Abstract
4-Chloro-N-(2-methyl-1-indolinyl)-3-sulfamoylbenzamide (indapamide), an indoline-containing diuretic drug, has recently been evaluated in a large Phase III clinical trial (ADVANCE) with a fixed-dose combination of an angiotensin-converting enzyme inhibitor, perindopril, and shown to significantly reduce the risks of major vascular toxicities in people with type 2 diabetes. The original metabolic studies of indapamide reported that the indoline functional group was aromatized to indole through a dehydrogenation pathway by cytochromes P450. However, the enzymatic efficiency of indapamide dehydrogenation was not elucidated. A consequence of indoline aromatization is that the product indoles might have dramatically different therapeutic potencies. Thus, studies that characterize dehydrogenation of the functional indoline of indapamide were needed. Here we identified several indapamide metabolic pathways in vitro with human liver microsomes and recombinant CYP3A4 that include the dehydrogenation of indapamide to its corresponding indole form, and also hydroxylation and epoxidation metabolites, as characterized by liquid chromatography/mass spectrometry. Indapamide dehydrogenation efficiency (Vmax/Km = 204 min/mM) by CYP3A4 was approximately 10-fold greater than that of indoline dehydrogenation. In silico molecular docking of indapamide into two CYP3A4 crystal structures, to evaluate the active site parameters that control dehydrogenation, produced conflicting results about the interactions of Arg212 with indapamide in the active site. These conflicting theories were addressed by functional studies with a CYP3A4R212A mutant enzyme, which showed that Arg212 does not seem to facilitate positioning of indapamide for dehydrogenation. However, the metabolites of indapamide were precisely consistent with in silico predictions of binding orientations using three diverse computer methods to predict drug metabolism pathways.
Indapamide is a traditional diuretic drug used to treat mild and moderate hypertension. A recent large Phase III clinical trial (11,140 patients in the ADVANCE trial in 20 countries) of a fixed-dose combination of indapamide and the angiotensin-converting enzyme inhibitor perindopril showed significantly reduced risks of major vascular events in patients with type 2 diabetes mellitus (Patel et al., 2007). The original Phase I study of indapamide in the early 1980s reported that the only detected metabolite in human blood was dehydroindapamide. The major metabolites in urine were 5-hydroxyindapamide and its glucuronic acid conjugate, together with 4-chloro-3-sulfamoylbenzoic acid, a metabolite formed through the hydrolysis of indapamide (Kitao et al., 1982). In another clinical study, the disposition of 14C-labeled indapamide at the C-2 position of its indoline ring showed a similar metabolic profile (Klunk et al., 1983). However, the pathways of cytochrome P450 (P450)-catalyzed indapamide biotransformation have not been elucidated. We have recently shown that the dehydrogenation of indoline to indole was catalyzed by several P450s, and CYP3A4 had the highest “aromatase” activity (Sun et al., 2007). Therefore, indapamide, an indoline derivative, would possibly be metabolized to dehydroindapamide (the indole form of indapamide) by CYP3A4 through a similar dehydrogenation mechanism.
P450-catalyzed dehydrogenation of 3-substituted indoles causes toxicities by forming reactive metabolites that inactivate P450 enzymes directly or covalently bind to other protein and DNA nucleophilic residues (Regal et al., 2001; Kassahun et al., 2005; Sun and Yost, 2008). Hence, the potential consequences of indoline dehydrogenation are 2-fold: 1) its indole metabolite can be bioactivated by an additional dehydrogenation step if it is a 3-substituted indoline; and 2) the pharmacological activities of product indoles can be significantly different from indolines (Sun et al., 2007). The first reports about indapamide biotransformation reported that it was metabolized extensively in humans, with less than 10% parent compound eliminated unchanged in the urine (Klunk et al., 1983). One major metabolite, 5-hydroxyindapamide, showed similar antioxidant activity to indapamide (Tamura et al., 1990), but it is unknown whether 5-hydroxyindapamide and the other major metabolite, dehydroindapamide, are similar to indapamide in antihypertensive activity. These metabolites could contribute significantly to the pharmacological effects of indapamide, only act as inactive products, or even serve as substrates for subsequent metabolism.
