Abstract
Sleep abnormalities are common in severe emphysema, and include poor sleep quality, the development of nocturnal oxygen desaturation, and the presence of coexistent obstructive sleep apnea. With lower baseline oxygenation and abnormal respiratory mechanics in patients with severe emphysema, alterations in ventilatory control and respiratory muscle function that normally occur during sleep can have profound effects, and contribute to the development of sleep abnormalities. The impact on quality of life, cardiopulmonary hemodynamics, and overall survival remains uncertain. In addition, treatment for chronic obstructive pulmonary disease and its effect on sleep abnormalities have demonstrated conflicting results. More recently, as part of the National Emphysema Treatment Trial, lung volume reduction surgery has been shown to improve both sleep quality and nocturnal oxygenation in emphysema. Although indications for performing an overnight polysomnogram in patients with emphysema have been debated, recommendations have been presented. Future studies investigating disease mechanism and response to therapy in patients with sleep abnormalities and severe emphysema are warranted.
Keywords: emphysema, sleep, hypoventilation, apnea, oxygenation
Patients with severe emphysema commonly have distinct abnormalities related to sleep that include poor sleep quality (1–4) and the development of nocturnal oxygen desaturation (NOD) (1, 3–8). Patients with emphysema often complain of difficulty with initiating and maintaining sleep (3), and objective measurements have demonstrated increased sleep latency, decreased total sleep time, and an increased number of nocturnal arousals (1–4). These findings contribute to the excessive daytime sleepiness and early morning awakenings reported in these patients (9–11). As part of the National Emphysema Treatment Trial (NETT), sleep quality and nocturnal oxygenation have been examined in patients with severe emphysema who were being evaluated for lung volume reduction surgery (LVRS) (4, 12).
Episodes of NOD are more pronounced during REM sleep (2, 5–7), and can develop despite an awake PaO2 > 60 mm Hg. Although predictors for the development of NOD have been identified (3, 7, 8, 13–18), its effect on pulmonary hemodynamics and overall survival are still uncertain. In addition, patients with emphysema with coexistent obstructive sleep apnea (OSA), often referred to as the “overlap syndrome,” also demonstrate NOD. This review examines the physiological variables that affect sleep quality and nocturnal oxygenation in severe emphysema. In addition, therapeutic interventions, including LVRS, and their impact on sleep quality and overall survival are reviewed. Finally, limitations in the current literature and open questions that remain to be answered are discussed.
PHYSIOLOGICAL ALTERATIONS DURING SLEEP
Ventilation is normally controlled by a combination of two systems: a metabolic system responsible for the automatic changes directly related to gas exchange, and a behavioral system responsible for the voluntary changes originating from cortical and forebrain structures. At sleep onset, input from the behavioral system decreases, and the metabolic control system, which was active but influenced by the behavioral control system during wakefulness, acts as the primary controller of the respiratory system. There are associated changes in ventilatory control and thoracoabdominal muscle activity that are sleep stage specific. Ventilatory responses to both hypoxia and hypercapnia are decreased, with the largest decrease noted during REM sleep (19, 20). In addition, during non-REM sleep, intercostal muscle activity and thus rib cage contribution to breathing, as well as diaphragmatic muscle activity, are increased (21). Despite an increase in respiratory muscle activity, minute ventilation decreases during non-REM sleep because of a decrease in tidal volume. A decrease in upper airway dilator muscle activity, resulting in an increase in upper airway resistance, appears to be responsible (22). As a result, PaCO2 increases 3 to 10 mm Hg, and PaO2 decreases 2 to 8 mm Hg (23).
During REM sleep, there is a decrease in rib cage contribution to breathing due to a marked decrease in intercostal muscle activity (21). Tidal volume is maintained by an increase in diaphragmatic muscle activity. Upper airway resistance, which would be expected to be highest during REM sleep because of pharyngeal dilator muscle atonia, has been less investigated. Results suggest an increase in upper airway resistance similar to that seen during non-REM sleep (24). The breathing pattern during REM sleep is irregular, with sudden changes in respiratory amplitude and frequency observed (23). Overall, tidal volume and minute ventilation are decreased during what is referred to as phasic REM sleep, when rapid eye movements are noted to be abundant.
RESPIRATORY ALTERATIONS DURING SLEEP AND THEIR CONSEQUENCES IN EMPHYSEMA
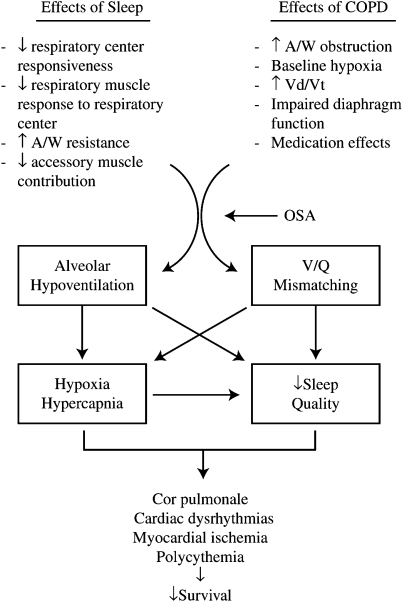
Alterations in ventilatory control and respiratory muscle function during sleep become important in patients with chronic obstructive pulmonary disease (COPD) who have lower baseline oxygenation and abnormal respiratory mechanics. These factors contribute to the development of NOD and poor sleep quality, which may lead to the long-term sequelae of polycythemia, cor pulmonale, arrhythmias, myocardial stress, and possibly decreased survival (Figure 1). However, evidence to support many of these relationships is still lacking.
Figure 1.
Potential consequences due to changes in ventilatory control and respiratory muscle function during sleep when they occur in patients with underlying chronic obstructive pulmonary disease (COPD). A/W = airways; OSA = obstructive sleep apnea; 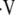 d/
d/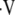 t = ratio of dead space volume to tidal volume.
t = ratio of dead space volume to tidal volume.
