Abstract
The pro-inflammatory potential of β-hairpin peptide hydrogels (MAX1 and MAX8) was assessed in vitro by measuring the cellular response of J774 mouse peritoneal macrophages cultured on the hydrogel surfaces. An enzyme-linked immunosorbent assay (ELISA) was used to measure the level of TNF-α, a pro-inflammatory cytokine, secreted by cells cultured on the gel surfaces. Both bulk and thin films of gels did not elicit TNF-α secretion from the macrophages. In addition, live/dead assays employing laser scanning confocal microscopy (LSCM) and phase-contrast light micrographs show the hydrogel surfaces are non-cytotoxic toward the macrophages and allow the cells to adopt healthy morphologies. When macrophages were activated with lipopolysaccharide (LPS), a known bacterial pathogen that activates an innate immune response, an increase in the TNF-α titers by two orders of magnitude was observed. On LPS induction, macrophages displayed a decrease in cell density, enlarged nuclei, and an increase in cytoplasmic granularity, all characteristics of activated macrophages indicating that the cells are still capable of reacting to insult. The data presented herein indicate that MAX1 and MAX8 gels do not elicit macrophage activation in vitro and suggest that these materials are excellent candidates for in vivo assessment in appropriate animal models.
Keywords: Hydrogel, Peptide, Self-assembly, Tissue engineering, Macrophage, Immunogenicity
1. Introduction
Over the last several decades, hydrogels have been developed as drug delivery devices [1], biologically [2] and synthetically derived adhesives [2–4], space-filling agents [5] and as three-dimensional scaffolds for cellular growth [6]. Hydrogels are attractive candidates for such applications due to their high water content and porosity. In addition, hydrogels can be designed to be environmentally responsive to fit the needs of the desired application [7,8].
Biomaterial design criteria can vary depending on the intended use. However, biocompatibility is a necessary requirement regardless of the application. Biocompatibility studies typically necessitate in vivo experiments involving animal models that are costly, time consuming and can be limiting for academic laboratories generating even a moderate number of materials for evaluation. To address some of these limitations, Bhatia et al. recently reported a facile in vitro screening process to assess both the cytotoxicity and the pro-inflammatory potential of biomedical materials [4]. Although inflammation is a normal process in wound healing, prolonged inflammation due to a non-compliant material may result in poor wound healing; in extreme cases, the implant must be removed [9,10]. In this screen, the pro-inflammatory nature of a tissue adhesive is assayed by measuring the response of macrophages that have come into contact with the material. This in vitro assay can hasten material development by narrowing the field of potential candidates for eventual implantation into animal models, thus increasing the economic efficiency of biocompatibility assessment and decreasing the number of animals required for the studies. We have taken advantage of this screen to evaluate the pro-inflammatory potential of peptide hydrogel materials developed in our laboratories.
Macrophages perform critical roles in the immune system to regulate homeostasis and act as effector cells at the site of a foreign body [11]. In an inflammatory response, these cells become activated to up-regulate the secretion of many regulatory biochemical cues, such as the classic pro-inflammatory cytokines (tumor necrosis factor (TNF-α), interleukins (IL-1β, and IL-6, IL-8, to name a few)), nitric oxide and arachidonic acid metabolites (platelet-activating factor and prostaglandins) [12]. Of these biochemical cues, TNF-α serves as one of the key regulators. Therefore an in vitro assay that measures the release of soluble TNF-α from mouse peritoneal macrophages co-cultured with candidate materials can provide an early indication of a material’s potential to elicit a pro-inflammatory response in animal models. J774 peritoneal macrophages are employed in this assay since they are a well-studied cell line that have been used extensively to assess biological response to particles in vitro according to the American Standard Test Methods (ASTM) standard test 1903–98.
Recently, we have reported the design of β-hairpin peptides that form rigid hydrogels in response to specific stimuli. The principle design strategy is to link peptide conformation with a self-assembly event that ultimately leads to hydrogel formation (Fig. 1A). MAX1 is a 20 amino acid peptide that when folded adopts an amphiphilic β-hairpin containing a type II′ β-turn (-VDPLPT-), flanked by two β-strands that contain alternating valine and lysine residues (Fig. 1B) [13,14].
Fig. 1.
Model for folding and self-assembly of β-hairpin peptides in response to DMEM cell culture media. (A) Addition of DMEM to a solution of unfolded peptide triggers intramolecular folding into a β-hairpin. This β-hairpin conformation subsequently self-assembles via lateral and facial associations affording a rigid hydrogel with a non-covalently crosslinked fibrillar supra-molecular structure. (B) Peptide sequences of MAX1 and MAX8.
