Abstract
Freshly isolated hepatocytes are widely accepted as the “gold standard” for providing reliable data on drug uptake across the sinusoidal (basolateral) membrane. However, the suitability of freshly isolated hepatocytes in suspension to assess efflux by canalicular (apical) proteins or predict biliary excretion in the intact organ is unclear. After collagenase digestion, hepatocytes rapidly lose polarity, but localization of canalicular transport proteins in the first few hours after isolation has not been well characterized. In this study, immunostaining and confocal microscopy have provided, for the first time, a detailed examination of canalicular transport protein localization in freshly isolated rat hepatocytes fixed within 1 h of isolation and in cells cultured for 1 h. Organic anion transporting polypeptide 1a1 (Oatp1a1) was expressed in all hepatocytes and distributed evenly across the basolateral membrane; there was no evidence for colocalization of Oatp1a1 with P-glycoprotein (P-gp) or multidrug resistance-associated protein 2 (Mrp2). In contrast, P-gp and Mrp2 expression was lower than Oatp1a1 and confined to junctions between adjacent cells, intracellular compartments, and “legacy” network structures at or near the cell surface. P-gp and Mrp2 staining was more predominant in regions adjacent to former canalicular spaces, identified by zonula occludens-1 staining. Functional analysis of rat hepatocytes cultured for 1 h demonstrated that the fluorescent anion and Mrp2 substrate, 5-(and-6)-carboxy-2′,7′-dichlorofluorescein (CDF), accumulated in cellular compartments; compartmental accumulation of CDF was sensitive to (E)-3-[[[3-[2-(7-chloro-2-quinolinyl)ethenyl]phenyl]-[[3-dimethylamino)-3-oxopropyl]thio]methyl]thio]-propanoic acid (MK571, Mrp inhibitor) and was not observed in hepatocytes isolated from Mrp2-deficient rats. Drug efflux from freshly isolated hepatocytes as an estimate of apical efflux/biliary excretion would give an inaccurate assessment of true apical elimination and, as such, should not be used to make in vivo extrapolations.
Reliable in vitro models to predict hepatic accumulation, distribution, metabolism, and excretion of drug candidates are important tools in preclinical drug development. Many of the currently available models are excellent predictors of in vivo hepatic and biliary clearance (Obach et al., 1997; Ghibellini et al., 2007), and several well established models exist to investigate hepatic drug metabolism and elimination. Freshly isolated hepatocytes in suspension contain a complete complement of drug metabolizing enzymes, remain viable for several hours, and possess relevant drug transport proteins (Hengstler et al., 2000; Gebhardt et al., 2003). Although suspensions of both freshly isolated and cryopreserved hepatocytes are accepted widely as the “gold standard” for providing reliable data on drug uptake across the sinusoidal (basolateral) membrane (Sandker et al., 1994; Hirano et al., 2004; Lam et al., 2006), there is no evidence to support the suitability of this model to accurately predict apical efflux (e.g., biliary excretion). After enzymatic and/or mechanical disruption, hepatocytes rapidly lose their polarity (Groothuis et al., 1981; Talamini et al., 1997). Examination of cultured hepatocytes 18 to 24 h after isolation indicated that canalicular proteins were internalized, the fluorescent glutathione conjugate, glutathione bimane, was accumulated in intracellular vesicles of hepatocytes from Wistar but not Mrp2-deficient (TR−) rats (Oude Elferink et al., 1993), and the apical efflux transport proteins, P-gp and Mrp2, were internalized (Hoffmaster et al., 2004; Zhang et al., 2005). Despite this, some investigators have reported that P-gp function is measurable in suspensions of isolated hepatocytes (Lam and Benet, 2004; Lam et al., 2006), although others have not reached the same conclusion (Jorgensen et al., 2007). The present study was designed to determine whether freshly isolated, suspended hepatocytes in the first hour after isolation can be used to assess accurately the extent of drug efflux by canalicular proteins. Data presented in this article provide, for the first time, clear evidence that P-gp and Mrp2 are rapidly internalized after isolation, remain confined to junctions between cell couplets, or reside on “legacy” canalicular networks of cells, which represent regions with concentrated tight junction protein expression where there was once cell-to-cell contact and a functional canalicular network. Thus, these transporters are largely unavailable to mediate efflux from the freshly isolated hepatocyte.
