammation, even after smoking cessation. This has led to the concept that an abnormal inflammatory response to cigarette smoke leads to the development of COPD in the susceptible individual.
The spectrum of COPD is vast, with chronic bronchitis at one end and emphysema on the other, with the majority of patients existing somewhere in the middle. The reasons for the development of different phenotypes with the same exposure to cigarette smoke left us shrugging our shoulders for many years, and they continue to occupy COPD investigators. But in the last decade great strides have been made in our understanding of airway inflammation and pathology, the immune response to cigarette smoke, and inflammatory biomarkers. This review will discuss the most recent progress on selected topics in COPD pathobiology and inflammation.
PATHOLOGY OF COPD AND PATHOPHYSIOLOGIC CONSEQUENCES
Emphysema
Emphysema is a pathologic term defined as the abnormal permanent enlargement of airspaces distal to the terminal bronchioles, accompanied by destruction of their walls and without obvious fibrosis (6). Two main subtypes exist: centrilobular emphysema (CLE) and panlobular emphysema (PLE). CLE affects the lobules around the central respiratory bronchioles (7, 8), and is the primary pathologic subtype associated with cigarette smoke–induced COPD. This pattern of emphysema is typically more prominent in the upper lung zones (9, 10). PLE uniformly affects the entire secondary lobule (11). This subtype differs from CLE, as it is associated with α1-antitrypsin deficiency and is more prominent in the lower lung zones (10).
The clinical consequences of emphysema have long been established. Airways are normally tethered open by the elastic recoil of lung parenchyma. Emphysema, however, reduces the amount of interstitial and alveolar attachments (12–14), which predisposes to collapse during exhalation (15). Supporting evidence is provided by the observation that lung volume reduction surgery (LVRS) improves elastic lung recoil and also lung function (16). In addition, hyperinflation and air trapping in emphysematous lung causes external airway compression and obstruction.
Airways Disease
A crucial pathologic feature of COPD is airway inflammation and remodeling. This process primarily occurs at the level of the small airways, defined as bronchioles that are less than 2 mm in diameter. Niewoehner and colleagues first demonstrated that inflammatory changes were present in the peripheral airways of young smokers who died suddenly outside of the hospital, indicating that early structural changes in the small airways developed before the diagnosis of COPD was established (17). Subsequently COPD investigators turned their attention to the inflammatory infiltrate and pathology of the small airways and verified that small airway remodeling was critical to COPD pathogenesis (18–20).
Epithelial Abnormalities
Typical airway pathologic changes are shown in Figure 1. Chronic exposure to cigarette smoke damages the airway epithelium, leading to squamous metaplasia. This abnormality is also seen in smokers without COPD, and likely does not contribute significantly to airflow obstruction. On a macroscopic level, epithelial layer thickness increases incrementally as disease severity worsens (20). This assessment does not clarify whether this is secondary to epithelial cell hyperplasia, hypertrophy, or mucous metaplasia, but it is likely that all three factors contribute. The degree of epithelial remodeling, however, is not uniform in all patients with COPD; a study of 25 LVRS patients found that small airway epithelial thickness was highly variable in a homogeneous group of patients with advanced emphysema (21).
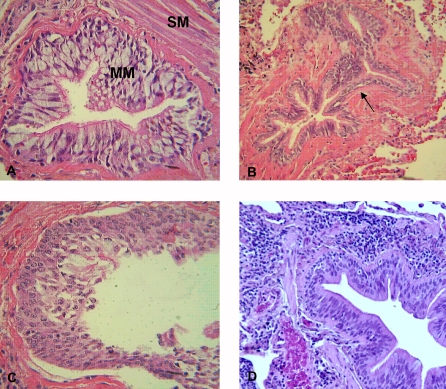
Figure 1.
Examples of airway remodeling in COPD. A represents mucous metaplasia (MM) of the epithelium and smooth muscle hypertrophy (SM). B represents peribronchial fibrosis (black arrow). C shows squamous metaplasia. D shows an inflammatory infiltrate of lymphocytes in the adventitia of a bronchiole.
An important component of epithelial remodeling is mucous metaplasia, a process in which mucus is overproduced in response to inflammatory signals (see Figure 2) (22). Mucous metaplasia has been found in the small airways of patients with COPD, and significantly contributes to airflow obstruction (20, 23, 24). This may develop from cigarette smoke exposure by itself (25, 26), acute and/or chronic viral infection (27), or inflammatory cell activation of mucin gene transcription (28).
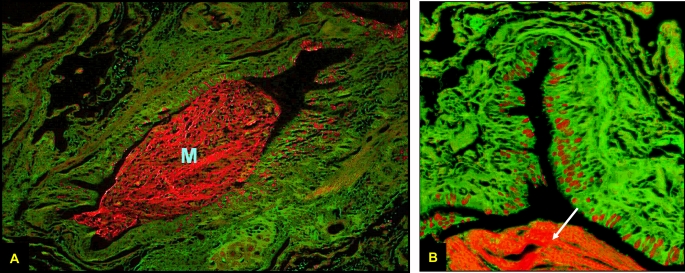
Figure 2.
Periodic Acid Fluorescent Schiff stain of a small airway from a patient with advanced emphysema. The entire airway is seen at ×10 magnification in A and a quadrant of the airway at ×40 in B. Mucin granules are shown in red along the apical border of the epithelium. Note the large intralumenal mucin plug (M) in A, also noted in B (white arrow).
Chronic mucus hypersecretion accelerates lung function decline (29) and predisposes to hospitalization and infection (30). Recently, small airway mucus obstruction has been associated with worse LVRS postoperative survival (31). Mucous metaplasia causes airflow obstruction by several mechanisms: increased mucus hypersecretion causes lumenal occlusion (20); epithelial layer thickening encroaches on the airway lumen (32); and increased mucus alters surface tension of the airway, thereby predisposing it to expiratory collapse (33).
