Abstract
A variety of studies have documented alterations in 5-HT1A receptor binding sites in the brain of subjects with major depressive disorder (MDD). The recently identified transcription factor, nuclear deformed epidermal autoregulatory factor (NUDR/Deaf-1) has been shown to function as a transcriptional modulator of the human 5-HT1A receptor gene. The present study was undertaken to document the regional and cellular localization of NUDR in the human prefrontal cortex and to examine the levels of NUDR and 5-HT1A receptor protein in prefrontal cortex of female and male depressed and control subjects. NUDR immunoreactivity was present in neurons and glia across cortical layers and was colocalized with 5-HT1A receptor immunoreactive neurons. NUDR immunoreactivity as measured by western blot was significantly decreased in the prefrontal cortex of female depressed subjects (42%, p=0.02) and unchanged in male depressed subjects relative to gender-matched control subjects. Similarly, 5-HT1A receptor protein level was significantly reduced in the prefrontal cortex of female depressed subjects (46%, p=0.03) and unchanged in male depressed subjects as compared to gender-matched control subjects. Reduced protein expression of NUDR in the prefrontal cortex of female subjects with MDD may reflect a functional alteration in this transcription factor, which may contribute to the decrease in 5-HT1A receptors observed in the same female subjects with MDD. In addition, the gender-specific alterations in cortical NUDR and 5-HT1A receptors protein could represent an underlying biological mechanism associated with the higher incidence of depression in women.
Keywords: 5-HT1A receptors, female, major depression, prefrontal cortex, transcription factor
Introduction
Clinical and human postmortem studies have provided evidence supporting the hypothesis that serotonin neurotransmission is reduced in major depressive disorder (MDD), with specific alterations noted in serotonin receptor subtypes. The 5-HT1A receptor is important since these receptors may function as somatodendritic autoreceptors on serotonin neurons and thereby regulate the activity of dorsal raphe neurons. In addition, 5-HT1A receptors are also located postsynaptically on neurons and glia in the prefrontal cortex (PFC) and limbic structures (Sotelo etal., 1990; Riad et al., 2000; Hoyer et al., 2002). Moreover, serotonin-selective reuptake inhibitors (SSRI's) are believed to exert at least part of their antidepressant effects by a desensitization of 5-HT1A receptors. It is also important to note that previous studies have reported that estrogen reduces 5-HT1A autoreceptor binding sites, mRNA levels and basal and stimulated [35S]GTP-γ-S binding in the dorsal raphe nucleus and decreased postsynaptic 5-HT1A receptor binding sites in the hypothalamus (Lu and Bethea, 2002). Thus, estrogen treatment appears to modulate 5-HT1A receptor expression in distinct brain regions.
In vivo imaging studies have reported alterations in 5-HT1A receptors in the brain of depressed patients. For example, Sargent et al.(2000) used positron emission tomography (PET) with the selective 5-HT1A receptor antagonist, [11C] WAY-100635, and reported decreased 5-HT1A receptor binding potential (BP) in the frontal, temporal and limbic cortices of male subjects with MDD. Bhagwagar et al. (2004) noted the decrease in 5HT1A binding potential throughout the cortex and limbic structures persisted in MDD despite clinical remission. Meltzer and colleagues (2004) saw no changes in 5-HT1A receptor binding potential in PFC but noted a decrease in 5-HT1A receptor binding in the dorsal raphe nuclei (DRN) of late-life depressives.
Although some studies of human postmortem tissue have also reported alterations in 5-HT1A receptors in specific brain regions in suicide or subjects with MDD, the results have not been consistent. Stockmeier and colleagues (1998) reported elevated radioligand binding to presynaptic 5-HT1A autoreceptors in specific subnuclei of the midbrain dorsal raphe of suicide victims with MDD. However, Arango et al. (2001) subsequently reported a decrease in 5-HT1A receptor “binding capacity” in the dorsal raphe of depressed suicide victims relative to controls. Yet a recent report from these investigators contradicts their earlier finding and reveals an increase in 5-HT1A autoreceptor binding sites in the rostral dorsal raphe of suicide subjects (Boldrini et al., 2007) which is consistent with the original Stockmeier et al.(1998) report. Several other groups have measured postsynaptic 5-HT1A receptors in the PFC of depressed suicide victims but again with disparate results. Matsubara et al. (1991) reported an increase in 5-HT1A receptors in PFC areas 8,9 of so-called non-violent suicide victims that were not psychiatrically characterized. Also, Arango et al. (1995) reported elevated binding to 5-HT1A receptor binding sites in the ventrolateral PFC only of uncharacterized suicide victims. In contrast, Lopez-Figueroa et al. (2004) found decreased 5-HT1A receptor mRNA levels in the dorsolateral PFC in MDD and Hsiung et al. (2003) reported reductions in 5-HT1A receptor signaling in the occipital cortex of depressed suicide victims. Stockmeier et al. (1997), however, found no changes in binding to 5-HT1A receptors in area 10 of the PFC of suicide victims with MDD. Although these reports contain conflicting results, they suggest that alterations in 5-HT1A receptors do exist in the brain of depressed subjects.
Recent data reveal several single nucleotide polymorphisms (SNPs) of the 5-HT1A receptor gene, although not all these mutations have a high frequency in the general population or an association with a psychiatric illness (Erdmann et al., 1995; Kawanishi et al., 1998; Wu and Comings, 1999; Arias et al., 2002). Recently, a novel C(-1019)G polymorphism in the 5-HT1A receptor gene promoter was identified (Lemonde et al., 2003). This polymorphism is prevalent in the normal population (Wu and Comings, 1999) and the G(-1019) allele is two-fold more frequent in subjects with MDD and four-fold enriched in suicide victims relative to matched control subjects (Lemonde et al., 2003). Recently, a nuclear protein complex, nuclear deformed epidermal autoregulatory factor-1 (Deaf-1) or the human homolog, NUDR, was identified that binds to the C(-1019) allele of the 5-HT1A receptor and represses the transcription activity of the C(-1019) allele of the 5-HT1A receptor promoter (Lemonde et al., 2003; Albert and Lemonde, 2004). This binding does not occur with the G(-1019) allele of the 5-HT1A receptor promoter. In vitro cell culture studies showed that this transcriptional repression results in a significant decrease in endogenous 5-HT1A receptor mRNA, protein and binding sites (Lemonde et al., 2003). Immunocytochemical studies revealed that NUDR is localized to neurons in the rodent raphe nuclei, hippocampus and the frontal cortex and is co-localized with 5-HT1A receptors in these brain regions (Lemonde et al., 2003). However, recent data in non-serotonergic cells indicated that NUDR functions as an enhancer rather than repressor of 5-HT1A receptors (Czesak et al., 2006). These data suggest that cell-specific regulation by NUDR may underlie region specific alterations in the expression of 5-HT1A receptors.
Given the transcriptional regulatory role of NUDR on 5-HT1A receptors, the colocalization of NUDR and 5-HT1A receptors in neurons, the interaction of NUDR with the C(-1019) polymorphism of the 5-HT1A receptor gene, and the substantial evidence of alterations in 5-HT1A receptors in depressed subjects, we sought to determine whether alterations in NUDR may exist in the brain of depressed individuals, whether this may be associated with alterations in 5-HT1A receptors, and whether gender-specific alterations in these two proteins may exist in subjects with depression. The present study was designed to quantify NUDR and 5-HT1A receptor protein levels in area 10 of the PFC of female and male subjects diagnosed with major depressive disorder and in psychiatrically-normal control subjects matched for gender.
