Abstract
The processing of affective material is known to be modulated by serotonin (5-HT), but few studies have used neurophysiological measures to characterize the effect of changes in 5-HT on neural responses to emotional stimuli. We used functional magnetic resonance imaging to investigate the effect of acute tryptophan depletion, which reduces central 5-HT synthesis, on neural responses to emotionally-valenced verbal stimuli. Though no participants experienced significant mood change, emotional information processing was substantially modified following 5-HT depletion. A behavioral bias towards positive stimuli was attenuated following depletion, which was accompanied by increased haemodynamic responses during the processing of emotional words in several subcortical structures. Inter-individual differences in tryptophan depletion-elicited anxiety correlated positively with the caudate bias towards negative stimuli. These data suggest that 5-HT may play an important role in mediating automatic negative attentional biases in major depression, as well as resilience against negative distracting stimuli in never-depressed individuals.
Keywords: Serotonin (5-HT); acute tryptophan depletion (ATD); functional magnetic resonance imaging (fMRI); emotion, affective go/no-go test (AGNG); caudate
INTRODUCTION
The central serotonergic system plays a major role in the regulation of emotional behavior, and is strongly implicated in the pathophysiology of major depression (Schatzberg et al. 2002). However, the neurobiological mechanisms by which serotonin (5-HT) affects emotional processing remain poorly understood. Here we used acute tryptophan depletion (ATD) in order to study the effects of a temporary reduction in 5-HT synthesis on behavioral and neural responses to emotional stimuli in healthy volunteers, using functional magnetic resonance imaging (fMRI).
ATD has proved an instructive paradigm in studying how 5-HT modulates mood, emotional processing and cognition in both patient populations and healthy volunteers (Bell et al. 2005). ATD has been used for over two decades as an experimental tool to cause a temporary and reversible reduction in 5-HT synthesis in humans and experimental animals. This reduction is achieved by selectively excluding the essential amino acid tryptophan, the precursor to 5-HT, from the diet (Young et al. 1985). The most well-known effect of ATD is to produce a temporary recurrence of some depressive symptoms in a proportion of patients who have previously experienced a major depressive episode but who are euthymic at the time of testing (Booij et al. 2002). This effect appears to be most marked in patients maintained on selective serotonin reuptake inhibitors (SSRIs), though mood change in unmedicated remitted major depressive disorder patients has also been reported (Neumeister et al. 2004).
Typically, ATD does not result in mood change in volunteers without a history of affective illness. Nevertheless, many studies have reported that ATD impairs performance on neuropsychological tasks that have an emotional component in healthy humans. For example, it has been reported that ATD impaired decision-making on gambling games (Rogers et al. 1999; Rogers et al. 2003), attenuated motivation on a reinforced speeded reaction-time task (Cools et al. 2005a; Roiser et al. 2006), impaired recognition of emotional expressions (Harmer et al. 2003) and resulted in a negative bias on an emotional inhibitory control paradigm, the Affective Go/No-go test (AGNG) (Murphy et al. 2002). Interestingly, such a negative bias on the AGNG has also been found in depressed patients studied under basal conditions (i.e. without manipulation of serotonergic function: Erickson et al. 2005; Murphy et al. 1999). A follow-up study using fMRI suggested that in depressed individuals this bias was mediated by altered activity in the ventral anterior cingulate cortex (ACC) and other ventromedial prefrontal and subcortical structures (Elliott et al. 2002). These medial prefrontal cortical areas form part of a “visceromotor” network, which participates in modulating the behavioral and visceral responses to emotional stimuli (Ongur and Price 2000); notably, the same areas have consistently been implicated in the pathophysiology of depression (Drevets 2000).
Emerging evidence suggests that even in individuals who do not experience mood change following perturbation of the 5-HT system, changes to the neural processing of emotional information still occur, particularly in the visceromotor network and connected subcortical structures. In one recent study healthy volunteers administered the SSRI citalopram (to acutely elevate intrasynaptic 5-HT concentrations) showed reduced hemodynamic responses in the amygdala to threat-related facial expressions (Harmer et al. 2006). Complementing this finding, other studies demonstrated that decreasing serotonergic function via ATD increased hemodynamic responses in the amygdala to threat-related facial expressions in vulnerable individuals (Cools et al. 2005b; van der Veen et al. 2007).
However, no published study to date has investigated the effect of ATD on emotionally valenced verbal stimuli. Understanding how dysfunctional 5-HT transmission might alter the neural processing of emotional stimuli is an important step in integrating neurochemical and neuroanatomical explanations of MDD. Furthermore, since healthy volunteers show a bias towards positive words in attentional tasks (Erickson et al. 2005), such an understanding may shed light on the neural mechanisms underlying resilience to negative stimuli in never-depressed healthy individuals.
Therefore, in the present study we aimed to characterize the effect of a temporary reduction in 5-HT availability on neural and behavioral responses to emotionally valenced verbal stimuli, using the AGNG, in healthy volunteers. We predicted that ATD would lead to a negative emotional bias on the AGNG, and hypothesised that this negative emotional bias would be mediated by altered activity in the orbital and medial PFC regions implicated in processing the emotional salience of sensory stimuli and in organizing behavioral and visceromotor responses to such stimuli, together with anatomically-related areas of the ventral striatum, thalamus, cingulate gyrus, and temporal lobe (amygdala, hippocampus, parahippocampal cortex, and superior and middle temporal gyri) (Kondo et al. 2005; Ongur et al. 2003; Ongur and Price 2000).
MATERIALS AND METHODS
Participants
Right-handed healthy volunteers between 18 and 50 years of age were recruited by newspaper advertisement in the Washington, D.C. metropolitan area. Volunteers were screened for medical and psychiatric disorders by medical history, physical examination, laboratory testing (including drug screening), neuromorphological magnetic resonance imaging (MRI) scanning, electrocardiogram, the Structured Clinical Interview for DSM-IV (SCID; Spitzer et al. 2002), and a semi-structured interview with a psychiatrist. The Family Interview for Genetic Studies (Maxwell 1992) was used to screen for family history of psychiatric disorders. Volunteers were excluded from participation if they had: 1) current or past psychiatric disorders (DSM-IV criteria); 2) first-degree relatives with mood or anxiety disorders; 3) major medical or neurological disorders; 4) exposure to psychotropic drugs or other medications likely to affect cerebral physiology, vascular function or anatomy within 3 weeks, or illicit drugs within one year; 5) alcohol abuse within 1 year or lifetime history of alcohol or drug dependence (DSM-IV criteria); 6) tobacco use within 3 months; 7) current pregnancy or breast feeding; 8) general MRI exclusions. For female participants, the menstrual phase was determined by home urine ovulation kits to detect the mid-cycle lutenizing hormone surge (Clear Plan Easy; Whitehall Laboratories; Madison, NJ, USA), and testing in the week prior to menstruation or during the first 4 days of menses was avoided. All participants provided informed consent following a full explanation of the procedures and purpose of the study, as approved by the National Institute of Mental Health (NIMH) Institutional Review Board.
Experimental procedure
Participants attended two amino acid challenge sessions, separated by at least 1 week, in a double-blind, placebo-controlled, crossover design. Participants fasted for at least 8 hours prior to the challenge. On the tryptophan depletion day (TRP-) participants were administered 70 white capsules containing L-isoleucine (4.2 g), L-leucine (6.6 g), L-lysine (4.8 g), L-methionine (1.5 g), L-phenylalanine (6.6 g), L-threonine (3.0 g), and L-valine (4.8 g), and 4 pink capsules containing lactose (1.2 g) (total amino acid load: 31.5g). On the sham depletion day (TRP+) the amino acid mixture in the 70 white capsules was the same, but the pink capsules contained L-tryptophan (1.2 g) (total amino acid load 32.7g, 3.67% L-tryptophan). Both researchers and participants were blind as to the identity of the pink capsules, which were prepared by a pharmacist who was not involved in any other experimental procedure.
A 15 ml blood sample was obtained via an indwelling intravenous cannula immediately preceding amino acid ingestion to determine baseline (T0) plasma tryptophan and other large neutral amino acid (LNAA) concentrations. Baseline mood ratings (Hamilton Depression Rating Scale (HAM-D: Hamilton 1960), Hamilton Anxiety Rating Scale (HAM-A: Hamilton 1959), State-Trait Anxiety Inventory (STAI: Spielberger et al. 1970), Profile of Mood States (POMS: McNair et al. 1971) and Automatic Thoughts Questionnaire (ATQ: Hollon and Kendall 1980)), and vital signs (pulse and blood pressure) were also obtained.
For the next 5 hours participants rested on the ward, and either watched television, read or slept. Participants continued to fast, but were allowed to drink water. Between 4.5 and 5 hours following amino acid ingestion (T5), a second blood sample was obtained and vital signs and mood measures were repeated. Participants then underwent an MRI scan lasting 60–90 minutes, including a high-resolution T1-weighted structural sequence and three echo-planar imaging (EPI) sequences, during which participants performed the AGNG. Details of all scanning procedures and the cognitive activation paradigm are provided below.
Following the MRI scan, participants returned to the ward for a final (T7) blood sample and mood assessment. Participants were then provided with a protein-rich meal of their choice, and returned home. Participants were contacted the day after each challenge to inquire whether they had experienced any persistent mood change.
Cognitive activation paradigm (Affective Go/No-go test)
Participants performed the AGNG during functional MRI (fMRI) on each study day. The AGNG task used the same conditions, stimuli and stimulus timings as described for the task of Elliott et al (2000; 2002), but was modified slightly from this original task by splitting the task into 3 separate runs. Briefly, in each block words of two different emotional valences (either positive, negative or neutral) were presented quickly in the middle of the screen, one after the other, in a random sequence. Ten words of each valence were presented, each remaining on the screen for 300 ms, with a 900 ms gap between each word. Immediately before each block, participants read instructions on-screen for 6 seconds, which informed them that should respond to each word of a particular valence (the target valence) and not respond to each word of the other valence (the distractor valence). Participants responded using a button-box in the scanner, and each button press was recorded. A 1200 ms gap was inserted immediately after the offset of the instructions, prior to the onset of the first word in each block to ensure that participants were able to read the first word. Each block therefore lasted 25.2 seconds, with a rest period (18 seconds fixation cross followed by 6 seconds of instructions) preceding each block.
