Abstract
We examined visual search for color singleton targets, whose shape was discriminated. Critically, we varied the reward priority of singleton colors (correct fast performance for red singletons was worth more “bonus points” than for green singletons, or vice-versa), while testing whether ERP signatures of visual selection can be affected by distinct reward priorities for different target types, even when every target has to be selected for report. The N2pc component was earlier and larger for high- versus low-reward targets. This reward influence on the N2pc correlated with the subject-by-subject impact of reward level on efficiency of behavioral performance. Later post-selection processing was also affected by target reward-level. These results demonstrate that visual selection of task-relevant item is rapidly modulated by reward-related priorities, even when both types of target have to be selected for response.
Keywords: Affective value, Visual selection, Singleton search, Event-related potentials
Visual processing does not depend solely on current retinal inputs, but is known to be modulated also by attentional factors (i.e., task-relevant versus task-irrelevant status of competing inputs) and by emotional factors (i.e., affective or reward-related status of those inputs), which might be conceived broadly as reflecting ‘cold’ or ‘hot’ aspects of cognition respectively. Vuilleumier and Driver (2007) recently reviewed the extensive literature on each of these distinct topics, i.e., not only for attentional modulation of visual processing, but also for the largely separate literature on possible emotion-related modulation of visual processing. Numerous functional neuroimaging and ERP studies have now documented attentional (task-relevance) influences upon visual processing (e.g., Kastner & Ungerleider, 2000; Luck, Woodman, & Vogel, 2000, for reviews). Other studies have analogously documented some affective influences on visual processing (e.g., Lane, Chua, & Dolan, 1999; Phelps & LeDoux, 2005; Surguladze et al., 2003; Eimer & Kiss, 2007; Vuilleumier & Pourtois, 2007). Such findings raise several new questions about how attentional modulation of visual processing may relate to more affective influences (see Vuilleumier & Driver, 2007).
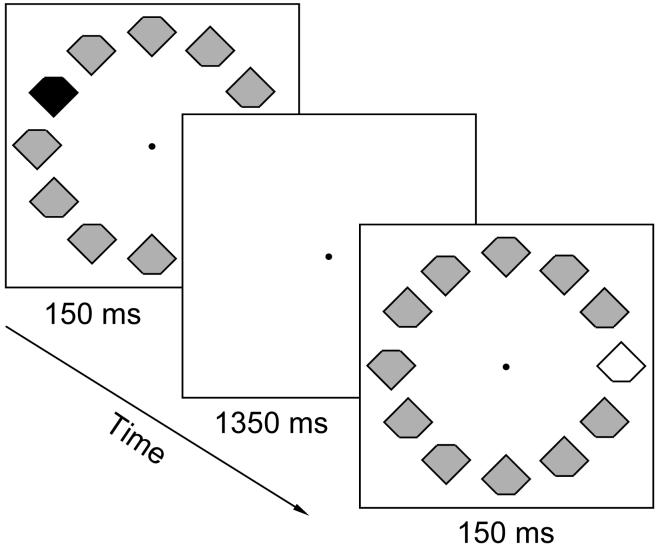
Here we examined a visual-search paradigm that is now particularly well characterized in relation to attentional influences (or putatively ‘cold cognition’, cf. Teasdale, 1993). We studied possible ‘hot cognition’, reward-related influences upon this paradigm, for the first time to our knowledge. The basic paradigm involved search for target singletons that have a unique color within each display (e.g., red among gray, or green among gray; see Figure 1), and might thereby attract attention automatically (e.g., Theeuwes, 1991). Similarly to several previous behavioral and ERP studies (e.g., Bravo & Nakayama, 1992; Mazza, Turatto, Umiltà, & Eimer, 2007), our participants had to judge a notch (here at top or bottom) of the singleton target on each trial (see also Kristjánsson, Vuilleumier, Malhotra, Husain, & Driver, 2005; Kristjánsson, Vuilleumier, Schwartz, Macaluso, & Driver, 2007). Our novel manipulation was that we varied the reward level of red singletons relative to green. For some participants, correct fast performance for red targets was worth more “bonus points” (see below) than for green targets, while the reverse applied for other participants, in a counterbalanced manner. In this way we could examine how reward-related priorities might influence performance in the visual search task. Importantly, we also acquired EEG data, allowing us to determine for the first time whether our manipulation of reward-related priorities in a well-understood visual-search paradigm would influence ERP signatures related to visual selection, and if so at which timepoints and for which components.
Figure 1.
Example search displays illustrating two successive trials. In each brief display, the target was the uniquely-colored singleton (unpredictably either red or green, among gray nontargets), and participants had to indicate whether the small notch on the singleton target was at its top or bottom. All items in the display had notches, randomly at top or bottom, regardless of notch-position for the target. Any red or green target (which never appeared together) had to be selected for judgement and speeded accurate response, but we varied reward-level for targets in one color versus the other (red or green, counterbalanced), in terms of the “bonus-points” that could accrue from fast-correct responses to them, with a possible 5 bonus-points for a target in the high-reward color, but only a possible 1 bonus-point for a target in the low-reward color. In the example search displays, one target color is shown in black and the other in white.
