Summary
Kinases and phosphatases regulate mRNA synthesis and processing by phosphorylating and dephosphorylating the C-terminal domain (CTD) of the largest subunit of RNA polymerase II. Fcp1 is an essential CTD phosphatase that preferentially hydrolyzes Ser2-PO4 of the tandem YSPTSPS CTD heptad array. Fcp1 crystal structures were captured at two stages of the reaction pathway: a Mg-BeF3 complex that mimics the aspartyl phosphate intermediate and a Mg-AlF4 − complex that mimics the transition state of the hydrolysis step. Fcp1 is a Y-shaped protein composed of an acylphosphatase domain located at the base of a deep canyon formed by flanking modules that are missing from the SCP (small CTD phosphatase) clade: an Fcp1-specific helical domain and a C-terminal BRCT (BRCA1 C-terminal) domain. The structure and mutational analysis reveals that Fcp1 and Scp1 (a Ser5-selective phosphatase) adopt different CTD binding modes; we surmise the CTD threads though the Fcp1 canyon to access the active site.
Introduction
The RNA polymerase II (Pol-II) CTD is composed of tandem heptapeptide repeats with the consensus sequence Y1S2P3T4S5P6S7. The CTD is essential for cell growth because it functions as a landing pad for myriad cellular proteins that regulate the initiation, elongation and termination steps of Pol-II transcription, modify chromatin structure, and catalyze or regulate RNA capping, splicing, and polyadenylation (Phatnani and Greenleaf, 2006). The CTD undergoes waves of serine phosphorylation and dephosphorylation during the transcription cycle. This process entails both global changes in phosphate content – whereby Pol-II is hypophosphorylated in the preinitiation complex and hyperphosphorylated in the elongation complex – and variations in the positional distribution of phosphoserine within the heptad repeat. Initiation and early elongation are marked by a CTD that is enriched in Ser5-PO4 (S5P). As elongation proceeds, S5P decreases while Ser2-PO4 (S2P) increases and persists until Pol-II reaches the 3’ end of the transcription unit. Changes in CTD structure help orchestrate the ordered recruitment of RNA processing factors at both ends of the transcription unit (Ho and Shuman, 1999; Cho et al., 2001a; Glover-Cutter et al., 2008).
The vast combinatorial complexity of the CTD phosphorylation array comprises a “CTD code” (Buratowski, 2003) that conveys information to CTD-binding proteins, some of which recognize particular phosphorylation patterns within phased heptad elements (Fabrega et al., 2003). The CTD structure is sculpted by the opposing actions of CTD-specific kinases and phosphatases that have varying positional specificity and act at different stages of the transcription cycle. At the end of the cycle, Pol-II is globally dephosphorylated to prepare the enzyme for the next round of transcription.
The principal CTD-specific serine phosphatase is Fcp1 (Chambers and Kane, 1996; Archambault et al., 1997). Fcp1 is conserved among eukarya and is essential in budding and fission yeast (Archambault et al., 1997; Kimura et al., 2002). A partial deficiency of human Fcp1 underlies an autosomal recessive developmental disorder (Varon et al., 2003). The conserved core of Fcp1 is composed of two essential modules: an FCP-homology (FCPH) domain near the N-terminus and a downstream BRCT (BRCA1 C-terminal) domain (Fig. 1A). Higher eukarya have an additional clade of small CTD phosphatases (SCPs) that consist of the FCPH domain, but lack the BRCT domain characteristic of the Fcp1 proteins (Yeo et al., 2003).
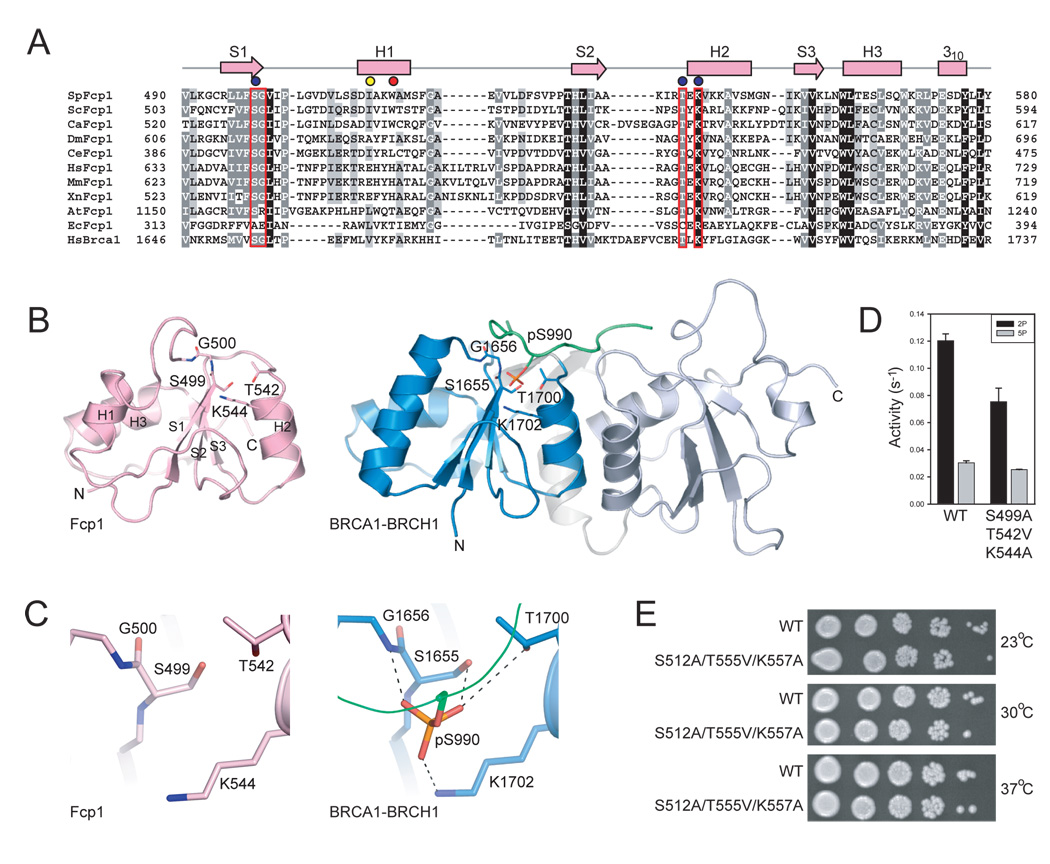
Figure 1. Fcp1 structure and domain organization.
A) S. pombe, S. cerevisiae, and H. sapiens Fcp1 family members aligned to H. sapiens Scp1. Amino acid positions numbered with domains color-coded: FCPH domains (blue), insertion domains (yellow), the linker helix between FCPH and BRCT domains (green), and BRCT domains (pink). Blue boxes indicate the TFIIF interaction helix position in ScFcp1 and HsFcp1. Question mark indicates that it is unknown whether SpFcp1 interacts with SpTFIIF. B) Orthogonal views of the Fcp1(140–580) structure as a ribbon with arrows for β-strands and wide-ribbons for helices. A transparent molecular surface envelops the Fcp1 structure. Domains colored as in (A). Helices in the yellow helical insertion domain are labeled (Fig. 2). N and C denote amino- and carboxy- termini. Asp170-BeF3 (stick representation) indicates the position of the Fcp1 active site. Arrows and distances denote the dimensions of the canyon.
Schizosaccharomyces pombe Fcp1 (SpFcp1; a 723-aa protein) is a well-characterized member of the Fcp1 family. It catalyzes metal-dependent hydrolysis of phosphoserine from the CTD in the context of native Pol-II, an isolated recombinant CTD polypeptide, or synthetic CTD phosphopeptides (Hausmann and Shuman, 2002; Suh et al., 2005). SpFcp1 forms a stable complex with phosphorylated recombinant CTD; it also binds to a non-CTD site on native Pol-II (Suh et al., 2005). SpFcp1 can dephosphorylate S2P and S5P CTD substrates although it displays a preference for S2P CTD substrates (Hausmann and Shuman, 2002). The minimal effective CTD substrate for SpFcp1 is a single heptad of phasing S5P6S7Y1S2PP3T4. The Tyr1 and Pro3 side chains that flank the S2P target are important determinants of Fcp1 activity (Hausmann et al., 2004).
Mutational analysis of SpFcp1 defined a constellation of essential amino acids within the FCPH domain that were posited to comprise the active site. Structure-activity relationships elaborated for SpFcp1 pointed strongly to its membership in the DxDxT superfamily of phosphotransferases (Hausmann and Shuman, 2002; 2003; Hausmann et al., 2004). DxDxT enzymes form a covalent enzyme-(aspartyl-Oδ-)-phosphate intermediate at the first aspartate of the DxDxT signature motif (Burroughs et al., 2006). The crystal structure of human Scp1 (a small CTD phosphatase) verified that the FCPH domain is an acylphosphatase family member and located the residues essential in Fcp1 at the Scp1 active site (Kamenski et al., 2004). A subsequent structure capturing Scp1 bound to a S5P CTD peptide illuminated the basis for the strong preference of Scp1 for hydrolysis of S5P versus S2P (Zhang et al., 2006).
