Abstract
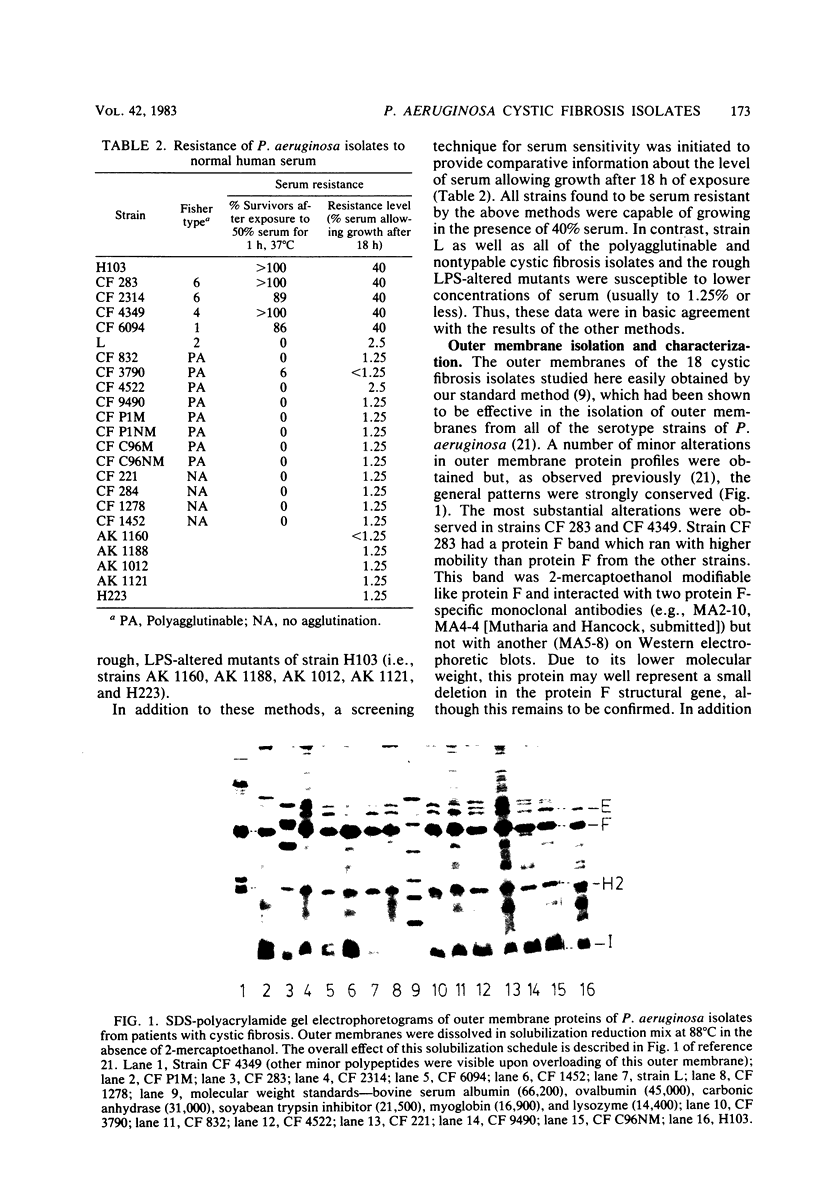
Twenty-six Pseudomonas aeruginosa strains from patients with cystic fibrosis were typed by the Fisher immunotyping scheme. Only 6 strains were agglutinated by a single typing serum, whereas 15 strains were agglutinated with more than one serum and 5 were not agglutinated by any serum. Neither the polyagglutinable nor the nonagglutinable strains were typable by hemagglutination inhibition or immunodiffusion, suggesting that these polyagglutinable strains did not express multiple serotype antigens, but were instead being agglutinated by antibody to nonserotype determinants. Four typable isolates were resistant to pooled normal human serum, whereas the 12 polyagglutinable and nonagglutinable isolates studied were very sensitive to normal human serum. The outer membranes of 16 strains were isolated and characterized. The data suggested, in general, strong conservation of outer membrane protein patterns. Lipopolysaccharides (LPS) were purified by a new technique which allowed isolation of both rough and smooth LPS in high yields. Three of four typable, serum-resistant strains examined had amounts of smooth, O-antigen-containing LPS equivalent to our laboratory wild type, P. aeruginosa PAO1 strain H103. In contrast, 10 of 12 polyagglutinable or nonagglutinable, serum-sensitive strains had very little or no smooth, O-antigen-containing LPS, and the other two contained less smooth LPS than our wild-type strain H103. In agreement with this data, five independent, rough, LPS O-antigen-deficient mutants of strain H103 were nontypable and serum sensitive. We suggest that the LPS defects described here represent a significant new property of many P. aeruginosa strains associated with cystic fibrosis.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chester I. R., Meadow P. M., Pitt T. L. The relationship between the O-antigenic lipopolysaccharides and serological specificity in strains of Pseudomonas aeruginosa of different O-serotypes. J Gen Microbiol. 1973 Oct;78(2):305–318. doi: 10.1099/00221287-78-2-305. [DOI] [PubMed] [Google Scholar]
- Darveau R. P., Hancock R. E. Procedure for isolation of bacterial lipopolysaccharides from both smooth and rough Pseudomonas aeruginosa and Salmonella typhimurium strains. J Bacteriol. 1983 Aug;155(2):831–838. doi: 10.1128/jb.155.2.831-838.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMatteo C. S., Hammer M. C., Baltch A. L., Smith R. P., Sutphen N. T., Michelsen P. B. Susceptibility of Pseudomonas aeruginosa to serum bactericidal activity. A comparison of three methods with clinical correlations. J Lab Clin Med. 1981 Oct;98(4):511–518. [PubMed] [Google Scholar]
- Doggett R. G. Incidence of mucoid Pseudomonas aeruginosa from clinical sources. Appl Microbiol. 1969 Nov;18(5):936–937. doi: 10.1128/am.18.5.936-937.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fick R. B., Jr, Naegel G. P., Reynolds H. Y. Use of Pseudomonas aeruginosa lipopolysaccharide immunoadsorbents to prepare high potency, mono-specific antibodies. J Immunol Methods. 1980;38(1-2):103–116. doi: 10.1016/0022-1759(80)90335-x. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Goldman R. C., Leive L. Heterogeneity of antigenic-side-chain length in lipopolysaccharide from Escherichia coli 0111 and Salmonella typhimurium LT2. Eur J Biochem. 1980;107(1):145–153. doi: 10.1111/j.1432-1033.1980.tb04635.x. [DOI] [PubMed] [Google Scholar]
- HIRSCH J. G., STRAUSS B. STUDIES ON HEAT-LABILE OPSONIN IN RABBIT SERUM. J Immunol. 1964 Jan;92:145–154. [PubMed] [Google Scholar]