Smooth Muscle Hypertrophy and Airway Wall Fibrosis
Some studies have found airway smooth muscle mass to be increased in COPD (18, 34, 35), whereas others have not (36–38). Whether or not airway hyperresponsiveness is a primary cause of fixed airflow obstruction continues to be a topic of debate. Regardless, chronic airway inflammation from cigarette smoke causes constriction and hypertrophy of even normal airway smooth muscle (24). This serves to increase airway wall thickness and therefore cause greater lumenal narrowing. In addition, the same degree of smooth muscle contraction in a thickened airway causes considerably greater airways resistance than in normal airways (39). Several investigators have found direct correlations with the degree of smooth muscle mass and airflow obstruction in COPD (20, 40), supporting the notion that smooth muscle hypertrophy not only is a significant pathologic finding but also that it contributes to the pathogenesis of COPD.
A prominent aspect of small airway remodeling is peribronchial fibrosis. Fibrosis of the membranous and respiratory bronchioles has been found to cause increased airway wall thickness in smokers compared with nonsmokers (40, 41). This phenomenon decreases airway elasticity, making it less responsive to bronchodilating agents. In addition, peribronchial fibrosis disrupts alveolar attachments to the airway, which predisposes the airway to expiratory closure. It is also possible that chronic inflammation weakens alveolar tissue and facilitates rupture from the airway.
The Interaction between Airways Disease and Emphysema
Figure 3 depicts two different pathophysiologic mechanisms of airflow obstruction. It is clear that both emphysema and loss of elastic recoil, as well as intrinsic airway abnormalities, synergistically contribute to disease severity. It is also becoming increasingly clear that these different pathologic abnormalities coexist in many patients, even if they demonstrate a clear clinical phenotype of emphysema or chronic bronchitis. Recent studies involving patients with emphysema have indeed revealed significant small airway disease. The degree of small airway pathology and small airways resistance have been shown to inversely correlate with postoperative changes in lung function after LVRS (21, 42), and to directly correlate with the severity of dynamic hyperinflation, a clinical phenomenon typically attributed to the degree of emphysema (43). These studies demonstrate that not only does significant small airway pathology exist in those that clinically and radiographically have an emphysematous phenotype but that it also affects outcome.
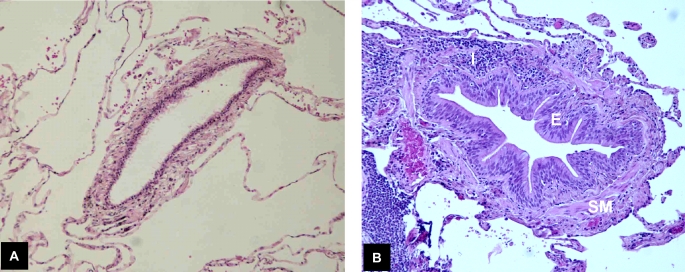
Figure 3.
Mechanisms of airflow obstruction in COPD. (A) Emphysema causes loss of alveolar attachments to the airway wall, predisposing it to expiratory collapse. (B) Small airway remodeling, as evidenced by epithelial thickening (E), smooth muscle hypertrophy (SM), and chronic airway inflammation (I), causes encroachment on the lumen, increased airway tone, reduced effectiveness of bronchodilators, and airway hyperresponsiveness.
LUNG IMMUNOLOGY AND INFLAMMATION IN COPD
Immunological Components in Disease Development
The inflammatory response observed in the lungs of patients with COPD is complex, and demonstrates evidence of the activation of both innate and acquired immune processes. In addition, the inflammatory disease pattern observed in COPD is diverse in many respects, and the accumulation of these inflammatory components appears to contribute to the lung injury in these patients, and serves as a self-perpetuating stimulus for further immune activation. It is clear that the disease process in the lungs of these individuals involves a robust migration of leukocytes, the production of inflammatory mediators, and the release of potentially destructive proinflammatory cytokines and proteases. The combined impact of activated leukocytes, endothelial cells, and epithelial cells is generation of a cocktail of chemoattractant mediators, including a number of chemokines, leading to the influx of additional inflammatory cells. The impact of these influences is to promote the activation of structural cells such as vascular endothelial cells and the very abundant epithelial cell populations, leading in turn to tissue remodeling and destruction. Finally, the immune response in the lungs appears to be characteristic of a T helper cell type I (Th1) acquired proinflammatory response in several respects. However, common features of the cellular response in the lungs of these patients elude convenient categorization, and we suggest that the diversity of the immunological response in the lungs is the result of activation of the newly defined Th17-type of acquired immune response. We will describe the characteristics of this form of inflammatory response in further detail below.
The Nature of Inflammatory and Cellular Infiltration in COPD
It is well established that there are certain common, general features of the immunological response in COPD. For example, studies of bronchial biopsy specimens from patients with mild to moderate COPD show an increase in infiltrating inflammatory cells compared with either nonsmokers or smokers who have not developed evidence of disease (44–46). The cellular composition of the infiltrate varies among patients, but there is typically an accumulation of neutrophils, macrophages, and CD8+ T cells. Smaller numbers of CD4+ T cells are also apparent, although these may be localized in certain anatomical sites (20). The mechanism by which CD8+ T cells are mobilized to the diseased lung is an intriguing issue, and is one which remains a critical consideration for further study. There is evidence that these cells express several proinflammatory chemokine receptors, including both CCR5 and CXCR3, but it is not at all certain that these chemokine receptors are responsible for the accumulation of CD8+ T cells in the lungs of these patients. The chemokine agonists for these receptors are reported to be expressed at elevated levels in the diseased tissue (47, 48), and the interferon (IFN)-inducible agonists for the chemokine receptors CXCR3, CXCL9, CXCL10, and CXCL11, are elevated in bronchial epithelial cells (47, 49, 50). Moreover, the chemokine CCL5 (a CCR5 agonist) is documented to be elevated in exacerbations of chronic bronchitis (48), and may promote the accumulation of both CCR5-expressing T cells and activated macrophages. Because the inflammatory response in this tissue is a chronic process, it is very likely that the attraction of leukocytes is complicated by the interactions of a large number of mediators produced by each of the sub-populations of infiltrating inflammatory cells and activated structural cells in the diseased lung. For example, the immigration of macrophages may provide a source of chemokines (such as CCL5) that recruit CD4+ T cells to the lung, and these may in turn attract the large numbers of CD8+ T cells, which are characteristic of the lung in COPD.