Words of each emotional valence could either be the targets or the distractors, creating six conditions: (1) happy targets, sad distractors; (2) happy targets, neutral distractors; (3) sad targets, happy distractors; (4) sad targets, neutral distractors; (5) neutral targets, happy distractors; (6) neutral targets, sad distractors. Two control conditions were also included, which contained only neutral words, where the target and distractor words were presented in different fonts (italic or normal): (7) neutral words in normal font targets, neutral words in italic font distractors; (8) neutral words in italic font targets, neutral words in normal font distractors. Participants performed 3 runs of the test, each lasting just under 7 minutes, in which all 8 conditions were presented once, in a pseudo-random order. A different order was used for each participant on each challenge day. Between each run participants rested for approximately 3 miutes.
MRI scanning
Participants were scanned during task performance using a 3T scanner (GE Signa, Milwaukee, WI). A total of 208 functional blood oxygenation-level dependent (BOLD) MRI images were acquired using an EPI pulse sequence (38 contiguous slices, TE = 23 msec; TR = 2000 msec; flip angle = 90°; field of view = 22 cm; 64 × 64 matrix; voxel dimensions = 3.5 × 3.44 × 3.44 mm). The first four images from each run were discarded to account for T1 equilibration. A high-resolution anatomical scan (spoiled gradient recalled - SPGR) was also acquired for every participant.
Biochemical measures
Plasma was separated by centrifugation and stored at −20°C. Plasma total amino acid concentrations (tyrosine, valine, phenylalanine, isoleucine, leucine and tryptophan) were measured by means of HPLC with fluorescence end-point detection and pre-column sample derivatisation adapted from the methods of Furst et al. (1990). Norvaline was used as an internal standard. The limit of detection was 5nmol/ml using a 10µl sample volume, and inter- and intra-assay coefficients of variation were <15% and <10%, respectively.
Data analysis
Mood, behavioral and biochemical data were analyzed using SPSS 14 (SPSS Inc, Chicago, IL, USA). This study employed a within-subjects, placebo-controlled crossover design. Therefore, repeated-measures ANOVA was employed where test assumptions were met (i.e. if data were normally distributed and variances were homogenous). For all measures, treatment (TRP+/TRP−) was entered as a within-subjects factor. For biochemical, mood and vital signs measures, time (T0/T5/T7) was entered as an additional within-subjects factor. For data from the AGNG, valence (Happy/Sad/Neutral) was entered as an additional within-subjects factor. On blocks where it was clear that participants had failed to attend to the task (i.e. 30% hit rate or lower), or had confused the targets and distractors (i.e. 30% hit rate or lower and 70% false alarm rate or higher), behavioral and BOLD data for that block were excluded from analysis.
Treatment order (TRP+/TRP− or TRP−/TRP+) was fully counterbalanced across subjects, but was initially entered as a between-subjects factor. If the main effect of treatment order and interaction of treatment order with treatment were found to be non-significant, data were collapsed across treatment order for subsequent analyses. Post-hoc analyses were carried out by constructing appropriate ANOVAs for each comparison of interest. In cases where there was a departure from the assumption of homogeneity of covariance in the repeated-measures ANOVA, an epsilon (ε) factor was calculated and used to adjust degrees of freedom accordingly, using the Huynh-Felt procedure (Howell 2002). As practice effects can confound crossover designs, between-subjects comparisons were conducted for first session only data if an interaction of drink order by treatment was found. Where appropriate, data were transformed prior to analysis as appropriate to reduce skew and stabilise variances, though data presented are untransformed values for clarity.
Analysis of BOLD fMRI data was performed using the general linear model within SPM5 (Wellcome Trust Centre for Neuroimaging, London, England; http://www.fil.ion.ucl.ac.uk/spm). Whole brain fMRI volumes were realigned to the fifth volume, co-registered with each participant's own SPGR scan, normalized to fit the Montreal Neurological Institute (MNI) standard brain template and smoothed using an 8mm full-width half-maximum Gaussian kernel. Low-frequency artifacts were removed using a high-pass filter at 1/128 Hz and global confounds were removed using global normalization. Temporal autocorrelation intrinsic to the fMRI time-series was corrected by pre-whitening using an AR(1) process. Single-subject main effect contrast maps were created by contrasting different emotional conditions (e.g. happy-sad target words) and different treatment conditions (i.e. tryptophan depletion-sham depletion conditions). Single-subject interaction maps were created by contrasting the difference maps between emotional conditions across the two treatment conditions [e.g. (happy-sad target words following sham depletion)-(happy-sad target words following tryptophan depletion)]. To correct for motion artifacts, the realignment parameters were modeled as regressors of no interest. We excluded from the fMRI analysis any runs on which participants exhibited movement of more than one voxel (3mm) translation or 2.5 degrees rotation. We excluded from the fMRI analysis any participants for whom we were unable to include data from at least two runs on each treatment day. Visual inspection of the single-subject interaction maps confirmed an absence of obvious motion artifacts in all data analyzed at the group level.
At the second level (group analysis), regions showing significant main effects or interactions were identified through random effects analysis of the beta images from the single-subject contrast maps. We confined the Discussion to regional changes that either remained significant (at p<0.05) after applying family-wise error correction for multiple comparisons, or consisted of clusters of ≥20 voxels for which the voxel level t-values corresponded to p<0.001 (uncorrected) located in regions included in our a priori hypotheses. However, to reduce the likelihood of Type II error we additionally report in the tables maxima reaching the p<0.001 (uncorrected) threshold and a minimum cluster size of 20 contained in any region. Coordinates were transformed from the MNI spatial array to the stereotaxic array of Talairach and Tournoux (1988) (http://imaging.mrccbu.cam.ac.uk/imaging/MniTalairach). Anatomical localization was performed with reference to the atlases of Talairach and Tournoux (1988) and Mai et al. (2003), and subregions of the ventral PFC were identified as described in Ongur et al. (2003).
Post-hoc analyses of interactions with treatment were conducted in SPSS 14. The beta values at the peak voxel in each significant cluster (representing a significant 2-way valence×treatment interaction) were extracted for each of the relevant within-subjects simple effects (e.g. happy-sad target words following sham depletion, happy-sad target words following tryptophan depletion) for each participant. T-tests were conducted on the beta values to assess the nature of the interaction.
To analyse how regional BOLD response to positive and negative words co-varied with inter-individual variability in the effect of ATD on mood and behavior at the time of scanning, we calculated changes score on the mood rating scales (i.e. tryptophan depletion – sham depletion) at T5 (i.e. just prior to scanning), and included these as regressors when calculating the valence × treatment interaction maps for happy relative to sad targets and distractors. Similar analyses were performed for emotional bias scores (i.e. happy-sad stimuli) for latency and commission errors on the AGNG.
RESULTS
Twenty participants (7 male) completed both study days. The mean age was 30.5 (±7.3) years, and the mean IQ was 122.9 (±10.5). Analyses of biochemical, mood, cardiovascular and behavioral data included all 20 participants unless otherwise stated.
Plasma amino acids
Due to occlusion of the venous cannulae, plasma samples could not be obtained from one participant at T5 and T7 on both days and three participants at T7 on both days. The following analyses are based on data from 19 participants. Administration of the TRP-mixture resulted in a decrease of 67% in the concentration of plasma tryptophan from T0 to T5, relative to an increase of 85% following TRP+ (treatment × time interaction: F1,18=58.7, p<0.001), and a decrease of 87% in the ratio of tryptophan to the other LNAAs (TRP:ΣLNAA) from T0 to T5, relative to a decrease of 37% following TRP+ (treatment × time interaction: F1,18=17.1, p<0.001). Analysis of the data at T7 confirmed that tryptophan availability to the brain remained low until after the scan following TRP-(see Table 1).
Table 1.
Plasma amino acid, cardiovascular and mood data at baseline, immediately prior to scanning and immediately following scanning
| TRP+ | TRP− | |||||
|---|---|---|---|---|---|---|
| T0 | T5 | T7 | T0 | T5 | T7 | |
| Plasma tryptophan (uM/l)a | 49.2 (15.5) | 91.7 (44.2) | 59.5 (23.0) | 51.0 (14.0) | 16.7 (11.5) | 21.8 (15.2) |
| Plasma tryptophan:ΣLNAA ratioa | 0.11 (0.062) | 0.070 (0.054) | 0.061 (0.062) | 0.12 (0.055) | 0.015 (0.013) | 0.025 (0.025) |
| Pulse (bpm) | 73.0 (11.2) | 71.1 (14.8) | 77.2 (10.5) | 74.8 (12.8) | 67.6 (10.7) | 72.0 (10.7) |
| Systolic blood pressure (mmHg) | 113.9 (11.7) | 114.9 (10.3) | 119.2 (7.7) | 115.0 (11.7) | 114.9 (12.6) | 123.4 (14.6) |
| Diastolic blood pressure (mmHg) | 67.2 (6.7) | 69.9 (9.6) | 74.3 (9.0) | 67.7 (8.1) | 66.6 (8.5) | 71.5 (9.0) |
| Hamilton Anxiety | 0.55 (0.94) | 1.5 (2.3) | 1.1 (1.3) | 0.65 (1.1) | 1.3 (1.8) | 1.3 (1.4) |
| Hamilton Depression | 0.60 (1.4) | 1.5 (2.0) | 0.95 (1.0) | 0.30 (0.66) | 1.5 (1.9) | 1.1 (1.5) |
| ATQ | 2.1 (2.7) | 1.2 (1.9) | 0.40 (0.82) | 1.7 (2.3) | 0.55 (1.2) | 0.30 (0.66) |
| POMS tension | 2.2 (2.3) | 2.5 (2.0) | 1.6 (1.8) | 1.8 (1.6) | 1.5 (1.2) | 1.9 (1.3) |
| POMS depression | 0.75 (2.2) | 0.25 (0.72) | 0.15 (0.49) | 1.1 (2.6) | 0.35 (1.2) | 0.50 (1.3) |
| POMS anger | 0.55 (1.1) | 0.45 (1.0) | 0.45 (1.1) | 0.80 (1.9) | 0.10 (0.31) | 0.25 (0.79) |
| POMS vigor | 19.6 (6.0) | 17.8 (6.0) | 17.5 (6.6) | 19.6 (5.8) | 16.9 (5.3) | 17.1 (5.9) |
| POMS fatigue | 2.1 (2.7) | 3.3 (4.4) | 3.4 (3.8) | 1.3 (1.8) | 2.6 (3.1) | 2.7 (3.1) |
| POMS confusion | 1.9 (1.9) | 2.4 (1.4) | 2.1 (1.5) | 2.1 (2.2) | 2.4 (1.7) | 2.4 (1.8) |
| STAI | 7.7 (8.8) | 8.2 (6.7) | 7.3 (6.2) | 6.8 (6.2) | 7.7 (4.9) | 7.4 (6.1) |
Treatment×time interaction significant at p<0.05.
