Abstract
Environmental and clinical Vibrio cholerae O-1 strains isolated from the U.S. Gulf Coast region were found to be lysogenic for a vibriophage which we have designated VcA-3. Comparison of VcA-3 with the previously described vibriophages VcA-1 and VcA-2 has shown that VcA-1 and VcA-3 are homoimmune, have extensive sequence homology, but have markedly different restriction endonuclease digestion patterns. VcA-3 was found to randomly integrate into the V. cholerae RV79 chromosome and to introduce stable auxotrophic mutations. We show that all U.S. Gulf Coast environmental and clinical isolates that are lysogenic for VcA-3, including both tox+ and tox- isolates, contain the prophage integrated at an identical chromosomal site. Given the known stability of temperate mutator bacteriophages, these results suggest that there is a clonal relationship among the V. cholerae O-1 strains examined in this study, including the tox+ and tox- isolates.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blake P. A., Allegra D. T., Snyder J. D., Barrett T. J., McFarland L., Caraway C. T., Feeley J. C., Craig J. P., Lee J. V., Puhr N. D. Cholera--a possible endemic focus in the United States. N Engl J Med. 1980 Feb 7;302(6):305–309. doi: 10.1056/NEJM198002073020601. [DOI] [PubMed] [Google Scholar]
- Brenner D. J., Fanning G. R., Johnson K. E., Citarella R. V., Falkow S. Polynucleotide sequence relationships among members of Enterobacteriaceae. J Bacteriol. 1969 May;98(2):637–650. doi: 10.1128/jb.98.2.637-650.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukhari A. I. Bacteriophage mu as a transposition element. Annu Rev Genet. 1976;10:389–412. doi: 10.1146/annurev.ge.10.120176.002133. [DOI] [PubMed] [Google Scholar]
- Costa J. J., Michel J. L., Rappuoli R., Murphy J. R. Restriction map of corynebacteriophages beta c and beta vir and physical localization of the diphtheria tox operon. J Bacteriol. 1981 Oct;148(1):124–130. doi: 10.1128/jb.148.1.124-130.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes J. C., Romig W. R. Complete and Defective Bacteriophages of Classical Vibrio cholerae: Relationship to the Kappa Type Bacteriophage. J Virol. 1975 May;15(5):1231–1238. doi: 10.1128/jvi.15.5.1231-1238.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
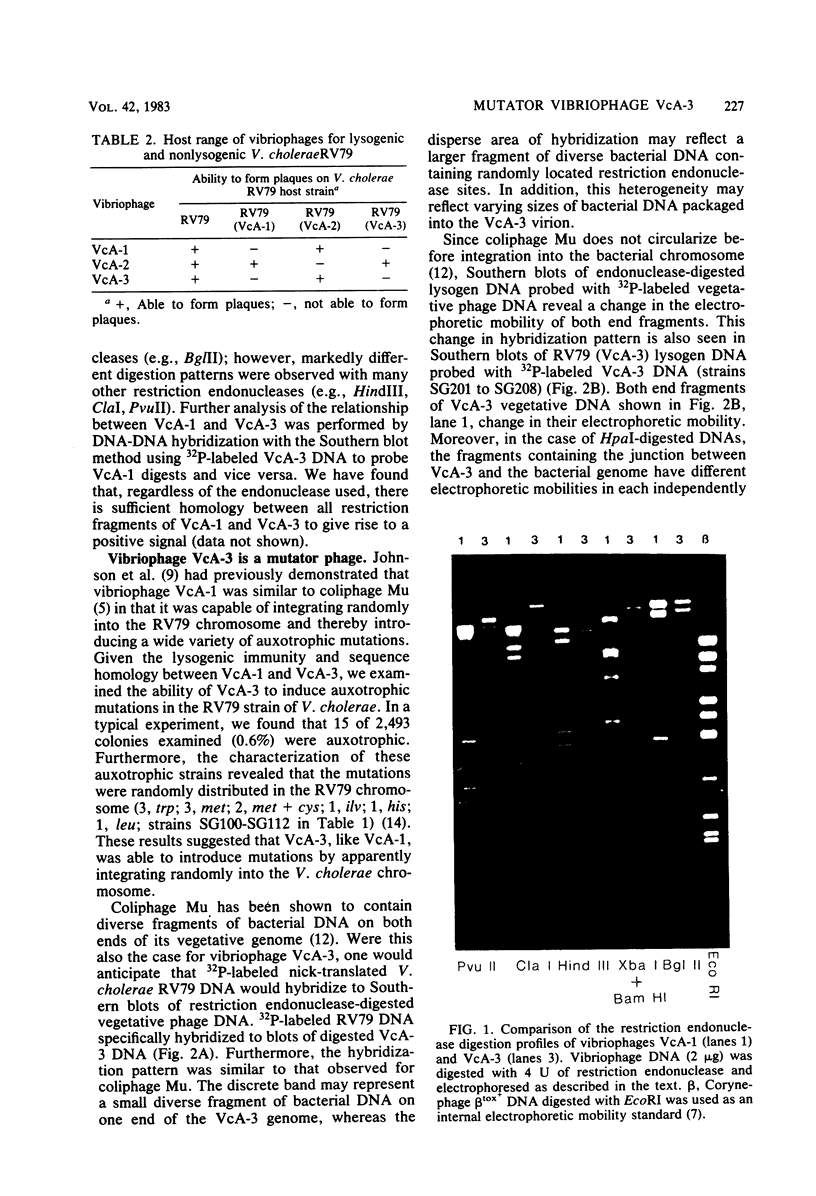
- Kaper J. B., Bradford H. B., Roberts N. C., Falkow S. Molecular epidemiology of Vibrio cholerae in the U.S. Gulf Coast. J Clin Microbiol. 1982 Jul;16(1):129–134. doi: 10.1128/jcm.16.1.129-134.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaper J. B., Moseley S. L., Falkow S. Molecular characterization of environmental and nontoxigenic strains of Vibrio cholerae. Infect Immun. 1981 May;32(2):661–667. doi: 10.1128/iai.32.2.661-667.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekalanos J. J., Moseley S. L., Murphy J. R., Falkow S. Isolation of enterotoxin structural gene deletion mutations in Vibrio cholerae induced by two mutagenic vibriophages. Proc Natl Acad Sci U S A. 1982 Jan;79(1):151–155. doi: 10.1073/pnas.79.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley S. L., Falkow S. Nucleotide sequence homology between the heat-labile enterotoxin gene of Escherichia coli and Vibrio cholerae deoxyribonucleic acid. J Bacteriol. 1980 Oct;144(1):444–446. doi: 10.1128/jb.144.1.444-446.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker C., Gauthier D., Tate A., Richardson K., Romig W. R. Expanded linkage map of Vibrio cholerae. Genetics. 1979 Feb;91(2):191–214. doi: 10.1093/genetics/91.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- TAYLOR A. L. BACTERIOPHAGE-INDUCED MUTATION IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1963 Dec;50:1043–1051. doi: 10.1073/pnas.50.6.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman J. B., DeWitt W. E., Thompson J., Muchnick C. N., Portnoy B. L., Feeley J. C., Gangarosa E. J. A case of cholera in Texas, 1973. Am J Epidemiol. 1974 Dec;100(6):487–498. doi: 10.1093/oxfordjournals.aje.a112061. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M., Benzinger R., Lawhorne L., Treiber G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology. 1970 Mar;40(3):734–744. doi: 10.1016/0042-6822(70)90218-7. [DOI] [PubMed] [Google Scholar]