At this time the role of CD8+ T cells in COPD is not clear, and one must consider the possibility that the CD8+ T cells in the diseased lung do not represent the most important inflammatory population, even though they are a relatively abundant population. Nevertheless, the simple presence of these cells almost certainly contributes a source of pathogenic mediators, which clearly have the potential to participate in lung tissue damage (51). Moreover, these cells possess the potential to promote the destruction of lung interstitium via the release of proteases such as perforin and granzyme, as well as cytotoxic cytokines such as tumor necrosis factor (TNF)-α. Indeed, recent evidence suggests that CD8+ T cells obtained from the sputum of patients with COPD produce elevated levels of perforin when compared with normal control subjects (52). Finally, CD8+ T cells may produce IFN-γ, which in turn induce the aforementioned CXCR3 agonists, and in this way set up a self-perpetuating cascade of CD8+ T cell mobilization in which these T cells continue to drive an accumulation of additional CXCR3-expressing inflammatory T cells.
Other inflammatory cells are routinely observed in the tissues of diseased lung, and are commonly observed in associated body fluids, such as bronchoalveolar lavage and induced sputum. For example, it should be appreciated that macrophages are the most abundant cell type recovered from bronchoalveolar lavage of patients with COPD, and macrophage number in the lavage fluid appears to correlate with disease severity (53). Simply for reasons of sheer cell number, the contribution of these cells should not be discounted. For example, these cells are the source of a number of potentially significant mediators, such as TNF-α, leukotriene B4 (LTB4), reactive oxygen species (ROS), and several chemokines including IL-8 and the very proinflammatory CCR2 agonist, CCL2 (51, 53). The production of this factor may be particularly critical because the production of CCL2 provides an opportunity for the recruitment of additional the proinflammatory sub-type of macrophages to the lungs. It is now clear that monocyte/macrophage populations can be distinguished based on their surface antigen expression, and functional activity (54). For example, recent reports suggest that at least two monocyte populations can be identified, based on the expression of CD14, 16, 62L, and the chemokine receptors CCR2 and CX3CR1 (54, 55). One population of cells, termed the “resident” monocyte/macrophage, expresses CD62L and CCR2 weakly and exhibits high CX3CR1 expression. While this population of CD16+ CX3CR+ cells appears to traffic to sites of inflammation (56), it has been suggested that CX3CR1-expressing monocytes arrest but do not transit through the blood vessel (56). The second population of cells expresses high CCR2 and CD62L, and is CX3CR1low/neg, and is termed the “inflammatory” monocyte/macrophage population. These cells preferentially traffic to sites of inflammation. The preferential recruitment of these proinflammatory cells provides an abundant source of a number of proteases including cathepsins K, L, and S, as well as MMPs-1, -2, -9, and -12, and the production of these proteases appears to be elevated in lung macrophages in patients with COPD both in vivo and ex vivo (57–61).
Neutrophils are an abundant population of inflammatory cells in the lungs of patients with COPD, and their contribution to the evolving disease should receive greater attention. Both sputum and bronchoalveolar lavage samples from patients with COPD typically show large numbers of neutrophils, and cigarette smoking has been shown to induce elevated numbers of circulating neutrophils, with sequestration of these cells in lung capillaries (62, 63). These cells are, in turn, mobilized to the lung interstitium in response to chemoattractants such as IL-8, and leukotriene B4 (LTB4), and both of these mediators are typically elevated in the airways of patients with COPD (64, 65). Neutrophils are a potent source of inflammatory mediators, including reactive oxidant species (ROS), lipid mediators with chemoattractant activity, and tissue proteases (66).
Cellular Basis for the Inflammatory Response in COPD
The cellular response associated with COPD has been most frequently (although not exclusively) attributed to the inflammatory Th1 (or Tc1 to designate a CD8+ T cell response in which proinflammatory cytokines are prominent) arm of the acquired immune system. However, recent advances in our understanding of effector T cell biology has revealed the existence of a second distinct subpopulation of CD4+ T cells with particularly potent proinflammatory functional activities (45). These cells are designated Th17 cells because of their capacity to produce IL-17A and IL-17F. The classical CD4+ effector T cell populations (Th1 and Th2) both fail to produce these cytokines, although IL-17 is also produced by a number of other hematopoietic cells including CD8+ cells, γδ T cells, NK cells, and neutrophils (67). The inflammatory activity of Th17 cells is apparently due to the production of a unique complex of proinflammatory cytokines, including IL-6, IL-17A, IL-17F, GM-CSF, and TNF-α. These cells also express receptors for both IL-18 and IL-23, cytokines typically produced by monocytes, macrophages, and dendritic cells. Very importantly, IL-23 serves as a growth factor to promote the activity of the Th17 population.
With respect to the lung, IL-17 is a potent inducer of IL-6 production by bronchial epithelial cells (68), and both IL-6 and IL-17 are strong inducers of mucins Muc5AC and Muc5B production by lung epithelial cells (69). In addition, IL-17 induces epithelial cells to release CXCL8, a chemokine that is important for the attraction of neutrophils (70), and mice genetically engineered to overexpress IL-17 in the alveoli exhibit lung inflammation (71). Finally, IL-17 is an inducer of IL-23 expression, a cytokine that serves to promote further Th17 activity and proliferation (72). This feedback loop of IL-17 stimulating IL-23 up-regulation which, in turn, promotes additional IL-17 production, could easily result in a self-perpetuating condition of chronic and recalcitrant inflammation. This indeed may help explain the phenomenon of persistent airway inflammation in COPD, despite smoking cessation.