Although deeply insightful, the Scp1 structures do not illuminate the salient properties of Fcp1. Whereas Fcp1 is a pan-eukaryal CTD-specific phosphatase that plays an essential general role in Pol-II transcription, Scp1 has a narrower phylogenetic distribution and plays a supporting role as a suppressor of neuronal gene expression in non-neuronal cells (Yeo et al., 2005). The biological function of Scp1 is not yet causally linked to dephosphorylation of Pol-II and there is some evidence that SCPs might function by dephosphorylating transcription components other than the CTD (Wrighton et al., 2006). SpFcp1, though it prefers S2P to S5P by about ~6-fold, has ample ability to dephosphorylate S5P CTD (Hausmann et al., 2004) and other Fcp1 orthologs have even less site preference (Lin et al., 2002). By contrast the catalytic efficiency of Scp1 is 70-fold higher at S5P than at S2P (Zhang et al., 2006). Most notably, Fcp1 requires both the FCPH and the BRCT domain for activity in vitro and in vivo (Hausmann and Shuman, 2002; Bang et al., 2006), whereas Scp1 is composed only of an FCPH domain.
To gain insight to the unique features of Fcp1, we crystallized a catalytically active version of SpFcp1 and determined its structure. We report a previously unobserved domain architecture for Fcp1 and visualize the enzyme at two points along the reaction coordinate: one mimicking the phosphoaspartate intermediate and another mimicking the transition state of the hydrolysis reaction. We exploit the structures to guide new mutational analyses and studies of Fcp1’s substrate specificity.
Results and Discussion
Fcp1 structure and overall domain organization
A segment of SpFcp1 from amino acids (aa) 140 to 580 suffices for full enzymatic activity in vitro (Hausmann and Shuman, 2003). SpFcp1(140–580) protein was purified and crystallized in the presence of BeCl2, NaF, MgCl2, and a non-phosphorylated 10-aa CTD peptide. SAD data collected from a SeMet-containing crystal was used to calculate phases based on six selenium positions. A model was built into this map and refined at 2.15 Å resolution (Rcrys/Rfree = 0.212/0.236; Table I).
Table 1.
Crystallographic Data Statistics
| Data Statistics | Native (BeF3) | Native (AlF4−) | SeMet (BeF3) |
|---|---|---|---|
| Source | APS 24ID-C | APS 24ID-C | APS 24ID-C |
| Wavelength (Å) | 0.979 | 0.979 | 0.979 |
| Resolution (Å) | 50 – 2.15 (2.23–2.15) | 50 – 2.10 (2.18–2.10) | 50 – 3.50 (3.63–3.50) |
| Space Group | P212121 | P212121 | P212121 |
| Unit Cell (Å) a, b, c | 53.7, 88.4, 113.2 | 55.2, 80.7, 89.3 | 53.8, 89.3,113.0 |
| # of observations | 461401 | 430799 | 201276 |
| # of reflections | 29320 | 23771 | 13260 |
| Completeness (%) | 97.4 (94.7) | 99.1 (98.1) | 99.6 (99.4) |
| Mean I/σI | 17.0(1.7) | 14.3 (5.0) | 5.1 (2.7) |
| Rmergea | 6.0 (34.0) | 6.9 (23.9) | 16.9 (35.2) |
| Cut-off criteria I/σI | 0 | 0 | −0.5 |
| FOM (SHARP) | 0.25 | ||
| FOM (Solomon) | 0.81 | ||
| Refinement Statistics | |||
| Resolution Limits (Å) | 30-2.15 (2.28–2.15) | 30-2.10 (2.23–2.10) | |
| # of reflections | 28751 | 23733 | |
| (working/test) | (27301/1450) | (22544/1189) | |
| Rworkb/Rfree | 21.1 (31.0)/23.4 (36.3) | 22.0 (24.4)/25.2 (28.8) | |
| # atoms protein/water | 2979/163 | 2902/175 | |
| B-factors protein/water | 50.0/54.4 | 33.8/38.4 | |
| Bond r.m.s deviations length (Å)/angles (°) | 0.006/1.22 | 0.006/1.3 | |
| Ramchandran plotc | |||
| Most favored | 290 (87.1%) | 282 (87.3%) | |
| Additionally allowed | 41 (12.3%) | 38 (11.8%) | |
| Generously allowed | 2 (0.6%) | 3 (0.9%) | |
| Disallowed region | 0 (0%) | 0 (0%) |
Rmerge = ∑hkl ∑i|I(hkl)i - <I(hkl)>|/ ∑hkl∑i <I(hkl)i>.
Rwork = ∑hkl |Fo(hkl)-Fc(hkl)|/ ∑hkl |Fo(hkl)|, where Fo and Fc are observed and calculated structure factors, respectively.
Calculated using the program PROCHECK
Numbers in parentheses indicate statistics for the high-resolution data bin for x-ray and refinement data.
SpFcp1 is a Y-shaped protein consisting of three discrete structural domains (Fig. 1). At the base of the Y is the FCPH domain (colored light blue in Fig. 1), which adopts a mixed α/β-fold formed by polypeptide segments 164–181, 203–317, and 441–478. An N-terminal α-helix (aa 140–163; colored gray) is located at the bottom tip of the stem of the Y. An α-helical domain (colored yellow) comprises the left arm of the Y. The four α-helices of this domain are derived from two peptide segments (aa 182–202 and 318–440) inserted between elements of the FCPH domain. The right arm of the Y is the BRCT domain (aa 490–580, colored pink), which consists of a three-stranded β-sheet surrounded by three α-helices and a 310-helix (Fig. 1 and Fig 4). The FCPH and BRCT domains are connected by a linker α-helix (colored green in Fig. 1). The active site (defined by the Asp-BeF3 adduct and Mg2+) is located within the FCPH domain at the base of a deep canyon, the sides of which are formed by the helical insertion and BRCT domains. The canyon is 20 Å wide near the top and narrows to 10 Å near the base. The canyon is 30 Å deep and stretches nearly 35 Å along the base (Fig. 1B).
Figure 4. Fcp1 BRCT domain and comparison to BRCA1.
A) Structure-based amino acid alignment of the SpFcp1 BRCT domain to the proximal BRCT domain of human BRCA1 protein expanded to include BRCT domains of nine other Fcp1 orthologs with SpFcp1 secondary structure elements above the sequence. Gaps indicated by (−). Side chain identity/similarity denoted by shading (black conserved in all; grey conserved in most). BRCA1 amino acids that coordinate the phosphoserine residue of BRCH1 (panel B) outlined in red. Results of mutational analysis indicated by color-coded circles as in Fig. 2. B) Ribbon diagram for SpFcp1 BRCT (left) and human BRCA1 tandem BRCT domains bound to a BRCH1 phosphopeptide (green; phospho-serine stick representation; PDB 1T29). N and C indicate N- and C-terminal residues for BRCT domains, respectively. C) Close-up views of the putative phosphopeptide binding site in SpFcp1 and the actual phosphoserine binding site in BRCA1. Side chain contacts to phosphoserine shown as dashed lines. D) Phosphatase activities of wild-type Fcp1 and Fcp1-S499A/T542V/K544A with triheptad S2P and S5P CTD substrates. Error bars (one standard deviation) calculated from three independent experiments performed in triplicate. E) Serial dilutions of S. cerevisiae fcp1Δ strains bearing indicated FCP1 alleles spotted on YPAD agar grown at 23°C (top), 30°C (middle), and 37°C (bottom).
No appreciable electron density was observed for Fcp1 aa 327–396 although SDS-PAGE analysis of a dissolved crystal confirmed it contained Fcp1(140–580) (not shown). To determine the relevance of the missing segment, an internal deletion mutant of Fcp1 (Δ330–393) was produced in which aa 330–393 were removed to fuse the two flanking segments (aa 149–329 and 394–580). Fcp1Δ330–393 protein retained full phosphatase activity in vitro for S2P and S5P CTD substrates (Fig. S1); thus, the structure is that of a catalytically active phosphatase. We proceeded to crystallize Fcp1Δ330–393 in the presence AlCl3, NaF and MgCl2 and determined this structure at 2.10 Å resolution (Rcrys/Rfree = 0.216/0.231; Table I).