Mood-rating scales
The mean ATQ score decreased over the course of each study day, while the mean HAM-A and HAM-D scores increased from T0 to T5 on each study day, remaining elevated until T7. These increases were small but statistically significant (main effect of time: ATQ – F2,38=9.6, p=0.002, ε=0.72; HAM-A - F2,38=4.8, p=0.023, ε=0.77; HAM-D - F2,38=6.1, p=0.05). However, no effect of time was apparent on any subscale of the POMS or the STAI, and the treatment × time interaction was non-significant for all mood measures (see Table 1), suggesting that the subjective emotional state of the participants was not differentially affected by TRP− relative to TRP+. No participant experienced mood or anxiety changes that persisted beyond the day of testing.
Cardiovascular measures
Blood pressure and pulse data were available for 11 participants at T0 and T5, but only for 8 participants at T0, T5 and T7. Blood pressure and pulse remained stable from T0 to T5, and were unaffected by treatment (F<1 for both main effect of time and treatment × time interaction for all measures). A similar pattern of results was apparent at T7 (see Table 1).
Behavior on the Affective Go/No-go task
Behavioral data are presented in Table 2. Analysis of reaction time data revealed that participants responded significantly more quickly to emotional targets than to neutral targets (conditions (1+3) – conditions (5+6): F1,19=69.8, p<0.001). This effect was apparent for both the contrast of happy vs neutral targets (condition 1 – condition 6: F1,19=47.9, p<0.001) and sad vs neutral targets (condition 3 – condition 5: F1,19=45.3, p<0.001). However, participants responded with similar speed to happy and sad targets (condition 2 – condition 4: F<1). In none of the analyses of latency data was an interaction with treatment apparent (p>0.2 for all interactions).
Table 2.
Behavior on the Affective Go/No-go test
| Measure | Condition | TRP+ | TRP− |
|---|---|---|---|
| Reaction time (msec) | |||
| Happy targets, sad distractors | 601.8 (59.4) | 613.6 (58.7) | |
| Happy targets, neutral distractors | 611.0 (69.7) | 633.5 (62.7) | |
| Sad targets, happy distractors | 611.8 (75.0) | 610.7 (67.6) | |
| Sad targets, neutral distractors | 615.1 (63.4) | 632.8 (66.0) | |
| Neutral targets, happy distractors | 684.2 (67.0) | 695.1 (59.9) | |
| Neutral targets, sad distractors | 673.7 (90.3) | 685.1 (63.2) | |
| Commission errors per block | |||
| Happy targets, sad distractors | 0.47 (0.65) | 0.41 (0.53) | |
| Happy targets, neutral distractors | 0.60 (0.71) | 0.63 (0.88) | |
| Sad targets, happy distractors | 0.93 (1.3) | 0.49 (0.62) | |
| Sad targets, neutral distractors | 0.55 (0.64) | 0.71 (0.86) | |
| Neutral targets, happy distractorsa | 1.7 (1.2) | 1.5 (0.83) | |
| Neutral targets, sad distractorsa | 1.0 (0.88) | 1.4 (1.5) | |
| Omission errors per block | |||
| Happy targets, sad distractors | 0.83 (1.2) | 0.74 (0.96) | |
| Happy targets, neutral distractors | 1.1 (1.5) | 1.3 (1.2) | |
| Sad targets, happy distractors | 0.68 (1.3) | 0.68 (1.1) | |
| Sad targets, neutral distractors | 0.87 (0.97) | 0.96 (1.2) | |
| Neutral targets, happy distractors | 1.1 (1.3) | 1.1 (1.2) | |
| Neutral targets, sad distractors | 1.3 (1.7) | 1.2 (1.3) |
Following sham depletion, participants made significantly more commission errors to happy distractors than sad distractors. Following tryptophan depletion this affect was attenuated and non-significant
Analysis of commission error rates revealed no main effect of emotional vs neutral distractors on inappropriate responding (conditions (1+3) – conditions (2+4): F<1). Participants made significantly more inappropriate responses to happy distractors than to sad distractors (condition 5 – condition 6: F1,19=5.9, p=0.025). Planned comparisons revealed that this positive emotional bias was only apparent following TRP+ (t19=3.1, p=0.005), and was non-significant following TRP− (t19<1). However, the interaction between treatment and valence fell short of significance (F1,19=2.5, p=0.13).
The pattern of omission errors mirrored that of the reaction time data. Participants missed significantly more neutral targets than emotional targets (conditions (1+3) – conditions (5+6): F1,19=24.5, p<0.001), an effect that was apparent for both the contrast of happy vs neutral targets (condition 1 – condition 6: F1,19=11.3, p<0.001) and sad vs neutral targets (condition 3 – condition 5: F1,19=9.79, p<0.001). However, participants responded with similar accuracy to happy and sad targets (condition 2 – condition 4: F1,19=2.2, p=0.16). In none of analyses of omission error data was an interaction with treatment apparent (p>0.2 for all interactions).
fMRI data
Three participants’ data were excluded due to excessive head movement. Therefore the following analyses are based on data from 17 participants. Participants showed a trend towards greater translational head movement per run in the sham depletion than the tryptophan depletion condition (sham depletion: x=0.23±0.10 mm; y=0.52±0.20 mm; z=0.67±0.30 mm; tryptophan depletion: x=0.23±0.09 mm; y=0.48±0.14 mm; z=0.55±0.36 mm; F(1,16)=3.3, p=0.088). There was no difference between the treatment conditions for rotational head movement per run (sham depletion: roll=0.69±0.43 degrees; pitch=0.34±0.16 degrees; yaw=0.28±0.12 degrees; tryptophan depletion: roll=0.63±0.42 degrees; pitch=0.37±0.13 degrees; yaw=0.27±0.11 degrees; F<1).
Table 3 and Table 4 list regions showing task-related differences averaging across treatment condition (i.e. main effects), while Table 5 and Table 6 list regions where the task-related regional response was modulated by treatment (i.e. interactions).
Table 3.
Regions showing a main effect (i.e. independent of depletion condition) of emotional content on regional BOLD response to target stimuli
| Region | Laterality | Stereotaxic coordinates |
Cluster size | Z value | ||
|---|---|---|---|---|---|---|
| Emotional>neutral targets | X | Y | Z | |||
| Posterior cingulate C | M | 0 | −51 | 25 | 1483† | 5.00 *b |
| Ventromedial frontal polar C | M | −4 | 58 | −1 | 270† | 4.98*b |
| Mid-cingulate G | M | 2 | − 16 | 21 | 85 | 4.17a |
| Pregenual anterior cingulate C | M | −4 | 45 | 16 | 123 | 3.78b |
| Superior temporal G | R | 48 | −63 | 27 | 47 | 3.62b |
| Neutral>emotional targets | ||||||
| Dorsal anterior cingulate C | R | 6 | 12 | 44 | 521† | 5.41*a |
| Dorsolateral PFC | L | −42 | 3 | 26 | 1318† | 4.99*a |
| Lateral orbitofrontal C | R | 42 | 27 | −5 | 372† | 4.73a |
| Inferior temporal G | L | −44 | −59 | −14 | 131 | 4.54a |
| Dorsolateral prefrontal C | R | 50 | 36 | 20 | 1343† | 4.52a |
| Fusiform G | R | 38 | −51 | −19 | 56 | 4.30a 37 |
| Medial cerebellum | L | −8 | −79 | −28 | 116 | 4.06a |
| Superior parietal C | R | 40 | −41 | 41 | 106 | 4.06a |
| Occipital C | R | 44 | −69 | −12 | 79 | 3.86a |
| Superior parietal C | L | −22 | −66 | 44 | 206† | 3.84a |
| Dorsolateral prefrontal C | R | 38 | 3 | 53 | 21 | 3.78a |
| Parietal C | R | 28 | −58 | 47 | 99 | 3.67a |
| Occipital C | R | 28 | −70 | 29 | 41 | 3.60a |
| Happy>sad targets | ||||||
| Occipital C | R | 8 | −80 | −4 | 23 | 3.92b |
| Ventrolateral prefrontal C | R | 51 | 19 | 25 | 42 | 3.92a |
| Medial cerebellum | L | −12 | −79 | −28 | 20 | 3.82a |
| Ventrolateral prefrontal C | L | −44 | 35 | −2 | 75 | 3.61a |
| Medial cerebellum | R | 10 | −77 | −16 | 27 | 3.45a |
Coordinates correspond to the stereotaxic array ofTalairach and Tournoux (1988) and denote the distance in mm from the anterior commissure, with positive×= right of midline, positive y = anterior to the anterior commissure, and positive z = dorsal to a plane containing both the anterior and the posterior commissures.