The Th17 population possesses very strong inflammatory properties, and has been shown to play an critical role in a number of inflammatory and autoimmune diseases, including psoriasis, rheumatoid arthritis, multiple sclerosis, inflammatory bowel disease, and airway inflammation (45, 71, 73). In studies using experimental animal models, mice that fail to express intact IL-23 receptors are resistant to the induction of several autoimmune diseases involving the central nervous system, and IL-17 knockout mice are resistant to the induction of autoimmune encephalitis or collagen-induced arthritis (74–76). Some investigators posit that COPD may be an autoimmune disease. In a study in which human umbilical vein endothelial cells were injected into rats, the animals developed antibodies active against these endothelial cells but also to lung capillary endothelial cells. Interestingly, these animals also developed pathologic evidence of emphysema (77). Similarly, transfer of CD4+ cells or serum from emphysematous animals to wild-type animals also resulted in the development of emphysema (77). Given the established role of Th17 in other autoimmune diseases, it is reasonable to infer that the Th17 population is also critical to the development of COPD. To date, the Th17 arm of T cell immunity is a component of the immune response that has not received adequate attention in COPD. The very potent proinflammatory activity of these cells, and their established role in other inflammatory disease states, supports the need for greater attention to this T cell sub-type.
Biomarkers for the Evaluation of Disease
A number of biomarkers have been identified that may be useful for an understanding of the immunopathogenesis of COPD. In addition, biomarker determinations might have diagnostic value for assessing the progress of the disease. A large number of cytokines, chemokines, proteases, acute phase proteins, and other mediators have been evaluated in the blood, bronchoalveolar lavage fluid, sputum, and lung tissue from patients with COPD, and it should be clear at this time that no single biomarker is likely to be uniquely informative about this disease. However, it is likely that for certain sub-populations of patients, a set of two or more biomarkers may have diagnostic or predictive value. While a complete review of each of these mediators is beyond the scope of this paper, we will briefly describe some of the more informative biomarkers.
Evidence at this point indicates that the biomarker that provides the most significant correlation with COPD disease severity is C-reactive protein (CRP). CRP is a circulating pentraxin, produced primarily by hepatocytes as a part of the acute phase response, and has been reported to induce proinflammatory cytokine and chemokine expression, and up-regulate adhesion molecules, which are likely to promote lung inflammation (78). However, it is not clear at this time whether CRP is more than just a barometer of the intensity of the inflammatory process, or an actual participant in the pathogenesis of the disease. Importantly, levels of CRP in the blood correlate well with future risk of morbidity and mortality in COPD (79, 80).
Additional proinflammatory mediators that appear to be useful biomarkers to evaluate the intensity of the disease process include IL-6, TNF-α, IL-10, and the chemokines IL-8 and CCL18/PARC (pulmonary and activation-regulated chemokine), which are elevated in COPD (79, 81–83). These factors are not unique to COPD, or necessarily specific for any single part of the inflammatory process in this disease, and may be produced in response to many inflammatory stimuli, including infectious agents and other types of tissue injury. Nevertheless, further analysis is necessary to determine the precise role that these (and other) biomarkers may play in the etiology of the disease.
IL-8 is produced by a number of cell populations, including activated bronchial epithelial cells, macrophages, and neutrophils. It is a potent chemoattractant for neutrophils and monocytes, and depending on the anatomical location, may contribute to the mobilization of these leukocyte populations into the lung. In some studies, the levels of IL-8 are increased in induced sputum of patients with COPD, and not surprisingly, the levels appear to correlate with the proportion of neutrophils (84), and are also increased in the sputum during exacerbations (85, 86).
TNF-α is an extremely potent proinflammatory mediator, and has the capacity to induce inflammation by both direct and indirect mechanisms. The activation of TNF-α receptors in many circumstances induces the production and release of a number of inflammatory mediators, and several of these mediators in turn induce additional inflammatory effects. This includes a broad spectrum of proinflammatory cytokines, chemokines, proteases, and adhesion molecules. A recent study by Churg and coworkers (87) using TNF receptor knockout mice shows that TNF-α is a critical contributor to cigarette smoke–induced emphysema.
IL-6 is also a potent proinflammatory cytokine, produced by a diverse set of cell populations, and exerts inflammatory effects by activating both leukocytes and structural cells including pulmonary epithelial cells. The levels of IL-6 are increased in the induced sputum, bronchoalveolar lavage, and blood of patients with COPD, and here again, levels have been reported to be even higher during exacerbation (79, 88, 89). IL-6 is a part of the acute phase response, and appears to be a potent inducer of CRP expression in the liver.
IL-10 is a cytokine that has been considered by many to be primarily anti-inflammatory because of its inhibitory activity for the Th1-type of CD4+ T cells. A recent report by Barcelo and colleagues (90) has shown that CD8+ T cells that produce IL-10 can be identified in the bronchoalveolar lavage of patients with COPD, and the numbers of these cells were found to be significantly elevated when compared with healthy smokers or nonsmokers. The role of these cells in COPD remains uncertain, but they may serve as a natural down-regulator of proinflammatory cytokine expression produced to protect inflamed tissues from the destructive actions of proinflammatory cytokines. Recent evidence based on analysis of bronchial biopsies shows an elevation of IL-10 expression in patients with COPD (91). Preliminary results from our laboratories suggest that an elevation in the blood level of IL-10, in individuals with TNF-α levels above a minimal threshold, correlate with the severity of COPD (92).
AREAS FOR FUTURE RESEARCH
The development of emphysema and airways disease is a complex process that involves significant interaction between proinflammatory stimuli and recruitment of neutrophils, macrophages, and lymphocytes, leading to the destruction of lung parenchyma and remodeling of multiple components of the airway wall. Although the inflammatory processes involved are becoming clearer, the reasons why individuals develop different clinical phenotypes are not. The answer may lie in genetic polymorphisms that confer not only disease susceptibility but also phenotypic expression. As our understanding of lung inflammation grows, future research should focus on how these new concepts in the inflammatory response to cigarette smoke interact in the susceptible individual to cause various aspects of COPD pathology.