Methods
Subjects
All procedures in our study were approved by the Institutional Review Board of the University of Mississippi Medical Center and University Hospitals of Cleveland. Human brain specimens were obtained in the course of routine autopsies conducted at the Cuyahoga County Coroner's Office, Cleveland, OH, after obtaining written consent from the legally-defined next-of-kin. Blood and urine samples from all subjects were examined by the coroner's office for psychotropic medications and substances of abuse. Subjects included 13 female and 11 male subjects diagnosed with MDD, and one male subject with dysthymia. The 13 female and 12 male control subjects never met criteria for an Axis I illness and had no history of a neurological disorder. Each depressed subject was matched with a control subject for gender and as closely as possible for age and post-mortem interval. Some of the pairs were also matched for race. The demographics for each subject are summarized in Tables 1.
Table 1.
Demographic characteristic of the subjects
| Pair | Sex/Race Age [y] | PMI [h] | pH | Cause of death | Sex/Race Age [y] | PMI [h] | pH | Cause of death | Episodesa S/M | Age of onset [y] | Duration of MDD [y] |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | MDD | ||||||||||
| 1 | F/B/58 | 12 | 6.2 | HCD | F/W/63 | 18 | 6.3 | Heart | M | 20 | 43 |
| 2 | F/B/67 | 16 | 6.4 | CSHD | F/W/67 | 17 | 6.7 | Artherosclerotic aneurism | M | 18 | 49 |
| 3 | F/B/51 | 22 | 6.3 | Heart | F/W/50 | 23 | 6.8 | Hanging | M | 25 | 25 |
| 4 | F/W/50 | 27 | 6.7 | Heart | F/W/50 | 28 | 6.5 | ACD | M | 30 | 20 |
| 5 | F/W/83 | 25 | 6.7 | Heart | F/W/87 | 24 | 6.6 | Aortic aneurism | M | 46 | 41 |
| 6 | F/W/65 | 26 | 6.2 | Heart | F/W/73 | 17 | 6.6 | Aortic aneurism | M | 72 | 1 |
| 7 | F/W/38 | 11 | 6.5 | HCD | F/W/38 | 12 | 6.4 | Drug overdose | M | 25 | 13 |
| 8 | F/B/80 | 21 | 6.8 | HCD | F/W/78 | 25 | 6.9 | Jumper | M | 45 | 33 |
| 9 | F/W/46 | 24 | 6.3 | Homicide | F/W/38 | 24 | 6.5 | Drug overdose | M | 30 | 8 |
| 10 | F/W/23 | 11 | 6.8 | MVA | F/W/25 | 17 | 6.7 | Hanging | M | 18 | 7 |
| 11 | F/W/78 | 11 | 6.4 | Heart disease | F/W/72 | 19 | 6.6 | Drowning | S | 33 | 39 |
| 12 | F/W/49 | 29 | 6.6 | Heart disease | F/W/48 | 24 | 6.1 | CO poisoning | M | 34 | 14 |
| 13 | F/W/44 | 32 | 6.7 | Heart | F/W/36 | 25 | 6.8 | Astmatic brochitis | S | 20 | 16 |
|
| |||||||||||
| 14 | M/W/71 | 24 | 6.8 | Cardiac rupture | M/W/74 | 24 | 6.9 | Hanging | M | 72 | 2 |
| 15 | M/W/44 | 6 | 6.7 | Aortic aneurysm | M/W/45 | 8 | 6.7 | Multiple knifing | S | 43 | 2 |
| 16 | M/W/59 | 6 | 6.8 | Heart | M/W/62 | 5 | 6.7 | Hanging | M | 43 | 19 |
| 17 | M/W/27 | 17 | 6.9 | Homicide | M/B/30 | 18 | 6.9 | SIGSW chest | M | 27 | 3 |
| 18 | M/W/73 | 22 | 6.7 | Heart | M/W/74 | 25 | 6.7 | SIGSW head | M | 50 | 24 |
| 19 | M/W/58 | 21 | 6.8 | Heart disease | M/W/53 | 29 | 6.7 | SIGSW head | M | 28 | 25 |
| 20 | M/W/47 | 17 | 6.9 | Heart | M/W/47 | 11 | 6.8 | SIGSW head | M | 20 | 27 |
| 21 | M/W/47 | 25 | 6.1 | Pulmonary embolism | M/B/46 | 17 | 6.3 | Homicide | S | 45 | 1 |
| 22 | M/B/53 | 23 | 6.8 | Electrocution | M/W/54 | 23 | 6.2 | CO | M | 51 | 3 |
| 23 | M/W/57 | 10 | 6.7 | Heart | M/W/59 | 12 | 6.8 | Hanging* | 56 | 3 | |
| 24 | M/W/82 | 16 | 6.7 | Aneurysm | M/W/82 | 12 | 6.5 | CO | M | 73 | 9 |
| 25 | M/B/27 | 11 | 6.8 | Asthma | M/W/36 | 11 | 6.9 | Undetermined | M | 35 | 1 |
F - female; M-male; W - white; B- black; PMI - post-mortem interval in hours; Episodes – S-single; M-multiple; AAHCD- Hypertensive cardiac disease; MVA-motor vehicle accident; ACD- Atherosclerotic cardiovascular disease; CSHD - Coronary sclerotic heart disease;
Mean ± SEM of female controls: Age (56 ± 5 years); PMI (20 ± 2 hours); pH (6.5 ± 0.06). Mean ± SEM of female MDD: Age (56 ± 5 years); PMI (21 ± 1 hours); pH (6.6 ± 0.06); Onset of depression: (32.0±4.2years; Mean ± SEM); Duration of depression: (23.7 ± 4.3years; Mean ± SEM);
Mean ± SEM of male controls: Age (54 ± 5 years); PMI (16.5 ± 2 hours); pH (6.7 ± 0.06); Mean ± SEM of male MDD: Age (55 ± 4.5 years); PMI (17 ± 2 hours); pH (6.7 ± 0.07); Onset of depression: (45.3 ± 4.8 years; Mean ± SEM); Duration of depression: (9.9 ± 3.0 years; Mean ± SEM);
Subject diagnosed as dysthymia alone (no MDD).
Retrospective, informant-based psychiatric assessments were performed for all depressed and control subjects as previously described (Stockmeier et al., 2004). About three months after the death of the subjects, a trained interviewer administered the Schedule for Affective Disorders and Schizophrenia: lifetime version (SADS-L, Spitzer, 1978) to knowledgeable next-of-kin of 18 of the depressed subjects, as previously described (Stockmeier et al., 1997). The Structured Clinical Interview for DSM-IV Psychiatric Disorders (SCID) was administered to next-of-kin of the seven remaining depressed subjects (First et al., 1996). Axis I psychopathology was independently assessed by a clinical psychologist and psychiatrist and consensus diagnosis was reached in conference using information from the interview, previous hospitalizations and doctors' records, and the coroner's office. Responses from the subjects evaluated with the SADS-L were also recorded in the SCID, and regardless of the structured diagnostic interview used, all subjects met criteria for MDD based on the Diagnostic and Statistical Manual of Mental Disorders-IV (DSM-IV, American Psychiatric Association, 1994). Twenty three subjects met DSM-IV criteria for a major depressive episode within the last month of life and MDD was in partial remission for one subject.
All 13 female depressed subjects met DSM-IV criteria for an Axis I diagnosis of MDD. Twelve female subjects met criteria for MDD within the last month of life and one female subject was in partial remission during the last month. Eleven female subjects had multiple depressive episodes during their life and 2 female subjects had a single episode. Alcohol dependence in one female subject and alcohol abuse in another were in remission at the time of death. Among the 13 female depressed subjects, 11 had a prescription for an antidepressant medication at the time of death, although postmortem toxicology screening revealed the presence of only diazepam in one subject. The mean (± SEM) age of onset of depression was 32.0 ± 4.2 years and the average duration of illness was 23.7 ± 4.3 years.