A sizeable ERP literature has already studied singleton visual search (e.g., Eimer & Kiss, 2008; Hickey, McDonald, & Theeuwes, 2006; Kiss, Jolicœur, Dell'Acqua, & Eimer, in press; Luck & Hillyard, 1994a, 1994b; Schubö, Schröger, Meinecke, & Müller, 2007), but hitherto without considering possible reward-related influences. Such work has identified the N2pc component as an important correlate of visual target selection. The N2pc is an enhanced negativity at posterior electrodes contralateral to a target within an array of multiple items, typically emerging ∼180-220 ms after display onset, and thought to reflect attentional selection of a target item from among distractors (typically, selection as a task-relevant item that needs to be judged and reported, e.g., Luck & Hillyard, 1994a, 1994b; see also Eimer, 1996; Girelli & Luck, 1997; Woodman & Luck, 1999). Brain source-analyses based on MEG recordings have implicated extrastriate visual cortex in the N2pc induced by task-relevant items, with some possible contribution from posterior parietal cortex in the earliest phase of this component (e.g., Hopf et al., 2000). As an on-line electrophysiological marker, the N2pc can be used as a tool to study the timecourse of attention selection during visual search (e.g., Woodman & Luck, 1999, 2003).
A later ERP component, again lateralized with respect to a target item appearing among nontargets, has also been reported (Vogel & Machizawa, 2004; see also Dell'Acqua, Sessa, Jolicœur, & Robitaille, 2006; Mazza et al., 2007; McCollough, Machizawa & Vogel, 2007) as a sustained posterior contralateral negativity (SPCN), typically arising at ∼350-400 ms after display onset. This component is thought to reflect additional processing of target stimuli after their attentional selection, including possible maintenance in visual short-term memory.
Here we examined whether the N2pc and/or SPCN components would be affected or not by our novel manipulation of reward-level for different types of singleton target in the visual search task (see Figure 1). Because both the red and green color singletons were salient target stimuli that had to be selected, judged and responded to, both these types of target should elicit an N2pc. In terms of bottom-up physical salience, as opposed to reward-level, the two target types were equated here not only via equiluminance, but importantly via full counterbalancing with respect to reward level. Thus, in terms of traditional ‘cold cognition’ factors, both types of target had to be selected and responded to, and were fully equivalent (after counterbalancing) in terms of their physical features. They thus differed only in terms of their reward level (see below), and thus any reward-related prioritization they might receive. If selection of salient color singletons, as reflected by the N2pc component, is exclusively determined by physical salience and/or the requirement to be explicitly judged, this component should be equivalent regardless of reward level for the two target-types here. If instead, however, the rapid selection process that the N2pc signifies can be sensitive to reward-related prioritization, we would expect a faster and/or increased N2pc to emerge specifically for the high-reward targets, despite their equivalence to the alternative target type in all other respects. Similar arguments apply for post-perceptual encoding of target stimuli into visual short-term memory, as reflected by the SPCN component. Finally, by testing for any subject-by-subject correlations between the impact of reward level upon behavior, and upon the N2pc (or SPCN), we could seek any reward-related brain-behavior relationships, for the first time in the context of singleton visual search.
Method
Participants
Eighteen paid volunteers participated, with three excluded due to excessive eye movements. One further participant was excluded because of exceptionally slow responses to one target type (see below). Thus, 14 participants (six male, mean age 26.9 years) remained in the sample. Two were left-handed, and all reported normal or corrected visual acuity. All participants gave informed consent in accord with local ethics, received a bonus payment of £5 (see below), and were fully debriefed at the end.
Stimuli and procedure
Stimuli were presented on a 17-inch computer monitor at a viewing distance of 60 cm. A central gray fixation point (0.3° × 0.3° visual angle) was present throughout. On each trial, a circular array of 12 diamonds, each with a ‘notch’ at top or bottom (see Figure 1) was presented against a black background, all at 4° from the fixation point. Each diamond subtended 1° × 1° (disregarding the 0.35° notch that was randomly at top or bottom). Each array contained 11 gray distractor diamonds and one uniquely colored (singleton) target diamond, which was unpredictably red for half the trials and green for the others. Gray, red, and green diamonds were physically equiluminant (14.1 cd/m2). Red or green target singletons could appear equiprobably at any one of the positions around the virtual 4° circular array, except at top or bottom (because the critical ERP contrasts relied on the hemifield of the singleton target, see below). Each search array was presented for 150 ms (too brief for saccades during it, thus further encouraging central fixation). The interval between the onset of successive search displays was 1500 ms. The experiment comprised 16 blocks of 40 trials each, plus one initial practice block that was not analyzed.