Abbreviations: BA 10P – Brodmann Area 10 polar (see Ongur et al. 2003); C – cortex; G – gyrus; L – left; M – midline; R – right
peak voxel significant at p<0.05 (FWE corrected for multiple comparisons)
cluster significant at p<0.05 (corrected in terms of spatial extent)
parameter estimates positive relative to rest
parameter estimates negative relative to rest. Italic font denotes that the maximum was contained within an a priori specified region of interest.
Table 4.
Regions showing a main effect (i.e. independent of depletion condition) of emotional content on regional BOLD response to distractor stimuli
| Region | Laterality | Stereotaxic coordinates |
Cluster size | Z value | ||
|---|---|---|---|---|---|---|
| Emotional>neutral distractors | X | Y | Z | |||
| Cerebellum | L | −24 | −46 | −30 | 23 | 3.9a |
| Occipital C | L | −10 | −53 | −7 | 32 | 3.68b |
| Ventromedial frontal polar C | R | 6 | 49 | −7 | 40 | 3.38b |
| Neutral>emotional distractors | ||||||
| Ventrolateral prefrontal C | L | −44 | 33 | 8 | 976† | 5.38*a |
| Ventrolateral prefrontal C | R | 46 | 31 | 8 | 724† | 5.05*a |
| Superior parietal C | R | 28 | −50 | 41 | 105 | 4.74a |
| Anterior cingulate sulcal C | M | 4 | 29 | 35 | 612† | 4.67a |
| Superior parietal C | L | −24 | −68 | 40 | 244† | 4.4a |
| Superior parietal C | R | 28 | −68 | 29 | 61 | 4.01a |
| Occipital C | L | −40 | −72 | −3 | 60 | 3.93a |
| Parieto-occipital transition zone | L | −30 | −81 | 19 | 72 | 3.82b |
| Medial cerebellum | L | −12 | −77 | −25 | 31 | 3.78a |
| Occipital C | L | −38 | −79 | 4 | 32 | 3.69a |
| Medial cerebellum | R | 16 | −79 | −26 | 102 | 3.67a |
| Precentral G | L | −40 | 3 | 26 | 25 | 3.61a |
Abbreviations and interpretation of stereotaxic coordinates as in Table 3.
peak voxel significant at p<0.05 (FWE corrected for multiple comparisons)
cluster significant at p<0.05 (corrected in terms of spatial extent)
parameter estimates positive relative to rest
parameter estimates negative relative to rest. Italic font denotes that the maximum was contained within an a priori specified region of interest.
Table 5.
Regions where the effect of emotional content on BOLD response to target stimuli was modulated by tryptophan depletion (i.e. treatment × condition interactions)
| Region | Laterality | Stereotaxic coordinates |
Cluster size | Z value | ||
|---|---|---|---|---|---|---|
| Emotional-neutral targets TRP−>TRP+ | X | Y | Z | |||
| Parietal operculum | L | −48 | −34 | 24 | 88 | 4.88a |
| Ventral putamen | L | −32 | −13 | 3 | 223† | 4.09a |
| Parahippocampal gyrus | R | 32 | −39 | −11 | 78 | 4.02b |
| Anterior insula/putamen | R | 30 | 8 | 5 | 148 † | 3.66a |
| Medial cerebellum | L | −16 | −46 | −28 | 27 | 3.6a |
| Thalamus | R | 16 | −23 | −2 | 36 | 3.55a |
| Parietal operculum | R | 36 | −28 | 14 | 26 | 3.49b |
| Putamen | L | −24 | 10 | 5 | 69 | 3.46a |
| Hippocampal formation | L | −24 | −29 | −7 | 22 | 3.46a |
| Amygdala/ ventral globus pallidus | L | −20 | −8 | −3 | 28 | 3.4a |
| Emotional-neutral targets TRP+>TRP− | ||||||
| Dorsolateralprefrontal C | R | 40 | 35 | 31 | 23 | 3.86c |
| Happy-sad targets TRP+>TRP− | ||||||
| Superior temporal G | R | 57 | −27 | 3 | 95 | 4.22d |
Abbreviations: TRP − and TRP + refer to the tryptophan depletion and sham depletion conditions, respectively; other abbreviations and interpretation of stereotaxic coordinates as in Table 3.
cluster significant at p<0.05 (corrected in terms of spatial extent)
Interaction characterised by increased BOLD response to emotional targets following tryptophan depletion (see Figure 1b)
Interaction characterized by an attenuation of deactivation to emotional targets following tryptophan depletion
Interaction characterized by increased BOLD response to neutral targets following tryptophan depletion (see Figure 1d)
Interaction characterized by greater BOLD response to happy targets than sad targets following sham depletion, with the opposite pattern of response following tryptophan depletion. Italic font denotes that the maximum was contained within an a priori specified region of interest.
Table 6.
Regions where the effect of emotional content on BOLD response to distractor stimuli was modulated by tryptophan depletion (i.e. treatment × condition interactions)
| Region | Laterality | Stereotaxic coordinates |
Cluster size | Z value | ||
|---|---|---|---|---|---|---|
| Emotional-neutral distractors TRP−>TRP+ | X | Y | Z | |||
| Superior temporal G | R | 38 | −55 | 18 | 127 | 4.26a |
| Posterior cingulate C | L | −24 | −53 | 19 | 356† | 4.21a |
| Medial cerebellum | L | −14 | −70 | −32 | 70 | 4.12a |
| Posterior hippocampus | L | −26 | −31 | 0 | 134 | 4.07a |
| Dorsal caudate | L | −16 | −3 | 24 | 135 | 3.98a |
| Inferior temporal G | R | 36 | −9 | −21 | 51 | 3.59a |
| Frontal operculum | R | 34 | 1 | 17 | 77 | 3.57a |
| Precentral gyrus | R | 32 | −23 | 44 | 50 | 3.5b |
| Ventrolateral prefrontal C | R | 34 | 28 | 12 | 36 | 3.5a |
| Caudate | R | 14 | 9 | 16 | 26 | 3.47a |
| Post central G | L | −22 | −29 | 46 | 26 | 3.43a |
| Frontal operculum | R | 34 | −14 | 25 | 25 | 3.24a |
| Emotional-neutral distractors TRP+>TRP− | ||||||
| Anterior cingulate sulcal C | M | 2 | 25 | 36 | 170 † | 3.73c |
| Happy-sad distractors TRP+>TRP− | ||||||
| Posterior cingulate C | M | 4 | −32 | 16 | 27 | 3.39d |
Abbreviations and interpretation of stereotaxic coordinates as in Table 3.
cluster significant at p<0.05 (corrected in terms of spatial extent)
Interaction characterised by increased BOLD response to emotional distractors following tryptophan depletion (see Figure 2b)
Interaction characterized by an attenuation of deactivation to emotional distractors following tryptophan depletion
Interaction characterized by increased BOLD response to neutral distractors following tryptophan depletion (see Figure 2d)
Interaction characterized by greater BOLD response to happy targets than sad distractors following sham depletion, with the opposite pattern of response following tryptophan depletion. Italic font denotes that the maximum was contained within an a priori specified region of interest.
Emotional relative to neutral targets
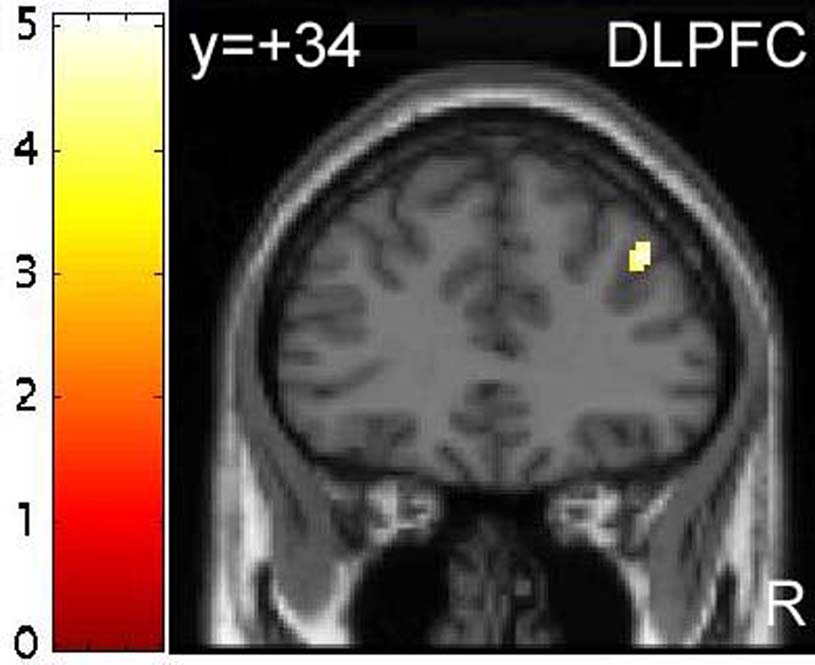
Regions showing increased BOLD response to emotional relative to neutral targets (conditions (1+3) – conditions (5+6)), included posterior cingulate and ventromedial PFC (BA 10P - Ongur et al. 2003), both of which reached significance at p<0.05 (corrected). Additionally, areas of the pregenual anterior cingulate (BA 32ac - Ongur et al. 2003), mid-cingulate cortex and superior temporal gyrus (STG) also showed increased BOLD response to emotional relative to neutral targets. Performing the reverse contrast revealed increased BOLD response to neutral relative to emotional targets in the left dorsolateral PFC (DLPFC) and right dorsal anterior cingulate cortex (dACC), both of which achieved significance at p<0.05 (corrected), as well as right DLPFC, right parietal cortex, right lateral orbitofrontal cortex (OFC) and left inferior temporal gyrus (see Table 3).