Eleven male depressed subjects met DSM-IV criteria for an Axis I diagnosis of MDD, and all 11 met this criteria for MDD in the last month of life. One depressed male subject met clinical criteria for dysthymia. Nine male subjects had multiple depressive episodes during their life and 2 male subjects had a single episode. Two male subjects had a history of alcohol abuse and one of benzodiazepine abuse, although these disorders were in remission at the time of death. Among the 12 depressed male subjects, eight had a prescription for an antidepressant medication at the time of death, although postmortem toxicology screening revealed only the presence of temazepam. The mean (± SEM) age of onset of depression was 45.3 ± 4.8 years and the average duration of illness was 9.9 ± 3.1 years.
Tissue sampling
The study was carried out on blocks of tissue which were dissected approximately 1-2 cm from the frontal pole of the right hemisphere. The tissue blocks consisted of Brodmann's area 10, however, some portions of the blocks also contained cytoarchitectonic features of adjacent areas 11, 47 or 9. In order to assure that sections or tissue punches were collected consistently from area 10, two 30μm thick sections were cut from each block and stained for Nissl prior to sampling. Microscopic examination distinguished area 10 from the adjacent prefrontal areas by the presence of a very broad, densely packed layer IV, large size of neurons in layers III, V and VI and by relatively similar width of layers III and V (Rajkowska and Goldman, 1995).
Immunohistochemistry
Immunohistochemistry was used to examine the expression and laminar distribution of NUDR in the human PFC of normal control subjects. Frozen 30 μm sections from PFC (area 10) were fixed in 4% paraformaldehyde (in 0.05M phosphate-buffered saline, PBS) for 1h at room temperature, preincubated in 5% normal horse serum in PBS for 30 min and then incubated for 24h at 4°C in the same solution containing rabbit anti-NUDR polyclonal antibody (1:50, Lemonde et al., 2003). Sections were washed in PBS and incubated for 4h at room temperature in biotinylated horse anti-rabbit IgG (1:200; Vector Laboratories) in PBS buffer. After incubation, the sections were processed using the Vectastain ABC immunoperoxidase kit (Vector) for 24h at 4°C. Antibody distribution was visualized using 3,3′-diaminobenzidine tetrahydrochloride (DAB; 0.05%, Sigma).
Immunofluoroscence
Immunofluoroscence was used to examine the cellular expression of NUDR and colocalization with 5-HT1A receptor in the human PFC. Frozen 20 μm sections from PFC (area 10) were subjected to double fluorescent immunolabeling procedure to detect in individual sections simultaneously NUDR and the neuronal marker - neuronal nuclei (NeuN), NUDR and astrocytic marker – glial fibrillary acidic protein (GFAP), NUDR and 5-HT1A receptor. Each section was incubated overnight with the rabbit anti-NUDR polyclonal antibody (1:50) and a mouse anti-GFAP monoclonal antibody (1:1000; Chemicon), a mouse anti-NeuN monoclonal antibody (1:1000; Chemicon) or a mouse anti-5-HT1A polyclonal antibody (1:100, Affinity Bioreagent) respectively. After washes in TBS, sections were incubated for 90 min with a mixture of a goat anti-mouse antibody conjugated with the fluorochrome Cy5 (1: 200; Jackson Immunochemicals) and a goat anti-rabbit antibody conjugated to fluorochrome Cy2 (1:200; Jackson Immunochemical) and washed again before coverslipping. Omission of the primary or the secondary antibody resulted in the absence of immunostaining. The cellular localization of immunofluoroscence was analyzed using a Nikon confocal microscope.
Western blot
Immunolabeling of NUDR and the 5-HT1A receptor were determined in tissue punches from PFC (area 10). Equal volumes of protein samples containing mostly membrane and nuclear fraction (30ug of protein) were resolved on 12.5% sodium dodecyl sulfate polyacrylamide gel and blotted on nitrocellulose membrane. The blots were incubated overnight at 4°C with affinity-purified primary rabbit anti-NUDR polyclonal antibody that was generated to an amino-terminal peptide from NUDR (1:5000, Lemonde et al., 2003) and with rabbit anti -5-HT1A receptor polyclonal antibody (1:2000; Aviva System Biology). Antibody specificity experiments revealed that preincubation of the primary 5-HT1A receptor antibody with a specific 5-HT1A peptide (Aviva System Biology), and preincubation of the primary NUDR antibody with a specific NUDR peptide completely blocked immunoreactivity. As a control for transfer and sample loading, anti–β-actin monoclonal antibody was used (1:5000, Chemicon). Immunoreactivity of NUDR and 5-HT1A receptor was investigated in pairs of depressed and control subjects matched for age, gender and PMI. Each subject pair was immunoblotted in duplicate. The relationship between optical density values and the concentrations of NUDR and 5HT1A receptor immunoreactivities respectively was determined by loading increasing concentrations of sample onto gels and immunoblotting with anti-NUDR or anti-5HT1A receptor antibody. Relative optical density values of immunoreactive bands were measured and presented as a function of protein concentration. The relationship between optical density and protein concentrations was linear (Figure 1).
Figure 1.
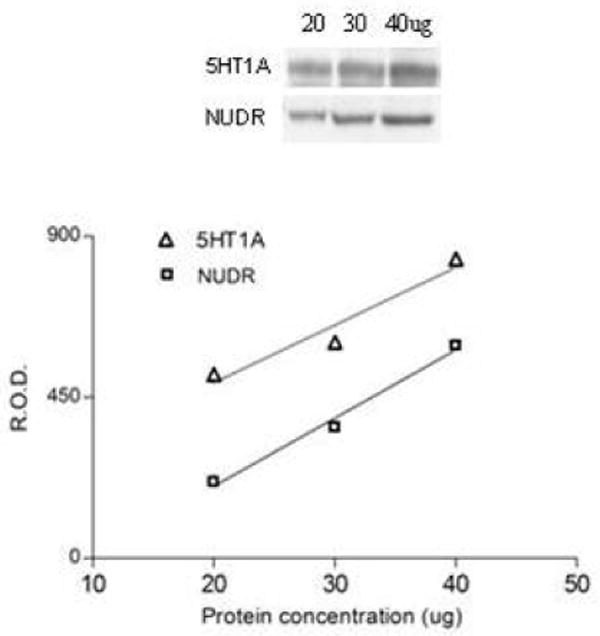
Relationship between the optical density values of NUDR and 5-HT1A receptor immunoreactivity and increasing total protein concentrations (20, 30, 40ug) of human PFC.
Relative optical density of NUDR and 5-HT1A bands were analyzed using imaging software (MCID Elite 7.0; Imaging Research, St. Catherines, Ontario, Canada) and normalized by the optical density of the corresponding β-actin band. The anti-NUDR and anti-5-HT1A receptor antibody-detected bands on the gel corresponded to a molecular weight of 59kDa and 56kDa, respectively.
Genotype analysis
Genomic DNA extracted was from brain samples of the 13 female and 12 male depressed subjects and 13 female and 12 male matched controls used for protein study. DNA was isolated from the brain tissue using the QIAamp DNA Mini Kit (Qiagen). The protocol was followed exactly, starting with 25mg of frozen tissue and eluting the DNA with 50μL of buffer AE. All DNA samples were stored at -20°C. Genotyping of all DNA samples for the 5-HT1A C(-1019)G SNP was performed by Q-PCR using TaqMan SNP Genotyping Assay #C_11904666_10 (Applied Biosystems). The genotyping reaction was carried out in a 10μL final volume of reaction mixture containing: 1μL of DNA, 5μL of TaqMan Universal PCR Master Mix, 0.5μL of the appropriate 20X TaqMan SNP Genotyping Assay Mix, and 3.5μL of nuclease-free water. Additionally, duplicate no template controls (nuclease-free water instead of DNA) were run in order to detect contamination of the reaction mixture. The Q-PCR run was performed using the Rotor-Gene 3000 Cycler (Corbett Research, Australia). The thermocycler program was as follows: 95°C for10 min (enzyme activation step); 40 cycles: 92°C for 15 sec, 60°C for 1 min; hold at 25°C. Genotypes were then determined using the Allelic Discrimination function of the Rotor-Gene software (version 6). The 5-HT1A Taqman assay was validated by genotyping 20 samples previously analyzed by manual sequencing (Lemonde et al., 2003).