When MAX1 is dissolved in low ionic strength aqueous solutions, it exists in an ensemble of random coil conformations due to electrostatic repulsions between positively charged lysine side chains. However, the addition of DMEM cell culture media, which contains approximately 160 mM salt, screens this charge and allows the peptide to fold [15,16]. Once folded, the β-hairpin self-assembles into a mechanically rigid hydrogel. Several analogs of MAX1 have been designed to undergo hydrogelation in response to a variety of other stimuli including pH [14], temperature [17] and light [18]. DMEM-mediated folding and self-assembly yield gels with surfaces that support cellular attachment and proliferation of NIH3T3 cells [15] and are non-hemolytic toward human red blood cells [19]. In addition, the surfaces of MAX1 gels also display antibacterial activity against a variety of gram (−) and gram (+) bacteria [19].
Although cells can be cultured on the surface of MAX1 gels, three-dimensional cellular encapsulation was non-optimal due to slow gelation kinetics, which led to non-homogenous cellular distribution [20]. Therefore, MAX8 (Fig. 1B) was designed to display enhanced hydrogelation kinetics. This was achieved by replacing a lysine with a glutamic acid to decrease the net positive charge on the hydrophilic face of MAX1 by two charge units. This point amino acid substitution results in a lower amount of positive charge that needs to be screened and facilitates rapid hydrogelation in response to the addition of cell culture media. At concentrations as low as 0.5 wt%, MAX8 forms rigid hydrogels capable of encapsulating cells within the hydrogel network with homogenous distribution [20].
An attractive feature of both MAX1 and MAX8 hydrogels is their ability to undergo a shear-thin and self-healing process [20]. For example, hydrogelation can be triggered directly in a syringe resulting in a rigid hydrogel. Depressing the syringe plunger applies a shear force that thins the gels allowing them to flow through an attached catheter. Upon exiting the catheter, the low viscosity gel immediately recovers forming a rigid hydrogel. When cells are encapsulated in MAX8 gels, this minimally invasive delivery method can be used to efficiently introduce cells into a host.
Extensive research towards the development and characterization of these self-assembling peptide-based hydrogels suggests that they are attractive candidates for a variety of tissue engineering applications due to their micro-porosity, rigidity, high water content (>98%), cytocompatibility and shear-thin/self-healing properties. However, for clinical use, it is essential that when implanted, these hydrogels do not elicit an innate immune response leading to inflammation and rejection by the host. Herein, we report results from an in vitro evaluation of the inflammatory potential of these hydrogels prior to undertaking extensive in vivo animal studies.
2. Experimental methods
2.1. General methods
PL-Rink amide resin was purchased from Polymer Laboratories. Trifluoroacetic acid (TFA), piperidine, thioanisole, ethanedithiol, N,N-diisopropylethylamine, HEPES, 1,8-diazabicyclo[5.4.0]undec-7-ene and anisole were purchased from Acros. Side-chain protected Fmoc-amino acids were purchased from Nova Biochem. 1H-Benzotriazolium 1-[bis(dimethylamino)methylene]-5chloro-,hexafluorophosphate (1-),3-oxide (HCTU) was purchased from Peptides International. 1-Methyl 2-pyrro-lidone was purchased from VWR and acteonitrile, acetic anhydride and fetal bovine serum (FBS) were purchased from Fisher Scientific. Dulbecco’s Modified Eagles Medium (DMEM) was purchased from Sigma Aldrich. DMEM supplemented with 25 mM HEPES was purchased from Gibco. The live/dead assay was purchased from Molecular Probes. J774 mouse peritoneal macrophages were purchased from ATCC and the Quantikine Mouse TNF-α/TNFSF1A immunoassay was purchased from R&D Systems, Inc.
2.2. Peptide synthesis
Peptides were synthesized on PL-Rink amide resin via an automated ABI 433A peptide synthesizer employing standard Fmoc-protocol and HCTU activation. The resulting dry resin-bound peptides were cleaved and side-chain deprotected for 2 h under an N2 atmosphere using a TFA:thioanisole:ethanedithiol:anisole (90:5:3:2) cocktail. Filtration of the resin followed by ether precipitation afforded crude peptides that were purified by RP-HPLC. MAX8 was further treated by dissolving in water and re-lyophilizing twice. See Supporting Information for purification gradients, analytical HPLC chromatograms and ESI(+) Mass Spectra.
2.3. Hydrogel preparation
Peptide stock solutions of 4 wt% MAX1 and 2 wt% MAX8 were prepared by dissolving the lyophilized peptide in a solution of 25 mM HEPES at pH 7.4. 75 μL of this solution was added to wells of a 48 well tissue culture treated polystyrene plate. An equal volume of DMEM supplemented with 25 mM HEPES, pH 7.4 was added to the peptide solutions to initiate self-assembly, resulting in 150 μL of the 2 wt% MAX1 and 1 wt% MAX8 hydrogels (~2 mm in height). Prior to the assay, 400 μL of DMEM with 25 mM HEPES was added to the top of the hydrogels and allowed to equilibrate overnight in an incubator at 37 °C, 5% CO2.