The Fcp1 FCPH domain and its relationship to Scp1 and other aspartyl-phosphatases
The crystal structure confirms that Fcp1 is a member of the DxDxT aspartyl-phosphotransferase superfamily that includes the structurally and biochemically characterized enzymes Methanococcus phosphoserine phosphatase (PSP) (Cho et al., 2001b; Wang et al., 2002), Lactococcus β-phosphoglucomutase (Lahiri et al., 2003), Bacteroides hexose phosphate phosphatase (Lu et al., 2008), and bacteriophage T4 polynucleotide 5’-kinase/3’-phosphatase (Pnkp) (Zhu et al., 2007). The FCPH domain comprises a central five-stranded parallel β-sheet of topology β-6,5,1,7,8 surrounded by four α-helices (α4, α5, α8, and α9) and a 310-helix (Fig. 1B). A three-stranded antiparallel β-sheet of topology β-2,4,3 is inserted after β1. This sheet, which projects out from the globular core to form the base of the left arm of the Y-shaped Fcp1 protein, is a distinctive Fcp1 variant of the β-hairpin “flap” element observed in many DxDxT family proteins (Burroughs et al., 2006). The DxDxT active site motif is located in the loop connecting strands β1 and β2. The structures of the FCPH domains of Fcp1 and Scp1 are similar, as expected. A tertiary structure alignment of Fcp1 and Scp1 highlighted a 2.1 Å rmsd at 167 Cα positions. Scp1 shares with Fcp1 the three-stranded antiparallel β-sheet emanating from the FCPH core. Although the folds are conserved, only 24% amino acid identity exists in regions of homology between Fcp1 and Scp1 (Fig. 2A).
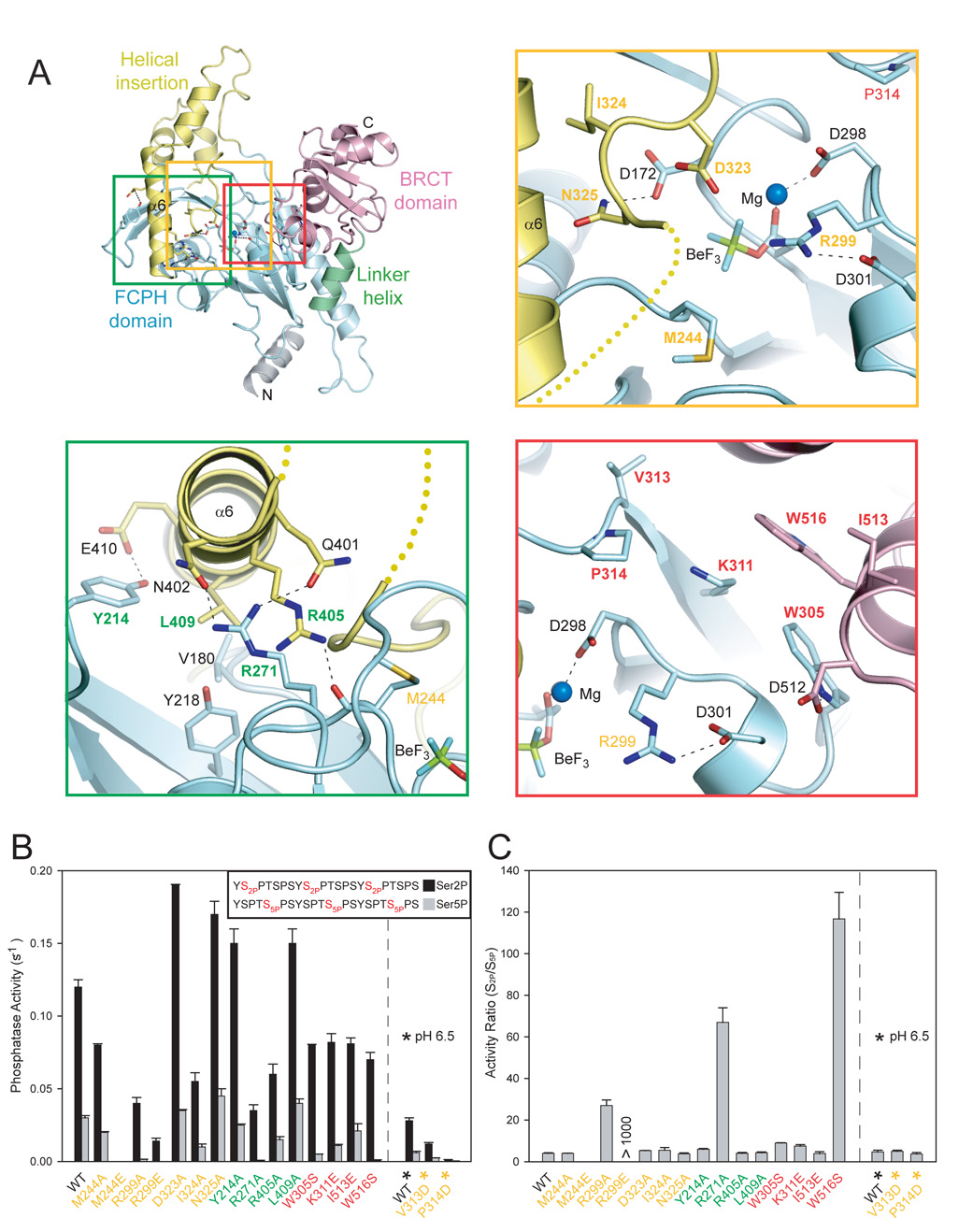
Figure 2. Primary structure and mutational effects on Fcp1 function in vitro.
A) Aligned amino acid sequences for Fcp1 FCPH domains from S. pombe, S. cerevisiae, C. albicans, D. melanogaster, C. elegans, H. sapiens, M. musculus, X. laevis, A. thaliana, and E. cuniculi and H.sapiens Scp1. SpFcp1 and Scp1 secondary structure elements are color-coded as in Fig. 1 above the sequence or shown in grey below the sequence, respectively (helices as bars; strands as arrows). Short alignment gaps indicated by (−). The disordered 72-amino acid loop (SpFcp1 aa 326-397) denoted as a break (//) in the alignment. Results of previous and current mutational analysis of SpFcp1 are indicated by circles above selected amino acids colored according to the relative activities of the respective mutants as specified by the color bar above panel B. B) Stereo diagram of the Fcp1 active site depicting amino acids subjected to mutational analysis. Side chains as sticks color-coded according to the phosphatase activity of the respective alanine mutant. Magnesium and water are depicted as blue and red spheres, respectively. Atomic contacts indicated by dashed lines. The polypeptide backbone is represented by a thin ribbon.
The key distinction between Fcp1 and other DxDxT enzyme structures is the nature of the so-called “flap” and “C1 cap” modules (Burroughs et al., 2006) inserted into the FCPH domain between strand β1 and helix α4. In Methanococcus PSP and many other DxDxT proteins, the C1 cap consists of a four-helix bundle (Wang et al., 2002; Lahiri et al., 2003; Burroughs et al., 2006). By contrast, Fcp1 has a three-stranded β-sheet insert that is embellished further by two α-helices (α2 and α3) placed between strands β2 and β3 (Fig. 2A). The Fcp1 FCPH domain is punctuated by a second modular insert, located distal to strand β8, that is analogous to the so-called “C2 cap” seen in a subset of DxDxT proteins (Burroughs et al., 2006). In Fcp1, this insert is large (aa 318–440) and it includes two α-helices (aa 397–428; α6 and α7) plus the disordered segment from residues 326–397 (Fig. 2A). Fcp1 helices α2, α3, α6 and α7 are not present in Scp1.
In contrast to Scp1, wherein the three-stranded β-sheet is surface-exposed, the Fcp1 β-sheet is buried under a discrete structural domain formed by the α-helices of the C1 and C2 cap modules (Fig. 1B). This marks a critical difference between Fcp1 and Scp1, because Scp1 utilizes its three-stranded β-sheet to bind the CTD and this surface is not available for CTD interaction in the Fcp1 structure. Note that all Fcp1 orthologs have analogs of the two “cap” modules inserted at equivalent position in their core FCPH domains, though the inserts vary in size and primary structure (Fig. 2A).
Active site architecture and structure of an analog of the acylphosphate intermediate
It is proposed that SpFcp1 remodels the CTD by a two-step reaction involving formation and hydrolysis of a phospho-enzyme intermediate (Fig. 3A). In the first step, the Asp170 Oδ acts as the nucleophile to attack the phosphorus atom of the CTD phosphoserine, resulting in formation of an acylphosphate intermediate and expulsion of the dephosphorylated CTD product. In the second step, a water nucleophile attacks the acylphosphate, resulting in formation of the inorganic phosphate product and expulsion of Asp170. Consistent with this scheme, replacement of Asp170 by alanine, asparagine or glutamate abolishes Fcp1 phosphohydrolase activity (Hausmann and Shuman, 2002; Suh et al., 2005).
Figure 3. CTD dephosphorylation and close-up views of the Fcp1 active site.