Participants were instructed to report whether the notch on each singleton target was at its top or bottom by pressing a spatially analogous key (i.e., one key was located above the other) with left or right index finger. Assignment of hands to response keys was reversed after half of the blocks, with order of that assignment counterbalanced across subjects. Hence overall the responding hand was not a critical factor. Our critical manipulation concerned the reward-level of red or green singleton targets, as instructed to participants before the task. Participants were informed that they could earn a “bonus payment” by accumulating a sufficient number of “bonus points” for fast and correct responses (In fact, our local ethics required that all participants received the same £5 bonus payment on completion of the study, which they all did, but which all merited; see below). They were told that five bonus points could be earned for a fast-correct response to any target in the high-reward color, and one bonus point for fast-correct responses to any target in the other color. For half of the participants, the high-reward color was red, for the other half it was green. After each block, feedback was given on the total number of bonus points earned in that block. Participants were encouraged to aim for a score of at least 60 bonus points per block. For each block, bonus points were awarded for each trial with a correct response and also a reaction time (RT) faster than the median RT of all correct responses in that block. Five points were awarded for each such fast-correct response to the high-reward target color, one point for each fast-correct response to the less rewarding color.
This scoring system motivated participants to aim for accuracy as well as speed (since no bonus points were ever awarded for error trials). To exclude any potential strategy of deliberately delaying correct responses to low-reward targets, we examined the full RT distributions for all subjects, and found only one with substantial outlier, exceptionally slow RTs specifically for the low-reward targets. The full debriefing revealed that this same participant was the only one to report using a go-slow strategy on low-reward trials, so they were excluded. To ensure further that participants did not simply trade speed for accuracy in relation to reward-level, we analysed all our behavioral results in terms of the well-established measure of inverse-efficiency (e.g., Kennett, Eimer, Spence, & Driver, 2001; Townsend & Ashby, 1983), which is calculated as mean RT divided by the proportion of correct responses for each participant. Hence both speed and accuracy were always taken into account when considering behavioral performance here.
EEG data acquisition and analysis
EEG was recorded from 23 Ag-AgCl electrodes mounted in an elastic cap at standard positions according to the 10/20 system. Horizontal eye movements (HEOG) were measured from two electrodes placed at outer canthi of the eyes. Amplifier bandpass was 0-40 Hz, with 250 Hz sampling rate. Scalp electrodes were referenced to left earlobe during recording. The right earlobe was recorded as an additional channel, and all channels were re-referenced off-line to averaged earlobes. All electrode impedances were kept below 5 kΩ. Continuous EEG was segmented from 100 ms before stimulus onset to 500 ms post-stimulus. Epochs containing horizontal eye movements (HEOG exceeding ±25 μV), eye blinks (Fpz ±60 μV), or movement artifacts (±80 μV at all other electrodes) were eliminated from further analyses. Only trials with a correct behavioral response were analyzed for EEG.
EEG waveforms were averaged separately for each combination of target color (high- or low-reward) and target position (left or right). N2pc and SPCN components were measured at lateral posterior electrodes PO7/PO8 where their amplitudes were maximal (see also McCollough et al., 2007). Mean amplitude values were computed within two post-stimulus-onset time windows. One corresponded to the early portion of the N2pc component (180-230 ms) that reflects rapid attentional selection (e.g., Eimer & Kiss, 2008), the other covered the interval where the SPCN was present (360-500 ms), in accord with previous research from our lab using a similar paradigm (Mazza et al., 2007) albeit without the critical reward manipulation introduced here. Repeated-measures ANOVAs were conducted on mean amplitudes with the factors of target reward level (high/low), contralaterality (electrode site ipsilateral or contralateral to target location), and target side (left/right hemifield). Onset latency of the N2pc was determined as the timepoint at which the voltage value on the ascending flank of individual N2pc difference waveforms exceeded an a priori criterion of −1.0 μV (though alternative approaches to defining N2pc onset gave the same results, see below). Effect sizes were calculated as standardized mean difference d when t-tests were used. When ANOVAs were used, they were calculated as partial eta-squared (ηp2), to determine the proportion of the effect plus error variance attributable to the effect. For all statistical tests, we report the probability of replicating an effect in the same direction (prep) rather than traditional p values. Values of prep of .9 and above correspond to significant conventional p-values (see Killeen, 2005).
Results
Behavioral data
RTs were computed from correct responses within 3 standard deviations (SD) from their mean RT, obtained separately for each reward level and participant. This criterion eliminated only 1% of correct trials, with no difference in exclusion rate between high- and low-reward targets. Across participants, RTs were faster to high- than low-reward targets (group means of 490 vs. 508 ms; t(13) = 4.8, prep = .99, d = 1.3). Error rates were 5.7% and 4.9% for high- and low-reward targets respectively (t(13) = 1.2, prep = .87, d = −0.33). To guard against possible speed-accuracy trade-offs, RT and error-rates were combined for each participant into the single parameter of inverse efficiency (Townsend & Ashby, 1983), calculated as the mean correct RT divided by the proportion of correct responses, separately for each condition. Inverse efficiency was lower (i.e., performance was more efficient) for high-reward as compared to low-reward targets (525 vs. 539 ms; t(13) = 3.7, prep = .99, d = 1.0).