NOCTURNAL OXYGEN DESATURATION
Prevalence of NOD in Emphysema
Patients with emphysema can develop significant NOD despite adequate oxygenation during wakefulness. This effect is more pronounced among those with low baseline oxygenation (1, 16), can be more severe than desaturation during maximal exercise (16), and may be seen more commonly in patients with the “blue bloater” than the “pink puffer” phenotype (25). However, significant NOD has also been reported in patients with COPD with mild daytime hypoxia. Koo and coworkers (5) studied 15 patients with COPD with a mean FEV1 of 0.96 L and found a mean decrease in PaO2 of 13.5 mm Hg and an increase in PaCO2 of 8.3 mm Hg during sleep, despite an awake PaO2 exceeding 60 mm Hg. Other studies have shown decreases in SaO2 during sleep, with the most severe episodes occurring during REM sleep (2, 6, 7). In one of the largest of these studies, Fletcher and coworkers (7) showed that 27% of 135 patients with COPD with awake PaO2 exceeding 60 mm Hg had REM-associated NOD. Although patients with REM-associated NOD had a lower awake PaO2 and higher awake PaCO2, these parameters were not predictive in identifying patients with NOD. In our study of 16 NETT patients (4), the mean SaO2 was 90 ± 6% and the lowest SaO2 during the night was 83 ± 8%. The percentage total sleep time (TST) with an SaO2 less than 90% was 37 ± 45%.
Mechanisms of NOD in Emphysema
Several mechanisms have been proposed to explain the development of NOD in emphysema (26–30). Alveolar hypoventilation appears to play a major role, especially during REM sleep. This was demonstrated by Becker and coworkers (26) in a study of nine patients with underlying emphysema. Compared with wakefulness, minute ventilation decreased 16% during non-REM sleep and 32% during REM sleep, predominantly because of a decrease in tidal volume, measured with a pneumotachograph. Hudgel and Devodatta (27) noted that desaturators had a greater decrease in functional residual capacity compared with nondesaturators, suggesting that a maldistribution of ventilation may be a contributing factor. However, Ballard and coworkers (28) reported no change in lung volume during sleep in patients with emphysema, and believed that the decrease in minute ventilation was related to an increase in upper airway resistance and a decrease in respiratory neuromuscular output. More recently, O'Donoghue and coworkers (29) noted a similar preservation of lung volume during sleep in hypercapnic patients with COPD, despite a decrease in tidal volume and minute ventilation. Finally, changes in the distribution of ventilation–perfusion ratios may contribute to NOD, suggested by the fact that mild increases in PaCO2 (and thus hypoventilation) could not account for the more significant decreases in PaO2 during sleep (30). However, changes in ventilation–perfusion ratios were not measured during the night. Therefore, a number of mechanisms may be responsible for the development of NOD, with hypoventilation, as occurs normally during sleep, playing an important role in patients with abnormal respiratory mechanics and lower baseline awake oxygenation.
Consequences of NOD in Emphysema
Potential consequences of NOD include the development of cor pulmonale. In other patient populations, pulmonary hypertension has been associated with NOD (31). In patients with emphysema, Coccagna and Lugaresi (32) noted a progressive acute increase in mean pulmonary artery pressures across all sleep stages in 12 patients with COPD, with the most significant increase noted in REM sleep. The increase in pulmonary artery pressure was associated with the decrease in PaO2 and less so with the increase in PaCO2. Whether these acute changes lead to the development of chronic pulmonary hypertension is still controversial. Fletcher and coworkers (33) demonstrated that systolic and mean pulmonary artery pressures, as well as pulmonary vascular resistance, were higher in patients with NOD compared with those without NOD. However, in a larger study, Chaouat and coworkers (17) noted no difference in mean pulmonary artery pressure in patients with and without NOD, and nocturnal oxygenation was not predictive of the presence or absence of pulmonary hypertension. The presence of coexisting obstructive sleep apnea (overlap syndrome), may increase the risk of developing cor pulmonale.
The relationship between NOD and sleep quality remains poorly defined. Because of the decrease in output from the reticular activating system and responsiveness to hypercapnia and especially hypoxia during sleep, subjects can remain asleep even when arterial saturation decreases to approximately 70% (19). We studied 16 patients with COPD with an emphysema-predominant phenotype as part of the NETT and found no significant correlation between changes in sleep quality (as measured by TST and sleep efficiency, respectively) and changes in nocturnal oxygenation during the night, including mean oxygen saturation (r = 0.1, P = 0.8 and r = 0.1, P = 0.9), lowest oxygen saturation (r = 0.4, P = 0.3 and r = 0.1, P = 0.9), and percentage TST with an oxygen saturation less than 90% (r = 0.1, P = 0.8 and r = 0.2, P = 0.6) (4).
Survival may also be affected by the presence of NOD in emphysema. In a retrospective study of 169 patients with emphysema, Fletcher and coworkers (34) noted significantly decreased survival among patients with NOD compared with those without NOD. However, this retrospective study was based on two different definitions of NOD, and this and other studies have not demonstrated improved survival on correction of the NOD (see below). Therefore, a definite relationship between NOD and the development of chronic pulmonary hypertension, sleep quality, and survival is yet to be determined.