To optimize hepatic clearance of new agents, it is important to understand the molecular features that control their metabolism. One protocol to accomplish this goal could be to characterize the active site residues of CYP3A4 that direct the hydroxylation and dehydrogenation of indapamide. Indapamide is slightly more lipophilic than indoline (logD7.4 = 2.1 versus 1.6), which would lead to slightly higher affinity for nonspecific binding to CYP3A4. However, indapamide contains more hydrogen bond donors and acceptors that should enhance its specific interactions with the active site residues of CYP3A4.
Molecular docking approaches have been widely used in the pharmaceutical industry to elucidate the interactions between ligands and receptors, and thus to accelerate the hit-to-lead discovery of new drugs. Successful examples include the discovery of estrogen receptor β inhibitors as a preventative therapy against age-related neurodegenerative diseases using Cambridge Crystallographic Data Center (CCDC; Cambridge, UK) genetic optimization for ligand docking (GOLD) 2.0 dockings (Zhao and Brinton, 2005); signal transducers and activators of transcription 3 SH2 domain antagonists for the treatment of breast cancer using UCSF (San Francisco, CA) DOCK 4.0 (Song et al., 2005); dipeptidyl peptidase IV S1 pocket antagonists for the treatment of type 2 diabetes using FlexX dockings (Bio-SolveIT, Sankt Augustin, Germany) (Rummey et al., 2006); thyroid receptor inhibitors for the treatment of hyperthyroidism using ICM virtual library screening (Molsoft, La Jolla, CA) (Schapira et al., 2003); and novel modes of inhibition of HIV integrase for the treatment of multidrug-resistant HIV using AutoDock (Scripps Research Institute, La Jolla, CA) combined with molecular dynamics simulations (Schames et al., 2004). These molecular docking approaches search for an optimal range of compound physiochemical properties to address a disease target (or drug target space). However, to provide comprehensive optimized parameters and advance these compounds for drug development, an optimal range of pharmacokinetic properties [or absorption, distribution, metabolism, and excretion (ADME) space] should also be considered, i.e., only the compounds within the overlapping region of target space and ADME space become drug candidates (Smith et al., 2006). If the ADME space is defined early in the discovery stage, ranking the hit series for hit-to-lead steps would prioritize the drug discovery program, enhance the late-stage survival rate, and shorten the total drug development cycle time (Jorgensen, 2004; Schnecke and Bostrom, 2006). We believe molecular docking approaches will eventually become key strategies for ADME scientists to virtually screen the ADME space of drugs, to prioritize the hit series, and to make correct decisions about hit-to-lead discoveries and drug redesign.
The primary objective of the present work was to determine the metabolic pathways of indapamide by CYP3A4 in vitro, with particular emphasis on the ratio of dehydrogenation to oxygenation pathways. In addition, the interaction between indapamide and the active site of CYP3A4 was predicted by comparing several molecular docking programs in silico, through which several putative active site residues for indapamide biotransformation were implied. Excellent correlations between in vitro and in silico indapamide metabolism were established, which suggest the molecular docking approach is a very useful tool to study drug metabolism and ADME-based drug redesign.
Materials and Methods
Materials. Indapamide, NADPH, reduced glutathione, ammonium acetate, formic acid, Celite, Darco, and human microsomal epoxide hydrolase were purchased from Sigma-Aldrich (St. Louis, MO). All the other chemicals for synthesis and analysis were analytical reagents or equivalent and obtained in the highest grade commercially available.