Thin films of 1 wt% MAX8 were prepared by pre-forming the hydrogel in a spray apparatus. After ~30 min, the gel was shear-thin sprayed into the wells of a 48 well plate resulting in films approximately 50–250 μm in thickness.
2.4. Cell culture
J774 mouse peritoneal macrophages were cultured in DMEM supplemented with 10% FBS, 5 mM L-Glutamine, and 50 μg/mL gentamicin (complete medium) at 37 °C, 5% CO2. Due to the possibility of trypsin’s ability to cleave cellular membrane associated receptors, the macrophages were dislodged from the flask surface using a cell scraper and dispensing into new T75 flasks. Media was changed every 3 days and cells were only used between passages 3 and 7 for the assays.
2.5. Cellular immunoassay for TNF-α
J774 mouse peritoneal macrophages were dislodged from the maintenance flask using a cell scraper and counted with a hemacytometer using trypan blue exclusion. The cell solution was centrifuged at 1500 rpm for 5 min to obtain a cell pellet, at which time the media was removed by aspiration and the cells re-suspended in complete medium to obtain a final concentration of 105,300 cells/mL. The following were added to the wells of the TCTP plate and to the 2 wt% MAX1 and 1 wt% MAX8 hydrogel surfaces: 190 μL of complete media, 190 μL of the cell suspension and 20 μL of DMEM resulting in a final volume of 400 μL containing 20,000 cells/well. For LPS induced macrophage activation, samples were prepared as stated above except, the 20 μL addition of DMEM contained 25 μg/mL and 100 μg/mL LPS. The final volume for each sample was 400 μL with final LPS concentrations of 1.25 μg/mL and 5 μg/mL LPS and 20,000 cells/well. After 48 h in culture, the supernatant was removed from each sample and the concentration of TNF-α was determined by using an ELISA per the manufacturer’s instructions. A correction factor was used to account for the additional volume occupied by the hydrogels (see diffusion assay for calculation). N = 3 for all data shown in Fig. 2.
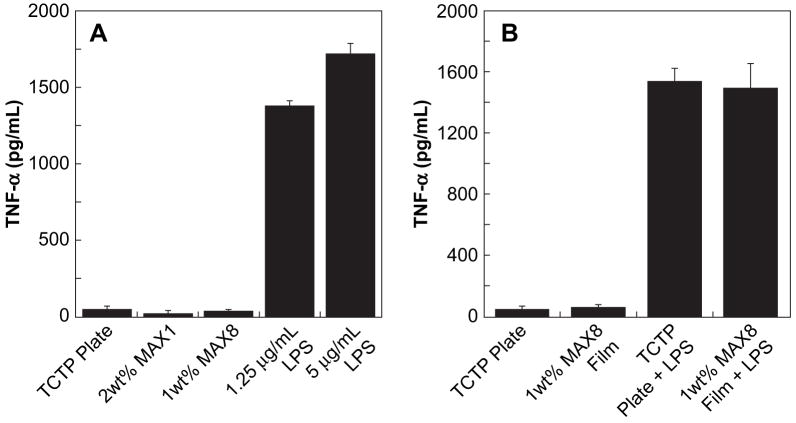
Fig. 2.
(A) ELISA monitoring TNF-α release from J774 macrophages after 48 h of culture on: TCTP control surface, 2 wt% MAX1 hydrogel, 1 wt% MAX8 hydrogel, TCTP control + 1.25 μg/mL LPS, and TCTP control + 5 μg/mL LPS. (B) ELISA monitoring TNF-α release from J774 macrophages after 48 h of culture on: TCTP control, 1 wt% MAX8 film, TCTP control + 1.25 μg/mL LPS, and 1 wt% MAX8 film + 1.25 μg/mL LPS. Initial cell seeding density was 20,000 for all samples, N = 3.
2.6. Macrophage viability and morphology
Immediately following the ELISA, the viability of the macrophages was assessed using a live/dead assay. A stock solution containing both 1 μM calcein AM and 2 μM ethidium homodimer in DMEM with 25 mM HEPES was prepared according to the live/dead assay package instructions. To each well, 200 μL of this stock was added and all samples were imaged using a Zeiss 510 laser scanning confocal microscope at a magnification of 10×. The software package “Image J” was used to measure the diameter of the cells on the TCTP plate, 2 wt% MAX1 and 1 wt% MAX8 hydrogel surfaces (N = 30). Due to the large variations in cell diameter for the macrophages cultured in the presence of LPS, the upper and lower values were reported for two sets of experiments performed in triplicate (N = 6).