Predictors of NOD in Emphysema
Because of the complexity of measuring NOD, many investigators have attempted to determine which physiological parameters measured during wakefulness might predict NOD (3, 7, 8, 12, 13, 15–18). Cormick and coworkers noted that NOD was related to waking values of SaO2 and PaCO2, but not to any other measurements of daytime lung function (3). Fletcher and coworkers found a combination of high daytime PaCO2 and low PaO2 to be significantly related, but not very predictive of NOD in 135 patients with emphysema (7), with similar findings noted in a subset of patients who were followed up a mean of 40 months later (15). Connaughton and coworkers studied 97 patients with severe emphysema and noted that both the daytime PaCO2 and PaO2 were significantly related to NOD (13). Bradley and coworkers identified awake SaO2 and PaCO2 as independent variables related to NOD; these variables accurately predicted NOD in a group of 26 patients with COPD (8). Mulloy and McNicholas (16) found that PaO2, and Chaouat and coworkers (17) identified PaCO2, as predictive of NOD in similar groups of patients. Heijdra and coworkers (18) demonstrated that, in addition to awake gas exchange, FEV1 and maximal inspiratory muscle strength correlated with NOD. Despite a noted relationship, the clinical utility of these awake physiological variables as they relate to NOD remains uncertain.
SLEEP QUALITY IN EMPHYSEMA
Prevalence and Characteristics
Patients with emphysema have low quality of life scores compared with normative samples (35). These findings apply to both general and disease-specific quality of life measures. However, few studies have examined the extent to which disturbed sleep contributes to these low quality of life scores (3, 36). Indicators of poor sleep quality include the subjective complaints of difficulty falling and staying asleep, morning tiredness, early awakenings, and excessive daytime sleepiness (3, 9–11). Cormick and coworkers (3) queried 50 patients with emphysema about their perception of sleep quality over a 6-month period and found that 72% complained of daytime sleepiness, 32% reported impaired daytime concentration, and 28% complained of early morning headaches. In a survey of more than 2,000 emphysema patients, Klink and coworkers (10) noted that the presence of respiratory symptoms, specifically cough or sputum production and wheezing, was associated with disturbed sleep, including difficulty initiating and maintaining sleep, as well as daytime sleepiness. If both symptoms were present, 53% of patients reported insomnia symptoms and 23% complained of daytime sleepiness. A similar association between insomnia complaints and the presence of respiratory symptoms was noted by Dodge and coworkers (11) in a survey of more than 1,600 patients with obstructive lung disease. Insomnia was more prevalent in those patients with reported cough, dyspnea, or wheezing. Finally, in a questionnaire to 734 patients greater than 65 years of age, Bellia and coworkers (9) noted that nocturnal awakening, morning tiredness, and early awakenings were more frequent in patients with either asthma or emphysema, as compared with age-matched control subjects who were free of respiratory disease.
Assessment of Sleep Quality in Emphysema
Objective measurement of sleep quality includes determination of sleep latency, the TST during the night, sleep efficiency (total sleep time/time in bed), and the arousal index. Other variables assessed objectively include the percentage spent in each sleep stage, including slow wave sleep (SWS) and REM sleep. Objective assessment of sleep quality by polysomnography has been performed predominantly in small cohorts of patients with severe emphysema. Calverley and coworkers (2) studied 20 patients with severe COPD and found increased sleep latency, a decreased amount of uninterrupted sleep, and decreases in SWS and REM sleep, when compared with healthy control subjects. Fleetham and coworkers (6) found decreased TST, an increased number of arousals, and decreased SWS and REM sleep in 15 patients with severe COPD compared with historical control subjects. Wynne and coworkers (1) studied seven patients with severe COPD, and noted similar findings of a decreased TST, with 30% of the night spent awake. In 16 patients with severe COPD, Cormick and coworkers (3) found that subjective complaints of difficulty initiating and maintaining sleep were in significant agreement with objective measurements of poor sleep quality as indicated by a TST of only 208 minutes and increased arousal index. More recently, we studied 16 patients (10 males; age [mean ± SD], 63 ± 6 yr) with severe COPD (FEV1% predicted, 28 ± 10%) who were evaluated for LVRS as part of the NETT (4). Patients underwent a full-night polysomnogram while on room air following an acquaintance night study to compensate for a “first night” effect. As compared with historical age-matched control subjects, sleep quality was poor, with a TST of 203 ± 100 minutes (mean ± SD), and a sleep efficiency of only 50 ± 24%. There was an increase in the number of arousals during the night and an increase in stage 1 sleep and decreases in SWS and REM sleep.
Consequences of Poor Sleep Quality in Emphysema
Despite subjective and objective findings of poor sleep quality in emphysema, Orr and coworkers (36) noted no evidence of daytime sleepiness, as measured with a multiple sleep latency test (mean sleep latency, 11 ± 4 min), in 14 patients with severe COPD. However, the study involved only a small number of patients, and none of the patients had any subjective complaints of daytime sleepiness. Other studies have noted a lack of subjective daytime sleepiness, despite the presence of objectively measured poor sleep quality (37). In addition, it is unknown at the present time whether poor sleep quality in patients with emphysema has an effect on other parameters of daytime function, such as neurocognition, or psychomotor vigilance.
Mechanisms Responsible for Poor Sleep Quality
In a survey of patients 65 years and older with obstructive airway disease due to asthma or COPD, Bellia and coworkers (9) found no correlation between sleep scores associated with insomnia complaints and severity of airflow obstruction (percent predicted FEV1). The only independent correlates for sleep scores were having underlying depression or arthritis. However, the study included patients with underlying asthma, with no comparison made between the control subjects who were free of respiratory disease and those with strictly COPD. Therefore, whether there is a relationship between sleep quality and the severity of underlying disease is yet to be determined (12).
A number of medications used in the treatment of COPD could affect sleep quality. Veale and coworkers (38) noted no change in sleep quality in 14 patients with COPD treated with a sustained-release form of salbutamol. The effects of theophylline on sleep quality have been more variable, with some studies demonstrating an impairment in sleep quality (39), whereas others have noted no significant effect (40–42). Anticholinergics such as ipratropium may improve sleep quality (43). More recently, McNicholas and coworkers (44) demonstrated that the long-acting anticholinergic tiotropium did not affect sleep quality after 4 weeks of treatment.