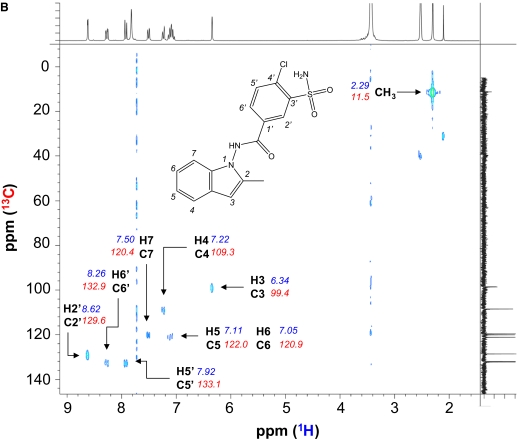
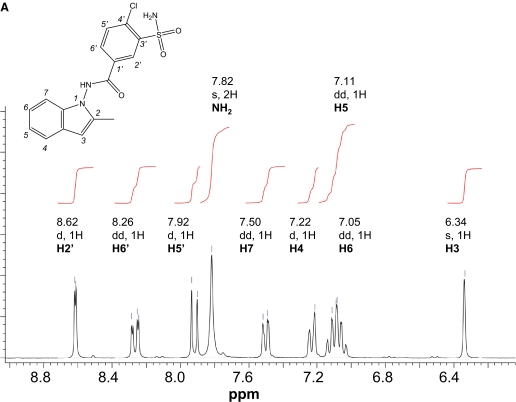
Synthesis of Dehydroindapamide. Dehydroindapamide was synthesized through the oxidation of indapamide by either MnO2 or 2,3-dichloro-5,6-dicyanobenzoquinone (DDQ). For MnO2-catalyzed reaction, MnO2 (1 g) was added to 200 mg of indapamide in acetone (30 ml). The mixture was stirred at room temperature for 2 h, filtered over a plug of Celite (∼2 g) and Darco (200 mg), and then rinsed with 1 volume of acetone. After evaporation, the residue was crystallized using methanol/CH2Cl2/ethyl acetate, from which grayish crystals were obtained, and checked by liquid chromatography/mass spectrometry (LC/MS) and 1H NMR. For the DDQ-catalyzed reaction, DDQ (80 mg) was added to 100 mg of indapamide in CH2Cl2 (50 ml) and acetone (300 ml). The reaction was fast, and DDQ was discolored quickly (10 min); then the mixture was filtered over neutral alumina grade III, and the eluate was purified by thin layer chromatography on silica gel (Merck, Darmstadt, Germany; CHCl3/acetone 8:2, Rf = 0.5). The synthesis yielded approximately 80 mg of dehydroindapamide. 1H NMR [250 MHz Bruker (Newark, DE), D6-dimethyl sulfoxide (DMSO), δ in ppm, aromatic region shown in Fig. 1A]: 11.82 (s,1 H, NH), 8.62 (d, 1 H, J = 1.9 Hz, H2′), 8.26 (dd, 1 H, J = 1.9, 8.5 Hz, H6′), 7.92 (d, 1 H, J = 8.5 Hz, H5′), 7.82 (s, 2 H, NH2), 7.50 (dd, 1 H, J = 6.9, 1.9 Hz, H7), 7.22 (d, 1 H, J = 7.4 Hz, H4), 7.11 (dd, 1 H, J = 6.9, 7.7 Hz, H5), 7.05 (dd, 1 H, J = 7.4, 6.9 Hz, H6), 6.34 (s, 1 H, H3), 2.29 (s, 3 H, CH3). 13C NMR [BB, δ in ppm, heteronuclear multiple bond correlation (HMBC) spectrum shown in Fig. 1B]: 11.5 (CH3), 99.4 (C3), 109.3 (C4), 120.4 (C7), 120.9 (C6), 122.0 (C5), 126.3, 126.6, 129.6 (C2′), 131.6 (q), 132.9 (C6′), 133.1 (C5′), 135.4 (q), 136.9 (q), 137.8 (q), 142.5 (q), 165.5 (CO).
Fig. 1.
Continued.