A) Two-step Fcp1 reaction pathway. Step 1 is the attack of the active-site aspartate on the phosphorus of phospho-CTD to form an acylphosphate intermediate. Step 2 entails a nucleophilic attack by water on the acylphosphate, resulting in formation of the inorganic phosphate product. B) Stereo view of the active site of Fcp1-Mg-BeF3 complex that mimics the acylphosphate intermediate. C) Stereo view of the Fcp1-Mg-AlF4 − complex that mimics the step 2 transition state. Side chains are depicted in stick representation; Mg and water are denoted by blue and red spheres, respectively. The proposed nucleophilic water is labeled Wat*. Atomic contacts indicated by dashed lines.
An analog of the covalent phospho-enzyme intermediate was captured by crystallizing Fcp1 in the presence of BeCl2, NaF and MgCl2 (Cho et al., 2001b). A covalent bond was observed between Fcp1 Asp170 Oδ1 and the beryllium atom of beryllofluoride (BeF3). The Asp–BeF3 adduct adopts the tetrahedral geometry and bond angles expected for the phosphoaspartate intermediate (Fig. 3B and Fig S2A). Electron density corresponding to the essential magnesium ion cofactor is seen at the center of an octahedral coordination complex (Fig. S2A). The six metal ligands are: the main-chain carbonyl of Asp172; Asp170 Oδ2; Asp298 Oδ1; a water coordinated by Asp297 Oδ1; another water coordinated by Asp298 Oδ2 and Asp323 Oδ1; and a fluorine atom of BeF3, which mimics a phosphate oxygen (Fig. 3A). The metal ion organizes constituents of the active site contributed by adjacent strands of the β sheet, in addition to participating directly in phosphoryl transfer chemistry by stabilizing the developing negative charge on the presumptive pentacoordinate transition state (see below). The position of the magnesium ion in Fcp1 and the nature of its octahedral ligands are similar to complexes observed in structures of Lactococcus β-phosphoglucomutase, human Scp1 and T4 Pnkp (Lahiri et al., 2003; Kamenski et al., 2004; Zhu et al., 2007). The importance of the SpFcp1 metal ligand complex is underscored by findings that replacement of Asp297 with alanine, glutamate or asparagine abolished SpFcp1 function, as did alanine and glutamate changes in lieu of Asp298; an asparagine at position 298 supported 5% of wild-type SpFcp1 activity (Hausmann and Shuman, 2003).
The Fcp1 structure reveals a network of ionic and polar contacts from the enzyme to the three fluorine atoms of BeF3 and the bridging oxygen to Asp170 (i.e., the phosphate oxygens). The phosphate ligands in the acylphosphate intermediate are: Thr243 Oγ; and Lys280 Nζ; and the main-chain amides of Leu171, Asp172 and Met244 (Fig. 3B). These protein atoms form an oxyanion hole to bind the CTD phosphoserine and to stabilize the transition state. Mutational studies have established that the Lys280 side chain is strictly essential; its replacement by alanine, arginine or glutamine abolishes SpFcp1 phosphohydrolase activity (Hausmann and Shuman, 2003). Whereas substitution of Thr243 with alanine or valine rendered SpFcp1 inactive, the conservative change to serine restored function to half the level of wild-type Fcp1 (Hausmann et al., 2004).
Crystals of the Fcp1-BeF3-Mg complex were grown in the presence of a nonphosphorylated CTD peptide in an effort to capture the structure of the step 1 product complex. However, there was no observable density for the CTD peptide in the vicinity of the active site, which likely reflects the ease of dissociation of the dephosphorylated CTD after the covalent intermediate is formed. Instead, we find a water in the active site located near the BeF3 adduct (Fig. 3B and Fig S2A). This water is 3.6 Å from the beryllium atom in an apical orientation to the bridging oxygen of Asp170 (water-Be-Oδ1 angle of 170°) (Fig. 3B). No such water was observed adjacent to the analogous BeF3 adduct in the crystal structure of human Scp1 (Kamenski et al., 2004).
In Fcp1, the distance and geometry of this water relative to the acylphosphate analog indicates that it corresponds to the water nucleophile for step 2 of the Fcp1 pathway (Fig. 3A). We surmise that the structure solved here exemplifies the Michaelis complex for the Fcp1 hydrolysis reaction. The water nucleophile is coordinated by the essential Asp172 side chain of the DxD172xT motif, which we invoke as a general base catalyst that activates the water for its attack on phosphoaspartate. Mutating Asp172 to alanine, asparagine, or glutamate abolished SpFcp1 phosphohydrolase activity (Hausmann and Shuman, 2002). Our structure is not consistent with the alternative mechanism proposed by Zhang et al. (2006), based on their structure of a phosho-CTD/Scp1 complex, that posits Scp1 reside Asp206 (equivalent to Asp297 in SpFcp1) as the general base. We are unable to credit the rationale for their proposal, which focuses on a putative water nucleophile that, in our view, is most unlikely to serve this function, given that: (i) there should be no water nucleophile in the Michaelis complex of enzyme bound to the phosphorylated CTD; rather, the bridging oxygen of the CTD phosphoserine is expected to occupy the same position that the water nucleophile occupies in step 2; (ii) the water pinpointed by Zhang et al. (2006) is orthogonal to the predicted acylphosphate intermediate, in a geometry that is not conducive to catalysis; (iii) Asp206 in Scp1 (and the Asp297 equivalent in Fcp1) coordinate a magnesium-bound water; and (iv) it is Asp98 in Scp1 (equivalent to Asp172 in Fcp1) that coordinates the bridging oxygen of phosphoserine – the leaving group in the step 1 reaction (Fig. S2C). We maintain that the present structure of Fcp1, plus the available structures of Scp1 and Methanococcus PSP (Wang et al., 2002), support the dual action of Asp172 as a general acid during step 1 acylphosphate formation (by donating a proton to the serine leaving group) and as a general base during the step 2 hydrolysis reaction.
Structure of a transition-state analog and evidence for an associative mechanism
An analog of the transition state of the hydrolysis step was trapped by co-crystallizing Fcp1Δ330–393 in the presence of AlCl3, NaF, and MgCl2. The planar aluminum fluoride (AlF4 −) sits at the center of a square pyramidal coordination complex with Asp170 Oδ at one apex and the attacking water at the other apical position (Fig. 3C). Although the Fcp1 transition state is postulated to have trigonal rather than square pyramidal geometry, we surmise that the AlF4 − complex resembles the proposed pentacoordinate phosphorane transition state of the hydrolysis reaction (Lahiri et al., 2003; Lu et al., 2008), insofar as it achieves a planar equatorial configuration around the aluminum (a mimetic of the phosphorus of phoshoaspartate) and the attacking water and the Asp170 Oδ leaving group subtend a 170° angle about the aluminum center. Because the aluminum atom is virtually equidistant from the leaving Asp170 Oδ (1.95 Å) and the attacking water (2.05 Å), and given that the distance from the water to the phosphorus-like center is shortened in the AlF4 − complex compared to the BeF3 adduct (3.6 Å) – while the contact of the water nucleophile with the Asp172 general base is maintained (2.6 Å) – we propose that Fcp1 catalyzes hydrolysis via an associative mechanism.
Although the octahedral magnesium complex is identical in the acylphosphate and transition state analog structures, one of the metal-bound waters makes a new contact to a planar fluorine (i.e. a nonbridging phosphate oxygen) in the transition state (Fig. 3C). Another noteworthy change in the AlF4 − transition state is the gained contact (3.0 Å) between a terminal guanidinium nitrogen of Arg299 and the same planar fluorines that interact with the metal-bound water (Fig. S2E). In the acylphosphate intermediate, the Arg299 side chain is relatively far (6.4 Å) from the nearest atom of BeF3 and held place by a bidentate salt bridge to Asp301 (Fig. S2D). This ion pair is severed during progression from the acylphosphate intermediate to the transition state, in which it is replaced by a longer water-mediated hydrogen bond, thus allowing Arg299 to approach the pentavalent phosphate.
Fcp1 structure accounts for extensive mutational data for the FCPH domain
Alanine scanning of SpFcp1 identified 11 amino acids as critical for phosphohydrolase activity in vitro: Asp170, Asp172, Thr174, Arg223, Tyr237, Thr243, Tyr249, Asp258, Lys280, Asp297, and Asp298. All are conserved among the Fcp1 orthologs aligned in Fig. 2A. Six are at the Fcp1 active site (Fig. 2B and Fig. 3), where they participate directly in covalent catalysis (Asp170), general acid-base catalysis (Asp172), coordination of the metal cofactor (Asp297, Asp298), or transition-state stabilization (Lys280, Thr243). Several functionally important side chains play key roles by virtue of their coordinating contacts to the aforementioned catalytic residues: (i) Thr174 donates a hydrogen bond to Asp170; (ii) Tyr249 donates a hydrogen bond to Asp172; (ii) Asp170 forms an ion pair with Lys280. By contrast, the Arg223, Tyr237, and Asp258 side chains are far away from the active site although each appears to stabilize the fold of the FCPH domain. Structure-activity relationships gleaned from the effects of conservative substitutions at essential residues are consonant with the atomic contacts of the native side chains visualized in the Fcp1 crystal structure.
