Abstract
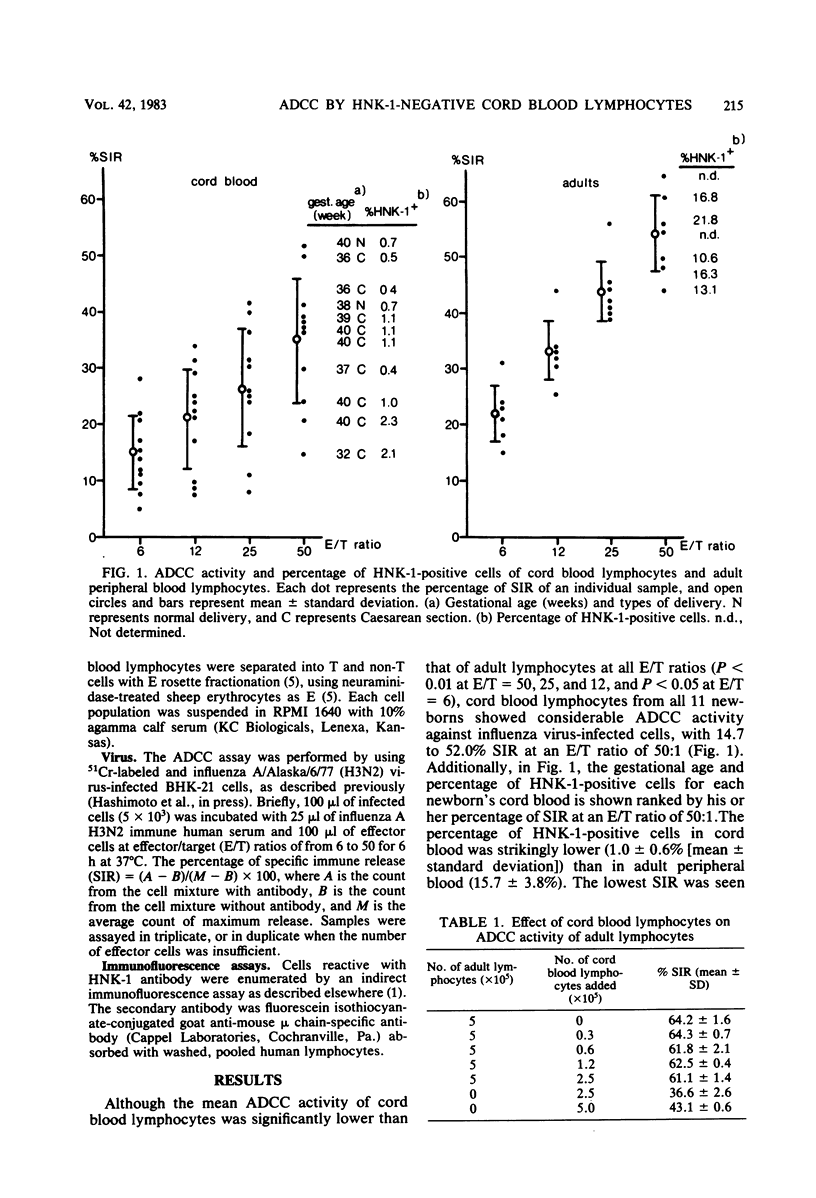
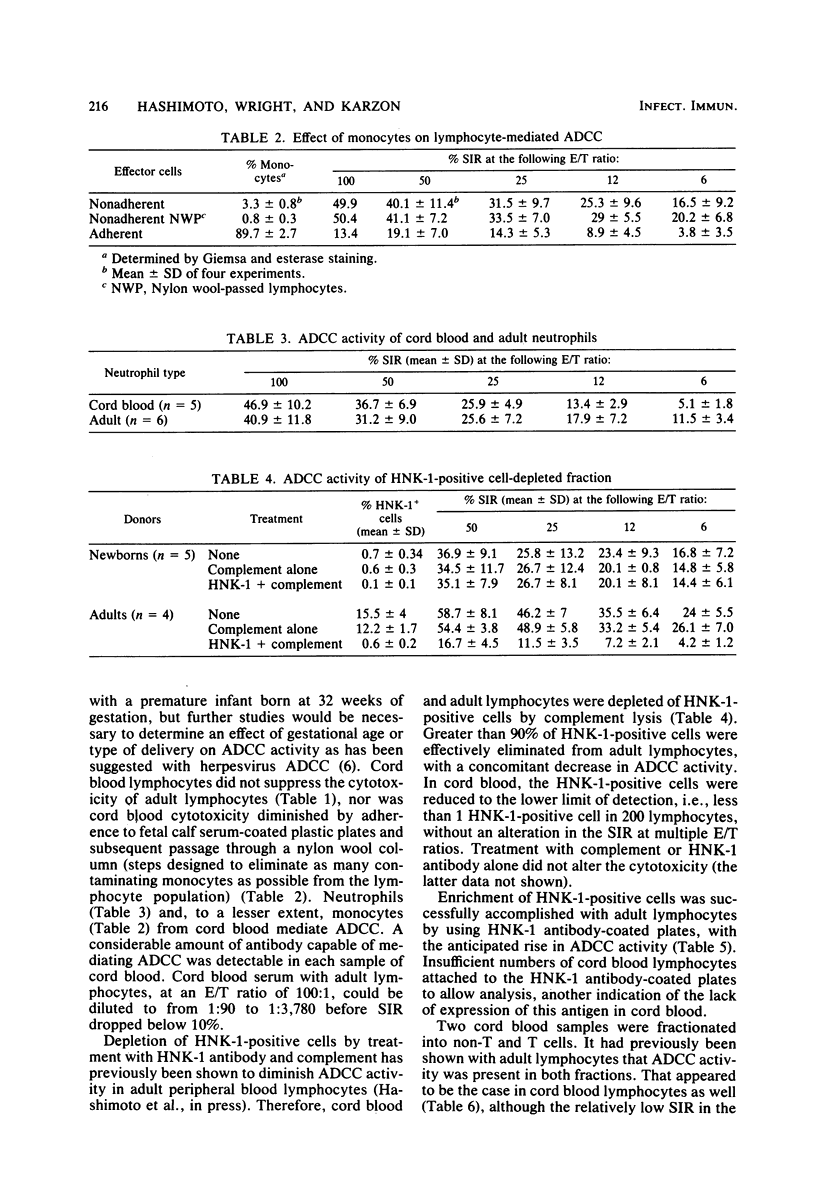
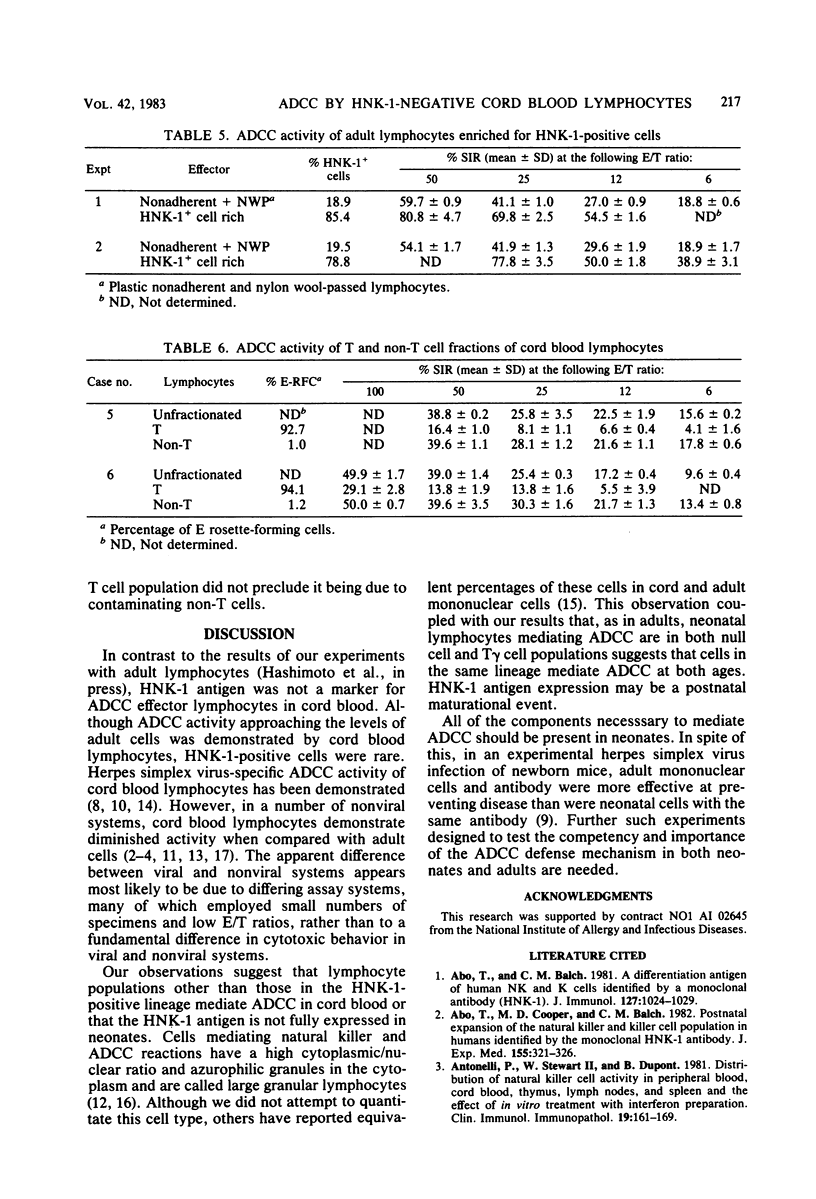
Cord blood lymphocytes, monocytes, and neutrophils from newborns were shown to mediate antibody-dependent cellular cytotoxicity (ADCC) against influenza virus-infected cells. Antibody mediating ADCC was detectable in cord plasma, indicating that all components necessary for ADCC against influenza virus-infected cells are present in newborns. Among adult lymphocytes, two effector cell populations of influenza ADCC are recognized: non-T and T gamma cells. Each of these cell types expresses an antigen recognized by monoclonal HNK-1 antibody. The proportion of HNK-1 antigen-positive lymphocytes in cord blood was markedly lower than in adult blood; furthermore, ADCC was mediated by cord blood lymphocytes which were HNK-1 negative. By lymphocyte fractionation, the effector lymphocytes in cord blood were, as in adults, non-T and T gamma cells, suggesting that HNK-1 antigen is not expressed on these cell lineages in newborns.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abo T., Balch C. M. A differentiation antigen of human NK and K cells identified by a monoclonal antibody (HNK-1). J Immunol. 1981 Sep;127(3):1024–1029. [PubMed] [Google Scholar]