Incubation of Indapamide with Human Liver Microsomes and Recombinant P450s. Pooled human liver microsomes and recombinant P450s, including CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP2E1, and CYP3A4, containing both P450 reductase and cytochrome b5 were purchased from BD Biosciences (San Jose, CA). A stock solution of indapamide was prepared in DMSO. The incubation mixtures contained indapamide (100 μM), human liver microsomes or recombinant P450 (25 pmol of P450 for each incubation), NADPH (2 mM), and potassium phosphate buffer (100 mM, pH 7.4) in a final volume of 100 μl. The final concentration of DMSO in the incubation media was less than 0.5%. Incubations were started by the addition of NADPH and terminated after 30 min with 2 volumes of ice-cold acetonitrile. The mixture was then vortexed, followed by centrifugation at 21,000g for 30 min to remove proteins. The supernatant was collected and analyzed by LC/MS. Incubations without NADPH were used as the negative controls. Incubations were also performed in the presence of glutathione (GSH) (4 mM) to trap electrophilic reactive metabolites and/or epoxide hydrolase (0.25 mg/ml) to hydrolyze potential epoxide intermediates formed during indapamide metabolism, using the same incubation conditions described above.
Fig. 1.
NMR spectra of synthetic dehydroindapamide: 1H NMR aromatic region of dehydroindapamide in D6-DMSO (A) and HMBC spectrum showing proton-carbon correlations (B) (1H on the horizontal axis and 13C on the vertical axis) obtained on a Bruker 250-MHz spectrometer. The vertical dotted lines on B at approximately 7.75, 3.38, and 1.40 ppm are instrumental noise.
Incubation of Indapamide with the CYP3A4R212A Mutant. The CYP3A4R212A mutant was created as described previously (Harlow and Halpert, 1997). CYP3A4 wild-type and the CYP3A4R212A mutant constructs containing C-terminal polyhistidine tags cloned into the pSE380 vector were used to characterize the importance of the Arg212 residue. The enzymes were expressed in Escherichia coli TOPP3 cells and purified as described previously (Domanski et al., 1998). The reconstituted system contained 50 pmol of purified P450, 100 pmol of recombinant NADPH-P450 reductase, 100 pmol of cytochrome b5, 0.04% sodium cholate, and 20 μg of lipid mix (equal weights of 1,2-dioleoyl-sn-glycero-3-phosphocholine, 1,2-dilauroyl-sn-glycero-3-phosphocholine, and 1,2-diacyl-sn-glycero-3-phospho-l-serine). The mixture was gently shaken at room temperature for 10 min. To the system was added potassium phosphate buffer (50 mM, pH 7.4), GSH (4 mM), MgCl2 (15 mM), and indapamide (100 μM) in a final volume of 500 μl. The mixture was preincubated at 37°C for 5 min, and the reaction was initiated by the addition of NADPH (2 mM). The reaction was allowed to proceed for 10 min at 37°C and then terminated as described above.
Kinetic Studies of Dehydroindapamide Formation. Quantitative analysis of dehydroindapamide formation by CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP2E1, and CYP3A4 was performed on an Agilent 1100 high-performance liquid chromatography (HPLC)/UV instrument (Agilent Technologies, Inc., Palo Alto, CA), including an autosampler and a diode-array UV-visible detector as described previously (Sun et al., 2007). In general, a series of incubations containing indapamide (0–500 μM), 25 pmol of P450 enzyme, and 2 mM NADPH in 0.1 M potassium phosphate buffer at pH 7.4 in a final volume of 100 μl were incubated for 10 min in a 37°C shaking water bath and stopped by adding ice-cold acetonitrile (1:1 v/v). The supernatant after centrifugation was collected and stored in a 4°C autosampler before injection onto a Phenomenex (Torrance, CA) Luna 5-μm C18 (150 × 2.00 mm, 5 μm) reverse-phase column at a flow rate of 0.2 ml/min. The mobile phase consisted of solution A (acetonitrile) and solution B (1 mM ammonium acetate). The gradient schedule was 0 min, 10% A; 5 min, 10% A; 35 min, 90% A; 40 min, 95% A; 45 min, 95% A; and 50 min, 10% A. The peak area (at 250 nm) of dehydroindapamide was integrated by the software (ChemStation, Agilent Technologies, Inc.) and quantitated by a standard curve of dehydroindapamide for kinetic analysis. The enzyme kinetic parameters Vmax and Km were calculated by a nonlinear least-squares regression fitting of the Michaelis-Menten equation V = Vmax × [S]/(Km + [S]) using the “Solver” function in Microsoft (Redmond, WA) Excel 2007.