ERP data
Figure 2 shows ERP waveforms elicited at PO7/8 for high-reward and low-reward target singletons. The N2pc component (enhanced negativity contralateral to the target, see Introduction) for the high-reward target appears slightly larger and earlier than that for the low-reward target. In addition, the subsequent SPCN component analogously seems more pronounced for the high-reward target. These effects can be seen more clearly in the difference waveforms shown in Figure 3 (top panel) obtained by subtracting ipsilateral from contralateral ERPs, separately for high- and low-reward targets. Statistical analyses confirmed the specific effects described below.
Figure 2.
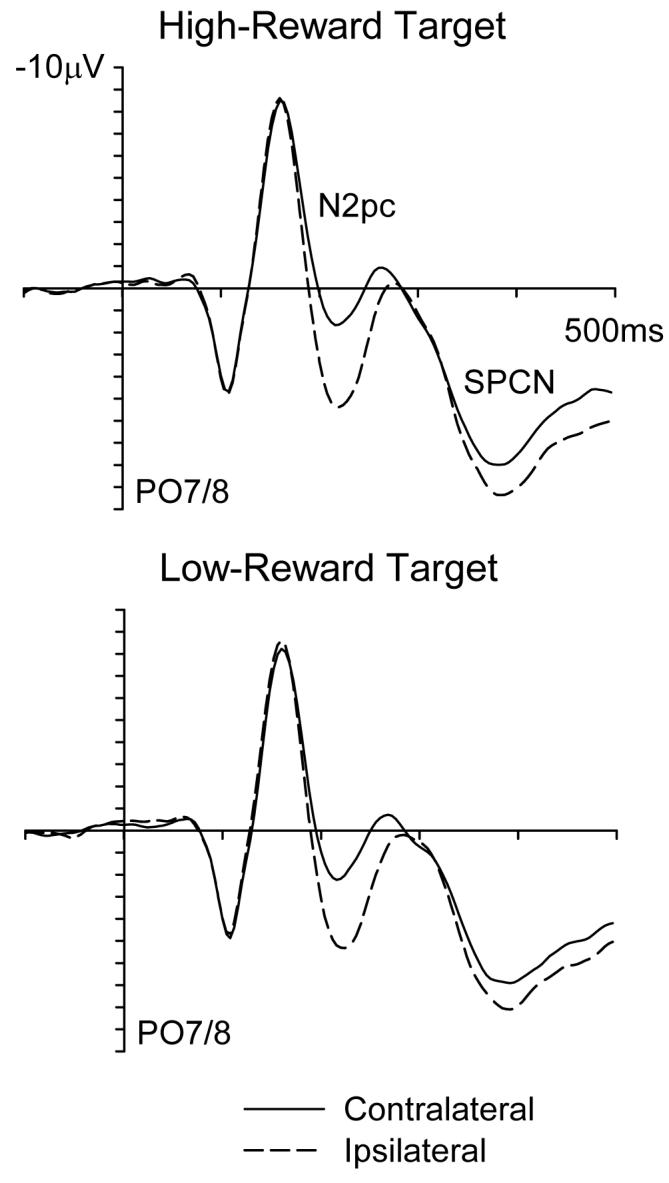
Grand average ERPs elicited at lateral posterior electrode sites (PO7/8) for search arrays containing a high-reward singleton (top panel) or a low-reward singleton instead (bottom panel). Separate waveforms are shown for electrodes contralateral (solid lines) or ipsilateral (dashed lines) to the target singleton. Note that high- and low-reward targets were physically equivalent (as the respective roles of red or green targets were counterbalanced over participants).
Figure 3.