- Abo T., Cooper M. D., Balch C. M. Postnatal expansion of the natural killer and keller cell population in humans identified by the monoclonal HNK-1 antibody. J Exp Med. 1982 Jan 1;155(1):321–326. doi: 10.1084/jem.155.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonelli P., Stewart W., 2nd, Dupont B. Distribution of natural killer cell activity in peripheral blood, cord blood, thymus, lymph nodes, and spleen and the effect of in vitro treatment with interferon preparation. Clin Immunol Immunopathol. 1981 May;19(2):161–169. doi: 10.1016/0090-1229(81)90059-3. [DOI] [PubMed] [Google Scholar]
- Campbell A. C., Waller C., Wood J., Aynsley-Green A., Yu V. Lymphocyte subpopulations in the blood of newborn infants. Clin Exp Immunol. 1974 Dec;18(4):469–482. [PMC free article] [PubMed] [Google Scholar]
- Frazier J. P., Kohl S., Pickering L. K., Loo L. S. The effect of route of delivery on neonatal natural killer cytotoxicity and antibody-dependent cellular cytotoxicity to herpes simplex virus-infected cells. Pediatr Res. 1982 Jul;16(7):558–560. doi: 10.1203/00006450-198207000-00013. [DOI] [PubMed] [Google Scholar]
- Kohl S., Frazier J. J., Greenberg S. B., Pickering L. K., Loo L. S. Interferon induction of natural killer cytotoxicity in human neonates. J Pediatr. 1981 Mar;98(3):379–384. doi: 10.1016/s0022-3476(81)80699-3. [DOI] [PubMed] [Google Scholar]