Identification of Metabolites by LC/MS and LC/Tandem MS. Indapamide and its NADPH-dependent metabolites by human liver microsomes and CYP3A4 were determined using LC/MS analysis. Analytes were chromatographed over a Phenomenex Luna 5-μm C18 (150 × 2.00 mm, 5 μm) reverse-phase column and analyzed using a Finnigan LCQ LC/MS system (Thermo Fisher Scientific, Waltham, MA). The mobile phase contained acetonitrile (solvent A) and 1 mM ammonium acetate (solvent B) with a flow rate of 0.25 ml/min. The gradient system was set as 0 min, 10% A; 5 min, 10% A; 35 min, 90% A; 40 min, 95% A; 45 min, 95% A; and 50 min, 10% A. Ionization of standard indapamide using direct infusion in both positive and negative modes showed that the negative mode had more than 20-fold higher sensitivity, and thus the electrospray mass spectra of indapamide and its metabolites were recorded in the negative mode on a Finnigan LCQ Advantage MAX mass spectrometer (Thermo Fisher Scientific). In the LC/MS scan, a range of m/z from 200 to 400 was selected with the following instrumental parameters: capillary temperature at 225°C, source voltage at 5000 V, capillary voltage at 21 V, sheath gas flow rate at 50 units, and aux/sweep gas flow at 18 units. Xcalibur 2.0 software (Thermo Fisher Scientific) was used for the analysis.
Liquid chromatography/tandem mass spectrometry (LC/MS/MS) experiments were carried out using a Sciex API 3000 triple quadrupole mass spectrometer (Applied Biosystems, Foster City, CA) with a Shimadzu (Kyoto, Japan) Prominence HPLC system and a CTC Analytics (Zwingen, Switzerland) HTS PAL autosampler. The corresponding m/z of indapamide and all the metabolites ([M-H]–) were selected for the collision-induced dissociation (CID) with the following instrumental parameters: source temperature at 450°C, capillary voltage at –4200 V, and nitrogen nebulizing, curtain, and collision gas set at arbitrary values of 9, 7, and 9, respectively. The collision energy was set as indapamide at 32.5%, dehydroindapamide at 39%, and the rest of metabolites at 35%. The mobile phase consisted of acetonitrile (solvent A) and 0.1% formic acid (solvent B), with a flow rate of 0.4 ml/min on an ACE C18 (30 × 2.1 mm) reverse-phase column (MAC-MOD Analytical, Inc.; Chadds Ford, PA). The gradient system was set as 0 min, 5% A; 0.5 min, 5% A; 1.8 min, 95% A; 2.5 min, 95% A; 2.8 min, 5% A and stop at 3.5 min, for fast analysis. The results were analyzed by Analyst 4.1 software (Applied Biosystems/MDS Sciex Instruments, Foster City, CA).
GSH adducts formed in the incubation were identified and characterized by the same Finnigan LCQ system, C18 column (150 × 2.00 mm), mobile phase, and gradient system as described above for LC/MS identification of the metabolites. Both product scan mode and neutral loss scan mode by scanning a neutral loss of 129 atomic mass units (amu) in a positive electrospray ionization were used to identify GSH adducts. The neutral loss scan of 129 amu by unknown metabolites provides a robust method to explore potential GSH adducts (Baillie and Davis, 1993). The instrumental parameters were set at capillary temperature at 225°C, source voltage at 5000 V, capillary voltage at 21 V, sheath gas flow rate at 50 units, and aux/sweep gas flow at 18 units, with the normalized collision energy set at 25%.