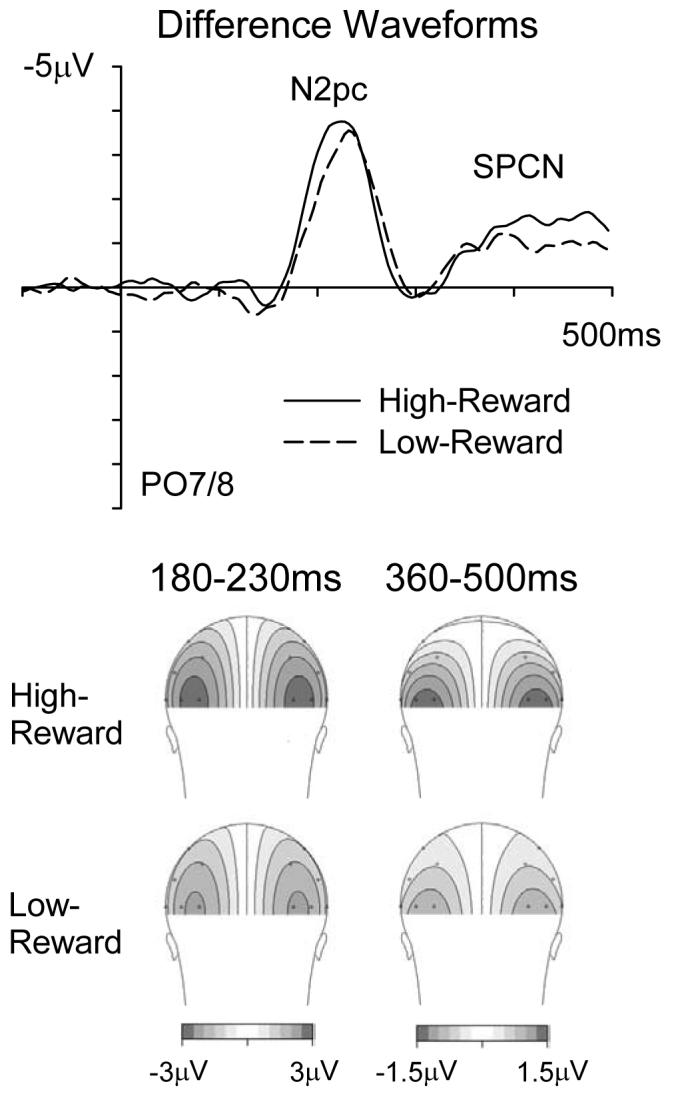
Top panel shows difference-waveforms obtained for contralateral-minus-ipsilateral ERPs (cf. Figure 2, which had plotted those ERPs separately), from electrodes PO7/8, shown separately for high-reward (solid line) and low-reward (dotted line) targets. The bottom panel shows the scalp topography of such difference waveforms in the N2pc (180-230 ms) or SPCN (360-500 ms) time windows, for high-reward (top row of topographies) and low-reward (bottom row) target singletons. These topographies were comparable regardless of reward level. Note the different scales used for N2pc and SPCN components.
N2pc (180-230 ms post-stimulus)
In the N2pc time window, a main effect of contralaterality, F(1,13) = 18.3, prep = .99, ηp2 = .584, confirmed that an N2pc was elicited in response to the color-singleton targets at PO7/8, as expected. Indeed, the N2pc was present for high-reward targets when considered alone, F(1,13) = 20.2, prep = .99, ηp2 = .608, and also for the low-reward targets, F(1,13) = 14.6, prep = .99, ηp2 = .529. But most importantly, there was a reward-level x contralaterality interaction, F(1,13) = 6.7, prep = .95, ηp2 = .341, because N2pc amplitudes were larger for high- than low-reward targets. Moreover, the onset of the N2pc (as defined by our a-priori criterion of −1.0 μV, see Methods) was earlier for the high- than low-reward target (187 versus 195 ms respectively, t(13) = 2.2, prep = .92, d = .52). The earlier onset of the N2pc for high- than low-reward targets was also confirmed when onset was defined instead as 50% of the peak amplitude measured in individual participants, t(13) = 2.5, prep = .94, d = .80.
The topographical maps in Figure 3 (bottom left) show that the scalp distribution of the N2pc component was very similar for high- and low-reward targets. To confirm that effects of reward-level on N2pc amplitudes and onset latencies were not restricted solely to electrodes PO7/8, additional analyses were conducted for all three lateral posterior electrode pairs (PO7/8, P7/8, P3/4), with electrode site now as an additional factor. A reward-level × contralaterality interaction, F(1,13) = 6.0, prep = .94, ηp2 = .315, indicated that N2pc amplitudes were generally larger for high-reward targets. There was also an electrode site × reward-level × contralaterality interaction, F(1,13) = 3.2, prep = .90, ηp2 = .196, as this reward effect was largest at PO7/8. However, analyses conducted separately for P7/8 and P3/4 revealed reward-level × contralaterality interactions for each of these electrode pairs also; F(1,13) = 4.9 and 5.8, prep = .92 and .94, ηp2 = .275 and .307, respectively. N2pc onset (defined by the −1.0 μV criterion) was earlier for high- than low-reward targets across all three posterior electrode pairs (190 versus 197 ms), as demonstrated by a main effect of reward-level, F(1,13) = 3.7, prep = .90, ηp2 = .223, with no electrode site × reward-level interaction (F < 1).
In summary, the N2pc emerged earlier and had a larger amplitude for high- than low-reward targets, despite their perfectly equated (fully counterbalanced) physical salience, and despite the fact that both target types had to be selected, judged and reported, with at least some reward/bonus-point obtained for fast-correct performance in either case. The impact on N2pc latency and amplitude can therefore only reflect the reward-level manipulation.