BOLD response to emotional relative to neutral targets was modulated by ATD in the left ventral putamen, left thalamus, left amygdala, right parahippocampal gyrus, bilateral parietal operculum and right anterior insula/putamen (see Table 5). Post-hoc analysis of the parameter estimates for these interactions revealed a greater BOLD response to emotional relative to neutral targets following TRP−, with either the opposite pattern of response or no difference between emotional and neutral targets following TRP+ (see Figures 1a & 1b).
Figure 1. Effect of tryptophan depletion on neural responses to emotional relative to neutral target words.
(A) Greater response to emotional relative to neutral target words following tryptophan depletion in the left putamen ([x=−24, y=10, z=5], peak Z score=3.46) and right insula/putamen ([x=30, y=8, z=5], peak Z score=3.66). (C) Greater response to neutral relative to emotional target words following tryptophan depletion in the right dorsolateral prefrontal cortex ([x=40,y=35,z=31, peak Z score=3.86). Effects in (A) and (C) were significant at p<0.001, minimum cluster size 20 voxels. Color bars indicate t-values and images are thresholded at p<0.001. (B and D) Plots of parameter estimates relative to rest for emotional and neutral target words under tryptophan and sham depletion conditions for peak voxels in the left putamen (B) and right dorsolateral prefrontal cortex (D). Error bars represent 1 SED between emotional and neutral targets.
BOLD response to emotional relative to neutral targets was also modulated by ATD in the right DLPFC, though the interaction was in the opposite direction (see Table 5). Post-hoc analysis of the parameter estimates for this interaction revealed a greater BOLD response to neutral relative to emotional targets following TRP−, with a difference in the same direction, but of lesser magnitude, following TRP+ (see Figures 1c & 1d).
Happy relative to sad targets
Increased BOLD response to happy relative to sad targets was apparent in the right DLPFC and left ventrolateral PFC (VLPFC) (see Table 3). BOLD response to happy relative to sad targets was modulated by ATD in the right STG (see Table 5). Post-hoc analysis of the parameter estimates revealed a greater BOLD response to happy relative to sad targets following TRP+, with the opposite pattern of response following TRP−.
Emotional relative to neutral distractors
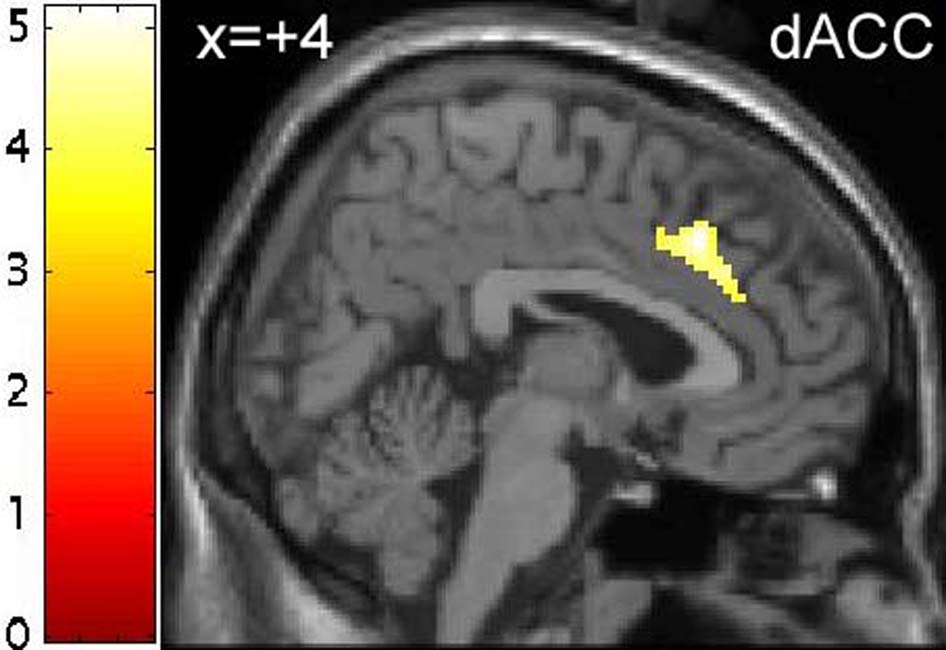
Within the a priori specified regions of interest, only the ventromedial PFC (BA 10P - Ongur et al. 2003) showed increased BOLD response to emotional relative to neutral distractors (conditions (1+3) – conditions (2+4)). However, performing the reverse contrast revealed a number of areas that showed increased BOLD response to neutral relative to emotional distractors, including bilateral VLPFC, which achieved significance at p<0.05 (corrected), as well as bilateral parietal cortex and right dACC (see Table 4).
BOLD response to emotional relative to neutral distractors was modulated by ATD in the right superior temporal gyrus, left posterior hippocampus, bilateral caudate, right inferior temporal gyrus, right VLPFC and left posterior cingulate (retrosplenial) cortex (see Table 6). Post-hoc analysis of the parameter estimates for these interactions revealed a greater BOLD response to emotional relative to neutral distractors following TRP−, with either the opposite pattern of response or no difference between emotional and neutral distractors following TRP+ (see Figures 2a & 2b).
Figure 2. Effect of tryptophan depletion on neural responses to emotional relative to neutral distractor words.
(A) Greater response to emotional relative to neutral distractor words following tryptophan depletion in the left hippocampus ([x=−26, y=−31, z=0], peak Z score=4.07). (C) Greater response to neutral relative to emotional target words following tryptophan depletion in dorsal anterior cingulate cortex ([x=2, y=25, z=36], peak Z score=3.73). Effects in (A) and (C) were significant at p<0.001, minimum cluster size 20 voxels. Color bars indicate t-values and images are thresholded at p<0.001. (B and D) Plots of parameter estimates relative to rest for emotional and neutral distractor words under tryptophan and sham depletion conditions for peak voxels in the left hippocampus (B) and dorsal anterior cingulate (D). Error bars represent 1 SED between emotional and neutral distractors.
BOLD response to emotional relative to neutral distractors was also modulated by ATD in the right dACC, though the interaction was in the opposite direction. The peak voxel of this interaction was contained within an area that showed a greater response to neutral relative to emotional distractors (see above and Table 4 & Table 6). Post-hoc analysis of the parameter estimates for the interaction qualified the main effect, revealing that the greater BOLD response to neutral relative to emotional distractors was only present following TRP−, with no differential response to emotional relative to neutral distractors following TRP+ (see Figures 2c & 2d).
Happy relative to sad distractors
No areas within the a priori defined ROIs showed a greater BOLD response to happy relative to sad distractors, or for the reverse contrast, at p<0.001 (uncorrected). The BOLD response to happy relative to sad distractors was modulated by ATD in the posterior cingulate cortex (see Table 6). Post-hoc analysis of the parameter estimates revealed a greater BOLD response to happy distractors compared to sad distractors following TRP+, with the opposite pattern of response following TRP−.
Correlation of mood and behavioral data with BOLD signal change following ATD
Change in T5 STAI score correlated significantly with change in BOLD response to happy relative to sad distractors following ATD in the right caudate [(−10,18,8), Z=4.48, cluster size 156; see Figures 3a & 3b]; participants who became more anxious following TRP− showed a greater response to negative relative to positive distractors following TRP−. A more spatially restricted relationship was also present in the left caudate. There were no regions in which change in anxiety score correlated significantly with change in BOLD response to happy relative to sad targets. There were also no areas in which change in BOLD response to happy relative sad stimuli following ATD correlated significantly with change in emotional bias in terms of either latency or commission errors.
Figure 3. Relationship between tryptophan depletion-elicited anxiety and tryptophan depletion-elicited bias towards negative distracting stimuli in the caudate.
(A) Across participants, tryptophan depletion-elicited anxiety covaried strongly with tryptophan depletion-elicited response to negative relative to positive distractors in the left caudate ([x=−10, y=18, z=8], peak Z score=4.48), with a similar, but more spatially restricted relationship, on the right. Participants who became more anxious following tryptophan depletion showed a greater caudate response to negative relative to positive distracting stimuli. The effect in the left caudate was significant at p<0.001, minimum cluster size 20 voxels. Color bars indicate t-values and the image is thresholded at p<0.005 for display purposes. (B) Correlation between tryptophan depletion-elicited change in parameter estimate at the peak voxel in the left caudate for the sad-happy distractors contrast and tryptophan depletion-elicited change in subjective anxiety rating (r=0.874, p<0.00001).
DISCUSSION
This is the first study to examine the effect of 5-HT depletion on neural and behavioral responses to emotional words. A highly significant attentional bias towards positive distractors evident under sham depletion was attenuated following tryptophan depletion, and this change was associated with increased neurophysiological responses to emotionally-valenced versus neutral words in the ventral striatum, hippocampal/parahippocampal cortex, anterior insula and VLPFC, and to negative versus positive words in the STG and posterior cingulate cortex. All of these regions have been implicated in the pathophysiology of major depression, a condition associated with both deficits in serotonergic function and loss of the normal attentional bias toward positive stimuli on the AGNG.
Most of the areas in which tryptophan depletion-increased neural responses to emotional relative to neutral stimuli receive moderate-to-high densities of serotonergic projections from the dorsal and/or median raphe nuclei (Jacobs and Azmitia 1992; Varnas et al. 2004). Multiple 5-HT receptor subtypes are present in each region, some of which when stimulated exert hyperpolarizing (i.e. inhibitory) effects on the neurons where they are expressed, and some of which exert depolarizing (i.e. excitatory) effects (Andrade 1998; Peroutka et al. 1990). Methods were not available that would allow non-invasive assessment of central 5-HT transmission or determination of the specific neurochemical mechanisms underlying the increased neurophysiological responsiveness to emotional stimuli observed during tryptophan depletion in these regions. However, it has been argued (Meeter et al. 2006) that the net effect of tonic 5-HT transmission is to hyperpolarize glutamatergic neurons (at least in the hippocampus), primarily via the 5-HT1A receptor, but also via 5-HT3 and 5-HT6 receptors. It is therefore conceivable that the cause of increased responsiveness of these subcortical structures to emotional stimuli following 5-HT depletion was a loss of inhibitory tone provided by these 5-HT receptor subtypes.