Statistical analysis
Data were analyzed using a matched pairs design. Each subject was measured at least twice so that there are two sources of potentially correlated observations, replicates within subjects and subjects within pairs. That is, data are considered multilevel with subjects representing the first level and pairs representing the second level. Statistical tests were performed separately for male and female groups. A maximum likelihood mixed models test was used to estimate parameters of the models, assuming pairs and subjects within pairs were random components (SAS, Little et al. 1996). As a first step, unadjusted models were fit to compare depressives versus controls without adjusting for potential confounders. Gender-specific adjusted models included the main effect for comparing depressives versus controls and covariates for age, PMI, pH and cause of case death (suicide vs. non-suicide). Interactions between the main effect, depressives versus controls, and each of the potentially confounding covariates was investigated and dropped from the model if it was not statistically significant. Results for the depressed and control groups are reported as mean ± standard error based on the mixed model. Results for main effects are considered significant if p<0.05.
Genotype frequencies between depressed and control subjects were compared by χ2 analysis with two-tailed p values; allele frequencies were compared by Fisher's exact test with two-tailed p values. Statistical analyses of the genotype data were performed with GraphPad Prism software using 95% confidence intervals.
Results
Laminar and cellular localization of NUDR immunoreactivity and colocalization with 5-HT1A receptor
The distribution of NUDR immunoreactivity was observed in cellular profiles in both prefrontal gray and white matter of PFC (area 10). In the gray matter, immunoreactivity was spread across all cortical layers with the highest abundance in superficial layer II and upper layer III (Figure 2B). Double fluorescence immunostaining performed for NUDR and neuronal marker NeuN as well as for NUDR and astrocytic marker GFAP showed that the majority of NUDR-positive cortical cells represent neurons (Figure 3A). However, NUDR immunoreactivity was also observed in some glial cells expressing GFAP (Figure 3B). In neurons, NUDR was localized to nuclear and perinuclear areas, whereas in glia, NUDR immunoreactivity was restricted to the nuclear area. Co-labeling of NUDR and 5-HT1A receptor revealed that most cortical cells expressing 5-HT1A receptors also contain NUDR protein. NUDR immunoreactivity was localized mostly to the nuclear and perinuclear areas whereas 5-HT1A receptors immunoreactivity was present in the cell body and neuronal processes (Figure 3C).
Figure 2.
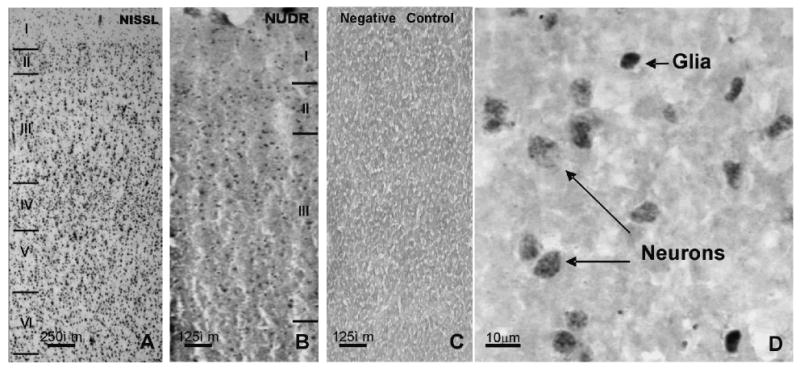
Laminar and cellular localization of NUDR-immunoreactivity in the human prefrontal cortical area 10. A: Nissl stained section of a non-psychiatric control subject showing the typical cytoarchitectonic features of area10 (wide layer IV, in the center of the cortical width, relatively narrow layer III). Image captured under the 2× objective. B: Distribution of NUDR immunoreactivity in the same subject (adjacent section) to the one shown in A. C: Negative control showing the complete lack of NUDR immunoreactivity in the absence of primary antibody on the section adjacent to that shown in B. Images B & C were captured under the 4× objective. D: High power (40× objective) micrograph of NUDR immunoreactivity showing the presence of immunoreactive product in both neurons and glial cells.
Figure 3.
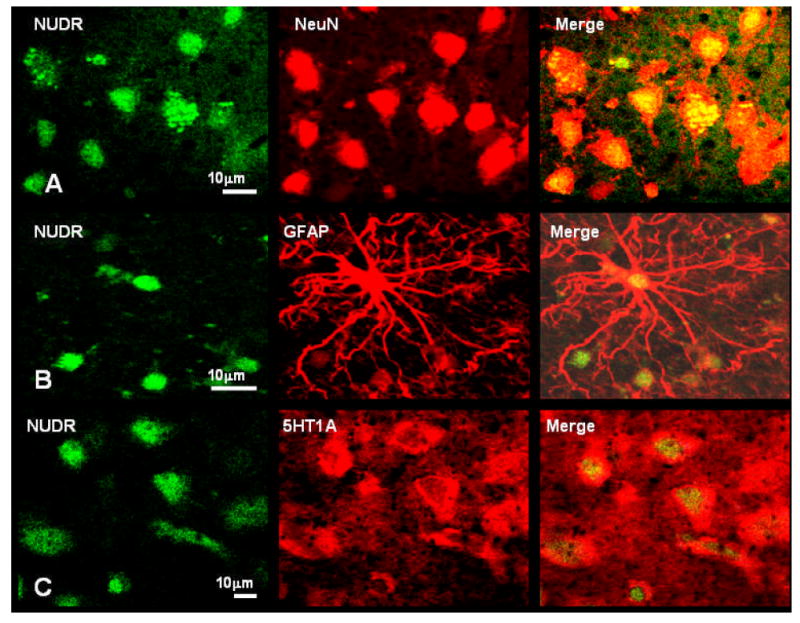
Colocalization of NUDR immunoreactivity in neurons and glia. A) Co-labeling of NUDR immunoreactivity (green) with the neuronal marker NeuN (red). NUDR protein (yellow) is localized to neuronal nuclei and perinuclear areas. B) Co-labeling of NUDR immunoreactivity (green) with the astrocytic marker GFAP (red). NUDR (yellow) is localized in glial nuclei. C) Colocalization of NUDR immunoreactivity (green) and immunoreactivity for 5-HT1A receptor (red). NUDR immunoreactivity (yellow) is present in nuclei of majority of cells expressing 5-HT1A receptor immunoreactivity.
Protein levels of NUDR
Representative immunoblots of NUDR and β-actin protein from control and depressed subjects are shown in Figure 4A. The results of the Western blot analyses of NUDR protein in PFC (area 10) of depressed and control subjects are summarized in Figure 5. The level of NUDR for each subject was determined as the ratio of the optical density of NUDR band to the optical density of the actin band. There was no significant difference between control and depressed subjects in the mean level of actin in both female (t12=0.49, p=0.63) and male groups (t11= 0.49, p=0.63). The mean protein level of NUDR in the PFC was significantly decreased by 42% in the female depressed group (0.52 ± 0.08, t12=2.64, P=0.022) compared to the matched female control group (0.89 ± 0.16, Figure 5A). Comparison of individual matched pairs revealed that the mean NUDR protein level was decreased in eight of the 13 depressed female subjects compared to the matched control subjects (Figure 5B). The mean NUDR protein level in the PFC of the male depressed subjects (0.47 ± 0.06, t10.2=0.33, P=0.474) was not significantly different from the matched male control subjects (0.51 ± 0.11, Figure 5C). In contrast to the female subjects, only five of the 12 depressed male subjects had lower levels of NUDR protein compared to the matched control subjects (Figure 5D).