The National Emphysema Treatment Trial (NETT) is supported by contracts with the National Heart, Lung, and Blood Institute (N01HR76101, N01HR76102, N01HR76103, N01HR76104, N01HR76105, N01HR76106, N01HR76107, N01HR76108, N01HR76109, N01HR76110, N01HR76111, N01HR76112, N01HR76113, N01HR76114, N01HR76115, N01HR76116, N01HR76118, and N01HR76119), the Centers for Medicare and Medicaid Services (CMS), and the Agency for Healthcare Research and Quality (AHRQ).
Conflict of Interest Statement: V.K. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. T.J.R. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. G.J.C. has served on Advisory Committees for Ortho-Biotech, Schering-Plough, Boehringer Ingelheim, Actelion, and Shire and Sepracor Pharmaceuticals. All of these sums are less than $2,500. He has received research grants from: Schering-Plough, Boehringer Ingelheim, Actelion, GlaxoSmithKline, Advanta, Daiichi Asubio, Pfizer, Roche and Sepracor Pharmaceuticals, Emphasys Medical, Inc., and Aeris Therapeutics. All research grant monies are deposited and controlled by Temple University.
References
- 1.Mannino DM, Homa DM, Akinbami LJ, Ford ES, Redd SC. Chronic obstructive pulmonary disease surveillance–United States, 1971–2000. MMWR Surveill Summ 2002;51:1–16. [PubMed] [Google Scholar]
- 2.Murray CJ, Lopez AD. Evidence-based health policy–lessons from the Global Burden of Disease Study. Science 1996;274:740–743. [DOI] [PubMed] [Google Scholar]
- 3.Fletcher C, Peto R. The natural history of chronic airflow obstruction. BMJ 1977;1:1645–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu X, Weiss ST, Rijcken B, Schouten JP. Smoking, changes in smoking habits, and rate of decline in FEV1: new insight into gender differences. Eur Respir J 1994;7:1056–1061. [PubMed] [Google Scholar]
- 5.American Thoracic Society. Cigarette smoking and health. Am J Respir Crit Care Med 1996;153:861–865. [DOI] [PubMed] [Google Scholar]
- 6.Piquette CA, Rennard SI, Snider GL. Chronic bronchitis and emphysema. In: Murray JF, Nadel JA, editors. Textbook of respiratory medicine, 3rd ed. Philadelphia, PA: W.B. Saunders; 2000. pp. 1188–1245.
- 7.McLean KH. The macroscopic anatomy of pulmonary emphysema. Australas Ann Med 1956;5:73–88. [DOI] [PubMed] [Google Scholar]
- 8.Leopold JG, Gough J. The centrilobular form of hypertrophic emphysema and its relation to chronic bronchitis. Thorax 1957;12:219–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heard BE. A pathological study of emphysema of the lungs with chronic bronchitis. Thorax 1958;13:136–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thurlbeck WM. The incidence of pulmonary emphysema, with observations on the relative incidence and spatial distribution of various types of emphysema. Am Rev Respir Dis 1963;87:206–215. [DOI] [PubMed] [Google Scholar]
- 11.Wyatt JP, Fischer VW, Sweet HC. Panlobular emphysema: anatomy and pathodynamics. Dis Chest 1962;41:239–259. [DOI] [PubMed] [Google Scholar]
- 12.Saetta M, Ghezzo H, Kim WD, King M, Angus GE, Wang NS, Cosio MG. Loss of alveolar attachments in smokers. A morphometric correlate of lung function impairment. Am Rev Respir Dis 1985;132:894–900. [DOI] [PubMed] [Google Scholar]
- 13.Saetta M, Shiner RJ, Angus GE, Kim WD, Wang NS, King M, Ghezzo H, Cosio MG. Destructive index: a measurement of lung parenchymal destruction in smokers. Am Rev Respir Dis 1985;131:764–769. [DOI] [PubMed] [Google Scholar]
- 14.Lamb D, McLean A, Gillooly M, Warren PM, Gould GA, MacNee W. Relation between distal airspace size, bronchiolar attachments, and lung function. Thorax 1993;48:1012–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petty TL, Silvers GW, Stanford RE. Radial traction and small airways disease in excised human lungs. Am Rev Respir Dis 1986;133:132–135. [DOI] [PubMed] [Google Scholar]
- 16.Sciurba FC, Rogers RM, Keenan RJ, Slivka WA, Gorcsan J III, Ferson PF, Holbert JM, Brown ML, Landreneau RJ. Improvement in pulmonary function and elastic recoil after lung-reduction surgery for diffuse emphysema. N Engl J Med 1996;334:1095–1099. [DOI] [PubMed] [Google Scholar]
- 17.Niewoehner DE, Kleinerman J, Rice DB. Pathologic changes in the peripheral airways of young cigarette smokers. N Engl J Med 1974;291:755–758. [DOI] [PubMed] [Google Scholar]
- 18.Bosken CH, Wiggs BR, Pare PD, Hogg JC. Small airway dimensions in smokers with obstruction to airflow. Am Rev Respir Dis 1990;142:563–570. [DOI] [PubMed] [Google Scholar]
- 19.Gelb AF, Hogg JC, Muller NL, Schein MJ, Kuei J, Tashkin DP, Epstein JD, Kollin J, Green RH, Zamel N, et al. Contribution of emphysema and small airways in COPD. Chest 1996;109:353–359. [DOI] [PubMed] [Google Scholar]
- 20.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 2004;350:2645–2653. [DOI] [PubMed] [Google Scholar]
- 21.Kim V, Criner GJ, Abdallah HY, Gaughan JP, Furukawa S, Solomides CC. Small airway morphometry and improvement in pulmonary function after lung volume reduction surgery. Am J Respir Crit Care Med 2005;171:40–47. [DOI] [PubMed] [Google Scholar]