Alternatively, it is possible that tryptophan depletion increased neural responses to emotional words via a decrease in activity in areas that regulate limbic activity. Both the DLPFC and dACC showed relatively attenuated activity to emotional words following tryptophan depletion (see Figure 1d & Figure 2d and Table 5 & Table 6). Both of these regions are thought to be important in regulating activity in limbic structures such as the amygdala and hippocampus (reviewed in Drevets and Price 2005). For example, individuals with major depression show increased amygdala activity and decreased DLPFC activity, as well as reduced effective connectivity between these two structures, in particular during emotional processing (Siegle et al. 2002). However, since direct projections from the dorsal PFC to the amygdala are relatively sparse, this regulation is presumably mediated by other regions, possibly more ventral aspects of the prefrontal cortex, which receives abundant projections from the dorsolateral and dorsomedial PFC (Ongur et al. 2003; Ongur and Price 2000). The pattern of findings that we observed, specifically increased limbic responses and decreased dorsal PFC responses to emotional stimuli following tryptophan depletion, suggests that 5-HT may modulate the “top-down” regulation of limbic circuits.
The specific anatomical regions where physiological responses to emotional stimuli increased during tryptophan depletion have been implicated in experimentally induced sadness (Drevets and Raichle 1992; Harmer et al. 2003; Mayberg et al. 1999) and major depression (Drevets et al. 1992). PET studies have demonstrated that glucose metabolism is increased and/or that grey matter volume is decreased in these structures in unipolar depressed individuals (Drevets and Price 2005). The metabolic activity in these areas appears to normalize during symptom remission. For example, physiological activity in the amygdala and anterior insula decreased following effective treatment with SSRIs in depressed patients (Drevets et al. 2002; Drevets and Raichle 1992; Mayberg et al. 1999), and also appeared decreased in the anterior insula and VLPFC in spontaneously remitted MDD individuals and healthy controls compared with depressed MDD individuals (Drevets et al. 1992). The anterior insula shares extensive anatomical connectivity with the amygdala, hippocampal/parahippocampal cortices, ventromedial striatum, and thalamus. The limbic-cortical-striatal-pallidal-thalamic circuit formed by these regions also interacts with other orbitomedial PFC, temporal lobe (e.g., superior temporal gyrus, parahippocampal cortex) and cingulate regions (mid- and posterior cingulate cortices) to form the visceromotor network, which modulates the endocrine, autonomic, and experiential aspects of emotional behaviour (Kondo et al. 2005; Ongur et al. 2003; Ongur and Price 2000).
The two regions in which tryptophan depletion increased BOLD response to negative relative to positive words, the superior temporal gyrus and posterior cingulate cortex, have also consistently been implicated in unipolar depression (Drevets et al. 2002; Nugent et al. 2006). Both areas share extensive, monosynaptic anatomical connections with the orbitomedial PFC regions associated with the visceromotor network (Ongur et al. 2003), and have been implicated in verbal processing, particularly the processing of emotionally-valenced verbal stimuli (Cato et al. 2004; Kuchinke et al. 2005; Maddock et al. 2003).
The most striking behavioral effect in this study was the highly significant positive bias on the AGNG. Participants made significantly more inappropriate responses to positive stimuli than negative stimuli. Within the context of AGNG tasks, such an increase in this type of error is interpreted as indicating that the distractor stimulus is capturing attention, reducing the capability for selectively attending to the relevant stimulus-set. Biases towards positive stimuli in healthy volunteers have been reported previously, in particular by Erickson and colleagues, who used a very similar AGNG task (Erickson et al. 2005). They suggested that this bias on the AGNG might represent a normal suppression of attention to negative stimuli. In contrast, the opposite bias (toward negative stimuli) was observed in unipolar depressives in the same study. Thus the normal attentional bias toward positive relative to negative stimuli was hypothesized to confer resilience against depression. In the present study, this positive bias was attenuated and non-significant following tryptophan depletion, though the interaction of attentional bias with depletion condition fell short of statistical significance. These data therefore tentatively suggest that serotonergic function may play a role in mediating resilience to negative stimuli in never-depressed healthy individuals.
When we included change in subjective anxiety state as a covariate in the analysis of neural responses to positive relative to negative distractors, a striking relationship was observed in the right caudate (and possibly also the left caudate, although the spatial extent on the left was below our significance threshold of 20-voxels). Participants who became more anxious following tryptophan depletion also showed an increase in neurophysiological response in the caudate to negative relative to positive distractors. Notably, BOLD signal response to emotional words was increased following tryptophan depletion in the same region. Data from human and animal studies implicate the caudate in modulating anxiety responses to stress or threat (reviewed in Charney and Drevets 2002). For example, studies in experimental animals suggested that dopamine release and turnover increased in the mesoaccumbens projections during mild-to-moderate stress, and in the nigrostriatal projections during more severe stress (Deutch and Roth 1990; Inoue et al. 1994). In humans, one study suggested that during amphetamine challenge the magnitude of DA release in the ventral striatum correlated inversely with anxiety ratings in healthy humans (Drevets et al. 2001). Moreover, the magnitude of dopamine release in the striatum has been shown to be modulated by 5-HT2A receptor stimulation (Pehek et al. 2006; Porras et al. 2002; Yan 2000), suggesting a mechanism through which the altered serotonergic function associated with tryptophan depletion may exert secondary effects on other neurotransmitter systems and thereby influence anxiety symptoms. Finally, the caudate responds preferentially to salient stimuli, whether or not such stimuli are associated with reward (Knutson et al. 2003; Schultz et al. 2003; Zink et al. 2006; Zink et al. 2004). Therefore it is possible that this relationship may reflect the increased saliency of negatively-valenced distractors in those participants who became more anxious following depletion.
Our findings replicated the results of Elliott and colleagues (2000), who reported that responding to emotional relative to neutral words resulted in increased BOLD response in the pregenual ACC in healthy humans. In the present study, for the same contrast using the same task, we found increased BOLD response in a pregenual ACC region situated close to that identified by Elliott and colleagues (2000) (Table 3). However, the parameter estimates in this region were negative relative to rest for both emotional and neutral targets, suggesting that the effect Elliott and colleagues reported may be mediated by an attenuation of deactivation to emotional words. Elliott et al. (2002) reported that patients with unipolar depression showed an attenuated hemodynamic response in the pregenual ACC to emotional targets. Our results suggest the hypothesis that depressed individuals show equivalent deactivation to emotional and neutral targets in the pregenual ACC, which may reflect a difficulty in regulating subcortical activity in response to emotional stimuli. However, in the present study this effect in the pregenual ACC was of similar magnitude in the sham depletion and tryptophan conditions, suggesting that this finding in depression did not simply reflect a deficit in 5-HT transmission, and may instead have resulted from the depressed mood or the pathophysiology associated with MDD, since few of our participants reported mood change following tryptophan depletion.
The lack of effect of tryptophan depletion on subjective mood state in these never-depressed, healthy humans with no first-degree relatives with mood or anxiety disorders is consistent with previous research (Bell et al. 2005). Few studies that have not specifically selected volunteers with a personal or family history of mood disorders have reported emotional changes following tryptophan depletion. However, despite the lack of subjective effects on mood, tryptophan depletion attenuated a bias towards positive distractors, and resulted in a bias in the neurophysiological response towards emotional stimuli in limbic-cortical-striatal-pallidal-thalamic circuits previously implicated in normal and pathological emotional processing (Phillips et al. 2003a; 2003b). These effects of tryptophan depletion on behavioral and neural responses to other types of emotional stimuli in the absence of subjective mood effects are consistent with several other reports in the literature, which found tryptophan depletion-induced changes in the hemodynamic responses to emotionally expressive face stimuli or to spurious negative feedback provided during probabilistic reversal learning (Cools et al. 2005a; Harmer et al. 2003; Murphy et al. 2002; van der Veen et al. 2007).
Several limitations of our study merit comment. Firstly, although we used a relatively homogenous sample of healthy volunteers with no personal history of psychiatric disease and no first degree relatives with mood or anxiety disorders, we included fewer than 20 participants in the neuroimaging analyses. Therefore these data should be treated with caution until independently replicated. Secondly, it is possible that vascular effects may have confounded the neuroimaging results; 5-HT receptors are known to be involved in vasoconstriction and vasodilation (Saxena and Villalon 1990), although different receptors exert distinct effects on vascular tone. However, the interactions between depletion condition and emotional valence that we observed were not simply confined to areas that showed a main effect, which might be expected if 5-HT depletion had simply increased or decreased the amplitude of the haemodynamic response function independent of neural activity.
Thirdly, participants showed a trend towards greater translational head movement per run in the sham depletion than the tryptophan depletion condition. However, the average difference in translational movement per run between the treatment conditions was approximately 0.15mm, representing approximately one twentieth of a voxel. Furthermore, our statistical model included the movement parameters for each run at the subject level, which should have removed most of the variance in the contrast images that was associated with head movement. Therefore we do not believe that the interpretation of our fMRI data is likely to be confounded by differences in head movement between the treatment conditions.
Finally, participants in the present study made more omission errors when responding to neutral words than when responding to emotional words, suggesting that the neutral and emotional conditions were imprecisely matched for difficulty. This effect was unexpected, since the neutral and emotional words were carefully matched for imageability, length and frequency, and were identical to those used by Elliott and colleagues (2000), who did not report such an effect. However, this result may explain the increased hemodynamic response we observed to neutral relative to emotional stimuli in the dorsolateral PFC and dACC, effects also not reported by Elliott et al (2000), since these regions have been implicated in error-likelihood signaling (Brown and Braver 2005). To avoid this confound, future studies might benefit from employing event-related designs so that trials on which participants responded incorrectly could be excluded from analysis.