Figure 4.
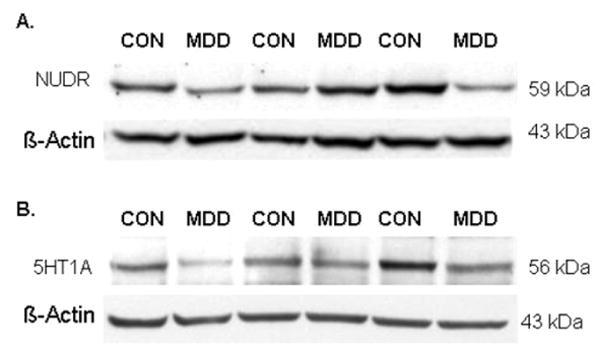
Representative Western blots showing the immunolabeling of NUDR (A) and 5-HT1A receptor (B) and β-actin in PFC of 3 pairs of control and matched MDD subjects.
Figure 5.
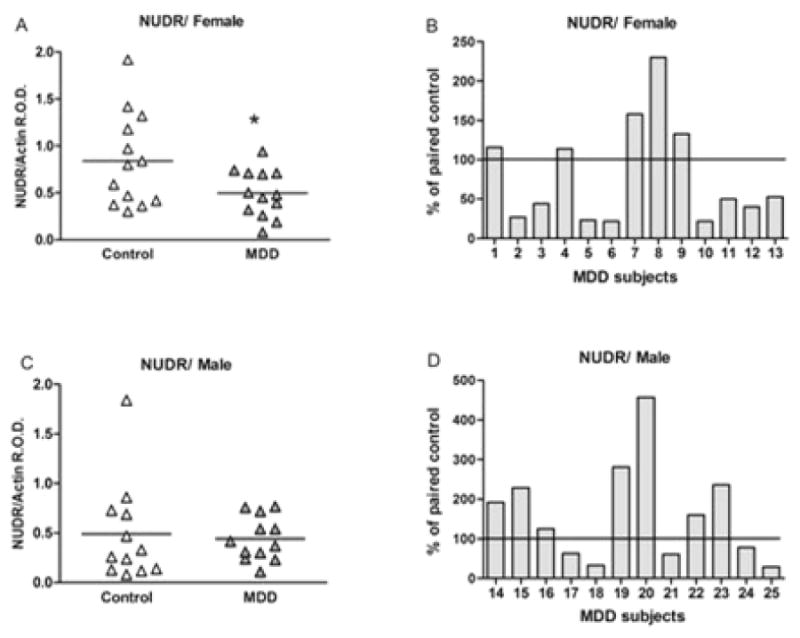
NUDR protein levels in PFC of 13 female (A, B) and 12 male (C, D) control and MDD subjects expressed as the mean ± SEM of NUDR/actin R.O.D. Data were analyzed using a maximum likelihood mixed models test. Female subject groups: control subjects (0.89 ± 0.16) compared to the matched MDD subjects (0.52 ± 0.08, t12=2.64, *P=0.022). Male subject groups: control subjects (0.51 ± 0.11) compared to the matched MDD subjects (0.47 ± 0.06, t10.2=0.33, P=0.474). NUDR levels in PFC of female (B) and male (D) depressive subjects expressed as percentage of values from paired control subjects.
Protein level of 5-HT1A receptor
Representative immunoblots of 5-HT1A and β-actin protein from control and depressed subjects are shown in Figure 4B. The results of the Western blot analyses of 5-HT1A protein in PFC (area 10) of depressed and control subjects are summarized in Figure 6. The level of 5-HT1A for each subject was determined as the ratio of the optical density of 5-HT1A band to the optical density of the actin band. There was no significant difference between control and depressed subjects in the protein level of actin in both female (t12=0.49, p=0.63) and male group (t22=0.01, p=0.99). The mean protein level of the 5-HT1A receptor in the PFC was significantly decreased by 46% in the female depressed group (0.31 ± 0.04, t12.8=2.43, P=0.030) compared to the matched female control group (0.58 ± 0.09) (Figure 6A). Comparison of individual matched pairs revealed that the mean 5-HT1A receptor protein level was reduced in eleven of the thirteen depressed female subjects compared to the matched control subjects (Figure 6B). No significant difference in the level of 5-HT1A receptor protein was found in the PFC of male depressed subjects (0.40 ± 0.04, t11.3=0.39, P=0.702) relative to the matched male control subjects (0.42 ± 0.04, Figures 6C). Comparison of individual matched pairs showed that only five of the twelve depressed male subjects had lower levels of 5-HT1A receptor protein compared to the matched control subjects (Figure 6D).
Figure 6.
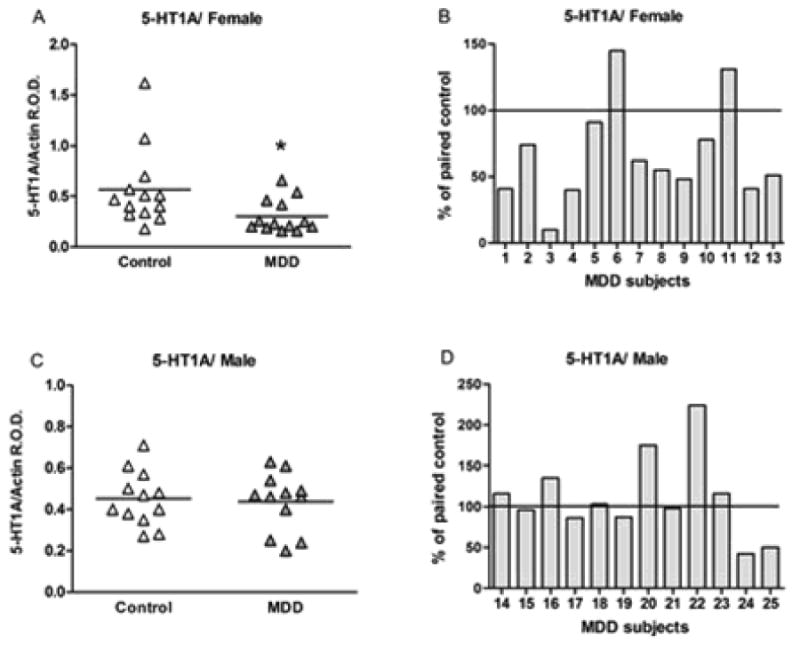
5-HT1A receptor levels in PFC of 13 female (A, B) and 12 male (C, D) control and depressive subjects expressed as the mean ± SEM of 5-HT1A/actin R.O.D. Data were analyzed using a maximum likelihood mixed models test. Female subject groups: control subjects (0.58 ± 0.09) compared to the matched MDD subjects (0.31 ± 0.04, t12.8=2.43, *P=0.030). Male subject groups: control subjects (0.42 ± 0.04) compared to the matched MDD subjects (0.40 ± 0.04, t11.3=0.39, P=0.702). 5-HT1A receptor levels in PFC of female (B) and male (D) depressive subjects expressed as percentage of values from paired control subjects.
Effects of covariates on NUDR and 5-HT1A receptors
The effect of the potential confounding variables such as age, PMI, brain pH, cause of death (suicide or non-suicide), onset of depression or duration of depression on protein levels of NUDR and 5-HT1A receptor was evaluated in the female and male subject groups using the adjusted mixed models test. There were no statistically significant differences in age, PMI and pH between female and male groups and there were no significant changes in the results after adjusting for age, PMI, brain pH as well as cause of death. There was a statistically significant difference between female and male MDD in the onset of depression (t23=2.08; p=0.048) and duration of depression (t23=2.56; p=0.017). However, there is no statistical evidence to support effects of these potentially confounding variables on changes in protein levels of NUDR and 5-HT1A receptors related to depression.