Therefore, although sleep quality appears to be poor in patients with emphysema, the mechanism responsible, and the association between nighttime findings and daytime function, has yet to be determined.
OVERLAP SYNDROME
Because of the prevalence of both COPD and OSA in the general population, both disorders are bound to occur together, what has been coined the “overlap syndrome.” Chaouat and coworkers (45) found an obstructive pattern on spirometry in 11% of patients with a known diagnosis of OSA. PaO2 was lower and PaCO2 higher in the overlap group and mean nocturnal SaO2 was significantly lower despite a lack of difference in the apnea–hypopnea index. A blunted central respiratory response in these patients, as measured by CO2 rebreathing, may explain some of these findings (46). More recently, as part of the Sleep Heart Health Study, Sanders and coworkers (47) reported that 22 and 14% of patients with mild obstructive airway disease (mean FEV1/FVC, 64%) had an apnea–hypopnea index greater than 10 and greater than 15, respectively. However, the prevalence of OSA was similar in the groups with and without obstructive airway disease. In addition, the risk for NOD in patients with coexistent obstructive airway disease and OSA was equal to the combined risk from each disorder alone (47). Regarding prognosis, patients with OSA who fall into the overlap group experience higher mortality despite treatment with continuous positive airway pressure therapy (48).
NOD is theorized to be worse in patients with OSA and COPD. This may be because oxygen stores are lower in diseased lungs than in normal lungs. Thus, for a given apnea period, desaturation should be worse in patients with overlap syndrome than in patients with only OSA but normal lungs. Therefore, in patients with overlap syndrome, the consequences of NOD might be expected to be greater. However, there are few data to address this concern.
TREATMENT
Oxygen Therapy
Both the British Medical Research Council (MRC) Long-Term Domiciliary Oxygen Therapy Trial (49) and the Nocturnal Oxygen Therapy Trial (NOTT) (50) evaluated the effects of oxygen therapy in patients with COPD with severe hypoxemia (mean PaO2, 49–51 and 51–52 mm Hg, respectively). In the British study, patients were assigned to continuous oxygen (at least 15 h/d) versus no oxygen therapy, and in the NOTT, patients received continuous oxygen (average, 18 h/d) versus nocturnal oxygen (average, 12 h/d). The continuous oxygen therapy groups in both studies demonstrated improved survival, but the number of patients in each study was small.
In a relatively small study of patients with moderate hypoxemia (PaO2, 56–69 mm Hg), Gorecka and coworkers (51) noted no difference in survival at 3 years between patients prescribed supplemental oxygen (average, 14 h/d) compared with a control group. In addition, Fletcher and coworkers (52) noted no difference in survival in patients with COPD with NOD and an awake PaO2 > 60 mm Hg, who were randomized to nocturnal oxygen at 3 L/minute or a sham control for 36 months. However, there was an improvement in pulmonary hemodynamics noted in the oxygen therapy group. Similar results regarding survival were noted by Chaouat and coworkers (53) in patients with mild to moderate hypoxemia (PaO2, 56–69 mm Hg) who were randomized to nocturnal oxygen therapy versus control and monitored for up to 60 months. In addition to no difference in survival, there was no difference in developing a need for conventional long-term oxygen therapy, or in the degree of change in pulmonary hemodynamics. A similar lack of change in pulmonary hemodynamics has been reported in an uncontrolled study (54).
The effect of oxygen therapy on sleep quality has been examined in only a limited number of studies. Calverley and coworkers (2) noted that the addition of oxygen led to a decrease in sleep latency, an increase in the duration of uninterrupted sleep, and an increase in REM sleep. However, Fleetham and coworkers (6) noted no change in TST, distribution of sleep stages, or frequency of arousals during the night with the addition of oxygen. Therefore, beneficial effects of nocturnal oxygen therapy on sleep quality, pulmonary hemodynamics, and survival remain unproven at the present time.
Medications
As noted above, bronchodilator medications have shown mixed results regarding their effects on sleep quality, with some demonstrating improvement (43), no significant effect (40–42, 44), or deterioration in sleep quality (39). However, despite their diverse effects on sleep quality, the majority have demonstrated an improvement in nocturnal oxygen saturation (39, 41, 43, 44) and lung function (39–44). Regarding treatment of insomnia symptoms in patients with emphysema, benzodiazepines should be avoided, even in normocapnic patients, because of their effect on ventilatory drive and worsening nocturnal hypoxemia (55). However, some of the newer nonbenzodiazepine imidazopyridine compounds, such as zolpidem, have been shown to be safer in patients with less severe COPD (56, 57).
Respiratory Muscle Training
Because of the presence of nocturnal hypoventilation, Heijdra and coworkers (18) hypothesized that respiratory muscle weakness may be contributing to the decrease in minute ventilation. They found a significant correlation between measurements of nocturnal oxygenation and both maximal inspiratory mouth and transdiaphragmatic pressures. As a result, Heijdra and coworkers (58) evaluated the effects of inspiratory muscle training on nocturnal oxygenation. Compared with a sham control group, patients in the inspiratory muscle–training group demonstrated an improvement in inspiratory muscle strength, which correlated with a noted improvement in nocturnal oxygenation. However, these studies involved only a small number of patients, and the improvement in mean nocturnal SaO2 was minimal (1.9 ± 2.2%).
Noninvasive Positive-Pressure Ventilation
The use of noninvasive positive-pressure ventilation (NIPPV) has been shown to be beneficial both during an acute exacerbation of COPD (59) as well as in selected groups of patients with stable chronic emphysema (60, 61). We previously demonstrated that NIPPV in a group of stable hypercapnic patients with COPD, as compared with a sham control group, improved sleep quality without an associated improvement in nocturnal gas exchange (60). These findings suggested that factors other than improvement in gas exchange, such as unloading inspiratory muscles or effects on central drive, might play a role. Other long-term trials have demonstrated improvements in sleep quality (62) and gas exchange (61, 62) and a decrease in hospital admissions and office visits (61). As a result, guidelines have been developed for the use of NIPPV in patients with stable COPD (63).