Alanine scanning also identified 16 amino acids within the SpFcp1 FCPH domain that are nonessential for activity, although they are conserved in the Fcp1 clade (Hausmann and Shuman, 2003; Hausmann et al., 2004). Thirteen of the nonessential residues are located outside the active site: Lys163, His177, Arg164, Lys221, Glu238, His240, Lys247, Arg267, Ser270, Arg271, Asp272, Asp273, and Asp301 (Fig. 2B and Fig. 3) while three are proximal to the active site: Gln173, Arg299, and Asp233. Additional studies of the role for some of these residues in enforcing CTD specificity will be presented below.
The Fcp1 BRCT domain and its interface with the FCPH domain
The Fcp1 BRCT domain (aa 490–580) consists of a three-stranded (S1, S2 and S3) parallel β-sheet flanked on one side by two α-helices (H1 and H3) and a 310-helix and, on the other side, by a single α-helix (H2) (Fig. 4). The BRCT domain forms the right arm of the Y-shaped protein and packs closely against the FCPH domain, burying a total surface area of 1500 Å2 in the interface between domains (Fig. 1B). Asp512, Lys515 and Trp516 from BRCT α-helix (H1) and Ser567 and Trp569 from BRCT α-helix (H3) (Fig. S3) engage in a network of van der Waals interactions and water-mediated hydrogen bonds with amino acid side chain and main-chain atoms of two segments of the FCPH domain: (i) Asp304, Trp305, Lys311, and Val313 flanking the β8 strand; and (ii) Arg448 and Val452 in helix α8. There is a single direct hydrogen bond across the interface from Trp305 Nε to Asp512 Oδ (Fig. S3).
Mutational analysis of the BRCT domain
BRCT domains are found in a variety of proteins that bind phosphopeptides (Manke et al., 2003). The prototype BRCA1 includes two tandem BRCT domains that interact in head-to-tail fashion, a configuration observed in other DNA repair enzymes that form homo- or heterodimeric BRCT interactions (Dulic et al., 2001; Zhang et al., 1998). Phosphopeptide binding usually occurs across the dimer interface of tandem or dimeric BRCT domains, with insertion of the phosphorylated amino acids into a specific binding pocket of one of the BRCT modules (Shiozaki et al., 2004; Clapperton et al., 2004; Williams et al., 2004). Yu et al. (2003) reported that a recombinant protein consisting of GST fused to the BRCT domain of human Fcp1 (aa 587–785) was able to recover phosphorylated Pol-II from a mammalian cell extract in an affinity pull-down Western blot assay. They also found that the GST-hFcp1-BRCT protein bound to a biotinylated, doubly phosphorylated CTD Ser2,5P peptide in a similar assay. Yu et al. (2003) also reported that W710R mutation in GST-hFcp1-BRCT protein abolished its interaction with phospho-CTD. We note that the equivalent residue is the ScFcp1 BRCT domain is Trp561, which is located in the hydrophobic core of the BRCT domain (Fig S3). We suspect its replacement by arginine would disrupt the BRCT fold in Fcp1.
The phospho-CTD recognition property attributed to the isolated BRCT domain is remarkable, given that most BRCT-phosphopeptide interactions involve tandem BRCT domains. It is conceivable that forced dimerization of Fcp1 BRCT in the context of the GST fusion protein conferred binding properties on the BRCT domain that do not apply to the native Fcp1 protein, which, in the case of SpFcp1, is clearly a monomeric enzyme, both in solution for full-length SpFcp1, and in crystallo for Fcp1(140–580). The studies of Scp1 establish that the BRCT domain is not required for an acylphosphatase-type enzyme to act on a CTD substrate. However, because the BRCT domain is essential for Fcp1 activity, and the basis for its essentiality is not clear, we tested whether the well-defined phosphopeptide-binding pocket common to other BRCT domains (Shiozaki et al., 2004; Clapperton et al., 2004; Williams et al., 2004) contributes to SpFcp1 function.
The SpFcp1 BRCT domain and the proximal BRCT domain of human BRCA1 were superimposed with 1.9 Å rmsd over 79 Cα positions, with 25% side chain identity (Figs. 4A and B). In the complex of BRCA1, bound to its phosphorylated partner BRCH1, the phosphoserine residue is coordinated by BRCT residues Ser1655, Gly1656, Thr1700 and Lys1702 (Figs. 4B and C). The corresponding SpFcp1 residues Ser499, Gly500, Thr542 and Lys544 occupy similar spatial positions (Fig. 4C) and are conserved among Fcp1 family members (Fig. 4A). A triple-mutant SpFcp1 protein containing S499A, T542V, and K544A was produced and assayed for phosphatase activity with synthetic triheptad CTD substrates containing S2P or S5P in each heptad repeat. As expected, the specific activity of the wild-type SpFcp1 was higher with S2P than S5P (Fig. 4D). The triple-mutation in the BRCT phosphate-binding pocket had little impact on dephosphorylation at S5P and caused only a modest decrement (33%) in dephosphorylation at S2P (Fig. 4D).
To evaluate the effects on these mutations on Fcp1 function in vivo, missense changes were introduced, singly or in combination, at the equivalent Ser512, Thr555, and Lys557 positions of S. cerevisiae FCP1 and tested by plasmid shuffle for their ability to complement growth of a yeast fcp1Δ p(CEN URA3 FCP1) strain. Introduction of two mutant alleles, D180N or Y276A, could not complement growth of the fcp1Δ strain (Fig. S4A). S. cerevisiae Fcp1 D180N and Y276A mutations are equivalent to the D170N and Y249A mutations that inactivate SpFcp1. In contrast, none of the individual or compound mutations in the BRCT domain phosphopeptide-binding pocket affected growth on rich medium at 23°, 30° or 37°C (Fig. 4E and Fig. S4B). Whole-cell extracts of the mutant S. cerevisiae strains were separated by SDS-PAGE and analyzed by Western blot to determine steady state levels of Pol-II phosphorylation using rabbit polyclonal antibodies raised against CTD epitopes that contained either Ser2P and Ser5P (Bethyl Laboratories), however no significant differences were observed when these data were quantified (data not shown). These results suggest that the putative BRCT phosphopeptide-binding site is not essential for Fcp1 function in vivo or in vitro under the conditions tested.
Insights to differences in CTD recognition between Scp1 and Fcp1
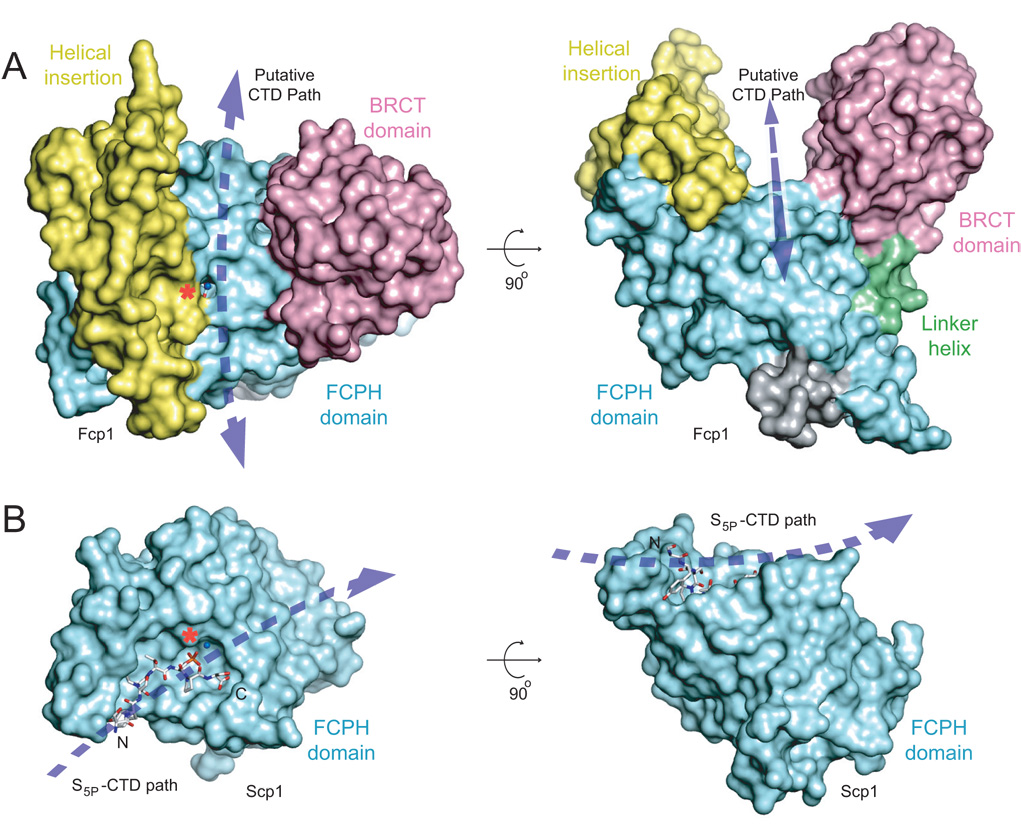
The basis for phospho-CTD recognition by Scp1 was elucidated by recent crystal structures in which CTD-Ser5P substrates were trapped in complexes with an Scp1-D96N mutant that is unable to form the acylphosphate intermediate (Zhang et al., 2006). The CTD binding site of Scp1 is an exposed shallow surface pocket (Fig. 5 and Fig S5) formed by amino acids that project out from the three-stranded β-sheet (Fig. S6A). Inspection of surface models of superimposed Scp1 and Fcp1 structures reveals that the path taken by the CTD on the Scp1 surface (Fig. 5B) is simply not available in Fcp1, because it is occluded on the left by the Fcp1-specific helical domain (in yellow in Fig. 5A and Fig. S5A) and on the right by the BRCT domain (in pink Fig. 5A). Instead, the Fcp1 surface model suggests that an extended CTD would engage Fcp1 by threading the canyon in an orientation orthogonal to that observed in the Scp1-CTD complex (Fig. 5B).