In summary, 5-HT depletion increased BOLD response to emotional stimuli generally in many structures implicated in unipolar depression, and increased responses specifically to negative stimuli in areas implicated in the processing of emotionally-valenced verbal information. Furthermore, inter-individual variability in the increase in subjective anxiety following tryptophan depletion was strongly correlated with the increase in hemodynamic activity in the caudate in response to negative distracting stimuli. These data suggest that 5-HT depletion subtly influences the neural processing of emotionally-valenced stimuli, in particular increasing responses to emotional stimuli in subcortical structures such as the amygdala, caudate and parahippocampal gyrus, and biasing activity towards negative stimuli in the posterior cingulate and temporal cortex. Notably, these changes occurred even in the absence of overt effects on participants’ mood state, although tryptophan depletion did attenuate a bias towards positive distracting stimuli. These data suggest a neural mechanism by which hypofunction of the 5-HT system, such as is hypothesized to exist in MDD, may result in a maladaptive bias towards negative stimuli, by increasing responsivity to emotional stimuli in subcortical limbic structures and biasing emotional information processing in the temporal cortex and posterior cingulate cortex towards negative stimuli, and may also provide insight into the neural modulation of resilience in never-depressed healthy volunteers.
ACKNOWLEDGEMENTS
This research was supported by the Intramural Research Program of the NIMH. JPR was supported by the NIH-Cambridge Health Science Scholars Program. We thank Judy Starling for preparation of the amino acid mixtures, Mike Franklin for analysis of the plasma amino acids, Harvey Iwamoto for programming the AGNG, Rebecca Elliott and Judy Rubinsztein for kindly providing the original word lists for the AGNG, Jeanette Black and Renee Hill for radiographer support, the nurses of ward 5 South-West for their dedicated clinical support, Karl Friston for guidance regarding fMRI analysis and Predrag Petrovic for helpful discussion of the manuscript. We thank Joan Williams and Paul Carlson for help with recruitment and clinical support. Finally, we would like to thank all the volunteers who participated in this study.
Footnotes
DISCLOSURE/CONFLICT OF INTEREST
The authors declare that over the past three years JPR and BJS have received compensation for consultancy work from Cambridge Cognition Ltd., who now own the behavioral version of the AGNG.
JPR has received compensation from Cambridge University.
GH has received compensation from Fundacion Lilly, Spain.
WCD has received compensation from Saint Vincent Catholic Medical Rights, the University of Maryland, Imedex, IntraMed Educational Group, CME Incorporated, Pfizer Inc./ Medcon, the Neuroscience Education Institute, the Society of Nuclear Medicine, the American Neuropsychiatric Association, Carroll Hospital, Wisconsin Medical School/ Current Medical Direction, Inc., the Foundation for Advanced Education in the Sciences, Carilion Health Systems, Roanoke VA, the Medical College of Ohio, Mt. Sinai School of Medicine, Washington University, St. Louis University, the Karolinska Institute, Laureate Psychiatric Clinic and Hospital (Tulsa), Assistance Publique, Hopitaux de Paris (sponsored by unrestricted educational grant from Servier), Photosound/ Mind Matters (sponsored by unrestricted educational grant from Sanofi-Aventis), the University of California at San Diego, the Neuroscience Right, Zurich, of the University of Zurich, Zurich Switzerland.
BJS has received compensation from Massachusetts General Hospital, the International Conference on Cognitive Dysfunction in Schizophrenia and Mood Disorders, the Medical Research Council Neurosciences & Mental Health Board, the Science Co-ordination Team for the Foresight Project on Mental Capital and Wellbeing (Office of Science and Innovation, Department of Trade and Industry), and the journal Psychological Medicine.
REFERENCES
- Andrade R. Regulation of membrane excitability in the central nervous system by serotonin receptor subtypes. Ann N Y Acad Sci. 1998;861:190–203. doi: 10.1111/j.1749-6632.1998.tb10191.x. [DOI] [PubMed] [Google Scholar]
- Bell CJ, Hood SD, Nutt DJ. Acute tryptophan depletion. Part II: clinical effects and implications. Aust N Z J Psychiatry. 2005;39:565–574. doi: 10.1080/j.1440-1614.2005.01628.x. [DOI] [PubMed] [Google Scholar]
- Booij L, Van der Does W, Benkelfat C, Bremner JD, Cowen PJ, Fava M, Gillin C, Leyton M, Moore P, Smith KA, Van der Kloot WA. Predictors of mood response to acute tryptophan depletion. A reanalysis. Neuropsychopharmacology. 2002;27:852–861. doi: 10.1016/S0893-133X(02)00361-5. [DOI] [PubMed] [Google Scholar]
- Brown JW, Braver TS. Learned predictions of error likelihood in the anterior cingulate cortex. Science. 2005;307:1118–1121. doi: 10.1126/science.1105783. [DOI] [PubMed] [Google Scholar]
- Cato MA, Crosson B, Gokcay D, Soltysik D, Wierenga C, Gopinath K, Himes N, Belanger H, Bauer RM, Fischler IS, Gonzalez-Rothi L, Briggs RW. Processing words with emotional connotation: an FMRI study of time course and laterality in rostral frontal and retrosplenial cortices. J Cogn Neurosci. 2004;16:167–177. doi: 10.1162/089892904322984481. [DOI] [PubMed] [Google Scholar]
- Charney DS, Drevets WC. The Neurobiological Basis of Anxiety Disorders. In: Davis KL, Charney DS, Coyle JT, Nemeroff CB, editors. Neuropsychophrmacology. Philedelphia: The Fifth Generation of Progress. Lippencott, Williams and Wilkins; 2002. pp. 910–930. [Google Scholar]
- Cools R, Blackwell A, Clark L, Menzies L, Cox S, Robbins TW. depletion disrupts the motivational guidance of goal-directed behavior as a function of trait impulsivity. Neuropsychopharmacology. 2005a;30:1362–1373. doi: 10.1038/sj.npp.1300704. [DOI] [PubMed] [Google Scholar]
- Cools R, Calder AJ, Lawrence AD, Clark L, Bullmore E, Robbins TW. Individual differences in threat sensitivity predict serotonergic modulation of dala response to fearful faces. Psychopharmacology (Berl) 2005b;180:670–679. doi: 10.1007/s00213-005-2215-5. [DOI] [PubMed] [Google Scholar]
- Deutch AY, Roth RH. The determinants of stress-induced activation of the prefrontal cortical dopamine system. Prog Brain Res. 1990;85:367–402. doi: 10.1016/s0079-6123(08)62691-6. discussion 402-3. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Neuroimaging studies of mood disorders. Biol Psychiatry. 2000;48:813–829. doi: 10.1016/s0006-3223(00)01020-9. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Bogers W, Raichle ME. Functional anatomical correlates of antidepressant drug treatment assessed using PET measures of regional glucose metabolism. Eur Neuropsychopharmacol. 2002;12:527–544. doi: 10.1016/s0924-977x(02)00102-5. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Gautier C, Price JC, Kupfer DJ, Kinahan PE, Grace AA, Price JL, Mathis CA. Amphetamine-induced dopamine release in human ventral striatum correlates with euphoria. Biol Psychiatry. 2001;49:81–96. doi: 10.1016/s0006-3223(00)01038-6. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JC. Neuroimaging and Neuropathological Studies of Mood Disorders. In: Licinio J, Wong ML, editors. Biology of Depression: From Novel Insights to Therapeutic Strategies. Weinheim, Germany: Wiley-VCH Verlag GmbH & Co.; 2005. pp. 427–466. [Google Scholar]
- Drevets WC, Raichle ME. Neuroanatomical circuits in depression: implications for treatment mechanisms. Psychopharmacol Bull. 1992;28:261–274. [PubMed] [Google Scholar]
- Drevets WC, Videen TO, Price JL, Preskorn SH, Carmichael ST, Raichle ME. A functional anatomical study of unipolar depression. J Neurosci. 1992;12:3628–3641. doi: 10.1523/JNEUROSCI.12-09-03628.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R, Rubinsztein JS, Sahakian BJ, Dolan RJ. Selective attention to emotional stimuli in a verbal go/no-go task: an fMRI study. Neuroreport. 2000;11:1739–1744. doi: 10.1097/00001756-200006050-00028. [DOI] [PubMed] [Google Scholar]
- Elliott R, Rubinsztein JS, Sahakian BJ, Dolan RJ. The neural basis of mood-congruent processing biases in depression. Arch Gen Psychiatry. 2002;59:597–604. doi: 10.1001/archpsyc.59.7.597. [DOI] [PubMed] [Google Scholar]
- Erickson K, Drevets WC, Clark L, Cannon DM, Bain EE, Zarate CA, Jr, Charney DS, Sahakian BJ. Mood-congruent bias in affective go/no-go performance of unmedicated patients with major depressive disorder. Am J Psychiatry. 2005;162:2171–2173. doi: 10.1176/appi.ajp.162.11.2171. [DOI] [PubMed] [Google Scholar]
- Furst P, Pollack L, Graser TA, Godel H, Stehle P. Appraisal of four pre-column derivatization methods for the high-performance liquid chromatographic determination of free amino acids in biological materials. J Chromatogr. 1990;499:557–569. doi: 10.1016/s0021-9673(00)97000-6. [DOI] [PubMed] [Google Scholar]
- Hamilton M. The assessment of anxiety states by rating. British Journal of Medical Psychology. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmer CJ, Mackay CE, Reid CB, Cowen PJ, Goodwin GM. Antidepressant drug treatment modifies the neural processing of nonconscious threat cues. Biol Psychiatry. 2006;59:816–820. doi: 10.1016/j.biopsych.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Rogers RD, Tunbridge E, Cowen PJ, Goodwin GM. Tryptophan depletion decreases the recognition of fear in female volunteers. Psychopharmacology (Berl) 2003;167:411–417. doi: 10.1007/s00213-003-1401-6. [DOI] [PubMed] [Google Scholar]