LVRS
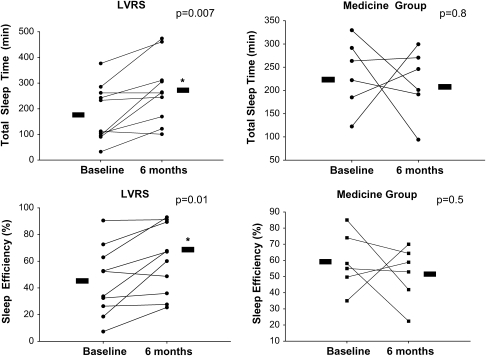
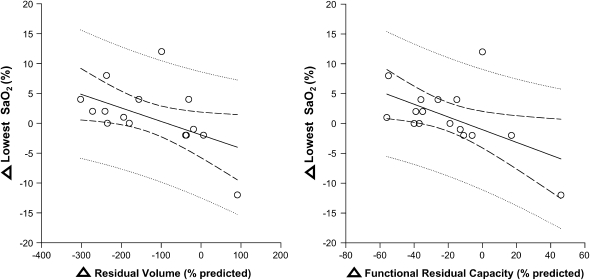
Before the NETT, LVRS had been demonstrated to improve a number of awake physiologic parameters, including respiratory mechanics, gas exchange, and exercise tolerance. We hypothesized that, in association with improvements in respiratory mechanics, LVRS would result in improvement in sleep quality and NOD in severe emphysema. Therefore, as an ancillary study for the NETT, we conducted a prospective, randomized controlled trial, with 6 patients randomized to optimal medical therapy and 10 patients randomized to LVRS and continued optimal medical therapy (4). All patients had an acquaintance night and baseline polysomnogram, with repeat testing at 6 months. The LVRS group, but not the medical group, demonstrated a significant improvement in sleep quality, as measured by TST, sleep efficiency, and arousal index (Figure 2). In addition, nocturnal oxygenation, as measured by the mean and lowest oxygen saturation, as well as the percentage TST with an SaO2 less than 90%, improved only in the LVRS group. The mechanism by which LVRS improved sleep quality was not conclusively determined. Improvements in nocturnal oxygenation were noted to correlate with improvements in airflow obstruction and inversely with improvements in air trapping and hyperinflation (Figure 3). However, improvement in nocturnal oxygenation did not appear to be responsible for the improvement in sleep quality, suggesting that other mechanisms, such as a decrease in hyperinflation and improvement in respiratory muscle function, may be responsible.
Figure 2.
Effects of lung volume reduction surgery (LVRS) and medical therapy on total sleep time and sleep efficiency after 6 months. There was a significant improvement in total sleep time and sleep efficiency in the LVRS group, with no change noted in the medical therapy group. Reprinted by permission from Reference 4.
Figure 3.
There was a significant inverse correlation between the change in lowest SaO2 during the night (from baseline to 6 mo) and changes in residual volume and functional residual capacity (n = 16; r = −0.5, P = 0.04 and r = −0.6, P = 0.03, respectively). Reprinted by permission from Reference 4.
INDICATIONS FOR A SLEEP STUDY
An American Thoracic Society and European Respiratory Society task force published a position paper on standards for the diagnosis and treatment in COPD (64). The group recommended that sleep studies be performed only under special circumstances, including when there a clinical suspicion for OSA, or if there are complications from hypoxemia that are unexplained on the basis of the awake arterial oxygen level, or if there is pulmonary hypertension that is out of proportion to the degree of airflow obstruction.
Limitations and Unanswered Questions
The current literature regarding sleep abnormalities in patients with emphysema has a number of limitations, with small numbers of patients being studied and often conflicting results. Therefore, a number of unanswered important questions remain, including determining whether NOD contributes to the development of chronic pulmonary hypertension and poor sleep quality, as well as whether NOD affects survival. In addition, it is yet to be determined whether poor sleep quality in these patients affects daytime function, including psychomotor vigilance and neurocognitive function. Other mechanistic issues include determining how interventions such as NIPPV and LVRS improve both sleep quality and nocturnal oxygenation. Finally, evaluating whether nocturnal oxygen therapy has an effect on sleep quality and survival will require a relatively large trial. It is hoped that some of these important questions can be answered in the upcoming multicenter National Institutes of Health–sponsored Long-term Oxygen Treatment Trial (LOTT).
CONCLUSIONS
In severe emphysema, sleep is often disturbed and of poor quality, both subjectively and objectively. In addition, nocturnal oxygen desaturation is common, even in patients who do not require oxygen as determined on the basis of awake values. The causes for disturbed sleep are probably multiple, with the severity of underlying disease possibly playing a role. In patients with mild to moderate hypoxemia, the effects of oxygen therapy are mixed, in terms of its effects on both pulmonary hemodynamics and sleep quality. More recently, LVRS has been shown to improve both sleep quality and nocturnal oxygenation in severe emphysema. However, the mechanism by which LVRS improves sleep quality is yet to be determined. A number of questions regarding NOD in COPD remain unanswered. These and other questions of interest will be answered only in future larger and well-controlled clinical trials.
The National Emphysema Treatment Trial (NETT) is supported by contracts with the National Heart, Lung, and Blood Institute (N01HR76101, N01HR76102, N01HR76103, N01HR76104, N01HR76105, N01HR76106, N01HR76107, N01HR76108, N01HR76109, N01HR76110, N01HR76111, N01HR76112, N01HR76113, N01HR76114, N01HR76115, N01HR76116, N01HR76118, and N01HR76119), the Centers for Medicare and Medicaid Services (CMS), and the Agency for Healthcare Research and Quality (AHRQ).