2.7. Partition assay for TNF-α in hydrogels
MAX1 (2 wt%) and 1 wt% MAX8 hydrogels were prepared as stated above. To the top of each hydrogel and the well of a TCTP plate (control), 400 μL of a 750 pg/mL stock solution of TNF-α was added and allowed to equilibrate for 2 h in the incubator at 37 °C, 5% CO2. Longer times were not investigated. The concentration of TNF-α above the hydrogel was quantified by an ELISA and the equation below was used to determine the percent TNF-α that partitioned into each hydrogel.
where Cabove hydrogel = concentration of TNF-α measured in the solution above the gel (400 μL) via the ELISA. The concentration of TNF-α was corrected to account for the volume occupied by the hydrogel (150 μL); Cblank = background absorbance; Ccontrol = concentration of TNF-α measured in solution in the absence of hydrogel.
3. Results and discussion
3.1. TNF-α secretion from macrophages on bulk gels
Prior to animal studies, an in vitro evaluation of the inflammatory potential of these peptide-based hydrogels was assessed by monitoring the cellular response of J774 mouse peritoneal macrophages cultured on MAX1 and MAX8 hydrogel surfaces. Fig. 2A shows data from an in vitro based enzyme-linked immunosorbent assay (ELISA) measuring the secretion of TNF-α from J774 mouse peritoneal macrophages. Serving as a negative control, the macrophages were cultured on a TCTP plate to ensure that pathogens were not inadvertently introduced during the assay, which would lead to elevated TNF-α titers. Additionally, it was necessary to ensure that the J774 mouse peritoneal macrophages used in this study release the inflammatory mediator, TNF-α, in response to an appropriate stimulus. Therefore, as a positive control, the macrophages were cultured in the presence of varying concentrations of lipopolysaccharide (LPS). LPS is the prominent component of the outer wall of gram (−) bacteria and is a potent initiator of macrophage activation [21,22].
J774 mouse peritoneal macrophages were introduced to the surfaces of the TCTP plate, 2 wt% MAX1 and 1 wt% MAX8 hydrogels at a cell loading density of 20,000 cells/well. This cell concentration was used to eliminate complications that may arise due to cell crowding and nutrient depletion at higher cell loading densities. After a culture period of 48 h, the cell culture media supernatants were analyzed via the ELISA to quantify the soluble TNF-α secreted by the macrophages. As expected, macrophages cultured on the TCTP plate exhibited a low level of TNF-α secretion (45 ± 6 pg/mL), verifying that the cells predominately reside in an unactivated state. Macrophages cultured on 2 wt% MAX1 and 1 wt% MAX8 hydrogel surfaces yielded TNF-α titers of 19 ± 5 pg/mL and 34 ± 2 pg/mL, respectively. In comparison to the negative control, it appears that the cells secrete a similar amount of TNF-α when cultured on the hydrogels. Introduction of LPS to macrophages cultured on the TCTP surface at concentrations of 1.25 and 5 μg/mL resulted in elevated levels of TNF-α reaching values of 1379 ± 36 pg/mL and 1732 ± 61 pg/mL, respectively. Additionally, this data show that exposure to increasing concentrations of LPS resulted in TNF-α release by the macrophages in a dose dependent manner. Taken together, the data in Fig. 2A show that the soluble TNF-α levels for macrophages cultured on the TCTP plate, MAX1 and MAX8 hydrogel surfaces are only 1–3% of the values found for the LPS induced cells. These results imply that macrophages cultured on the hydrogel surfaces do not secrete significant levels of TNF-α indicating that the surfaces may not be pro-inflammatory.
3.2. TNF-α secretion from macrophages on thin film gels
Due to the high water content (≥98%) and porous nature of the hydrogels, it is possible that secreted TNF-α can partition into the gels. This would lead to aberrantly low TNF-α titers since only the solution above the gel is used in the ELISA described above. The gels used to generate the data in Fig. 2A are ~2 mm thick, representing 27% of the total assay volume. Thus, there is an ample opportunity for a significant portion of secreted TNF-α to partition into the gel matrix. In fact, close examination of the data in Fig. 2A shows that macrophages cultured on the MAX1 and MAX8 hydrogel surfaces result in slightly lower secretion levels of TNF-α in comparison to the TCTP control (19 and 34 pg/mL for the gels versus 45 pg/mL for the control). To address these concerns, partition studies were performed in the absence of cells by allowing a solution of TNF-α to diffuse into the 2 wt% MAX1 and 1 wt% MAX8 hydrogels. After allowing sufficient time for diffusion, the concentration of TNF-α above the gels was determined by ELISA. These studies revealed that 45% (partition coefficient, Pgel/buffer = 0.82) and 22% (Pgel/buffer = 0.28) of the added TNF-α partitioned into the MAX1 and MAX8 hydrogels, respectively. This suggests that the titers observed in Fig. 2A could be slightly lower than expected. However, after taking into account the maximal hypothetical percentage of TNF-α that could partition into each hydrogel after being secreted by the cells, the TNF-α titers for the MAX1 and MAX8 hydrogels would be 35 ± 9 pg/mL and 44 ± 3 pg/mL, respectively. These values are still no larger than that observed for the negative control surface (45 pg/mL), strongly suggesting that these materials do not elicit a pro-inflammatory response by the macrophages.