Materials and Methods
Materials
Monoclonal mouse antibody against C219 (P-gp) was supplied by Covance Research Products, Inc. (Princeton, NJ). Rabbit-anti rat Oatp1a1 antibody was kindly provided by Dr. Peter Meier, University of Zurich, Switzerland. Monoclonal antibody to MRP2 (M2III-6) was supplied by Axxora, LLC (San Diego, CA). Zymed rabbit-anti ZO-1, 5-(and-6)-carboxy-2′,7′-dichlorofluorescein diacetate (CDF-DA), and Dulbecco’s modified Eagle’s medium without phenol red were supplied by Invitrogen (Carlsbad, CA). Type I rat tail collagen was supplied by BD Biosciences (San Jose, CA) and MK571 by Calbiochem (San Diego, CA). All other chemicals were obtained from commercial suppliers and were of the highest purity available.
Animals
Male wild-type Wistar (Charles River Laboratories, Raleigh, NC) or TR− Wistar (in-house breeding colony originally obtained from Dr. Mary Vore, University of Kentucky, Lexington, KY) rats (175–300 g) were used for hepatocyte isolation from whole liver. Animals had free access to water and food before surgery. All animal procedures were compliant with the guidelines of the University of North Carolina Institutional Animal Care and Use Committee.
Isolation and Culture of Rat Hepatocytes
Hepatocytes were isolated from male wild-type or TR− Wistar rats using a collagenase perfusion method as described previously (Liu et al., 1999). Hepatocyte viability was >85% as determined by trypan blue exclusion. Immediately after isolation, suspended hepatocytes were centrifuged for 5 min at 50g and then resuspended in phosphate-buffered saline (PBS) at ~106 cells/ml. Cells were allowed to attach to 35-mm glass-bottomed culture dishes (glass coverslip; 14-mm diameter, 0.16–0.19-mm thickness) (MatTek Corporation, Ashland, MA) previously coated with rat tail collagen solution (75 μl of type I collagen; 1.5 mg/ml, pH 7.4) at room temperature (RT) for 30 to 60 min. For the purpose of this study, cells allowed to attach to dishes at RT were described as “freshly isolated.” For the 1-h culture period, hepatocytes (0.75–1.5 × 106) were seeded in Dulbecco’s modified Eagle’s medium (supplemented with 5% fetal bovine serum, nonessential amino-acids, l-glutamine, penicillin/streptomycin, and 1 μM dexamethasone) on the collagen-coated 35-mm glass-bottomed culture dishes and cultured in a humidified incubator with 95% air/5% CO2.
Immunostaining
Both freshly isolated and cultured cells were washed three times with PBS and fixed in ice-cold acetone for 10 min at 4°C. Cells were washed three times with PBS and then blocked (30 min at RT) with PBS containing 5% (v/v) goat serum and 1% (w/v) BSA. Primary antibodies (P-gp, 1:10; Oatp1a1, 1:20; MRP2, 1:20; ZO-1, 1:50) were diluted in PBS containing 5% (v/v) goat serum and 0.1% (w/v) acetylated BSA, added to the cells, and incubated for 1 h at RT. Cells were washed with PBS (three times) for 10 min at RT with shaking. Secondary antibodies [diluted in PBS containing 5% (v/v) goat serum and 0.1% (w/v) acetylated BSA] were added to the cells and incubated for 1 h at RT [Alexa Fluor 488 goat anti-mouse IgG (H+L) (Invitrogen) for P-gp and Mrp2 (1:1000) and Alexa Fluor 543 goat anti-rabbit IgG (H+L) (Invitrogen) for Oatp1a1 and ZO-1 (1:500)]. After incubation, cells were washed three times with PBS for 10 min at RT with shaking. Cells were imaged using a Zeiss LSM 510 laser scanning confocal microscope (Carl Zeiss Inc., Thornwood, NY) with a Zeiss C-Apochromat 40×/1.2 water objective lens. The Alexa Fluor 488 and Alexa Fluor 543 images were acquired consecutively using, respectively, the laser lines at 488 nm (Argon laser, 2%) and 543 nm (HeNe laser, 20%) for excitation and 505 to 530 nm band pass and 650 nm long pass filters for emission. Negative control experiments showed no signal at the settings used to image specific fluorescence.