- Kohl S., Frazier J. P., Pickering L. K., Loo L. S. Normal function of neonatal polymorphonuclear leukocytes in antibody-dependent cellular-cytotoxicity to herpes simplex virus-infected cells. J Pediatr. 1981 May;98(5):783–785. doi: 10.1016/s0022-3476(81)80847-5. [DOI] [PubMed] [Google Scholar]
- Kohl S., Loo L. S., Pickering L. K. Protection of neonatal mice against herpes simplex viral infection by human antibody and leukocytes from adult, but not neonatal humans. J Immunol. 1981 Oct;127(4):1273–1275. [PubMed] [Google Scholar]
- Kohl S., Shaban S. S., Starr S. E., Wood P. A., Nahmias A. J. Human neonatal and maternal monocyte-macrophage and lymphocyte-mediated antibody-dependent cytotoxicity to cells infected with herpes simplex. J Pediatr. 1978 Aug;93(2):206–210. doi: 10.1016/s0022-3476(78)80497-1. [DOI] [PubMed] [Google Scholar]
- McConnachie P. R., Rachelefsky G., Stiehm E. R., Terasaki P. I. Antibody-dependent lymphocyte killer function and age. Pediatrics. 1973 Dec;52(6):795–800. [PubMed] [Google Scholar]
- Saksela E., Timonen T., Ranki A., Häyry P. Morphological and functional characterization of isolated effector cells responsible for human natural killer activity to fetal fibroblasts and to cultured cell line targets. Immunol Rev. 1979;44:71–123. doi: 10.1111/j.1600-065x.1979.tb00268.x. [DOI] [PubMed] [Google Scholar]