SPCN (360-500 ms post-stimulus)
The presence of the SPCN component was confirmed by a main effect of contralaterality, F(1,13) = 9.3, prep = .97, ηp2 = .418, during this later time window. This component was present for high-reward targets, F(1,13) = 11.9, prep = .98, ηp2 = .477, as well as for low-reward targets, F(1,13) = 6.2, prep = .94, ηp2 = .322, when these were considered alone. Similarly to the N2pc (but with later onset and extended time-course), the SPCN also importantly showed an interaction between reward level × contralaterality, F(1,13) = 6.6, prep = .95, ηp2 = .337, due to an enhanced SPCN for high-reward targets, see Figures 2 and 3. In addition, there was a main effect of reward value, F(1,13) = 4.9, prep = .92, ηp2 = .273, due to ERPs generally being more positive during the 360-500 ms time window for high-reward trials (see Figure 2). The scalp topography of the SPCN (as shown in Figure 3, bottom right) was comparable for high- and low-reward targets, with just its amplitude being enhanced for high-reward targets.
Brain-behavior relations for reward impact
To explore any relationship between enhanced behavioral efficiency and enhanced amplitudes of the lateralized ERP components, for high versus low-reward targets, we calculated Pearson correlations (and also Spearman nonparametric rank correlations, to minimize any influence from outliers) between the subject-by-subject size of the behavioral reward effect on inverse efficiency (i.e., for the most informative behavioral measure that combines RT and accuracy jointly), and the subject-by-subject size of the reward effect on N2pc or SPCN amplitudes at PO7/8. Remarkably, this revealed that the reward impact on performance was positively correlated with the N2pc amplitude effect (see Figure 4), r(13) = .61, prep = .95; Spearman's ρ(13) = .57, prep = .94. There were similar but less reliable trends for an analogous relationship between performance and SPCN amplitude in terms of reward effects, r(13) = .43; Spearman's ρ(13) = .43, both prep = .86.
Figure 4.
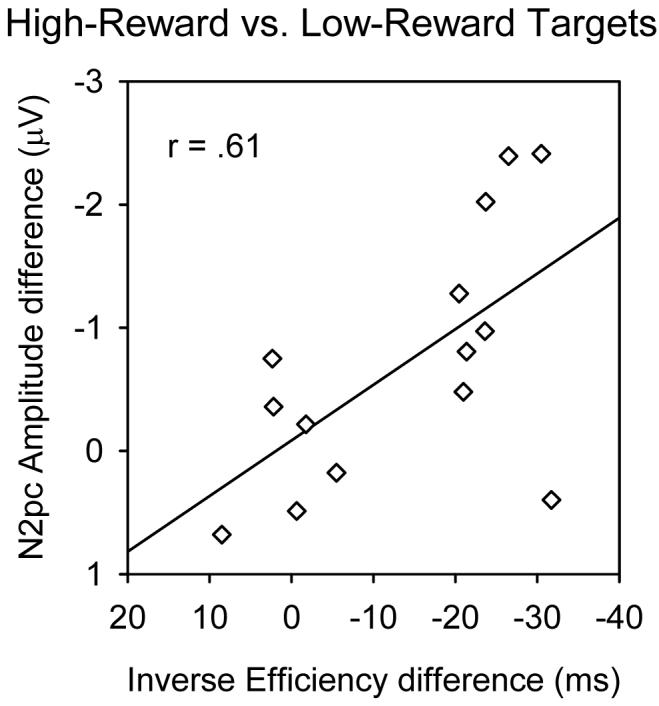
Scatterplot illustrating the consistent relationship (across participants, despite the occasional outlier) between the effects of reward-level on inverse efficiency and on N2pc amplitudes at PO7/8. Negative values indicate better behavioral performance (along the x-axis), and larger N2pc components (along the y-axis), for high-reward versus low-reward targets.
Discussion
Processing of visual stimuli can be modulated by task-relevance and by affective significance (see Driver & Vuilleumier, 2007, for one recent review), but as yet relatively little is known about the interplay between such aspects of cognition in visual selection. Moreover, any putatively ‘hot’, reward-related aspects have rarely been considered in the majority of work on visual search to date. Here we used electrophysiological markers of target selection (N2pc) and of post-selection processing (SPCN) to demonstrate for the first time that the reward level of targets can have systematic, and remarkably early, effects on visual selection in a singleton-search task. The N2pc component emerged earlier and was larger in amplitude for high- than low-reward targets, demonstrating that reward value can affect the efficiency of target selection within the first 200 ms of processing after stimulus onset. Moreover, the size of the reward effect on N2pc amplitude correlated positively with the corresponding behavioral impact of reward-level on performance efficiency.