Figure 5. Insights to CTD recognition.
A) Surface representations for SpFcp1 with domains colored as in Fig. 1. A red asterisk indicates the active site Asp170-BeF3-Mg. Proposed CTD path through the Fcp1 canyon indicated by a dashed line. B) Surface representation for the Scp1-CTD complex (Zhang et al., 2006; PDB 2GHT) aligned to SpFcp1 in A. The S5P-CTD ligand is depicted as a stick model with its N and C termini labeled. The dashed arrow indicates the CTD path on the Scp1 surface.
The inference that Scp1 and Fcp1 have fundamentally different CTD binding modes is underscored by reference to the Fcp1 equivalents of the amino acids that comprise CTD-binding site of Scp1 (Fig. S6). The CTD Tyr1, Pro3 and Thr4 side chains rest against a hydrophobic platform formed by Scp1 residues Phe106, Val118, Ile120, Val127, Leu155, and Tyr158 (Fig. S6A). The CTD Pro6 makes van der Waals contact with Scp1 Tyr188. The CTD Ser2 and Pro3 carbonyl oxygen atoms receive a bifurcated hydrogen bond contact from Scp1 Arg178. The CTD Tyr1 OH receives a hydrogen bond from Scp1 Lys157 Nζ (Fig. S6A). These contacts provide an anchor to enforce specificity for S5P CTD substrates (Zhang et al., 2006). By contrast, Fcp1 residues Val180, Leu207, Glu209, Tyr218, Thr246, and Tyr249 – corresponding to Scp1 Phe106, Val118, Ile120, Val127, Leu155, and Tyr158 – are engulfed in the Fcp1 hydrophobic core, where many of them engage in cross-domain contacts with the helical insert module (Fig. S6B). Fcp1 Arg271 corresponds to Scp1 CTD-binding residue Arg178, but in our structure, the Arg271 side chain tethers one of the helices of the helical insert via hydrogen bonds from the terminal guanidinium nitrogens to Gln401 Oε and Asn402 Oδ (Fig. S6B). Fcp1 residue Ala248 is analogous to the Scp1 Lys157 that contacts Tyr1 of the CTD. The upshot of this comparison is that the protein side chain determinants of CTD-binding and substrate specificity in Scp1 are either not present in Fcp1, or are otherwise engaged by interactions with the Fcp1 helical insert domain.
Mutagenesis and gatekeepers for CTD specificity
We initiated a new round of Fcp1 mutagenesis based on the structure to delineate potential determinants of the enzyme’s substrate specificity by targeting an ensemble of amino acids that: (i) mediate contacts between the FCPH and helical insert domain (Arg271, Arg405, Tyr214, Leu409), (ii) line the deep end of the canyon near the active site (Met244, Arg299, Asp323, Ile324, Asn325), and (iii) line the midline and rear of the canyon floor (Trp305, Lys311, Val313, Pro314, Ile513, Trp516) (Fig. 2A, Fig. 4A, Fig. 6A). Among these are several exposed hydrophobic residues that might contribute to interactions with hydrophobic residues in the CTD substrate. SpFcp1(140–580) proteins containing single substitutions were purified (Fig. S1C) and assayed for activity in parallel with triheptad CTD substrates phosphorylated uniquely at Ser2 or Ser5 to identify biased specificity phenotypes. (Prior mutational studies of SpFcp1 phosphatase activity utilized a tetraheptad CTDS2P,S5P peptide where both Ser2 and Ser5 were phosphorylated or a 10-mer CTD peptide containing only S2P.) We also assessed Fcp1 function in vivo by introducing a subset of mutations at equivalent positions of S. cerevisiae Fcp1, and then tested mutant FCP1 alleles by plasmid shuffle for their ability to complement growth of the fcp1Δ strain (Fig. S4C).
Figure 6. Structure-guided mutational analysis of Fcp1.
A) Ribbon trace of the SpFcp1 fold with domains colored as in Fig. 1. Yellow, green and red boxes outline three regions selected for mutational analysis; side chains selected for mutation are indicated by colored-coded labels in yellow, green or red. B) Specific activities of wild-type and mutant SpFcp1 for release of phosphate from 50 µM triheptad S2P and S5P CTD substrates. Residue labels on the x-axis are color-coded by regions as in A. Asterisk denote specific activity at pH 6.5. Error bars (one standard deviation) calculated from three independent experiments performed in triplicate (Fig. S1). C) CTD substrate preferences are indicated by plotting the S2P/S5P activity ratios calculated using data in panel B.
Phosphatase reaction mixtures contained 50 µM CTD phosphopeptide and increasing amounts of wild-type or mutant SpFcp1. From the slope of the titration curve for wild-type Fcp1, we calculated turnover numbers of 0.03 s−1 for CTD-S5P and 0.12 s−1 for CTD-S2P (Fig. S1A), values similar to that determined for full-length SpFcp1 hydrolysis of 25 µM tetraheptad CTDS2P,S5P substrate (0.19 s−1) (Hausmann and Shuman, 2003). Turnover numbers and ratios of S2P/S5P CTD phosphatase activities of the wild-type and mutant enzymes are presented in a bar graph format in Fig. 6B and Fig. 6C, respectively.
The Y214A, D323A, N325A, L409A, and I513E proteins were as active as or slightly more active than wild-type SpFcp1 in hydrolyzing phosphoserine at positions 2 and 5. SpFcp1 mutants M244A, W305S, K311E, V313D, I324A, R405A and I324A displayed one to two thirds of the wild-type Ser2 phosphatase activity and evinced roughly similar decrements in Ser5 phosphatase activity, such that the S2P/S5P ratios did not change significantly (Fig. 6C). Two mutant S. cerevisiae Fcp1 alleles (W332A and I352A corresponding to SpFcp1 Trp305 and Ile324) had no effect on yeast growth at 23°, 30° and 37°C (Fig. S4C). Due to their hydrophobic character and surface exposed position within the canyon, we explored the effects M244E and P314D substitutions on Fcp1 activity. M244E rendered the enzyme inactive in vitro and the corresponding mutant allele in S. cerevisiae Fcp1 was unable to support growth in vivo (Fig. 6C). P314D exhibited 4–5% of wild-type activity on both S2P and S5P CTD substrates (Fig. 6C), although the corresponding mutant allele in S. cerevisiae Fcp1 supported yeast growth at 23°, 30° and 37°C (Fig. S4C).
More instructive effects were observed for the R271A, R299A, and W516S mutations, which strongly suppressed Ser5 phosphatase activity (to 5%, 2%, and 2% of the wild-type level, respectively) but had relatively modest 2- to 3-fold effects on Ser2 phosphatase activity (Fig. 6B). Consequently, these mutations drastically skewed the S2P/S5P ratio (Fig. 6C), such that R271A, R299A, and W516S had a 17-fold, 7-fold or 29-fold greater preference for S2P than did wild-type Fcp1. These results suggest that Arg271, Arg299, and Trp516 can act as gatekeepers of Fcp1 substrate specificity, i.e., they are permissive for S5P when present and restrictive when removed. Thus, it is perhaps remarkable that equivalent mutations in S. cerevisiae Fcp1 (R298A, R326A, and W529S) had no apparent effect on yeast growth at any temperature tested (Fig. S4C), a result consonant with earlier studies suggesting that yeast Fcp1 is primarily a Ser2-specific CTD phosphatase in vivo (Cho et al., 2001a).