CDF Accumulation in Cultured Hepatocytes
Hepatocytes were cultured for 1 h (0.75 × 106 cells/dish), washed three times with Hanks’ balanced salt solution-HEPES, pH 7.4, and incubated for 20 min with or without 10 μM MK571 then with 1 μM CDF-DA for ~20 to 30 min at 37°C. The diacetate form of CDF, CDF-DA, readily diffuses across the plasma membrane where it is hydrolyzed to CDF, a fluorescent Mrp2 substrate (Zamek-Gliszczynski et al., 2003). Cells were imaged with a laser scanning confocal microscope as described previously. The CDF images were collected using the laser line at 488 nm (Argon laser, 0.05%) for excitation with a long-pass filter at 505 nm for emission.
Results
Examination of freshly isolated hepatocytes, fixed and immunostained for P-gp (C219) or Mrp2 with Oatp1a1 (Fig. 1, A and B), indicated that Oatp1a1 was distributed evenly on the basolateral membrane, whereas P-gp and Mrp2 staining was confined primarily to junctions between adjacent cells and intracellular compartments. Three-dimensional reconstructions suggested that both P-gp and Mrp2 were localized in cellular compartments at or near the cell surface and/or at junctions between cells referred to as legacy canalicular spaces. Slices in the z-plane (Fig. 1Aiii) of freshly isolated hepatocytes showed that neither P-gp nor Mrp2 was localized with Oatp1a1 on the basolateral membrane. Examination of hepatocytes cultured for 1 h before fixation (Fig. 2, A and B) showed similar distribution patterns, but internalization of P-gp and Mrp2 was more apparent. The expression and localization of P-gp and Mrp2 were investigated further by dual immunostaining with a ZO-1 antibody. In freshly isolated hepatocytes, P-gp (Fig. 1C) and Mrp2 (Fig. 1D) expression was very closely associated with ZO-1. ZO-1 expression seemed to be less organized in the cultured cells (Fig. 2, C and D). Cells cultured for 1 h were examined to determine whether the regions expressing Mrp2 in single hepatocytes were sealed compartments. CDF accumulation seemed to be diffuse within cells and also concentrated in cellular compartments (regions with bright punctate staining) thought to be fragments of legacy networks (Fig. 3A). CDF accumulation was not apparent in cells pretreated with MK571 (Fig. 3B) or in TR− (Mrp2-deficient) hepatocytes (Fig. 3C). Closer examination of these cells with differential interference contrast microscopy showed sealed structures at or near the cell surface where CDF accumulation was apparent (Fig. 3, D and E).
Fig. 1.
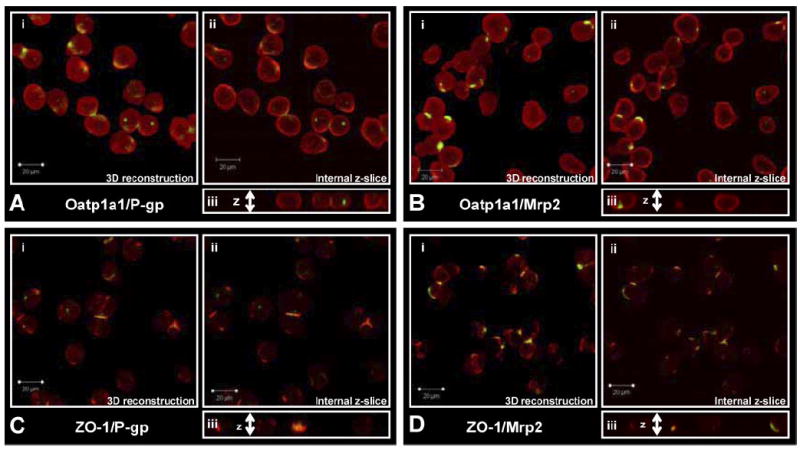
P-gp or Mrp2 staining with Oatp1a1 or ZO-1 in freshly isolated rat hepatocytes. i, three-dimensional reconstruction of z-stack (1-μm xy slices); ii, single internal 1-μM slice; and iii, cut-through of z-stack. A, Oap1a1 (red) and P-gp (green); B, Oatp1a1 (red) and Mrp2 (green); C, ZO-1 (red) and P-gp (green); D, ZO-1 (red) and Mrp2 (green).