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Wynne JW, Block AJ, Hemenway J, Hunt LA, Flick MR. Disordered breathing and oxygen desaturation during sleep in patients with chronic obstructive lung disease. Am J Med 1979;66:573–579. [DOI] [PubMed] [Google Scholar]
- 2.Calverley PMA, Brezinova V, Douglas NJ, Catterall JR, Flenley DC. The effect of oxygenation on sleep quality in chronic bronchitis and emphysema. Am Rev Respir Dis 1982;126:206–210. [DOI] [PubMed] [Google Scholar]
- 3.Cormick W, Olson LG, Hensley MJ, Suadners NA. Nocturnal hypoxaemia and quality of sleep in patients with chronic obstructive lung disease. Thorax 1986;41:846–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krachman SL, Chatila W, Martin UJ, Nugent T, Crocetti J, Gaughan J, Criner GJ. Effects of lung volume reduction surgery on sleep quality and nocturnal gas exchange in patients with severe emphysema. Chest 2005;128:3221–3228. [DOI] [PubMed] [Google Scholar]
- 5.Koo KW, Sax DS, Snider GL. Arterial blood gases and pH during sleep in chronic obstructive pulmonary disease. Am J Med 1975;58:663–670. [DOI] [PubMed] [Google Scholar]
- 6.Fleetham J, West P, Mezon B, Conway W, Roth T, Kryger M. Sleep, arousals, and oxygen desaturation in chronic obstructive pulmonary disease: the effect of oxygen therapy. Am Rev Respir Dis 1982;126:429–433. [DOI] [PubMed] [Google Scholar]
- 7.Fletcher EC, Miller J, Divine GW, Fletcher JG, Miller T. Nocturnal oxyhemoglobin desaturation in COPD patients with arterial oxygen tensions above 60 mmHg. Chest 1987;92:604–608. [DOI] [PubMed] [Google Scholar]
- 8.Bradley TD, Mateika J, Li D, Avendano M, Goldstein RS. Daytime hypercapnia in the development of nocturnal hypoxemia in COPD. Chest 1990;97:308–312. [DOI] [PubMed] [Google Scholar]
- 9.Bellia V, Catalano F, Scichilone N, Incalzi RA, Spatafora M, Vergani C, Rengo F. Sleep disorders in the elderly with and without chronic airflow obstruction: the SARA Study. Sleep 2003;26:318–323. [DOI] [PubMed] [Google Scholar]
- 10.Klink ME, Dodge R, Quan SF. The relation of sleep complaints to respiratory symptoms in the general population. Chest 1994;105:151–154. [DOI] [PubMed] [Google Scholar]
- 11.Dodge R, Cline MG, Quan SF. The natural history of insomnia and its relationship to respiratory symptoms. Arch Intern Med 1995;155:1797–1800. [PubMed] [Google Scholar]
- 12.Krachman SL, Criner GJ, Nugent T, Martin UJ, Chatila WM. Physiologic determinants of sleep quality in patients with severe emphysema [abstract]. Am J Respir Crit Care Med 2003;167:A234. [Google Scholar]
- 13.Connaughton JJ, Catterrall JR, Elton RA, Stradling JR, Douglas NJ. Do sleep studies contribute to the management of patients with severe chronic obstructive pulmonary disease? Am Rev Respir Dis 1988;138:341–344. [DOI] [PubMed] [Google Scholar]
- 14.Fletcher EC, Luckett RA, Miller T, Fletcher JG. Exercise hemodynamics and gas exchange in patients with chronic obstruction pulmonary disease, sleep desaturation and daytime PaO2 above 60 mmHg. Am Rev Respir Dis 1989;140:1237–1245. [DOI] [PubMed] [Google Scholar]
- 15.Fletcher EC, Scott D, Qian W, Luckett RA, Miller CC, Goodnight-White S. Evolution of nocturnal oxyhemoglobin desaturation in patients with chronic obstructive pulmonary disease and a daytime PaO2 above 60 mmHg. Am Rev Respir Dis 1991;144:401–405. [DOI] [PubMed] [Google Scholar]
- 16.Mulloy E, McNicholas WT. Ventilation and gas exchange during sleep and exercise in severe COPD. Chest 1996;109:387–394. [DOI] [PubMed] [Google Scholar]
- 17.Chaouat A, Weitzenblum E, Kessler R, Charpentier C, Ehrhart M, Levi-Valensi P, Zielinski J, Delaunois L, Cornudella R, Moutinho dos Santos J. Sleep-related O2 desaturation and daytime pulmonary haemodynamics in COPD patients with mild hypoxaemia. Eur Respir J 1997;10:1730–1735. [DOI] [PubMed] [Google Scholar]
- 18.Heijdra YF, Dekhuijzen PNR, van Herwaarden CLA, Folgering HTM. Nocturnal saturation and respiratory muscle function in patients with chronic obstructive pulmonary disease. Thorax 1995;50:610–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berthon-Jones M, Sullivan CE. Ventilatory and arousal responses to hypoxia in normal sleeping humans. Am Rev Respir Dis 1982;125:632–639. [DOI] [PubMed] [Google Scholar]
- 20.Berthon-Jones M, Sullivan CE. Ventilatory and arousal responses to hypercapnia in normal sleeping humans. J Appl Physiol 1984;57:59–67. [DOI] [PubMed] [Google Scholar]
- 21.Tabachnik E, Muller NL, Bryan AC, Levison H. Changes in ventilation and chest wall mechanics during sleep in normal adolescents. J Appl Physiol 1981;51:557–564. [DOI] [PubMed] [Google Scholar]
- 22.Berger RJ. Tonus of extrinsic laryngeal muscles during sleep and dreaming. Science 1961;134:840. [DOI] [PubMed] [Google Scholar]
- 23.Krieger J. Breathing during sleep in normal subjects. In: Kryger MH, Roth T, Dement WC, editors. Principles and practices of sleep medicine. Philadelphia, PA: W.B. Saunders; 2005. pp. 232–244.