C(-1019)G 5-HT1A promoter polymorphism with NUDR AND 5-HT1A protein level
Since the 5-HT1A C(-1019)G polymorphism blocks regulation by NUDR of 5-HT1A receptor expression and has been associated with depression, the allele distribution in female and male control and depressed subjects was determined as shown in Table 2. No statistically significant difference in the allele or genotype frequency was observed, although the male cohort showed a trend for association of the G(-1019) allele with depression that was too small to reach statistical significance. These data suggest that decreased expression of 5-HT1A receptors observed in depressed female subjects is not associated with the G(-1019) genotype, and may result from decreased NUDR expression.
Table 2.
5-HT1A C(-1019)G allele distribution in control and depressed subjects.
| Subject | N | C/C | C/G | G/G | Allele C | Allele G |
|---|---|---|---|---|---|---|
| Female | ||||||
| Control | 13 | 4 | 7 | 2 | 15 | 11 |
| Depressed | 13 | 5 | 6 | 2 | 16 | 10 |
|
| ||||||
| Male | ||||||
| Control | 12 | 6 | 4 | 2 | 16 | 8 |
| Depressed | 12 | 3 | 6 | 3 | 12 | 12 |
Genotype frequencies were analyzed using a two-tailed Chi-squared test and allele frequencies were analyzed using a two-tailed Fisher's exact test.
For genotype frequency of control versus depressed subjects: female subject group Chi-squared=0.1880; df=2; P=0.9103; male subject group Chi-squared=0.600, df=2; P=0.4493; all Chi-squared=0.3768; df=2; P=0.8283. For allele frequency of control versus depressed subjects: female, P=1.0; male, P=.3801; all, P=0.6845
Discussion
The present study found that the concentration of NUDR protein was significantly decreased in the PFC (area 10) in female subjects with major depressive disorder relative to matched female control subjects, but was unchanged in the PFC (area 10) of male depressed subjects compared to male controls. Furthermore, in the same cortical homogenates of the same female subjects with MDD, the protein levels of the 5-HT1A receptor were significantly reduced compared to the matched female controls, whereas male depressed subjects showed no change in 5-HT1A receptor protein levels in this cortical region. These findings represent the first examination in human postmortem tissue of NUDR, a transcriptional repressor of the 5-HT1A receptor gene, in prefrontal cortical specimens of subjects with MDD. But perhaps more importantly, our study documents a gender-specific alteration in both NUDR and 5HT1A receptor protein expression in the PFC of female subjects diagnosed with MDD.
NUDR is the human homolog of Drosophila Deaf-1, which was identified as a DNA-binding protein and potential gene regulator (Huggenvik et al., 1998). The sequence and functional similarities between NUDR and Deaf-1 suggest that NUDR may also play a similar role in gene regulation. Indeed, recent evidence has shown that NUDR represses both human and rat 5-HT1A receptor promoter- luciferase constructs. Furthermore, stable expression of NUDR significantly reduced the expression and binding of endogenous 5-HT1A receptors and mRNA levels in raphe cells, suggesting that NUDR negatively regulates both 5-HT1A receptor gene transcription and protein expression (Lemonde et al., 2003). However, a very recent report from Czesak et al. (2006) revealed that NUDR (Deaf-1) has a dual function; it acts as a transcriptional repressor of the 5-HT1A receptor in serotonergic cells, but functions as a transcriptional enhancer of the 5-HT1A receptor in non-serotonergic cells such as postsynaptic neurons. Therefore, depending on the synaptic localization of 5-HT1A receptors, NUDR may serve distinct functional roles.
In our case, NUDR protein expression was decreased in prefrontal cortex in depressed females. Therefore, if one assumes that NUDR is normally acting as a transcriptional enhancer in non-serotonergic, postsynaptic prefrontal cortical neurons, then the decrease in NUDR protein in the PFC may reduce its capacity to maintain or increase 5-HT1A receptor gene transcription and perhaps this contributes to the decrease in 5-HT1A receptors in the PFC of depressed female subjects.
It is important to note that our preliminary assessment of the regional and cellular localization of NUDR immunoreactivity in the human PFC was localized primarily to the superficial layers II and upper III. This laminar localization of NUDR is consistent with a previous report showing that the density of 5-HT1A receptor axon initial segment staining was highest in the superficial layers of Brodmann's area 46 (Cruz et al., 2004). NUDR immunoreactivity was also found in both neurons and glia, and previous studies by Rajkowska and colleagues (1999) found that both of these cell types are diminished in density in the PFC of younger and older subjects with MDD. Further studies will be required to determine the specific cellular profiles that exhibit the decrease in NUDR protein expression in depressed female subjects.
Our finding of a gender-specific decrease in 5-HT1A receptor protein in female subjects with MDD has not previously been reported, but it is in general agreement with two PET imaging reports with one conducted in elderly depressed patients and the other study in postpartum depressed women. Meltzer and colleagues (2004) reported reduced 5-HT1A receptor binding potential in the dorsal raphe nucleus and temporal limbic regions of elderly subjects with MDD. Although they did not find a significant gender difference perhaps due to their relatively small subject group, the majority of the depressed subjects were females. A more recent study by this same group found that 5-HT1A receptor binding potential was significantly decreased in the mesiotemporal, subgenual cingulate and lateral orbitofrontal cortices of postpartum depressed subjects relative to postpartum control subjects (Moses-Kolko et al., 2008). The alterations in pre- and postsynaptic 5-HT1A receptors in depressed women reveal specific serotonergic alterations and perhaps these alterations may contribute to the differential treatment response to antidepressant medications between genders (Baca et al., 2004; Kornstein et al., 2000, 2002). Our observation that 5-HT1A receptor protein levels were unchanged in the depressed male subjects relative to matched controls is consistent with two previous studies that were composed of predominantly male subjects reporting no significant alterations in 5-HT1A receptor binding sites (area 10, Stockmeier et al., 1997) or in the density of 5-HT1A receptor axon initial segments in the PFC of depressed subjects (Cruz et al., 2004).
It is unlikely that our main measures of NUDR and 5-HT1A receptor protein levels were influenced by antidepressant medication since toxicology screens were negative for antidepressants for all depressed subjects. However, since several of the depressed subjects had a prescription for an antidepressant at the time of death, questions may be raised regarding the compliance of these patients and the possible long-term effects of antidepressants on these serotonin markers.
The 5-HT1A receptor gene contains a novel C(-1019)G polymorphism and NUDR binds to the C(-1019) allele of the 5-HT1A receptor and represses the transcription activity, but this binding does not occur with the G(-1019) allele (Lemonde et al., 2003; Albert and Lemonde, 2004). It was recently reported that the G(-1019) allele is two-fold more frequent in depressed subjects and four-fold enriched in completed suicide victims relative to matched control subjects (Lemonde et al., 2003). Moreover, the G(-1019) allele of the 5-HT1A receptor gene is associated with reduced therapeutic response to antidepressant treatment and this effect is more pronounced in female than in male subjects (Lemonde et al., 2004). In a previous study, significant association of the G(-1019) allele with suicide was observed with a larger cohort of male subjects (Lemonde et al., 2003). Interestingly, our male cohort of depressed suicides also showed a trend for association with depression. The finding that the female subjects did not display an association with the G(-1019) allele is important, because it implies that the decrease in 5-HT1A receptors observed in these subjects is not explained by impaired repression by NUDR at the G-allele. Rather, the association of decreased NUDR and 5-HT1A receptor expression levels in depressed females suggests a causal link between dys-regulation of NUDR expression leading to decreased 5-HT1A receptor expression in cortex. This is consistent with the finding that NUDR activates receptor expression in non-serotonergic neurons that express post-synaptic 5-HT1A receptors; hence a decrease in NUDR would be expected to down-regulate post-synaptic receptor expression. The basis for decreased NUDR protein in depressed female subjects remains unclear as little is known of factors that regulate NUDR expression.