Fig. 2.
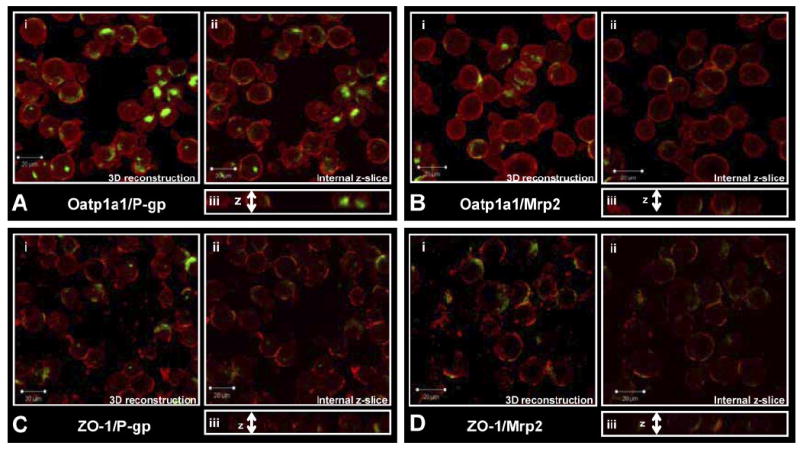
P-gp or Mrp2 staining with Oatp1a1 or ZO-1 in hepatocytes cultured for 1 h. i, three-dimensional reconstruction of z-stack (1-μm xy slices); ii, single internal 1-μM slice; and iii, cut-through of z-stack. A, Oap1a1 (red) and P-gp (green); B; Oatp1a1 (red) and Mrp2 (green); C, ZO-1 (red) and P-gp (green); D, ZO-1 (red) and Mrp2 (green).
Fig. 3.
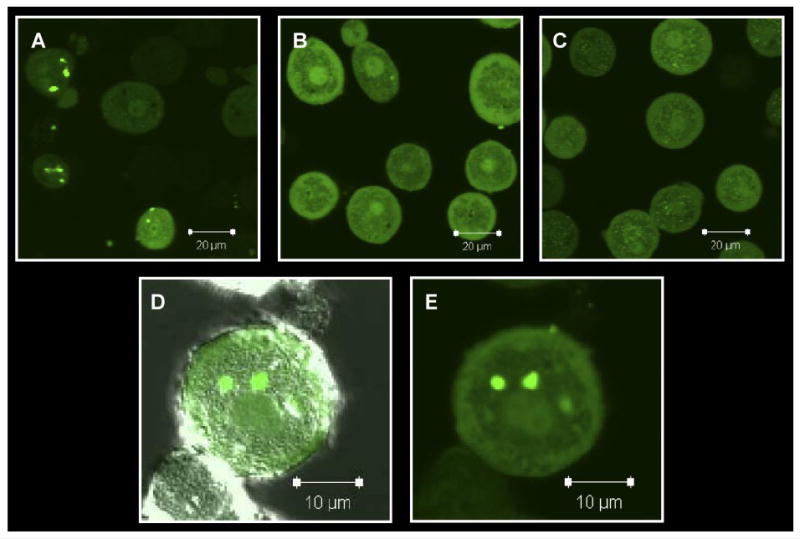
Accumulation of 1 μM CDF (green) in rat hepatocytes cultured for 1 h. A, wild-type cells; B, wild-type cells + 10 μM MK571; C, TR− cells; D, with and (E) without differential interference contrast microscopy. Representative images from multiple experiments.