These rapid influences of reward-level are all the more remarkable when one considers that all of our target stimuli were color singletons, which are often assumed to attract attention in a rapid bottom-up fashion due to high perceptual salience (e.g., Theeuwes, 1991). Some previous behavioral research (e.g., Folk et al., 1992) has shown that attentional capture by color singleton can be contingent on task-set. Our present combined behavioral and electrophysiological findings reveal for the first time that selection of color singletons can also be modulated by their reward status, potentially indicating another important source of top-down influence. A recent study by Della Libera and Chelazzi (2006) already provided some initial evidence for effects of reward on attentional processing. They reported negative priming effects (i.e., delayed responses to targets that served as distractors on the preceding trial) only following selections that received a high monetary reward, but not after selections that were poorly rewarded, indicating that attentional inhibition of distractors may be sensitive to reward contingencies. While those findings show that reward can affect the later consequences of selective attentional processing, our results demonstrate the speed and efficiency with which targets are selected can itself be modulated by their reward status. It might be argued that our manipulation of reward-level for alternative target types here affected participants' motivation, their prioritization, and/or the attentional effort allocated to one target type versus the other. But this would not undermine our main conclusion that target reward-level can lead to modulations of attentional selection that arise remarkably rapidly after display onset. Indeed, attentional effort might provide one potential bridge between putatively ‘hot’ (reward-related) and putatively ‘cold’ (selection for report) aspects of cognition, allowing reward-related priorities to modulate attentional performance.
Our further observation that SPCN amplitudes were larger for high- than low-reward targets implies that reward influences on visual search may not be restricted solely to initial selection of targets, but can also extend to subsequent, more in-depth processing, and possible maintenance in visual short-term memory. Thus reward-related influences on selective visual processing are evidently present both at relatively early stages of initial selection (as indicated by the N2pc) and for subsequent post-selection processing (as indicated by the SPCN).
In conclusion, our study reveals for the first time that singleton visual search is influenced not only by bottom-up stimulus salience (as emphasized, for example, by Theeuwes, 1991), and not only by relevance for report (see Folk et al., 1992), but can also be significantly modulated by the reward level of different target types. Reward influences on visual search can evidently arise relatively early in target selection, and go on to affect later stages of selective processing also. While reward had previously been shown to influence many aspects of behavior in an instrumental fashion (e.g. see Dayan & Balleine, 2002, for a review), its possible impact on rapidly selective visual processing has been overlooked hitherto. Such an influence was clearly revealed here for the first time, not only for behavioral performance, but also for electrophysiological markers of selective visual processing.
Acknowledgments
This study was supported by the Biotechnology and Biological Sciences Research Council (BBSRC) plus the following sources. ME holds a Royal Society-Wolfson Research Merit Award. JD is supported by the Wellcome Trust, the Medical Research Council (UK), and by a Royal Society Leverhulme-Trust Senior Research Fellowship.
References
- Dayan P, Balleine BW. Reward, motivation, and reinforcement learning. Neuron. 2002;36:285–298. doi: 10.1016/s0896-6273(02)00963-7. [DOI] [PubMed] [Google Scholar]
- Dell'Acqua R, Sessa P, Jolicœur P, Robitaille N. Spatial attention freezes during the attentional blink. Psychophysiology. 2006;43:394–400. doi: 10.1111/j.1469-8986.2006.00411.x. [DOI] [PubMed] [Google Scholar]
- Della Libera C, Chelazzi L. Visual selective attention and the effects of monetary rewards. Psychological Science. 2006;17:222–227. doi: 10.1111/j.1467-9280.2006.01689.x. [DOI] [PubMed] [Google Scholar]
- Eimer M. The N2pc component as an indicator of attentional selectivity. Electroencephalography and Clinical Neurophysiology. 1996;99:225–234. doi: 10.1016/0013-4694(96)95711-9. [DOI] [PubMed] [Google Scholar]
- Eimer M, Kiss M. Attentional capture by task-irrelevant fearful faces is revealed by the N2pc component. Biological Psychology. 2007;74:108–112. doi: 10.1016/j.biopsycho.2006.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eimer M, Kiss M. Involuntary attentional capture is determined by task set: Evidence from event-related brain potentials. Journal of Cognitive Neuroscience. 2008;20:1423–1433. doi: 10.1162/jocn.2008.20099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girelli M, Luck SJ. Are the same attentional mechanisms used to detect visual search targets defined by color, orientation, and motion? Journal of Cognitive Neuroscience. 1997;9:238–253. doi: 10.1162/jocn.1997.9.2.238. [DOI] [PubMed] [Google Scholar]