- Sato T., Fuse A., Kuwata T. Enhancement by interferon of natural cytotoxic activities of lymphocytes from human cord blood and peripheral blood of aged persons. Cell Immunol. 1979 Jul;45(2):458–463. doi: 10.1016/0008-8749(79)90406-4. [DOI] [PubMed] [Google Scholar]
- Shore S. L., Milgrom H., Wood P. A., Nahmias A. J. Antibody-dependent cellular cytotoxicity to target cells infected with herpes simplex viruses: functional adequacy in the neonate. Pediatrics. 1977 Jan;59(1):22–28. [PubMed] [Google Scholar]
- Tarkkanen J., Saksela E. Umbilical-cord-blood-derived suppressor cells of the human natural killer cell activity are inhibited by interferon. Scand J Immunol. 1982 Feb;15(2):149–157. doi: 10.1111/j.1365-3083.1982.tb00633.x. [DOI] [PubMed] [Google Scholar]
- Timonen T., Ortaldo J. R., Herberman R. B. Characteristics of human large granular lymphocytes and relationship to natural killer and K cells. J Exp Med. 1981 Mar 1;153(3):569–582. doi: 10.1084/jem.153.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uksila J., Lassila O., Hirvonen T. Natural killer cell function of human neonatal lymphocytes. Clin Exp Immunol. 1982 Jun;48(3):649–654. [PMC free article] [PubMed] [Google Scholar]