The specific reduction in both NUDR and 5-HT1A receptor expression in the PFC of depressed female subjects raises questions regarding the underlying biological mechanisms associated with these changes in women with MDD. The ovarian steroid hormones, estrogen and progesterone are biological factors that deserve consideration in this regard. Although currently there are no available data on the effects of estrogen or progesterone on NUDR, there is substantial evidence to support a role for estrogen in regulating the serotonin system and 5-HT1A receptors specifically. Studies of estrogen treatment in rodents have yielded inconsistent results with increased (Flugge et al., 1999), decreased (Osterlund et al., 2000), or unchanged (Clarke and Maayani, 1990; Frankfurt et al., 1994) postsynaptic 5-HT1A binding sites. On the other hand, ovariectomized non-human primates that were subsequently treated with estrogen or estrogen plus progesterone show a significant reduction in 5-HT1A autoreceptor binding sites, mRNA and basal and stimulated [35S]GTP-γ-S binding in the dorsal raphe nucleus and decreased postsynaptic 5-HT1A receptor binding sites in the hypothalamus (Lu and Bethea, 2002). Thus, estrogen treatment appears to modulate 5-HT1A receptor expression in distinct brain regions. Regarding the female subjects in our study, we have no evidence from either toxicology screens or from the structured interviews that any of the female subjects were receiving hormone therapy at the time of death. Also, the majority of the female subjects were over 45 yrs old (20/26), and only 6 of the females were younger than 45 yrs old. Therefore, most of the female subjects were likely perimenopausal or postmenopausal. Taken together, these characteristics suggest that circulating estrogen did not have a confounding effect on 5-HT1A receptor expression in our female subjects. However, this evidence does not exclude the possibility that ovarian hormones may play a role in the underlying mechanisms associated with the reduction in NUDR and 5-HT1A receptors in depressed women. Clearly, further studies are required to understand the biochemical interactions between estrogen, NUDR and 5-HT1A receptors and their role in the pathophysiology of major depressive disorder.
In summary, this report presents evidence of diminished protein expression of NUDR, a novel transcription factor, and of 5-HT1A receptors, the target protein regulated by NUDR, in the prefrontal cortex of female subjects with MDD. It is intriguing to speculate that alterations in NUDR in depressed women may contribute to altered serotonin neurotransmission and thereby constitute one biological mechanism that may contribute to the greater incidence of depression in women.
Acknowledgments
We acknowledge Dr. Javier Miguel-Hildalgo for assistance using the confocal microscope to generate the immunofluorescent photomicrographs and Tarsha Harris for technical assistance in punching tissue samples and preparation of proteins for assays. The assistance of Bryan Roth, M.D., Ph.D., in four of the psychiatric assessments is gratefully acknowledged. We are deeply appreciative of the assistance of the next-of-kin of the deceased and the gratefully acknowledge the assistance of the Cuyahoga County Coroner's Office, Cleveland, Ohio. This study was supported by grants from the National Institute of Mental Health: MH63187, MH67996, MH61578, National Center for Research Resources: RR17701 and the Canadian Institutes of Health Research.
Footnotes
Statement of Interest None
References
- Albert PR, Lemonde S. 5-HT1A receptors, gene repression, and depression: guilt by association. Neuroscientist. 2004;10:575–593. doi: 10.1177/1073858404267382. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders-IV. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Arango V, Underwood MD, Gubbi AV, Mann JJ. Localized alterations in pre and postsynaptic serotonin binding sites in the ventrolateral prefrontal cortex of suicide victims. Brain Research. 1995;688:121–133. doi: 10.1016/0006-8993(95)00523-s. [DOI] [PubMed] [Google Scholar]
- Arango V, Underwood MD, Boldrini M, Tamir H, Kassir SA, Hsiung S, Chen JJ, Mann JJ. Serotonin 1A receptors, serotonin transporter binding and serotonin transporter mRNA expression in the brainstem of depressed suicide victims. Neuropsychopharmacology. 2001;25:892–903. doi: 10.1016/S0893-133X(01)00310-4. [DOI] [PubMed] [Google Scholar]
- Arias B, Arranz MJ, Gasto C, Catalan R, Pintor L, Gutierrez B, Kerwin RW, Fananas L. Analysis of structural polymorphisms and C-1018G promoter variant of the 5-HT(1A) receptor gene as putative risk factors in major depression. Molecular Psychiatry. 2002;7:930–932. doi: 10.1038/sj.mp.4001146. [DOI] [PubMed] [Google Scholar]
- Baca E, Garcia-Garcia M, Porras-Chavarion A. Gender differences in treatment response to sertraline versus imipramine in patients with nonmelancholic depressive disorders. Progress in Neuropsychopharmacology and Biological Psychiatry. 2004;28:57–65. doi: 10.1016/S0278-5846(03)00177-5. [DOI] [PubMed] [Google Scholar]
- Bhagwagar Z, Rabiner EA, Sargent PA, Grasby PM, Cowen PJ. Persistent reduction in brain serotonin 1A receptor binding in recovered depressed men measured by positron emission tomography with [11C]WAY-100635. Molecular Psychiatry. 2004;9:386–392. doi: 10.1038/sj.mp.4001401. [DOI] [PubMed] [Google Scholar]
- Boldrini M, Underwood MD, Mann JJ, Arango V. Serotonin-1A autoreceptor binding in the dorsal raphe nucleus of depressed suicides. Journal of Psychiatric Research. 2007 doi: 10.1016/j.jpsychires.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke WP, Maayani S. Estrogen effects on 5-HT1A receptors in hippocampal membranes from ovariectomized rats: functional and binding studies. Brain Research. 1990;518:287–291. doi: 10.1016/0006-8993(90)90983-i. [DOI] [PubMed] [Google Scholar]
- Cruz DA, Eggan SM, Azmitia EC, Lewis DA. Serotonin1A receptors at the axonal initial segment of prefrontal pyramidal neurons in schizophrenia. American Journal of Psychiatry. 2004;161:739–742. doi: 10.1176/appi.ajp.161.4.739. [DOI] [PubMed] [Google Scholar]
- Czesak M, Lemonde S, Peterson EA, Rogaeva A, Albert PR. Cell-specific repressor or enhancer activities of Deaf-1 at a serotonin 1A receptor gene polymorphism. Journal of Neuroscience. 2006;26:1864–1871. doi: 10.1523/JNEUROSCI.2643-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdmann J, Shimron-Abarbanell D, Cichon S, Albus M, Maier W, Lichtermann D, Minges J, Reuner U, Franzek E, Ertl MA. Systematic screening for mutations in the promoter and the coding region of the 5-HT1A gene. American Journal of Medical Genetics. 1995;60:393–399. doi: 10.1002/ajmg.1320600509. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders- patient edition (ver2) Biometrics Research Department, New York State Psychiatric Institute; 1996. [Google Scholar]
- Flugge G, Pfender D, Rudolph S, Jarry H, Fuchs E. 5HT1A receptor binding in the brain of cyclic and ovariectomized female rats. Journal of Neuroendocrinology. 1999;11:243–249. doi: 10.1046/j.1365-2826.1999.00317.x. [DOI] [PubMed] [Google Scholar]