Discussion
Numerous studies have established that isolated hepatocyte suspensions are an excellent in vitro model to study both drug metabolism and drug uptake/accumulation. However, there is some debate regarding the suitability of this in vitro system to measure apical efflux/ canalicular excretion and to predict in vivo biliary excretion. During collagenase isolation, hepatocyte cell polarity is lost quickly but returns with time under optimal culture conditions (Groothuis et al., 1981; Talamini et al., 1997). Recent studies have confirmed the apical transport proteins, P-gp and Mrp2, are expressed correctly on the canalicular membrane of sandwich-cultured hepatocytes (by day 3) and efflux drugs into sealed canalicular networks (Hoffmaster et al., 2004; Zhang et al., 2005). However, the cellular localization of apical transport proteins immediately after isolation of hepatocytes remained to be established. In the present study, using immunostaining and transport of the fluorescent Mrp2 substrate, CDF, we demonstrated that P-gp and Mrp2 (Fig. 1) are confined primarily to sealed compartments or to junctions between cells immediately after isolation from rat liver. P-gp and Mrp2 (Fig. 2) internalization seemed to be more pronounced after 1 h of culture, in accord with the previously published data for Mrp2 in cell couplets (Roelofsen et al., 1998). Sealed tight junctions between hepatocytes form the basis for the development of biliary networks in vivo. After disruption of cell-to-cell contacts, tight junctions are disrupted; however, the tight junction protein ZO-1 remained highly organized at the former tight junction site (Fig. 1) with only a slight loss in organization apparent after 1 h of culture (Fig. 2). Initially, in freshly isolated hepatocytes, both P-gp and Mrp2 remained closely associated with ZO-1 (Fig. 1, C and D) in cell couplets and legacy canalicular networks of single cells. The staining of Oatp1a1 was diffuse across the surface of the basolateral membrane and did not seem to be colocalized with either P-gp or Mrp2 (Fig. 1, A and B).
Collagenase isolation of hepatocytes can result in a mixture of both single cells and cell couplets. Cell couplets have been used in multiple studies to examine biliary excretion into sealed compartments between cells (Boyer et al., 1990; Wilton et al., 1993). As shown in the present study, CDF accumulated within legacy network compartments (Fig. 3), indicating that these are sealed units expressing functional Mrp2. After synthesis, ATP-binding-cassette canalicular transport proteins translocate in a direct manner from Golgi, bypassing the basolateral membrane in immortalized cell lines (Kipp and Arias, 2000; Slimane et al., 2003). However, little work has been done on protein trafficking at early time points in freshly isolated hepatocytes, which may differ from cell lines. The apical plasma membrane marker dipeptidyl peptidase IV follows an indirect pathway to the canalicular membrane via sorting at the basolateral membrane in the polarized hepatic cell line WIF-B (Bastaki et al., 2002). Interestingly, in a mutant hepatocyte cell line (HepG2-AJ−), cells modified to lack E-cadherin and β-catenin dipeptidyl peptidase IV trafficked in a direct manner to either canalicular or basolateral membranes (Theard et al., 2007). In the polarized WIF-B9 cell line, bile salt export pump (canalicular transport protein) cycles in and out of the canalicular membrane, moving into intracellular pools of rab11a-positive endosomes, where it can translocate to the surface membrane as required (Wakabayashi et al., 2004). Internalized P-gp and Mrp2 in freshly isolated hepatocytes also may be held within endosomes until polarization is re-established, which occurs after culturing hepatocytes for 2 to 3 days in a sandwich configuration (Hoffmaster et al., 2004; Zhang et al., 2005). Indeed, in hepatocytes cultured for 18 to 24 h, canalicular proteins may be responsible for the accumulation of organic anions in intracellular vesicles (Oude Elferink et al., 1993). As knowledge and understanding of protein function, expression, and the mechanisms of protein trafficking increase, insight may be gained regarding why and how apical drug transport proteins are internalized after disruption of tight junctions and cell-to-cell contact. In addition, chemical modulation of protein trafficking pathways may allow redirection of apical proteins to the basolateral membrane to facilitate efflux studies. Even if canalicular proteins could be redirected to the basolateral membrane, discerning basolateral-mediated efflux via canalicular proteins (e.g., P-gp, Mrp2, Bcrp) from basolateral proteins (e.g., Oatp1a1, Mrp3) without the use of specific transport inhibitors would be difficult. Hepatocytes isolated from single, double, or multiple knockout transporter models might be useful for these types of studies.