- 22.Williams OW, Sharafkhaneh A, Kim V, Dickey BF, Evans CM. Airway mucus: from production to secretion. Am J Respir Cell Mol Biol 2006;34:527–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saetta M, Turato G, Baraldo S, Zanin A, Braccioni F, Mapp CE, Maestrelli P, Cavallesco G, Papi A, Fabbri LM. Goblet cell hyperplasia and epithelial inflammation in peripheral airways of smokers with both symptoms of chronic bronchitis and chronic airflow limitation. Am J Respir Crit Care Med 2000;161:1016–1021. [DOI] [PubMed] [Google Scholar]
- 24.Baraldo S, Saetta M, Cosio MG. Pathophysiology of the small airways. Semin Respir Crit Care Med 2003;24:465–472. [DOI] [PubMed] [Google Scholar]
- 25.Ebert RV, Terracio MJ. The bronchiolar epithelium in cigarette smokers: observations with the scanning electron microscope. Am Rev Respir Dis 1975;111:4–11. [DOI] [PubMed] [Google Scholar]
- 26.Deshmukh HS, Case LM, Wesselkamper SC, Borchers MT, Martin LD, Shertzer HG, Nadel JA, Leikauf GD. Metalloproteinases mediate mucin 5AC expression by epidermal growth factor receptor activation. Am J Respir Crit Care Med 2005;171:305–314. [DOI] [PubMed] [Google Scholar]
- 27.Holtzman MJ, Tyner JW, Kim EY, Lo MS, Patel AC, Shornick LP, Agapov E, Zhang Y. Acute and chronic airway responses to viral infection: implications for asthma and chronic obstructive pulmonary disease. Proc Am Thorac Soc 2005;2:132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burgel PR, Nadel JA. Roles of epidermal growth factor receptor activation in epithelial cell repair and mucin production in airway epithelium. Thorax 2004;59:992–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vestbo J, Prescott E, Lange P. Association of chronic mucus hypersecretion with FEV1 decline and chronic obstructive pulmonary disease morbidity. Copenhagen City Heart Study Group. Am J Respir Crit Care Med 1996;153:1530–1535. [DOI] [PubMed] [Google Scholar]
- 30.Prescott E, Lange P, Vestbo J. Chronic mucus hypersecretion in COPD and death from pulmonary infection. Eur Respir J 1995;8:1333–1338. [DOI] [PubMed] [Google Scholar]
- 31.Hogg JC, Chu FS, Tan WC, Sin DD, Patel SA, Pare PD, Martinez FJ, Rogers RM, Make BJ, Criner GJ, et al. Survival after lung volume reduction in chronic obstructive pulmonary disease: insights from small airway pathology. Am J Respir Crit Care Med 2007;176:454–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.James AL, Wenzel S. Clinical relevance of airway remodelling in airway diseases. Eur Respir J 2007;30:134–155. [DOI] [PubMed] [Google Scholar]
- 33.Macklem PT, Proctor DF, Hogg JC. The stability of peripheral airways. Respir Physiol 1970;8:191–203. [DOI] [PubMed] [Google Scholar]
- 34.Nagai A, West WW, Thurlbeck WM. The National Institutes of Health Intermittent Positive-Pressure Breathing trial: pathology studies: II. Correlation between morphologic findings, clinical findings, and evidence of expiratory air-flow obstruction. Am Rev Respir Dis 1985;132:946–953. [DOI] [PubMed] [Google Scholar]
- 35.Kuwano K, Bosken CH, Pare PD, Bai TR, Wiggs BR, Hogg JC. Small airways dimensions in asthma and in chronic obstructive pulmonary disease. Am Rev Respir Dis 1993;148:1220–1225. [DOI] [PubMed] [Google Scholar]
- 36.Cosio M, Ghezzo H, Hogg JC, Corbin R, Loveland M, Dosman J, Macklem PT. The relations between structural changes in small airways and pulmonary-function tests. N Engl J Med 1978;298:1277–1281. [DOI] [PubMed] [Google Scholar]
- 37.Petty TL, Silvers GW, Stanford RE, Baird MD, Mitchell RS. Small airway pathology is related to increased closing capacity and abnormal slope of phase III in excised human lungs. Am Rev Respir Dis 1980;121:449–456. [DOI] [PubMed] [Google Scholar]
- 38.Tiddens HA, Pare PD, Hogg JC, Hop WC, Lambert R, de Jongste JC. Cartilaginous airway dimensions and airflow obstruction in human lungs. Am J Respir Crit Care Med 1995;152:260–266. [DOI] [PubMed] [Google Scholar]
- 39.Lambert RK, Wiggs BR, Kuwano K, Hogg JC, Pare PD. Functional significance of increased airway smooth muscle in asthma and COPD. J Appl Physiol 1993;74:2771–2781. [DOI] [PubMed] [Google Scholar]
- 40.Finkelstein R, Fraser RS, Ghezzo H, Cosio MG. Alveolar inflammation and its relation to emphysema in smokers. Am J Respir Crit Care Med 1995;152:1666–1672. [DOI] [PubMed] [Google Scholar]
- 41.Wright JL, Hobson J, Wiggs BR, Pare PD, Hogg JC. Effect of cigarette smoking on structure of the small airways. Lung 1987;165:91–100. [DOI] [PubMed] [Google Scholar]
- 42.Ingenito EP, Evans RB, Loring SH, Kaczka DW, Rodenhouse JD, Body SC, Sugarbaker DJ, Mentzer SJ, DeCamp MM, Reilly JJ Jr. Relation between preoperative inspiratory lung resistance and the outcome of lung-volume-reduction surgery for emphysema. N Engl J Med 1998;338:1181–1185. [DOI] [PubMed] [Google Scholar]
- 43.Patel BJ, Marchetti N, Kim V, Gaughan JP, Criner GJ. Airway wall thickness correlates with dynamic hyperinflation in severe COPD [abstract]. Proc Am Thorac Soc 2006;3:A712. [Google Scholar]
- 44.Majo J, Ghezzo H, Cosio MG. Lymphocyte population and apoptosis in the lungs of smokers and their relation to emphysema. Eur Respir J 2001;17:946–953. [DOI] [PubMed] [Google Scholar]
- 45.Kikly K, Liu L, Na S, Sedgwick JD. The IL-23/Th(17) axis: therapeutic targets for autoimmune inflammation. Curr Opin Immunol 2006;18:670–675. [DOI] [PubMed] [Google Scholar]