An additional experiment was performed to circumvent the propensity of TNF-α to partition into the gel matrix. Fig. 2B shows TNF-α titers measured from macrophages cultured on thin films of MAX8. These films are about 50–250 μm thick and should have limited capacity to sequester large amounts of the cytokine. The data clearly show that macrophages cultured on films of MAX8 release no more cytokine than those cultured on the control TCTP surface. When LPS is added to cells on both the control and gel surfaces, significant cytokine release is realized. Importantly, the levels of detectable TNF-α were nearly the same for both positive controls indicating that the thin films of gel have limited TNF-α sequestration capacity.
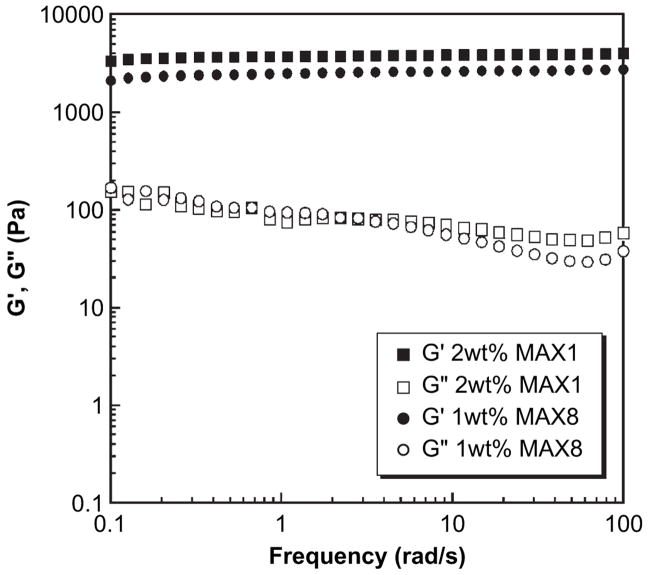
The partition studies bring to light an important consideration when using this assay to gauge the pro-inflammatory potential of a given material. It is necessary to consider the physical nature of the material and how it might influence the propensity of TNF-α to partition and thus avoid detection. In the case of the MAX1 gel, 45% of the added TNF-α partitions into the gel. Under physiological conditions, the net charge of monomeric MAX1 is +9; gels prepared from this peptide are highly electropositive. Soluble TNF-α exists as a trimer [23] whose isoelectric point has been predicted and measured to be anywhere from 5.3 to 6.8 [24]. The electrostatic driving force for this negatively charged cytokine to remain localized in the gel, after partitioning from the bulk solvent, should be high. Interestingly, when TNF-α is added to MAX8 gels, a significantly higher amount of the cytokine is measured above the gel matrix; that is, less is contained in the gel. The net charge of monomeric MAX8 is +7 and gels prepared from this peptide are significantly less electropositive than those prepared from MAX1. In addition, MAX1 gels are 2 wt% in peptide whereas MAX8 gels are 1 wt%; given the molecular weights, net peptide charge and peptide concentration in each gel, MAX1 gels are about 2.5 more positively charged than MAX8 gels. Although electrostatics is most likely the reason for the difference in the observed amount of cytokine retained by the gels, it is possible that differences in the physical nature of the two gels, such as crosslink density and mesh size, may be playing a role. However, both peptides undergo a similar mechanism of folding and self-assembly resulting in hydrogels comprising mono-dispersed fibrils with a solvent exposed, hydrophilic face consisting of mainly lysine residues (Fig. 1A). In addition, oscillatory rheology shows that 2 wt% MAX1 and 1 wt% MAX8 hydrogels exhibit similar mechanical rigidities. Fig. 3 shows dynamic frequency sweep data for these hydrogels where the storage (G′) and loss (G″) moduli are plotted as a function of frequency. Therefore, each gel should possess similar crosslink densities and thus mesh sizes within their networks, exerting a comparable affect on the mobility/diffusion of macromolecules [25]. This suggests that electrostatics is largely at play in explaining the preference of TNF-α to remain sequestered within MAX1 versus MAX8 gels.