The terminal nitrogens of Arg271 are occupied as hydrogen bond donors to Gln401 and Asn402 in the helical insert domain and are therefore unlikely to interact with the CTD. However, the proximal aliphatic linker of Arg271 is exposed on the enzyme surface in a pocket ~10 Å away from the BeF3 adduct and to the left of the putative CTD path (Fig. 5). It is conceivable that loss of the Arg271 side chain has a subtle effect on the position of the helical insert domain that in turn affects the CTD-binding surface to the detriment of CTD S5P. Trp516 is partially exposed within the interface between the BRCT and FCPH domains and could participate in contacts to the CTD, although it seems more likely that loss of the Trp516 side chain disfavors interaction with the S5P CTD by altering the position of the canyon wall by destabilizing the BRCT-FCPH domain interface.
The Arg299 side chain is a clearer candidate to interact directly with the CTD because its guanidinium group is exposed on the surface of the canyon floor immediately adjacent to the active site. Arg299 is conserved among most Fcp1 orthologs and is replaced by serine in human Scp1. As discussed above, Arg299 is mobile and contacts the AlF4 − transition state analog, but Arg299 is certainly not required for transition state stabilization during catalysis, as evinced by the high residual activity of the R299A mutant with CTD-S2P (Figs. 6C). To further explore the importance of this side chain, we analyzed the R299E mutation and found that it strongly suppressed Ser5 phosphatase activity (to undetectable levels, <1%), although it had a less severe effect on Ser2 phosphatase activity (down 8.5-fold) (Fig. 6B). The equivalent mutation in S. cerevisiae Fcp1 (R326E) exhibited a severe growth defect at all temperatures tested (Fig. S4C).
Conclusion
We have presented structures for Fcp1 trapped in two distinct states along the reaction pathway, one that mimics the aspartyl-phosphate intermediate and one that mimics the transition state for hydrolysis. These structures reveal the location of the nucleophilic water molecule and the aspartate general acid-base catalyst. In addition, the geometry of the transition state mimic suggests that Fcp1 catalyzes hydrolysis via an associative mechanism. Importantly, these structures now reconcile a large body of mutational data by elucidating the structural context for conserved Fcp1 amino acid residues involved in phosphoryl transfer chemistry and substrate binding.
The Fcp1 structures also reveal features unique to the Fcp1 clan of aspartyl-phosphatases, including its Y-shaped architecture, stand-alone BRCT domain, and residues that contribute to CTD specificity. Mutational analysis revealed that amino acids that line the canyon walls adjacent to the Fcp1 active site can act as molecular ‘gatekeepers’ to affect CTD specificity. The BRCT was suggested to bind CTD, but we show that its putative phosphopeptide binding site is not essential for Fcp1 function, either in vivo or in vitro. Rather, we believe that the BRCT domain is essential for catalytic activity in vitro because its domain buttresses residues in the FCPH domain adjacent to the active site that are important for catalytic activity.
Perhaps the most striking feature observed for Fcp1 is its Y-shaped architecture formed by the FCPH, helical insertion, and BRCT domains. This arrangement, combined with our mutational analysis and the observation that analogous surfaces used by Scp1 to bind CTD are occluded in the Fcp1 structure, suggests that an extended CTD must penetrate the Fcp1 canyon in an orthogonal orientation to that observed for Scp1.
Experimental Procedures
Crystallographic analysis
Fcp1-BeF3-Mg was obtained by incubating 200 µM Fcp1(140–580) –purified as described in the Supplementary Material – with 225 µM CTD peptide (SPSYSPTSPS), 300 µM BeCl2, 5 mM NaF and 5 mM MgCl2 on ice for 1 h then crystallized by sitting drop vapor diffusion against 22% PEG-4000, 100 mM Na-citrate (pH 5.6), 100 mM ammonium acetate, 5% hexane 1,6-diol, 5% aminocaproic acid, 5% propane-1,3-diol, 5 mM DTT. Fcp1-AlF4 −-Mg was obtained by incubating 250 µM Fcp1(149–580)-Δ330–393 with 500 µM AlCl3, 5 mM NaF and 5 mM MgCl2 on ice for 2 h prior to crystallization by hanging drop vapor diffusion against 20% PEG-3350, 190 mM ammonium formate (pH 7.0). Data statistics are provided in Table 1 and methods for data collection, reduction, phasing, and refinement are in Supplementary Material. Models generated with PyMol (DeLano, 2002).
Phosphatase assay
CTD substrates included two triheptad polypeptides in which either Ser5 or Ser2 positions were phosphorylated (Fig. S1A). Reaction mixtures (10 µl) containing 50 mM Tris-acetate (pH 5.5), 10 mM MgCl2, 50 µM CTD substrates and 0.05 to 0.8 µM wild-type or mutant SpFcp1 were incubated at 37°C for 45 min, quenched by adding 100 µl malachite green (BIOMOL), and incubated at 23°C for 30 min before measuring A620. SpFcp1 mutants V313D and P314D were compared to wild-type at their pH optimum (pH 6.5) because they precipitated in assays conducted at pH 5.5 (wild-type SpFcp1 pH optimum). Inorganic phosphate (Pi) was quantified by interpolating absorbance values to a standard curve derived from KH2PO4 diluted in reaction buffer.
Plasmid complementation
Mutational effects on Fcp1 function in vivo were tested by plasmid shuffle using a S. cerevisiae fcp1Δ strain that contained wild-type FCP1 on a CEN URA3 plasmid (Fig. S4). Wild-type and mutant FCP1 alleles inserted the pSE358 (CEN TRP1) plasmid were used to transform the fcp1Δ strain. FCP1 expression is under control of its endogenous promoter. Trp+ colonies were patched on agar medium containing 5-fluoroorotic acid (5-FOA) to select for loss of the FCP1 URA3 plasmid. Viable strains were grown in YPAD media (0.03% adenine) to an A600 of 0.5. Aliquots (3 µl) of serial 10-fold dilutions were spotted on YPAD plates and incubated for 3 days at 23°C or 2 days at 30°C or 37°C.
Supplementary Material
Acknowledgements
Use of the Advanced Photon Source (APS) is supported by the U.S. Dept. of Energy, Office of Science, Office of Basic Energy Sciences, under W-31-109-Eng-38. Use of NE-CAT beamline at Sector 24 is based upon research conducted at the Northeastern Collaborative Access Team beamlines of the APS, which is supported by award RR-15301 from the NCRR at the NIH. This work was supported by a Human Frontier Science Fellowship (A.G.) and by NIH grants GM52470 (S.S.) and GM61906 (C.D.L).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Structure factors and coordinates are deposited in the RCSB Protein Data Base with accession codes 3EF0 and 3EF1.