- Hancock R. E., Carey A. M. Outer membrane of Pseudomonas aeruginosa: heat- 2-mercaptoethanol-modifiable proteins. J Bacteriol. 1979 Dec;140(3):902–910. doi: 10.1128/jb.140.3.902-910.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Wieczorek A. A., Mutharia L. M., Poole K. Monoclonal antibodies against Pseudomonas aeruginosa outer membrane antigens: isolation and characterization. Infect Immun. 1982 Jul;37(1):166–171. doi: 10.1128/iai.37.1.166-171.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrell K. F., Kropinski A. M. Isolation and characterization of a bacteriophage specific for the lipopolysaccharide of rough derivatives of Pseudomonas aeruginosa strain PAO. J Virol. 1981 May;38(2):529–538. doi: 10.1128/jvi.38.2.529-538.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropinski A. M., Chan L., Milazzo F. H. Susceptibility of lipopolysaccharide-defective mutants of Pseudomonas aeruginosa strain PAO to dyes, detergents, and antibiotics. Antimicrob Agents Chemother. 1978 Mar;13(3):494–499. doi: 10.1128/aac.13.3.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropinski A. M., Kuzio J., Angus B. L., Hancock R. E. Chemical and chromatographic analysis of lipopolysaccharide from an antibiotic-supersusceptible mutant of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1982 Feb;21(2):310–319. doi: 10.1128/aac.21.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Martin D. R. Mucoid variation in Pseudomonas aeruginosa induced by the action of phage. J Med Microbiol. 1973 Feb;6(1):111–118. doi: 10.1099/00222615-6-1-111. [DOI] [PubMed] [Google Scholar]
- Mutharia L. M., Nicas T. I., Hancock R. E. Outer membrane proteins of Pseudomonas aeruginosa serotype strains. J Infect Dis. 1982 Dec;146(6):770–779. doi: 10.1093/infdis/146.6.770. [DOI] [PubMed] [Google Scholar]
- Pier G. B., Sidberry H. F., Zolyomi S., Sadoff J. C. Isolation and characterization of a high-molecular-weight polysaccharide from the slime of Pseudomonas aeruginosa. Infect Immun. 1978 Dec;22(3):908–918. doi: 10.1128/iai.22.3.908-918.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pier G. B., Thomas D. M. Lipopolysaccharide and high-molecular-weight polysaccharide serotypes of Pseudomonas aeruginosa. J Infect Dis. 1982 Feb;145(2):217–223. doi: 10.1093/infdis/145.2.217. [DOI] [PubMed] [Google Scholar]
- Salkinoja-Salonen M., Nurmiaho E. L. The effect of lipopolysaccharide composition on the ultrastructure of Pseudomonas aeruginosa. J Gen Microbiol. 1978 Mar;105(1):23–28. doi: 10.1099/00221287-105-1-23. [DOI] [PubMed] [Google Scholar]
- Schneider H., Griffiss J. M. A bactericidal microassay for testing serum sensitivity of Neisseria gonorrhoeae. J Immunol Methods. 1982 Oct 15;54(1):101–105. doi: 10.1016/0022-1759(82)90118-1. [DOI] [PubMed] [Google Scholar]
- Schneider H., Griffiss J. M., Williams G. D., Pier G. B. Immunological basis of serum resistance of Neisseria gonorrhoeae. J Gen Microbiol. 1982 Jan;128(1):13–22. doi: 10.1099/00221287-128-1-13. [DOI] [PubMed] [Google Scholar]
- Tanamoto K., Abe C., Homma J. Y., Kojima Y. Regions of the lipopolysaccharide of Pseudomonas aeruginosa essential for antitumor and interferon-inducing activities. Eur J Biochem. 1979 Jul;97(2):623–629. doi: 10.1111/j.1432-1033.1979.tb13152.x. [DOI] [PubMed] [Google Scholar]
- Thomassen M. J., Demko C. A. Serum bactericidal effect on Pseudomonas aeruginosa isolates from cystic fibrosis patients. Infect Immun. 1981 Aug;33(2):512–518. doi: 10.1128/iai.33.2.512-518.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Wilkinson S. G., Galbrath L. Studies of lipopolysaccharides from Pseudomonas aeruginosa. Eur J Biochem. 1975 Mar 17;52(2):331–343. doi: 10.1111/j.1432-1033.1975.tb04001.x. [DOI] [PubMed] [Google Scholar]
- Zierdt C. H., Williams R. L. Serotyping of Pseudomonas aeruginosa isolates from patients with cystic fibrosis of the pancreas. J Clin Microbiol. 1975 Jun;1(6):521–526. doi: 10.1128/jcm.1.6.521-526.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]