Freshly isolated suspensions of hepatocytes are a well established in vitro model to study drug transport and xenobiotic metabolism. Based on immunostaining profiles observed for the basolateral transport protein Oatp1a1, the suspended hepatocyte model is an excellent first choice for studying initial hepatic uptake of drugs. However, results from uptake studies may be confounded by basolateral efflux transport proteins or bidirectionality of uptake transporters (Mahagita et al., 2007). Clearly, an important limitation of the suspended hepatocyte model is the localization of canalicular drug transport proteins. Based on the present data, canalicular transport proteins are internalized or confined to junctions between adjacent cells, and therefore suspended hepatocytes are not an appropriate system to study apical efflux/canalicular excretion of drugs. Sandwich-cultured hepatocytes or canalicular membrane vesicles would be more suitable systems to estimate the contribution of apical proteins to efflux/biliary excretion (Nishida et al., 1991; Liu et al., 1999).
Acknowledgments
We thank Dr. Peter Meier, University of Zurich, Switzerland, for kindly providing the antibody to rat Oatp1a1 and Dr. Yiwei Rong for her technical expertise in the isolation of rat hepatocytes. Discussions with Dr. Leslie Z. Benet that led to the initiation of this project are gratefully acknowledged.
Research was supported by National Institutes of Health (NIH) Grant GM41935 and by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences (NIEHS). All data in this project were collected with the assistance of the Fluorescence Microscopy and Imaging Center at the NIEHS.
ABBREVIATIONS
- Mrp2
multidrug resistance-associated protein 2
- BSA
bovine serum albumin
- CDF
5-(and-6)-carboxy-2′,7′-dichlorofluorescein
- CDF-DA
5-(and-6)-carboxy-2′,7′-dichlorofluorescein diacetate
- Oatp1a1
organic anion transporting polypeptide 1a1
- PBS
phosphate-buffered saline
- MK571
(E)-3-[[[3-[2-(7-chloro-2-quinolinyl)ethenyl]phenyl]-[[3-dimethylamino)-3-oxopropyl]thio]methyl]thio]-propanoic acid
- P-gp
P-glycoprotein
- RT
room temperature
- TR−
Mrp2-deficient Wistar rat
- ZO-1
zonula occludens 1
Footnotes
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
References
- Bastaki M, Braiterman LT, Johns DC, Chen YH, Hubbard AL. Absence of direct delivery for single transmembrane apical proteins or their “Secretory” forms in polarized hepatic cells. Mol Biol Cell. 2002;13:225–237. doi: 10.1091/mbc.01-07-0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer JL, Phillips JM, Graf J. Preparation and specific applications of isolated hepatocyte couplets. Methods Enzymol. 1990;192:501–516. doi: 10.1016/0076-6879(90)92090-z. [DOI] [PubMed] [Google Scholar]
- Gebhardt R, Hengstler JG, Muller D, Glockner R, Buenning P, Laube B, Schmelzer E, Ullrich M, Utesch D, Hewitt N, et al. New hepatocyte in vitro systems for drug metabolism: metabolic capacity and recommendations for application in basic research and drug development, standard operation procedures. Drug Metab Rev. 2003;35:145–213. doi: 10.1081/dmr-120023684. [DOI] [PubMed] [Google Scholar]