References
- Archambault J, Chambers RS, Kobor MS, Ho Y, Cartier M, Bolotin D, Andrews B, Kane CM, Greenblatt J. An essential component of a C-terminal domain phosphatase that interacts with transcription factor IIF in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA. 1997;94:14300–14305. doi: 10.1073/pnas.94.26.14300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang W, Kim S, Ueda A, Vikram Mm, Yun D, Bressan RA, Hasegawa PM, Bohk J, Koiwa H. Arabidopsis carboxyl-terminal domain phosphatase-like isoforms share common catalytic and interaction domains but have distinct in planta functions. Plant Physiol. 2006;142:586–594. doi: 10.1104/pp.106.084939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buratowski S. The CTD code. Nature Struct. Biol. 2003;10:679–680. doi: 10.1038/nsb0903-679. [DOI] [PubMed] [Google Scholar]
- Burroughs AM, Allen KN, Dunaway-Mariano D, Aravind L. Evolutionary genomics of the HAD superfamily: understanding the structural adaptations and catalytic diversity in a superfamily of phosphoesterases and allied enzymes. J. Mol. Biol. 2006;361:1003–1034. doi: 10.1016/j.jmb.2006.06.049. [DOI] [PubMed] [Google Scholar]
- Chambers RS, Kane CM. Purification and characterization of an RNA polymerase II phosphatase from yeast. J. Biol. Chem. 1996;271:24498–24504. doi: 10.1074/jbc.271.40.24498. [DOI] [PubMed] [Google Scholar]
- Clapperton JA, Manke IA, Lowery DM, Ho T, Haire LF, Yaffe MB, Smerdon SJ. Structure and mechanism of BTCA1 BRCT domain recognition of phosphorylated BACH1 with implications for cancer. Nat. Struct. Mol. Biol. 2004;11:512–518. doi: 10.1038/nsmb775. [DOI] [PubMed] [Google Scholar]
- Cho E, Kobor MS, Kim M, Greenblatt J, Buratowski S. Opposing effects of Ctk1 kinase and Fcp1 phosphatase at Ser2 of the RNA polymerase II C-terminal domain. Genes Dev. 2001a;15:3319–3329. doi: 10.1101/gad.935901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HS, Wang W, Kim R, Yokota H, Damo S, Kim SH, Wemmer DE, Kustu S, Yan D. BeF3− acts as a phosphate analog in proteins phosphorylated on aspartate: structure of a BeF3− complex with phosphoserine phosphatase. Proc. Natl. Acad. Sci. USA. 2001b;98:8525–8530. doi: 10.1073/pnas.131213698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLano WL. The PyMOL molecular graphics system. San Carlos, CA, USA: DeLano Scientific; 2002. http://www.pymol.org. [Google Scholar]
- Dulic A, Bates PA, Zhang X, Martin SR, Freemont PS, Lindahl T, Barnes DE. BRCT domain interactions in the heterodimeric DNA repair protein XRCC1-DNA ligase III. Biochemistry. 2001;40:5906–5913. doi: 10.1021/bi002701e. [DOI] [PubMed] [Google Scholar]
- Fabrega C, Shen V, Shuman S, Lima CD. Structure of an mRNA capping enzyme bound to the phosphorylated carboxyl-terminal domain of RNA polymerase II. Mol. Cell. 2003;11:1549–1561. doi: 10.1016/s1097-2765(03)00187-4. [DOI] [PubMed] [Google Scholar]
- Glover-Cutter K, Kim S, Esponiosa J, Bentley DL. RNA polymerase II pauses and associates with pre-mRNA processing factors at both ends of genes. Nat. Struct. Mol. Biol. 2008;15:71–72. doi: 10.1038/nsmb1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausmann S, Erdjument-Bromage H, Shuman S. Schizosaccharomyces pombe carboxyl-terminal domain (CTD) phosphatase Fcp1: distributive mechanism, minimal CTD substrate, and active site mapping. J. Biol. Chem. 2004;279:10892–10900. doi: 10.1074/jbc.M312513200. [DOI] [PubMed] [Google Scholar]
- Hausmann S, Shuman S. Characterization of the CTD phosphatase Fcp1 from fission yeast: preferential dephosphorylation of serine 2 versus serine 5. J. Biol. Chem. 2002;277:21213–21220. doi: 10.1074/jbc.M202056200. [DOI] [PubMed] [Google Scholar]
- Hausmann S, Shuman S. Defining the active site of Schizosaccharomyces pombe C-terminal domain phosphatase Fcp1. J Biol Chem. 2003;278:13627–13632. doi: 10.1074/jbc.M213191200. [DOI] [PubMed] [Google Scholar]
- Ho CK, Shuman S. Distinct roles for CTD Ser2 and Ser5 phosphorylation in the recruitment and allosteric activation of mammalian capping enzyme. Mol. Cell. 1999;3:405–411. doi: 10.1016/s1097-2765(00)80468-2. [DOI] [PubMed] [Google Scholar]
- Kamenski T, Heilmeier S, Meinhart A, Cramer P. Structure and mechanism of RNA polymerase II CTD phosphatases. Mol. Cell. 2004;15:399–407. doi: 10.1016/j.molcel.2004.06.035. [DOI] [PubMed] [Google Scholar]
- Kimura M, Suzuki H, Ishihama A. Formation of a carboxy-terminal domain phosphatase (Fcp1)/TFIIF/RNA polymerase II (pol II) complex in Schizosaccharomyces pombe involves direct interaction between Fcp1 and the Rpb4 subunit of pol II. Mol. Cell. Biol. 2002;22:1577–1588. doi: 10.1128/mcb.22.5.1577-1588.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahiri SD, Zhang G, Dunaway-Mariano D, Allen KN. The pentacovalent phosphorus intermediate of a phosphoryl transfer reaction. Science. 2003;299:2067–2071. doi: 10.1126/science.1082710. [DOI] [PubMed] [Google Scholar]
- Lin PS, Dubois MF, Dahmus ME. TFIIF-associating carboxyl-terminal domain phosphatase dephosphorylates phosphoserines 2 and 5 of RNA polymerase II. J. Biol. Chem. 2002;277:45949–45956. doi: 10.1074/jbc.M208588200. [DOI] [PubMed] [Google Scholar]
- Lu Z, Dunaway-Mariano D, Allen KN. The catalytic scaffold of the haloalkanoic acid dehalogenase enzyme superfamily acts as a mold for the trigonal bipyramidal transition state. Proc. Natl. Acad. Sci. USA. 2008;105:5687–5692. doi: 10.1073/pnas.0710800105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manke IA, Lowery DM, Nguyen A, Yaffe MB. BRCT repeats as phosphopeptide-binding modules involved in protein targeting. Science. 2003;302:636–639. doi: 10.1126/science.1088877. [DOI] [PubMed] [Google Scholar]
- Phatnani HP, Greenleaf AL. Phosphorylation and functions of the RNA polymerase II CTD. Genes Dev. 2006;20:2922–2936. doi: 10.1101/gad.1477006. [DOI] [PubMed] [Google Scholar]
- Shiozaki EN, Gu L, Yan N, Shi Y. Structure of the BRCT repeats of BRCA1 bound to a BACH1 phosphopeptide: implications for signaling. Mol Cell. 2004;14:405–412. doi: 10.1016/s1097-2765(04)00238-2. [DOI] [PubMed] [Google Scholar]
- Suh MH, Ye P, Zhang M, Hausmann S, Shuman S, Gnatt AL, Fu J. Fcp1 directly recognizes the C-terminal domain (CTD) and interacts with a site on RNA polymerase II distinct from the CTD. Proc Natl Acad Sci U S A. 2005;102:17314–17319. doi: 10.1073/pnas.0507987102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varon R, Gooding R, Steglich C, Marns L, Tang H, Angelicheva D, Yong KK, Ambrugger P, Reinhold A, Morar B, et al. Partial deficiency of the C-terminal-domain phosphatase of RNA polymerase II is associated with congenital cataracts facial dysmorphism neuropathy syndrome. Nat Genet. 2003;35:185–189. doi: 10.1038/ng1243. [DOI] [PubMed] [Google Scholar]
- Wang W, Cho HS, Kim R, Jancarik J, Yokota H, Nguyen HH, Grigoriev IV, Wemmer DE, Kim SH. Structural characterization of the reaction pathway in phosphoserine phosphatase: crystallographic snapshots of intermediate states. J. Mol. Biol. 2002;319:421–431. doi: 10.1016/S0022-2836(02)00324-8. [DOI] [PubMed] [Google Scholar]
- Williams RS, Lee MS, Hau DD, Glover JN. Structural basis of phosphopeptide recognition by the BRCT domain of BRCA1. Nat. Struct. Mol. Biol. 2004;11:519–525. doi: 10.1038/nsmb776. [DOI] [PubMed] [Google Scholar]
- Wrighton KH, Willis D, Liu F, Lin X, Feng X. Small C-terminal domain phosphatases dephosphorylate the regulatory linker regions of Smad2 and Smad3 to enhance transforming growth factor-b signaling. J. Biol. Chem. 2006;281:38365–38375. doi: 10.1074/jbc.M607246200. [DOI] [PubMed] [Google Scholar]
- Yeo M, Lee SK, Lee B, Ruiz EC, Pfaff SL, Gill GN. Small CTD phosphatases function in silencing neuronal gene expression. Science. 2005;307:596–600. doi: 10.1126/science.1100801. [DOI] [PubMed] [Google Scholar]
- Yeo M, Lin PS, Dahmus ME, Gill GN. A novel RNA polymerase II C-terminal domain phosphatase that preferentially dephosphorylates serine 5. J. Biol. Chem. 2003;278:26078–26085. doi: 10.1074/jbc.M301791200. [DOI] [PubMed] [Google Scholar]
- Yu X, Chini CC, He M, Mer G, Chen J. The BRCT domain is a phospho-protein binding domain. Science. 2003;302:639–642. doi: 10.1126/science.1088753. [DOI] [PubMed] [Google Scholar]
- Zhang X, Morera S, Bates PA, Whitehead PC, Coffer AI, Hainbucher K, Nash RA, Sternberg MJ, Lindahl T, Freemont PS. Structure of an XRCC1 BRCT domain: a new protein-protein interaction module. EMBO J. 1998;17:6404–6411. doi: 10.1093/emboj/17.21.6404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Kim Y, Genoud N, Gao J, Kelly JW, Pfaff SL, Gill GN, Dixon JE, Noel JP. Determinants for dephosphorylation of the RNA polymerase II C-terminal domain by Scp1. Mol. Cell. 2006;24:759–770. doi: 10.1016/j.molcel.2006.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Smith P, Wang LK, Shuman S. Structure-function analysis of the 3' phosphatase component of T4 polynucleotide kinase/phosphatase. Virology. 2007;366:126–136. doi: 10.1016/j.virol.2007.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.