- 46.Saetta M, Baraldo S, Corbino L, Turato G, Braccioni F, Rea F, Cavallesco G, Tropeano G, Mapp CE, Maestrelli P, et al. CD8+ve cells in the lungs of smokers with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999;160:711–717. [DOI] [PubMed] [Google Scholar]
- 47.Saetta M, Mariani M, Panina-Bordignon P, Turato G, Buonsanti C, Baraldo S, Bellettato CM, Papi A, Corbetta L, Zuin R, et al. Increased expression of the chemokine receptor CXCR3 and its ligand CXCL10 in peripheral airways of smokers with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2002;165:1404–1409. [DOI] [PubMed] [Google Scholar]
- 48.Zhu J, Qiu YS, Majumdar S, Gamble E, Matin D, Turato G, Fabbri LM, Barnes N, Saetta M, Jeffery PK. Exacerbations of Bronchitis: bronchial eosinophilia and gene expression for interleukin-4, interleukin-5, and eosinophil chemoattractants. Am J Respir Crit Care Med 2001;164:109–116. [DOI] [PubMed] [Google Scholar]
- 49.Aksoy MO, Yang Y, Ji R, Reddy PJ, Shahabuddin S, Litvin J, Rogers TJ, Kelsen SG. CXCR3 surface expression in human airway epithelial cells: cell cycle dependence and effect on cell proliferation. Am J Physiol Lung Cell Mol Physiol 2006;290:L909–L918. [DOI] [PubMed] [Google Scholar]
- 50.Kelsen SG, Aksoy MO, Yang Y, Shahabuddin S, Litvin J, Safadi F, Rogers TJ. The chemokine receptor CXCR3 and its splice variant are expressed in human airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 2004;287:L584–L591. [DOI] [PubMed] [Google Scholar]
- 51.O'Donnell R, Breen D, Wilson S, Djukanovic R. Inflammatory cells in the airways in COPD. Thorax 2006;61:448–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chrysofakis G, Tzanakis N, Kyriakoy D, Tsoumakidou M, Tsiligianni I, Klimathianaki M, Siafakas NM. Perforin expression and cytotoxic activity of sputum CD8+ lymphocytes in patients with COPD. Chest 2004;125:71–76. [DOI] [PubMed] [Google Scholar]
- 53.MacNee W. Pathogenesis of chronic obstructive pulmonary disease. Proc Am Thorac Soc 2005;2:258–266. (discussion 290–291). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 2003;19:71–82. [DOI] [PubMed] [Google Scholar]
- 55.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol 2005;5:953–964. [DOI] [PubMed] [Google Scholar]
- 56.Ancuta P, Rao R, Moses A, Mehle A, Shaw SK, Luscinskas FW, Gabuzda D. Fractalkine preferentially mediates arrest and migration of CD16+ monocytes. J Exp Med 2003;197:1701–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Culpitt SV, Maziak W, Loukidis S, Nightingale JA, Matthews JL, Barnes PJ. Effect of high dose inhaled steroid on cells, cytokines, and proteases in induced sputum in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999;160:1635–1639. [DOI] [PubMed] [Google Scholar]
- 58.Finlay GA, O'Driscoll LR, Russell KJ, D'Arcy EM, Masterson JB, FitzGerald MX, O'Connor CM. Matrix metalloproteinase expression and production by alveolar macrophages in emphysema. Am J Respir Crit Care Med 1997;156:240–247. [DOI] [PubMed] [Google Scholar]
- 59.Molet S, Belleguic C, Lena H, Germain N, Bertrand CP, Shapiro SD, Planquois JM, Delaval P, Lagente V. Increase in macrophage elastase (MMP-12) in lungs from patients with chronic obstructive pulmonary disease. Inflamm Res 2005;54:31–36. [DOI] [PubMed] [Google Scholar]
- 60.Segura-Valdez L, Pardo A, Gaxiola M, Uhal BD, Becerril C, Selman M. Upregulation of gelatinases A and B, collagenases 1 and 2, and increased parenchymal cell death in COPD. Chest 2000;117:684–694. [DOI] [PubMed] [Google Scholar]
- 61.Selman M, Cisneros-Lira J, Gaxiola M, Ramirez R, Kudlacz EM, Mitchell PG, Pardo A. Matrix metalloproteinases inhibition attenuates tobacco smoke-induced emphysema in Guinea pigs. Chest 2003;123:1633–1641. [DOI] [PubMed] [Google Scholar]
- 62.Peleman RA, Rytila PH, Kips JC, Joos GF, Pauwels RA. The cellular composition of induced sputum in chronic obstructive pulmonary disease. Eur Respir J 1999;13:839–843. [DOI] [PubMed] [Google Scholar]
- 63.Rutgers SR, Postma DS, ten Hacken NH, Kauffman HF, van Der Mark TW, Koeter GH, Timens W. Ongoing airway inflammation in patients with COPD who Do not currently smoke. Chest 2000;117:262S. [DOI] [PubMed] [Google Scholar]
- 64.Tanino M, Betsuyaku T, Takeyabu K, Tanino Y, Yamaguchi E, Miyamoto K, Nishimura M. Increased levels of interleukin-8 in BAL fluid from smokers susceptible to pulmonary emphysema. Thorax 2002;57:405–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Traves SL, Culpitt SV, Russell RE, Barnes PJ, Donnelly LE. Increased levels of the chemokines GROalpha and MCP-1 in sputum samples from patients with COPD. Thorax 2002;57:590–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hiemstra PS, van Wetering S, Stolk J. Neutrophil serine proteinases and defensins in chronic obstructive pulmonary disease: effects on pulmonary epithelium. Eur Respir J 1998;12:1200–1208. [DOI] [PubMed] [Google Scholar]
- 67.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol 2007;25:821–852. [DOI] [PubMed] [Google Scholar]
- 68.Molet S, Hamid Q, Davoine F, Nutku E, Taha R, Page N, Olivenstein R, Elias J, Chakir J. IL-17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. J Allergy Clin Immunol 2001;108:430–438. [DOI] [PubMed] [Google Scholar]