- Ghibellini G, Vasist LS, Leslie EM, Heizer WD, Kowalsky RJ, Calvo BF, Brouwer KLR. In vitro-in vivo correlation of hepatobiliary drug clearance in humans. Clin Pharmacol Ther. 2007;81:406–413. doi: 10.1038/sj.clpt.6100059. [DOI] [PubMed] [Google Scholar]
- Groothuis GM, Hulstaert CE, Kalicharan D, Hardonk MJ. Plasma membrane specialization and intracellular polarity of freshly isolated rat hepatocytes. Eur J Cell Biol. 1981;26:43–51. [PubMed] [Google Scholar]
- Hengstler JG, Utesch D, Steinberg P, Platt KL, Diener B, Ringel M, Swales N, Fischer T, Biefang K, Gerl M, et al. Cryopreserved primary hepatocytes as a constantly available in vitro model for the evaluation of human and animal drug metabolism and enzyme induction. Drug Metab Rev. 2000;32:81–118. doi: 10.1081/dmr-100100564. [DOI] [PubMed] [Google Scholar]
- Hirano M, Maeda K, Shitara Y, Sugiyama Y. Contribution of OATP2 (OATP1B1) and OATP8 (OATP1B3) to the hepatic uptake of pitavastatin in humans. J Pharmacol Exp Ther. 2004;311:139–146. doi: 10.1124/jpet.104.068056. [DOI] [PubMed] [Google Scholar]
- Hoffmaster KA, Turncliff RZ, LeCluyse EL, Kim RB, Meier PJ, Brouwer KLR. P-glycoprotein expression, localization, and function in sandwich-cultured primary rat and human hepatocytes: relevance to the hepatobiliary disposition of a model opioid peptide. Pharm Res. 2004;21:1294–1302. doi: 10.1023/b:pham.0000033018.97745.0d. [DOI] [PubMed] [Google Scholar]
- Jorgensen L, Van Beek J, Lund S, Schousboe A, Badolo L. Evidence of Oatp and Mdr1 in cryopreserved rat hepatocytes. Eur J Pharm Sci. 2007;30:181–189. doi: 10.1016/j.ejps.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Kipp H, Arias IM. Intracellular trafficking and regulation of canalicular ATP-binding cassette transporters. Semin Liver Dis. 2000;20:339–351. doi: 10.1055/s-2000-9388. [DOI] [PubMed] [Google Scholar]
- Lam JL, Benet LZ. Hepatic microsome studies are insufficient to characterize in vivo hepatic metabolic clearance and metabolic drug-drug interactions: studies of digoxin metabolism in primary rat hepatocytes versus microsomes. Drug Metab Dispos. 2004;32:1311–1316. doi: 10.1124/dmd.32.11.. [DOI] [PubMed] [Google Scholar]
- Lam JL, Okochi H, Huang Y, Benet LZ. In vitro and in vivo correlation of hepatic transporter effects on erythromycin metabolism: characterizing the importance of transporter-enzyme interplay. Drug Metab Dispos. 2006;34:1336–1344. doi: 10.1124/dmd.106.009258. [DOI] [PubMed] [Google Scholar]
- Liu X, LeCluyse EL, Brouwer KR, Gan LS, Lemasters JJ, Stieger B, Meier PJ, Brouwer KLR. Biliary excretion in primary rat hepatocytes cultured in a collagen-sandwich configuration. Am J Physiol. 1999;277:G12–21. doi: 10.1152/ajpgi.1999.277.1.G12. [DOI] [PubMed] [Google Scholar]
- Mahagita C, Grassl SM, Piyachaturawat P, Ballatori N. Human organic anion transporter 1B1 (OATP1B1/OATP-C) and 1B3 (OATP1B3/OATP-8) function as bidirectional carriers and do not mediate GSH-bile acid co-transport. Am J Physiol Gastrointest Liver Physiol. 2007;293:G271–278. doi: 10.1152/ajpgi.00075.2007. [DOI] [PubMed] [Google Scholar]