Fig. 3.
Oscillatory rheology dynamic frequency sweep measurements of 2 wt% MAX1 and 1 wt% MAX8 hydrogels, 25 mM HEPES, DMEM at pH 7.4, 37 °C. (G′ = storage modulus, G″ = loss modulus). Strain = 0.2%, gap height = 0.5 mm.
3.3. Macrophage viability and morphology
Although the data presented thus far strongly suggest that the hydrogels are non pro-inflammatory, it is prudent to assess the cytotoxicity of the gels towards the macrophages. It is possible that the gel surfaces are inducing cell death resulting in minimal titers of cytokine. Fig. 4a–e shows live/dead assays performed with the same J774 macrophages that were used to generate the data in Fig. 2A. Immediately after the ELISA was performed, a solution containing calcein AM and ethidium homodimer was added to each sample well and cells imaged using laser scanning confocal microscopy (LSCM). Live cells fluoresce green due to ester hydrolysis of calcein AM to calcein, while dead cells fluoresce red due to DNA binding of ethidium homodimer in cells with compromised nuclear membranes. LSCM micrographs of macrophages cultured on 2 wt% MAX1 (Fig. 4b) and 1 wt% MAX8 (Fig. 4c) demonstrate that the gel surfaces are conducive to cellular attachment. When compared to the TCTP surface (Fig. 4a), cells cultured on MAX1 and MAX8 hydrogels displayed similar viabilities, densities and morphologies. In addition, the J774 mouse peritoneal macrophages cultured on the hydrogel surfaces past 48 h proliferate to confluence provided that the nutrients are replenished.
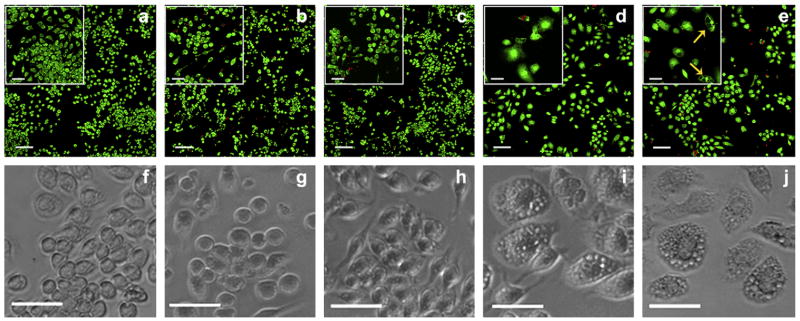
Fig. 4.
Cellular viability and morphology of J774 macrophages after 48 h cultured on: (a, f) TCTP control; (b, g) 2 wt% MAX1 hydrogel; (c, h) 1 wt% MAX8 hydrogel; (d, i) TCTP control + 1.25 μg/mL LPS; and (e, j) TCTP control + 5 μg/mL LPS. Top panels (a–e): LSCM images showing live/dead assays, scale bar is 100 μm. Inset shows magnification of the cells, scale bar is 50 μm. Green = live cells, Red = dead cells. Bottom panels (f–j): phase-contrast images of the cells showing cellular morphology, scale bar is 50 μm. Yellow arrows indicate the formation of vacuoles, N = 6.
Upon LPS induction, activated macrophages should exhibit drastic morphological alterations with a decrease in their rate of proliferation [26–28]. LSCM images of the positive controls consisting of the macrophages exposed to 1.25 μg/mL (Fig. 4d) and 5 μg/mL (Fig. 4e) LPS, revealed similar cellular viabilities in comparison to the TCTP plate. Close inspection of panels (d and e) reveal that the cells have increased in size and are less densely populated. In addition, the appearance of large vacuoles in response to LPS activation can be clearly observed in the inset of panel (e) [29].
Phase-contrast microscopy provides further evidence that cell morphology does not significantly change for cells cultured on the gels versus the control surface. Panels (f–h) show cells with spherical morphologies with slight spreading characteristic of unactivated cells. In contrast, when LPS is added (panels i and j), cells become noticeably enlarged with spread out and flattened morphologies. The average cellular diameters of the macrophages cultured on the TCTP plate, MAX1 and MAX8 hydrogels are 19.7 ± 2.9 μm, 20.1 ± 3.3 μm and 20.0 ± 2.5 μm, respectively. Conversely, LPS activated macrophages exhibit large variations in their cellular diameters ranging from 20 μm to as large as 60 μm. In addition, cells in the presence of LPS display enlarged nuclei and increased granularity throughout their cytoplasm. These are morphological characteristics of activated macrophages [26,27]. All images (both the live/dead assays and the phase-contrast micrographs) in Fig. 4 are representative of multiple experiments, where N = 6.