- 69.Chen Y, Thai P, Zhao YH, Ho YS, DeSouza MM, Wu R. Stimulation of airway mucin gene expression by interleukin (IL)-17 through IL-6 paracrine/autocrine loop. J Biol Chem 2003;278:17036–17043. [DOI] [PubMed] [Google Scholar]
- 70.Jones CE, Chan K. Interleukin-17 stimulates the expression of interleukin-8, growth-related oncogene-alpha, and granulocyte-colony-stimulating factor by human airway epithelial cells. Am J Respir Cell Mol Biol 2002;26:748–753. [DOI] [PubMed] [Google Scholar]
- 71.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol 2005;6:1133–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Langrish CL, McKenzie BS, Wilson NJ, de Waal Malefyt R, Kastelein RA, Cua DJ. IL-12 and IL-23: master regulators of innate and adaptive immunity. Immunol Rev 2004;202:96–105. [DOI] [PubMed] [Google Scholar]
- 73.Steinman L. A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nat Med 2007;13:139–145. [DOI] [PubMed] [Google Scholar]
- 74.Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 2003;421:744–748. [DOI] [PubMed] [Google Scholar]
- 75.Lubberts E, Koenders MI, Oppers-Walgreen B, van den Bersselaar L, Coenen-de Roo CJ, Joosten LA, van den Berg WB. Treatment with a neutralizing anti-murine interleukin-17 antibody after the onset of collagen-induced arthritis reduces joint inflammation, cartilage destruction, and bone erosion. Arthritis Rheum 2004;50:650–659. [DOI] [PubMed] [Google Scholar]
- 76.Murphy CA, Langrish CL, Chen Y, Blumenschein W, McClanahan T, Kastelein RA, Sedgwick JD, Cua DJ. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med 2003;198:1951–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Taraseviciene-Stewart L, Scerbavicius R, Choe KH, Moore M, Sullivan A, Nicolls MR, Fontenot AP, Tuder RM, Voelkel NF. An animal model of autoimmune emphysema. Am J Respir Crit Care Med 2005;171:734–742. [DOI] [PubMed] [Google Scholar]
- 78.Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest 2003;111:1805–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Man SF, Connett JE, Anthonisen NR, Wise RA, Tashkin DP, Sin DD. C-reactive protein and mortality in mild to moderate chronic obstructive pulmonary disease. Thorax 2006;61:849–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dahl M, Vestbo J, Lange P, Bojesen SE, Tybjaerg-Hansen A, Nordestgaard BG. C-reactive protein as a predictor of prognosis in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2007;175:250–255. [DOI] [PubMed] [Google Scholar]
- 81.Calikoglu M, Sahin G, Unlu A, Ozturk C, Tamer L, Ercan B, Kanik A, Atik U. Leptin and TNF-alpha levels in patients with chronic obstructive pulmonary disease and their relationship to nutritional parameters. Respiration 2004;71:45–50. [DOI] [PubMed] [Google Scholar]
- 82.Spruit MA, Gosselink R, Troosters T, Kasran A, Gayan-Ramirez G, Bogaerts P, Bouillon R, Decramer M. Muscle force during an acute exacerbation in hospitalised patients with COPD and its relationship with CXCL8 and IGF-I. Thorax 2003;58:752–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wedzicha JA, Seemungal TA, MacCallum PK, Paul EA, Donaldson GC, Bhowmik A, Jeffries DJ, Meade TW. Acute exacerbations of chronic obstructive pulmonary disease are accompanied by elevations of plasma fibrinogen and serum IL-6 levels. Thromb Haemost 2000;84:210–215. [PubMed] [Google Scholar]
- 84.Yamamoto C, Yoneda T, Yoshikawa M, Fu A, Tokuyama T, Tsukaguchi K, Narita N. Airway inflammation in COPD assessed by sputum levels of interleukin-8. Chest 1997;112:505–510. [DOI] [PubMed] [Google Scholar]
- 85.Crooks SW, Bayley DL, Hill SL, Stockley RA. Bronchial inflammation in acute bacterial exacerbations of chronic bronchitis: the role of leukotriene B4. Eur Respir J 2000;15:274–280. [DOI] [PubMed] [Google Scholar]
- 86.Gompertz S, O'Brien C, Bayley DL, Hill SL, Stockley RA. Changes in bronchial inflammation during acute exacerbations of chronic bronchitis. Eur Respir J 2001;17:1112–1119. [DOI] [PubMed] [Google Scholar]
- 87.Churg A, Wang RD, Tai H, Wang X, Xie C, Wright JL. Tumor necrosis factor-alpha drives 70% of cigarette smoke-induced emphysema in the mouse. Am J Respir Crit Care Med 2004;170:492–498. [DOI] [PubMed] [Google Scholar]
- 88.Bhowmik A, Seemungal TA, Sapsford RJ, Wedzicha JA. Relation of sputum inflammatory markers to symptoms and lung function changes in COPD exacerbations. Thorax 2000;55:114–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bucchioni E, Kharitonov SA, Allegra L, Barnes PJ. High levels of interleukin-6 in the exhaled breath condensate of patients with COPD. Respir Med 2003;97:1299–1302. [DOI] [PubMed] [Google Scholar]
- 90.Barcelo B, Pons J, Fuster A, Sauleda J, Noguera A, Ferrer JM, Agusti AG. Intracellular cytokine profile of T lymphocytes in patients with chronic obstructive pulmonary disease. Clin Exp Immunol 2006;145:474–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Panzner P, Lafitte JJ, Tsicopoulos A, Hamid Q, Tulic MK. Marked up-regulation of T lymphocytes and expression of interleukin-9 in bronchial biopsies from patients with chronic bronchitis with obstruction. Chest 2003;124:1909–1915. [DOI] [PubMed] [Google Scholar]
- 92.Ciccolella DE, Rogers TJ, Criner GJ. Systemic levels of IL-8 are elevated in patients with COPD exacerbation [abstract]. Proc Am Thorac Soc 2006;3:A626. [Google Scholar]