- Nishida T, Gatmaitan Z, Che M, Arias IM. Rat liver canalicular membrane vesicles contain an ATP-dependent bile acid transport system. Proc Natl Acad Sci U S A. 1991;88:6590–6594. doi: 10.1073/pnas.88.15.6590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obach RS, Baxter JG, Liston TE, Silber BM, Jones BC, MacIntyre F, Rance DJ, Wastall P. The prediction of human pharmacokinetic parameters from preclinical and in vitro metabolism data. J Pharmacol Exp Ther. 1997;283:46–58. [PubMed] [Google Scholar]
- Oude Elferink RPJ, Bakker CTM, Roelofson H, Middelkoop E, Ottenhoff R, Heijn M, Jansen PLM. Accumulation of organic anion in intracellular vesicles of cultured rat hepatocytes is mediated by the canalicular multispecific organic anion transporter. Hepatology. 1993;17:434–444. [PubMed] [Google Scholar]
- Roelofsen H, Soroka CJ, Keppler D, Boyer JL. Cyclic AMP stimulates sorting of the canalicular organic anion transporter (Mrp2/cMoat) to the apical domain in hepatocyte couplets. J Cell Sci. 1998;111:1137–1145. doi: 10.1242/jcs.111.8.1137. [DOI] [PubMed] [Google Scholar]
- Sandker GW, Weert B, Olinga P, Wolters H, Slooff MJ, Meijer DK, Groothuis GM. Characterization of transport in isolated human hepatocytes. A study with the bile acid taurocholic acid, the uncharged ouabain and the organic cations vecuronium and rocuronium. Biochem Pharmacol. 1994;47:2193–2200. doi: 10.1016/0006-2952(94)90255-0. [DOI] [PubMed] [Google Scholar]
- Slimane TA, Trugnan G, Van ISC, Hoekstra D. Raft-mediated trafficking of apical resident proteins occurs in both direct and transcytotic pathways in polarized hepatic cells: role of distinct lipid microdomains. Mol Biol Cell. 2003;14:611–624. doi: 10.1091/mbc.E02-08-0528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talamini MA, Kappus B, Hubbard A. Repolarization of hepatocytes in culture. Hepatology. 1997;25:167–172. doi: 10.1002/hep.510250131. [DOI] [PubMed] [Google Scholar]
- Theard D, Steiner M, Kalicharan D, Hoekstra D, van Ijzendoorn SC. Cell polarity development and protein trafficking in hepatocytes lacking E-cadherin/beta-catenin-based adherens junctions. Mol Biol Cell. 2007;18:2313–2321. doi: 10.1091/mbc.E06-11-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi Y, Lippincott-Schwartz J, Arias IM. Intracellular trafficking of bile salt export pump (ABCB11) in polarized hepatic cells: constitutive cycling between the canalicular membrane and rab11-positive endosomes. Mol Biol Cell. 2004;15:3485–3496. doi: 10.1091/mbc.E03-10-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilton JC, Coleman R, Lankester DJ, Chipman JK. Stability and optimization of canalicular function in hepatocyte couplets. Cell Biochem Funct. 1993;11:179–185. doi: 10.1002/cbf.290110305. [DOI] [PubMed] [Google Scholar]
- Zamek-Gliszczynski MJ, Xiong H, Patel NJ, Turncliff RZ, Pollack GM, Brouwer KLR. Pharmacokinetics of 5 (and 6)-carboxy-2′,7′-dichlorofluorescein and its diacetate promoiety in the liver. J Pharmacol Exp Ther. 2003;304:801–809. doi: 10.1124/jpet.102.044107. [DOI] [PubMed] [Google Scholar]
- Zhang P, Tian X, Chandra P, Brouwer KLR. Role of glycosylation in trafficking of Mrp2 in sandwich-cultured rat hepatocytes. Mol Pharmacol. 2005;67:1334–1341. doi: 10.1124/mol.104.004481. [DOI] [PubMed] [Google Scholar]