- Hollon SD, Kendall PC. Cognitive self-statements in depression: Development of an automatic thoughts questionnaire. Cognitive Therapy and Research. 1980;4:383–395. [Google Scholar]
- Howell DC. Statistical Methods for Psychology. 5th edn. London: Duxbury Press; 2002. [Google Scholar]
- Inoue T, Tsuchiya K, Koyama T. Regional changes in dopamine and serotonin activation with various intensity of physical and psychological stress in the rat brain. Pharmacol Biochem Behav. 1994;49:911–920. doi: 10.1016/0091-3057(94)90243-7. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Bennett SM, Adams CM, Hommer D. A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: characterization with rapid event-related fMRI. Neuroimage. 2003;18:263–272. doi: 10.1016/s1053-8119(02)00057-5. [DOI] [PubMed] [Google Scholar]
- Kondo H, Saleem KS, Price JL. Differential connections of the perirhinal and parahippocampal cortex with the orbital and medial prefrontal networks in macaque monkeys. J Comp Neurol. 2005;493:479–509. doi: 10.1002/cne.20796. [DOI] [PubMed] [Google Scholar]
- Kuchinke L, Jacobs AM, Grubich C, Vo ML, Conrad M, Herrmann M. Incidental effects of emotional valence in single word processing: an fMRI study. Neuroimage. 2005;28:1022–1032. doi: 10.1016/j.neuroimage.2005.06.050. [DOI] [PubMed] [Google Scholar]
- Maddock RJ, Garrett AS, Buonocore MH. Posterior cingulate cortex activation by emotional words: fMRI evidence from a valence decision task. Hum Brain Mapp. 2003;18:30–41. doi: 10.1002/hbm.10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai JK, Assheuer J, Paxinos G. Atlas of the human brain. 2nd edn. San Diego: Academic Press; 2003. [Google Scholar]
- Maxwell E. Family Interview for Genetic Studies (FIGS) A Manual for FIGS. Bethesda, Maryland: National Institute of Mental Health; Clinical Neurogenetics Branch, Intramural Research Program. 1992
- Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, Silva JA, Tekell JL, Martin CC, Lancaster JL, Fox PT. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156:675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppleman LF. Manual: Profile of mood states (POMS) San Diego: Educational & Industrial Testing Service; 1971. [Google Scholar]
- Meeter M, Talaminiq L, Schmitt JA, Riedel WJ. Effects of 5-HT on memory and the hippocampus: model and data. Neuropsychopharmacology. 2006;31:712–720. doi: 10.1038/sj.npp.1300869. [DOI] [PubMed] [Google Scholar]
- Murphy FC, Sahakian BJ, Rubinsztein JS, Michael A, Rogers RD, Robbins TW, Paykel ES. Emotional bias and inhibitory control processes in mania and depression. Psychol Med. 1999;29:1307–1321. doi: 10.1017/s0033291799001233. [DOI] [PubMed] [Google Scholar]
- Murphy FC, Smith KA, Cowen PJ, Robbins TW, Sahakian BJ. The effects tryptophan depletion on cognitive and affective processing in healthy volunteers. Psychopharmacology (Berl) 2002;163:42–53. doi: 10.1007/s00213-002-1128-9. [DOI] [PubMed] [Google Scholar]
- Neumeister A, Nugent AC, Waldeck T, Geraci M, Schwarz M, Bonne O, Bain EE, Luckenbaugh DA, Herscovitch P, Charney DS, Drevets WC. Neural and behavioral responses to tryptophan depletion in unmedicated patients with remitted major depressive disorder and controls. Arch Gen Psychiatry. 2004;61:765–773. doi: 10.1001/archpsyc.61.8.765. [DOI] [PubMed] [Google Scholar]
- Nugent AC, Milham MP, Bain EE, Mah L, Cannon DM, Marrett S, Zarate CA, Pine DS, Price JL, Drevets WC. Cortical abnormalities in bipolar disorder investigated with MRI and voxel-based morphometry. Neuroimage. 2006;30:485–497. doi: 10.1016/j.neuroimage.2005.09.029. [DOI] [PubMed] [Google Scholar]
- Ongur D, Ferry AT, Price JL. Architectonic subdivision of the human orbital and medial prefrontal cortex. J Comp Neurol. 2003;460:425–449. doi: 10.1002/cne.10609. [DOI] [PubMed] [Google Scholar]
- Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Pehek EA, Nocjar C, Roth BL, Byrd TA, Mabrouk OS. Evidence for the preferential involvement of 5-HT2A serotonin receptors in stress- and drug-induced dopamine release in the rat medial prefrontal cortex. Neuropsychopharmacology. 2006;31:265–277. doi: 10.1038/sj.npp.1300819. [DOI] [PubMed] [Google Scholar]
- Peroutka SJ, Schmidt AW, Sleight AJ, Harrington MA. Serotonin receptor "families" in the central nervous system: an overview. Ann N Y Acad Sci. 1990;600:104–112. doi: 10.1111/j.1749-6632.1990.tb16876.x. discussion 113. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biol Psychiatry. 2003a;54:504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biol Psychiatry. 2003b;54:515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- Porras G, Di Matteo V, Fracasso C, Lucas G, De Deurwaerdere P, Caccia S, Esposito E, Spampinato U. 5-HT2A and 5-HT2C/2B receptor subtypes modulate dopamine release induced in vivo by amphetamine and morphine in both the rat nucleus accumbens and striatum. Neuropsychopharmacology. 2002;26:311–324. doi: 10.1016/S0893-133X(01)00333-5. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Everitt BJ, Baldacchino A, Blackshaw AJ, Swainson R, Wynne K, Baker NB, Hunter J, Carthy T, Booker E, London M, Deakin JF, Sahakian BJ, Robbins TW. Dissociable deficits in the decision-making cognition of chronic amphetamine abusers, opiate abusers, patients with focal damage to prefrontal cortex, and tryptophan-depleted normal volunteers: evidence for monoaminergic mechanisms. Neuropsychopharmacology. 1999;20:322–339. doi: 10.1016/S0893-133X(98)00091-8. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Tunbridge EM, Bhagwagar Z, Drevets WC, Sahakian BJ, Carter CS. Tryptophan depletion alters the decision-making of healthy volunteers through altered processing of reward cues. Neuropsychopharmacology. 2003;28:153–162. doi: 10.1038/sj.npp.1300001. [DOI] [PubMed] [Google Scholar]
- Roiser JP, Blackwell AD, Cools R, Clark L, Rubinsztein DC, Robbins TW, Sahakian BJ. Serotonin transporter polymorphism mediates vulnerability to loss of incentive motivation following acute tryptophan depletion. Neuropsychopharmacology. 2006;31:2264–2272. doi: 10.1038/sj.npp.1301055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena PR, Villalon CM. Cardiovascular effects of serotonin agonists and antagonists. J Cardiovasc Pharmacol. 1990;15 Suppl 7:S17–S34. [PubMed] [Google Scholar]
- Schatzberg AF, Garlow SJ, Nemeroff CB. Molecular and cellular mechanisms in depression. In: Davis KL, Charney DS, Coyle JT, Nemeroff CB, editors. Neuropsychopharmacology. Philadelphia: The Fifth Generation Of Progress. Lippincott, Williams & Wilkins; 2002. pp. 1039–1050. [Google Scholar]
- Schultz W, Tremblay L, Hollerman JR. Changes in behavior-related neuronal activity in the striatum during learning. Trends Neurosci. 2003;26:321–328. doi: 10.1016/S0166-2236(03)00122-X. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, Thase ME, Stenger VA, Carter CS. Can't shake that feeling: event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biol Psychiatry. 2002;51:693–707. doi: 10.1016/s0006-3223(02)01314-8. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE. Manual for the state-trait anxiety inventory. Palo Alto, California: Consulting Psychologists Press; 1970. [Google Scholar]
- Spitzer MB, Gibbon M, Williams JBW. Stuctured Clinical Interview for DSM-IV-TR, Research Version, Patient Edition (SCID-I/P) New York: Biometrics Research, New York State Biometrics Institute; 2002. [Google Scholar]
- Talairach J, Tournoux P. Coplanar Stereotactic Atlas of the Human Brain. Stuttgart-New York: Thieme; 1988. [Google Scholar]
- van der Veen FM, Evers EA, Deutz NE, Schmitt JA. Effects of acute tryptophan depletion on mood and facial emotion perception related brain activation and performance in healthy women with and without a family history of depression. Neuropsychopharmacology. 2007;32:216–224. doi: 10.1038/sj.npp.1301212. [DOI] [PubMed] [Google Scholar]
- Varnas K, Halldin C, Hall H. Autoradiographic distribution of serotonin transporters and receptor subtypes in human brain. Hum Brain Mapp. 2004;22:246–260. doi: 10.1002/hbm.20035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan QS. Activation of 5-HT2A/2C receptors within the nucleus accumbens increases local dopaminergic transmission. Brain Res Bull. 2000;51:75–81. doi: 10.1016/s0361-9230(99)00208-7. [DOI] [PubMed] [Google Scholar]
- Young SN, Smith SE, Pihl RO, Ervin FR. Tryptophan depletion causes a rapid lowering of mood in normal males. Psychopharmacology (Berl) 1985;87:173–177. doi: 10.1007/BF00431803. [DOI] [PubMed] [Google Scholar]
- Zink CF, Pagnoni G, Chappelow J, Martin-Skurski M, Berns GS. Human striatal activation reflects degree of stimulus saliency. Neuroimage. 2006;29:977–983. doi: 10.1016/j.neuroimage.2005.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink CF, Pagnoni G, Martin-Skurski ME, Chappelow JC, Berns GS. Human striatal responses to monetary reward depend on saliency. Neuron. 2004;42:509–517. doi: 10.1016/s0896-6273(04)00183-7. [DOI] [PubMed] [Google Scholar]