4. Conclusion
Prior to extensive and costly in vivo studies, an in vitro based assay was performed to assess the potential pro-inflammatory nature of MAX1 and MAX8 hydrogel materials. J774 mouse peritoneal macrophages cultured on the surfaces of 2 wt% MAX1 and 1 wt% MAX8 hydrogels secreted minimal titers of the cytokine TNF-α. Cell viability and morphological studies indicate that the macrophages exist in their unactivated state when in contact with the gels. Positive controls were performed by adding LPS to the cells both in the absence and presence of hydrogel and indicate that the cells are capable of becoming activated upon insult. Taken together, the data indicate that MAX1 and MAX8 hydrogels do not elicit macrophage activation and suggest that these materials may not provoke an inflammatory immune response when implanted into animals. Based on previous work and the results presented herein, these hydrogels are excellent candidates for further in vivo studies due to their mechanical rigidity, porosity, cytocompatibility and their potential non-inflammatory nature.
Supplementary Material
HPLC Chromatograms and ESI-MS of peptides. Supplementary material associated with this article can be found in the online version, at 10.1016/j.biomaterials.2008.07.009.
Acknowledgments
This work was supported by the NIH-National Institute of Dental and Craniofacial research grant R01 DE01638601. We also thank Sujata Bhatia for insightful discussions.
References
- 1.Lee KY, Yuk SH. Polymeric protein delivery systems. Prog Polym Sci. 2007;32:669–97. [Google Scholar]
- 2.Reece TB, Maxey TS, Kron IL. A prospectus on tissue adhesives. Am J Surg. 2001;182:40S–4S. doi: 10.1016/s0002-9610(01)00742-5. [DOI] [PubMed] [Google Scholar]
- 3.Bhatia SK, Arthur SD, Chenault HK, Figuly GD, Kodokian GK. Polysaccharide-based tissue adhesives for sealing corneal incisions. Curr Eye Res. 2007;32:1045–50. doi: 10.1080/02713680701767876. [DOI] [PubMed] [Google Scholar]
- 4.Bhatia SK, Arthur SD, Chenault HK, Kodokian GK. Interactions of polysaccharide-based tissue adhesives with clinically relevant fibroblast and macrophage cell lines. Biotechnol Lett. 2007;29:1645–9. doi: 10.1007/s10529-007-9465-8. [DOI] [PubMed] [Google Scholar]
- 5.Drury JL, Mooney DJ. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials. 2003;24:4337–51. doi: 10.1016/s0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- 6.Hillel A, Shah P, Elisseeff J. Hydrogels in cell encapsulation and tissue engineering. In: Jenkins M, editor. Biomedical polymers. Cambridge, England: Woodhead; 2007. pp. 57–82. [Google Scholar]
- 7.Kopecek J. Hydrogel biomaterials: a smart future? Biomaterials. 2007;28:5185–92. doi: 10.1016/j.biomaterials.2007.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peppas NA, Hilt JZ, Khademhosseini A, Langer R. Hydrogels in biology and medicine: from molecular principles to bionanotechnology. Adv Mater. 2006;18:1345–60. [Google Scholar]
- 9.Babensee JE, Anderson JM, McIntire LV, Mikos AG. Host response to tissue engineered devices. Adv Drug Delivery Rev. 1998;33:111–39. doi: 10.1016/s0169-409x(98)00023-4. [DOI] [PubMed] [Google Scholar]
- 10.Anderson JM. Mechanisms of inflammation and infection with implanted devices. Cardiovasc Pathol. 1993;2:S33–41. [Google Scholar]
- 11.Xaus J, Comalada M, Valledor AF, Cardo M, Herrero C, Soler C, et al. Molecular mechanisms involved in macrophage survival, proliferation, activation or apoptosis. Immunobiology. 2001;204:543–50. doi: 10.1078/0171-2985-00091. [DOI] [PubMed] [Google Scholar]
- 12.Valledor AF, Comalada M, Xaus J, Celada A. The differential time-course of extracellular-regulated kinase activity correlates with the macrophage response toward proliferation or activation. J Biol Chem. 2000;275:7403–9. doi: 10.1074/jbc.275.10.7403. [DOI] [PubMed] [Google Scholar]
- 13.Rajagopal K, Ozbas B, Pochan DJ, Schneider JP. Probing the importance of lateral hydrophobic association in self-assembling peptide hydrogelators. Eur Biophys J. 2006;35:162–9. doi: 10.1007/s00249-005-0017-7. [DOI] [PubMed] [Google Scholar]