- 24.Wiegand DA, Latz B, Zwillich CW, Wiegand L. Geniohyoid muscle activity in normal men during wakefulness and sleep. J Appl Physiol 1990;69:1262–1269. [DOI] [PubMed] [Google Scholar]
- 25.De Marco FJ Jr, Wynne JW, Block AJ, Boysen PG, Tassan VC. Oxygen desaturation during sleep as a determinant of the blue bloated syndrome. Chest 1981;79:621–625. [DOI] [PubMed] [Google Scholar]
- 26.Becker HF, Piper AJ, Flynn WE, McNamara SG, Grunstein RR, Peter JH, Sullivan CE. Breathing during sleep in patients with nocturnal desaturation. Am J Respir Crit Care Med 1999;159:112–118. [DOI] [PubMed] [Google Scholar]
- 27.Hudgel DW, Devodatta P. Decrease in functional residual capacity during sleep in normal humans. J Appl Physiol 1984;57:1319–1322. [DOI] [PubMed] [Google Scholar]
- 28.Ballard RD, Clover CW, Suh BY. Influence of sleep on respiratory function in emphysema. Am J Respir Crit Care Med 1995;151:945–951. [DOI] [PubMed] [Google Scholar]
- 29.O'Donoghue FJ, Catcheside PG, Eckert DJ, McEvoy RD. Changes in respiration in NREM sleep in hypercapnic chronic obstructive pulmonary disease. J Physiol 2004;559:663–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Catterall JR, Calverley PMA, MacNee W, Warren PM, Shapiro CM, Douglas NJ, Flenley DC. Mechanism of transient nocturnal hypoxemia in hypoxic chronic bronchitis and emphysema. J Appl Physiol 1985;59:1698–1703. [DOI] [PubMed] [Google Scholar]
- 31.Minai OA, Pandya CM, Golish JA, Avecillas JF, McCarthy K, Marlow S, Arrolida AC. Predictors of nocturnal oxygen desaturation in pulmonary arterial hypertension. Chest 2007;131:109–117. [DOI] [PubMed] [Google Scholar]
- 32.Coccagna G, Lugaresi E. Arterial blood gases and pulmonary and systemic arterial pressure during sleep in chronic obstructive pulmonary disease. Sleep 1978;1:117–124. [DOI] [PubMed] [Google Scholar]
- 33.Fletcher EC, Luckett RA, Miller T, Costarangos C, Kutka N, Fletcher JG. Pulmonary vascular hemodynamics in chronic lung disease patients with and without oxyhemoglobin desaturation during sleep. Chest 1989;95:757–766. [DOI] [PubMed] [Google Scholar]
- 34.Fletcher EC, Donner CF, Midgren B, Zielinski J, Levi-Valensi P, Braghiroli A, Rida Z, Miller CC. Survival in COPD patients with daytime PaO2 > 60 mmHg with and without nocturnal oxyhemoglobin desaturation. Chest 1992;101:649–655. [DOI] [PubMed] [Google Scholar]
- 35.Kaplan RM, Ries AL, Reilly J. Mohsenifar: measurement of health-related quality of life in the National Emphysema Treatment Trial. Chest 2004;126:781–789. [DOI] [PubMed] [Google Scholar]
- 36.Orr WC, Shamma-Othman Z, Levin D, Lthman J, Rundell OH. Persistent hypoxemia and excessive daytime sleepiness in chronic obstructive pulmonary disease (COPD). Chest 1990;97:583–585. [DOI] [PubMed] [Google Scholar]
- 37.Saaresranta T, Irjala K, Aittokallio T, Polo O. Sleep quality, daytime sleepiness and fasting insulin levels in women with chronic obstructive pulmonary disease. Respir Med 2005;99:856–863. [DOI] [PubMed] [Google Scholar]
- 38.Veale D, Cooper BG, Griffiths CJ, Corris PA, Gibson GJ. The effect of controlled-release salbutamol on sleep and nocturnal oxygenation in patients with asthma and chronic obstructive pulmonary disease. Respir Med 1994;88:121–124. [DOI] [PubMed] [Google Scholar]
- 39.Mulloy E, McNicholas WT. Theophylline improves gas exchange during rest, exercise and sleep in severe chronic obstructive pulmonary disease. Am Rev Respir Dis 1993;148:1030–1036. [DOI] [PubMed] [Google Scholar]
- 40.Martin RJ, Pak J. Overnight theophylline concentrations and effects on sleep and lung function in chronic obstructive pulmonary disease. Am Rev Respir Dis 1992;145:540–544. [DOI] [PubMed] [Google Scholar]
- 41.Berry RB, Desa MM, Branum JP, Light RW. Effect of theophylline on sleep and sleep-disordered breathing in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis 1991;143:245–250. [DOI] [PubMed] [Google Scholar]
- 42.Mann GCW, Chapman KR, Ali SH, Darke AC. Sleep quality and nocturnal respiratory function with once-daily theophylline (Uniphyl) and inhaled salbutamol in patients with COPD. Chest 1996;110:648–653. [DOI] [PubMed] [Google Scholar]
- 43.Martin RJ, Bucher Bartelson B, Smith P, Hudgel DW, Lewis D, Pohl G, Koker P, Souhrada JF. Effect of ipratropium bromide treatment on oxygen saturation and sleep quality in COPD. Chest 1999;115:1338–1345. [DOI] [PubMed] [Google Scholar]
- 44.McNicholas WT, Calverley PM, Lee A, Edwards JC. Long-acting inhaled anticholinergic therapy improves sleeping oxygen saturation in COPD. Eur Respir J 2004;23:825–831. [DOI] [PubMed] [Google Scholar]
- 45.Chaouat A, Weitzenblum E, Krieger J, Ifoundza T, Oswald M, Kessler R. Association of chronic obstructive pulmonary disease and sleep apnea syndrome. Am J Respir Crit Care Med 1995;151:82–86. [DOI] [PubMed] [Google Scholar]