Molecular Docking Using AutoDock. AutoDock 3.05 was obtained from Scripps Research Institute. In AutoDock, the substrate indapamide was treated as a flexible ligand by modifying its rotatable torsions, but the template CYP3A4 was considered as a rigid receptor. Three-dimensional coordinates of CYP3A4 structure were acquired from Research Collaboratory for Structural Bioinformatics Protein Data Bank [PDB code: 1TQN, resolution: 2.05 Å (Yano et al., 2004) and 1W0E, resolution: 2.80 Å (Williams et al., 2004)]. The structure of indapamide was built using Chem3D Ultra10 (CambridgeSoft Corporation, Cambridge, MA), and its energy was minimized using the molecular mechanics method (MM2), which was then modified by AutoDockTools program (Scripps Research Institute) with the Gasteiger atomic charges assigned and flexible torsions defined. Two templates of CYP3A4, 1TQN and 1W0E, were modified for docking with polar hydrogens, Kollman partial charges, and solvation parameters added using AutoDockTools. To define the active site space in which indapamide moves, AutoGrid 3.06 (Scripps Research Institute) was performed, which precalculates grids of van der Waals, hydrogen bonding, electrostatics, torsion, and solvation interactions between CYP3A4 and indapamide (Morris et al., 1998). A grid box with sufficient space to cover the whole active site of CYP3A4 was defined with dimensions of 60 × 60 × 60 and a resolution of 0.375 Å in each dimension. Docking was accomplished on a Dell (Round Rock, TX) Precision 690 workstation with two 64-bit Dual-Core Intel (Santa Clara, CA) Xeon processors and Red Hat (Raleigh, NC) Enterprise Linux WS4 operating system. The maximum number of energy evaluations and generations was set at 250,000 and 27,000, respectively. The rates of gene mutation and crossover rate were set at 0.02 and 0.80, respectively. The rest of the parameters were set at their default values. AutoDock searched the globally optimized conformations and orientations using the Lamarckian genetic algorithm (LGA), a hybrid of a genetic algorithm with an adaptive local search (LC) method (Morris et al., 1998). Two thousand LGA runs (from 10 independent experiments with 200 LGA runs each) were conducted. At the end of all the docking experiments, a cluster analysis of 2000 pooled conformations was performed. Docking solutions with indapamide all-atom root mean square deviation (RMSD) within 2.0 Å of each other were clustered together using AutoDockTools, and the best ranked clusters by the lowest docking energy were selected for analysis.
Metabolism Prediction Using MetaSite. MetaSite 2.8.6 was obtained from Molecular Discovery Ltd. (Pinner, UK). Unlike the molecular docking programs, in MetaSite the GRID-based representation of CYP3A4 was precomputed and stored in the program (Cruciani et al., 2005). CYP3A4 was characterized using different probes, such as the hydrogen probe for active site volume, the water probe for hydrophilic regions, the DRY probe for hydrophobic interaction, the N1-amide nitrogen probe for hydrogen-bond donor interactions, the O-carbonyl oxygen probe for hydrogen-bond acceptor regions, and positive/negative charge probes for charge-charge electrostatic interactions in GRID molecular interaction fields. The distances between the heme iron of CYP3A4 and the different regions of the active site were plotted in a correlogram by MetaSite. For the substrate indapamide, all the atoms were classified into GRID probe categories and compared with CYP3A4 correlogram for a similarity score. The other component in MetaSite was the reactivity score, which was precalculated using an ab initio method on database fragments, and then applied to the fragmentized indapamide. The only input file was the structure of indapamide that was sketched by MetaSite and submitted for MetaSite prediction using default parameters. The metabolism prediction results were automatically produced by MetaSite.