- Frankfurt M, McKittrick CR, Mendelson SD, McEwen BS. Effect of 5,7-dihydroxytryptamine, ovariectomy and gonadal steroids on serotonin receptor binding in rat brain. Neuroendocrinology. 1994;59:245–250. doi: 10.1159/000126665. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacology Biochemistry Behavior. 2002;71:533–554. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- Hsiung SC, Adlersberg M, Arango V, Mann JJ, Tamir H, Liu KP. Attenuated 5-HT1A receptor signaling in brains of suicide victims: involvement of adenylyl cyclase, phosphatidylinositol 3-kinase, Akt and mitogen-activated protein kinase. Journal of Neurochemistry. 2003;87:182–194. doi: 10.1046/j.1471-4159.2003.01987.x. [DOI] [PubMed] [Google Scholar]
- Huggenvik JI, Michelson RJ, Collard MW, Ziemba AJ, Gurley P, Mowen KA. Characterization of a nuclear deformed epidermal autoregulatory factor-1 (DEAF-1)-related (NUDR) transcriptional regulator protein. Molecular Endocrinology. 1998;12:1619–1639. doi: 10.1210/mend.12.10.0181. [DOI] [PubMed] [Google Scholar]
- Kawanishi Y, Harada S, Tachikawa H, Okubo T, Shiraishi H. Novel mutations in the promoter and coding region of the human 5-HT1A receptor gene and association analysis in schizophrenia. American Journal of Medical Genetics. 1998;81:434–439. doi: 10.1002/(sici)1096-8628(19980907)81:5<434::aid-ajmg13>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Kornstein SG, Schatzberg AF, Thase ME, Yonkers KA, McCullough JP, Keitner GI, Gelenberg AJ, Davis SM, Harrison WM, Keller MB. Gender differences in treatment response to sertraline versus imipramine in chronic depression. American Journal of Psychiatry. 2000;157:1445–1452. doi: 10.1176/appi.ajp.157.9.1445. [DOI] [PubMed] [Google Scholar]
- Kornstein SG, Sloan DME, Thase ME. Gender-specific differences in depression and treatment response. Psychopharm Bull. 2002;36 3:99–112. [PubMed] [Google Scholar]
- Lemonde S, Turecki G, Bakish D, Du L, Hrdina PD, Bown CD, Sequeira A, Kushwaha N, Morris SJ, Basak A, Ou XM, Albert PR. Impaired repression at a 5-hydroxytryptamine 1A receptor gene polymorphism associated with major depression and suicide. Journal of Neuroscience. 2003;23:8788–8799. doi: 10.1523/JNEUROSCI.23-25-08788.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemonde S, Du L, Bakish D, Hrdina P, Albert PR. Association of the C(-1019)G 5-HT1A functional promoter polymorphism with antidepressant response. International Journal of Neuropsychopharmacology. 2004;7:501–506. doi: 10.1017/S1461145704004699. [DOI] [PubMed] [Google Scholar]
- Little RC, Milleken GA, Stroup WW, Wolfinger RD. SAS System for Mixed Models. Cary, NC: SAS Institute Inc.; 1996. [Google Scholar]
- Lopez-Figueroa L, Norton CS, Lopez-Figueroa MO, Rmellini-Dodel D, Burke S, Akil H, López JF, Watson SJ. Serotonin 5-HT1A, 5-HT1B and 5-HT2A receptor mRNA expression in subjects with major depression, bipolar disorder, and schizophrenia. Biological Psychiatry. 2004;55:225–233. doi: 10.1016/j.biopsych.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Lu NZ, Bethea CL. Ovarian steroid regulation of 5-HT1A receptor binding and G protein activation in female monkeys. Neuropsychopharmacology. 2002;27:12–24. doi: 10.1016/S0893-133X(01)00423-7. [DOI] [PubMed] [Google Scholar]
- Matsubara S, Arora RC, Meltzer HY. Serotonergic measures in suicide brain: 5-HT1A binding sites in frontal cortex of suicide victims. Journal of Neural Transmission General Section. 1991;85:181–194. doi: 10.1007/BF01244944. [DOI] [PubMed] [Google Scholar]
- Meltzer CC, Price JC, Mathis CA, Butters MA, Ziolko SK, Moses-Kolko E, Mazumdar S, Mulsant BH, Houck PR, Lopresti BJ, Weissfeld LA, Reynolds CF. Serotonin 1A receptor binding and treatment response in late-life depression. Neuropsychopharmacology. 2004;29:2258–2265. doi: 10.1038/sj.npp.1300556. [DOI] [PubMed] [Google Scholar]
- Moses-Kolko EL, Wisner KL, Price JC, Berga SL, Drevets WC, Hanusa BH, Loucks TL, Meltzer CC. Serotonin 1A receptor reductions in postpartum depression: a positron emission tomography study. Fertil Steril. 2008;89:685–692. doi: 10.1016/j.fertnstert.2007.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterlund MK, Halldin C, Hurd YL. Effects of chronic 17 beta-estradiol treatment on the serotonin 5-HT(1A) receptor mRNA and binding levels in the rat brain. Synapse. 2000;35:39–44. doi: 10.1002/(SICI)1098-2396(200001)35:1<39::AID-SYN5>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Goldman PS. Cytoarchitectonic definition of prefrontal areas in the normal human cortex: I. Remapping of areas 9 and 46 using quantitative criteria. Cerebral Cortex. 1995;5:307–322. doi: 10.1093/cercor/5.4.307. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Miguel-Hidalgo JJ, Wei J, Dilley G, Pittman SD, Meltzer HY, Overholser JC, Roth BL, Stockmeier CA. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biological Psychiatry. 1999;45:1085–1098. doi: 10.1016/s0006-3223(99)00041-4. [DOI] [PubMed] [Google Scholar]
- Riad M, Garcia S, Watkins KC, Jodoin N, Doucet E, Langlois X, el Mestikawy S, Hamon M, Descarries L. Somatodendritic localization of 5-HT1A and preterminal axonal localization of 5-HT1B serotonin receptors in adult rat brain. Journal of Comparative Neurology. 2000;417:181–194. [PubMed] [Google Scholar]
- Sargent PA, Kjaer KH, Bench CJ, Rabiner EA, Messa C, Meyer J, Gunn RN, Grasby PM, Cowen PJ. Brain serotonin1A receptor binding measured by positron emission tomography with [11C] WAY-100635: effects of depression and antidepressant treatment. Archives General Psychiatry. 2000;57:174–180. doi: 10.1001/archpsyc.57.2.174. [DOI] [PubMed] [Google Scholar]
- Sotelo C, Cholley B, EL Mestikawy S, Gozlan H, Hamon M. Direct immunohistochemical evidence of the existence of 5-HT1A autoreceptors on serotoninergic neurons in the midbrain raphe nuclei. European Journal of Neuroscience. 1990;2:1144–1154. doi: 10.1111/j.1460-9568.1990.tb00026.x. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Endicott J. Schedule for affective disorders and schizophrenia (SADS) Third. New York: New York State Psychiatric Institute; 1978. [Google Scholar]
- Stockmeier CA, Dilley GE, Shapiro LA, Overholser JC, Thompson PA, Meltzer HY. Serotonin receptors in suicide victims with major depression. Neuropsychopharmacology. 1997;16:162–173. doi: 10.1016/S0893-133X(96)00170-4. [DOI] [PubMed] [Google Scholar]
- Stockmeier CA, Shapiro LA, Dilley GE, Kolli TN, Friedman L, Rajkowska G. Increase in serotonin-1A autoreceptors in the midbrain of suicide victims with major depression-postmortem evidence for decreased serotonin activity. Journal of Neuroscience. 1998;18:7394–7401. doi: 10.1523/JNEUROSCI.18-18-07394.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockmeier CA, Mahajan GJ, Konick LC, Overholser JC, Jurjus GJ, Meltzer HY, Uylings HB, Friedman L, Rajkowska G. Cellular changes in the postmortem hippocampus in major depression. Biological Psychiatry. 2004;56:640–650. doi: 10.1016/j.biopsych.2004.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Comings DE. A common C-1018G polymorphism in the human 5-HT1A receptor gene. Psychiatric Genetics. 1999;9:105–106. doi: 10.1097/00041444-199906000-00010. [DOI] [PubMed] [Google Scholar]