- 46.Radwan L, Maszczyk Z, Koziorowski A, Koziej M, Cieslicki J, Sliwinski P, Zielinski J. Control of breathing in obstructive sleep apnoea and in patients with the overlap syndrome. Eur Respir J 1995;8:542–545. [PubMed] [Google Scholar]
- 47.Sanders MH, Newman AB, Haggerty CL, Redline S, Lebowitz M, Samet J, O'Connor GT, Punjabi NM, Shahar E. Sleep and sleep-disordered breathing in adults with predominantly mild obstructive airway disease. Am J Respir Crit Care Med 2003;167:7–14. [DOI] [PubMed] [Google Scholar]
- 48.Chaouat A, Wetzenblum E, Krieger J, Sforza I, Hammad H, Oswald M, Kessler R. Prognostic value of lung function and pulmonary haemodynamics in OSA patients treated with CPAP. Eur Respir J 1999;13:1091–1096. [DOI] [PubMed] [Google Scholar]
- 49.Medical Research Council Working Party. Long-term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Lancet 1981;1:681–686. [PubMed] [Google Scholar]
- 50.Nocturnal Oxygen Therapy Trial Group. Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: a clinical trial. Ann Intern Med 1980;93:391–398. [DOI] [PubMed] [Google Scholar]
- 51.Gorecka D, Gorzelak K, Sliwinski P, Tobiasz M, Zielinski J. Effect of long-term oxygen therapy on survival in patients with chronic obstructive pulmonary disease with moderate hypoxemia. Thorax 1997;52:674–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fletcher EC, Luckett RA, Goodnigth-White S, Miller CC, Qian W, Costarangos-Galarza C. A double-blind trial of nocturnal supplemental oxygen for sleep desaturation in patient with chronic obstructive pulmonary disease and a daytime PaO2 above 60 mmHg. Am Rev Respir Dis 1992;145:1070–1076. [DOI] [PubMed] [Google Scholar]
- 53.Chaouat A, Weitzenblum E, Kessler R, Charpentier C, Ehrahart M, Schott R, Levi-Valensi P, Zielinski J, Delaunois L, Cornudella R, et al. A randomized trial of nocturnal oxygen therapy in chronic obstructive pulmonary disease patients. Eur Respir J 1999;14:1002–1008. [DOI] [PubMed] [Google Scholar]
- 54.Zielenski J, Tobiasz M, Hawrylkiewicz I, Sliwinski P, Palasiewicz G. Effects of long-term oxygen therapy on pulmonary hemodynamics in COPD patients. Chest 1998;113:65–70. [DOI] [PubMed] [Google Scholar]
- 55.Block AJ, Dolly FR, Slayton PC. Does flurazepam ingestion affect breathing and oxygenation during sleep in patients with chronic obstructive lung disease? Am Rev Respir Dis 1984;129:230–233. [PubMed] [Google Scholar]
- 56.Girault C, Muir JF, Mihaltan F, Borderies P, De La Giclais B, Verdure A, Samson-Dollfus D. Effects of repeated administration of zolpidem on sleep, diurnal and nocturnal respiratory function, vigilance, and physical performance in patients with COPD. Chest 1996;110:1203–1211. [DOI] [PubMed] [Google Scholar]
- 57.Steens R, Pouliot Z, Millar T, Kryger M, George C. Effects of zolpidem and triazolam on sleep and respiration in mild to moderate chronic obstructive pulmonary disease. Sleep 1993;16:318–326. [DOI] [PubMed] [Google Scholar]
- 58.Heijdra YF, Dekhuijzen PN, van Herwaarden CL, Folgering HT. Nocturnal saturation improves by target-flow inspiratory muscle training in patients with COPD. Am J Respir Crit Care Med 1996;153:260–265. [DOI] [PubMed] [Google Scholar]
- 59.Antonelli M, Conti G, Rocco M, Bufi M, De Blasi RA, Vivino G, Gasparetto A, Meduri GU. A comparison of noninvasive positive pressure ventilation and conventional mechanical ventilation in patients with acute respiratory failure. N Engl J Med 1998;339:429–435. [DOI] [PubMed] [Google Scholar]
- 60.Krachman SL, Quaranta AJ, Berger TJ, Criner GJ. Effects of noninvasive positive pressure ventilation on gas exchange and sleep in COPD patients. Chest 1997;112:623–628. [DOI] [PubMed] [Google Scholar]
- 61.Jones SE, Packham S, Hebden M, Smith AP. Domiciliary nocturnal intermittent positive pressure ventilation in patients with respiratory failure due to severe COPD: long-term follow-up and effect on survival. Thorax 1998;53:495–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Elliott MW, Simonds AK, Carroll MP, Wedzicha JA, Branthwaite MA. Domiciliary nocturnal nasal intermittent positive pressure ventilation in hypercapnic respiratory failure due to chronic obstructive lung disease: effects on sleep and quality of life. Thorax 1992;47:342–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Goldberg A, conference facilitator; Leger P, Hill N, Criner G. Clinical indications for noninvasive positive pressure ventilation in chronic respiratory failure due to restrictive lung disease, COPD, and nocturnal hypoventilation: a consensus conference report. Chest 1999;116:521–534. [DOI] [PubMed] [Google Scholar]
- 64.Celli BR, Mac Nee W, Agusti A, Anzueto A, Berg B, Buist AS, Calverley PMA, Chavannes N, Dillard T, Fahy B, et al. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J 2004;23:932–946. [DOI] [PubMed] [Google Scholar]