- 14.Schneider JP, Pochan DJ, Ozbas B, Rajagopal K, Pakstis L, Kretsinger J. Responsive hydrogels from the intramolecular folding and self-assembly of a designed peptide. J Am Chem Soc. 2002;124:15030–7. doi: 10.1021/ja027993g. [DOI] [PubMed] [Google Scholar]
- 15.Kretsinger JK, Haines LA, Ozbas B, Pochan DJ, Schneider JP. Cytocompatibility of self-assembled β-hairpin peptide hydrogel surfaces. Biomaterials. 2005;26:5177–86. doi: 10.1016/j.biomaterials.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 16.Ozbas B, Kretsinger J, Rajagopal K, Schneider JP, Pochan DJ. Salt-triggered peptide folding and consequent self-assembly into hydrogels with tunable modulus. Macromolecules. 2004;37:7331–7. [Google Scholar]
- 17.Pochan DJ, Schneider JP, Kretsinger J, Ozbas B, Rajagopal K, Haines L. Thermally reversible hydrogels via intramolecular folding and consequent self-assembly of a de novo designed peptide. J Am Chem Soc. 2003;125:11802–3. doi: 10.1021/ja0353154. [DOI] [PubMed] [Google Scholar]
- 18.Haines LA, Rajagopal K, Ozbas B, Salick DA, Pochan DJ, Schneider JP. Light-activated hydrogel formation via the triggered folding and self-assembly of a designed peptide. J Am Chem Soc. 2005;127:17025–9. doi: 10.1021/ja054719o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salick DA, Kretsinger JK, Pochan DJ, Schneider JP. Inherent antibacterial activity of a peptide-based β-hairpin hydrogel. J Am Chem Soc. 2007;129:14793–9. doi: 10.1021/ja076300z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haines-Butterick L, Rajagopal K, Branco M, Salick D, Rughani R, Pilarz M, et al. Controlling hydrogelation kinetics by peptide design for three-dimensional encapsulation and injectable delivery of cells. Proc Natl Acad Sci U S A. 2007;104:7791–6. doi: 10.1073/pnas.0701980104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dobrovolskaia MA, Vogel SN. Toll receptors, CD14, and macrophage activation and deactivation by LPS. Microbes Infect. 2002;4:903–14. doi: 10.1016/s1286-4579(02)01613-1. [DOI] [PubMed] [Google Scholar]
- 22.Fujihara M, Muroi M, Tanamoto K, Suzuki T, Azuma H, Ikeda H. Molecular mechanisms of macrophage activation and deactivation by lipopolysaccharide: roles of the receptor complex. Pharmacol Ther. 2003;100:171–94. doi: 10.1016/j.pharmthera.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 23.Jones EY, Stuart DI, Walker NPC. Structure of tumor necrosis factor. Nature. 1989;338:225–8. doi: 10.1038/338225a0. [DOI] [PubMed] [Google Scholar]
- 24.Aggarwal BB. Structure of tumor necrosis factor and its receptor. Biotherapy. 1991;3:113–20. doi: 10.1007/BF02172083. [DOI] [PubMed] [Google Scholar]
- 25.Ozbas B, Rajagopal K, Schneider JP, Pochan DJ. Semiflexible chain networks formed via self-assembly of β-hairpin molecules. Phys Rev Lett. 2004;93:268106. doi: 10.1103/PhysRevLett.93.268106. [DOI] [PubMed] [Google Scholar]
- 26.Akassoglou K, Adams RA, Bauer J, Mercado P, Tseveleki V, Lassmann H, et al. Fibrin depletion decreases inflammation and delays the onset of demyelination in a tumor necrosis factor transgenic mouse model for multiple sclerosis multiple sclerosis. Proc Natl Acad Sci U S A. 2004;101:6698–703. doi: 10.1073/pnas.0303859101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bernatchez SF, Parks PJ, Gibbons DF. Interaction of macrophages with fibrous materials in vitro. Biomaterials. 1996;17:2077–86. doi: 10.1016/0142-9612(96)00014-2. [DOI] [PubMed] [Google Scholar]
- 28.Xaus J, Comalada M, Valledor AF, Lloberas J, Lopez-Soriano F, Argiles JM, et al. LPS induces apoptosis in macrophages mostly through the autocrine production of TNF-a. Blood. 2000;95:3823–31. [PubMed] [Google Scholar]
- 29.Wilson MR, Foucaud L, Barlow PG, Hutchison GR, Sales J, Simpson RJ, et al. Nanoparticle interactions with zinc and iron: implications for toxicology and inflammation. Toxicol Appl Pharmacol. 2007;225:80–9. doi: 10.1016/j.taap.2007.07.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
HPLC Chromatograms and ESI-MS of peptides. Supplementary material associated with this article can be found in the online version, at 10.1016/j.biomaterials.2008.07.009.