- Hickey C, McDonald JJ, Theeuwes J. Electrophysiological evidence of the capture of visual attention. Journal of Cognitive Neuroscience. 2006;18:604–613. doi: 10.1162/jocn.2006.18.4.604. [DOI] [PubMed] [Google Scholar]
- Hopf J-M, Luck SJ, Girelli M, Hagner T, Mangun GR, Scheich H, et al. Neural sources of focused attention in visual search. Cerebral Cortex. 2000;10:1233–1241. doi: 10.1093/cercor/10.12.1233. [DOI] [PubMed] [Google Scholar]
- Kastner S, Ungerleider LG. Mechanisms of visual attention in the human cortex. Annual Review of Neuroscience. 2000;23:315–341. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- Kennett S, Eimer M, Spence C, Driver J. Tactile-visual links in exogenous spatial attention under different postures: Convergent evidence from psychophysics and ERPs. Journal of Cognitive Neuroscience. 2001;13:462–478. doi: 10.1162/08989290152001899. [DOI] [PubMed] [Google Scholar]
- Killeen PR. An alternative to null-hypothesis significance tests. Psychological Science. 2005;16:345–353. doi: 10.1111/j.0956-7976.2005.01538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss M, Jolicœur P, Dell'Acqua R, Eimer M. Attentional capture by visual singletons is mediated by top-down task set: New evidence from the N2pc component. Psychophysiology. doi: 10.1111/j.1469-8986.2008.00700.x. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristjánsson Á, Vuilleumier P, Malhotra P, Husain M, Driver J. Priming of color and position during visual search in unilateral spatial neglect. Journal of Cognitive Neuroscience. 2005;17:859–873. doi: 10.1162/0898929054021148. [DOI] [PubMed] [Google Scholar]
- Kristjánsson Á, Vuilleumier P, Schwartz S, Macaluso E, Driver J. Neural basis for priming of pop-out during visual search revealed with fMRI. Cerebral Cortex. 2007;17:1612–1624. doi: 10.1093/cercor/bhl072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane RD, Chua PM-L, Dolan RJ. Common effects of emotional valence, arousal and attention on neural activation during visual processing of pictures. Neuropsychologia. 1999;37:989–997. doi: 10.1016/s0028-3932(99)00017-2. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Hillyard SA. Electrophysiological correlates of feature analysis during visual search. Psychophysiology. 1994a;31:291–308. doi: 10.1111/j.1469-8986.1994.tb02218.x. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Hillyard SA. Spatial filtering during visual search: Evidence from human electrophysiology. Journal of Experimental Psychology: Human Perception and Performance. 1994b;20:1000–1014. doi: 10.1037//0096-1523.20.5.1000. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Woodman GF, Vogel EK. Event-related potential studies of attention. Trends in Cognitive Sciences. 2000;4:432–440. doi: 10.1016/s1364-6613(00)01545-x. [DOI] [PubMed] [Google Scholar]
- Mazza V, Turatto M, Umiltà C, Eimer M. Attentional selection and identification of visual objects are reflected by distinct electrophysiological responses. Experimental Brain Research. 2007;181:531–536. doi: 10.1007/s00221-007-1002-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCollough AW, Machizawa MG, Vogel EK. Electrophysiological measures of maintaining representations in visual working memory. Cortex. 2007;43:77–90. doi: 10.1016/s0010-9452(08)70447-7. [DOI] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: From animal models to human behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Schubö A, Schröger E, Meinecke C, Müller HJ. Attentional resources and popout detection in search displays. NeuroReport. 2007;18:1589–1593. doi: 10.1097/WNR.0b013e3282efa08e. [DOI] [PubMed] [Google Scholar]
- Surguladze SA, Brammer MJ, Young AW, Andrew C, Travis MJ, Williams SCR, et al. A preferential increase in the extrastriate response to signals of danger. NeuroImage. 2003;19:1317–1328. doi: 10.1016/s1053-8119(03)00085-5. [DOI] [PubMed] [Google Scholar]
- Teasdale JD. Emotion and two kinds of meaning: Cognitive therapy and applied cognitive science. Behaviour Research and Therapy. 1993;31:339–354. doi: 10.1016/0005-7967(93)90092-9. [DOI] [PubMed] [Google Scholar]
- Theeuwes J. Cross-dimensional perceptual selectivity. Perception & Psychophysics. 1991;50:184–193. doi: 10.3758/bf03212219. [DOI] [PubMed] [Google Scholar]
- Townsend JT, Ashby FG. Stochastic modelling of elementary psychological processes. Cambridge University Press; New York: 1983. [Google Scholar]
- Vogel EK, Machizawa MG. Neural activity predicts individual differences in visual working memory capacity. Nature. 2004;428:784–751. doi: 10.1038/nature02447. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Driver J. Modulation of visual processing by attention and emotion: windows on causal interactions between human brain regions. Philosophical Transactions of the Royal Society. 2007;362:837–855. doi: 10.1098/rstb.2007.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P, Pourtois G. Distributed and interactive brain mechanisms during emotion face perception: evidence from functional neuroimaging. Neuropsychologia. 2007;45:174–194. doi: 10.1016/j.neuropsychologia.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Woodman GF, Luck SJ. Electrophysiological measurement of rapid shifts of attention during visual search. Nature. 1999;400:867–869. doi: 10.1038/23698. [DOI] [PubMed] [Google Scholar]
- Woodman GF, Luck SJ. Serial deployment of attention during visual search. Journal of Experimental Psychology: Human Perception and Performance. 2003;29:121–138. doi: 10.1037//0096-1523.29.1.121. [DOI] [PubMed] [Google Scholar]