Molecular Docking Using CCDC GOLD. GOLD 3.2 was obtained from CCDC. In the present work, CYP3A4 (PDB code: 1TQN) was prepared by adding hydrogens using Reduce (Duke University, Durham, NC), and the structure of indapamide was built using Chem3D as described above. Genetic algorithm was applied in GOLD that allows partial flexibility of CYP3A4 and full flexibility of indapamide, that is, the flexible dihedrals and ring geometries of indapamide and dihedrals of OH and NH groups of CYP3A4. The placement of indapamide was based on fitting points, which were added by GOLD to hydrogen-bonding groups on both CYP3A4 and indapamide. In addition, hydrophobic fitting points were also added in the active site of CYP3A4. The active site cavity was detected by LIGSITE algorithm in GOLD and defined as a sphere region of 20-Å radius above the heme iron, which contains sufficient space to cover the whole active site of CYP3A4. The docking poses of indapamide were ranked based on either GoldScore or ChemScore scoring functions that include a hydrogen-bond term, a 4 to 8 intermolecular van der Waals term, and a 6 to 12 intramolecular van der Waals term for the internal energy of indapamide, but ChemScore also estimated lipophilic and metal-ligand binding contributions. Two hundred genetic algorithm runs were conducted with docking parameters set as default values. The results were analyzed, and all the docking solutions were ranked and clustered at various RMSD values; 10 conformations of indapamide with the highest ranked scores were selected and visualized using SILVER (CCDC).
Molecular Docking Using UCSF DOCK. DOCK 6 was obtained from UCSF and compiled at Cygwin (Red Hat), a UNIX (The Open Group, San Francisco, CA) shell environment running on Windows (Microsoft). DOCK 6 and all the accessory programs were conducted in a ThinkPad T61 (Lenovo, Morrisville, NC) with Intel Core2 Duo CPU T7300 2.0 GHz. CYP3A4 (PDB code: 1TQN) was prepared using Dock Prep function in Chimera (UCSF) with solvent deleted and hydrogens and charges added. The structure of indapamide was built using Chem3D as described above, and both hydrogens and charges were added using Chimera. The molecular surface of CYP3A4 was generated by a DOCK accessory program DMS by rolling a sphere with the size of a water molecule over the van der Waal's surface of CYP3A4. A function within DOCK, Sphgen, was used to create a set of spheres based on the molecular surface of CYP3A4, and thus it detected all the cavities within CYP3A4 with a probe radius of 2.0 Å. The spheres were then visualized by Chimera, and the active site sphere was identified by its unique position, right above the heme and at the distal side of cysteine 442. The command Showbox was used to create the space sufficient to cover the whole CYP3A4 active site to define the location and size of the grid to be calculated using the program GRID, which precomputes the contact and electrostatic potentials for the active site of CYP3A4 at a grid spacing of 0.3 Å with the coefficient of the dielectric of 4.0 and the exponent and repulsive of attractive Lennard-Jones term for van der Waals potential of 6 and 12, respectively. The “anchor-and-grow” flexible ligand docking algorithm in DOCK 6 was applied for docking, in which the anchor part of indapamide was rigidly oriented in the active site. The location of each flexible bond was used to partition the indapamide molecule into rigid segments, and then the remaining flexible portion of indapamide was built. The geometric coordinates of the best pose were recorded for analysis.
Results
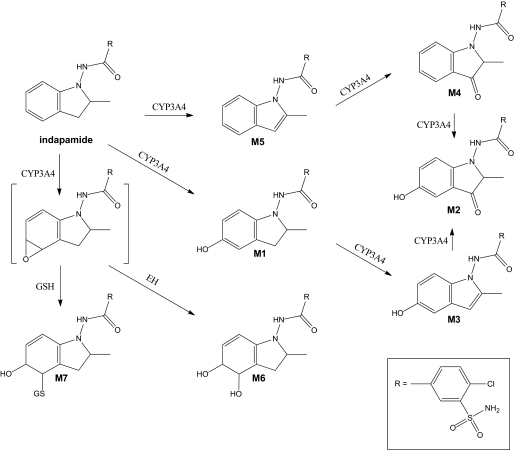
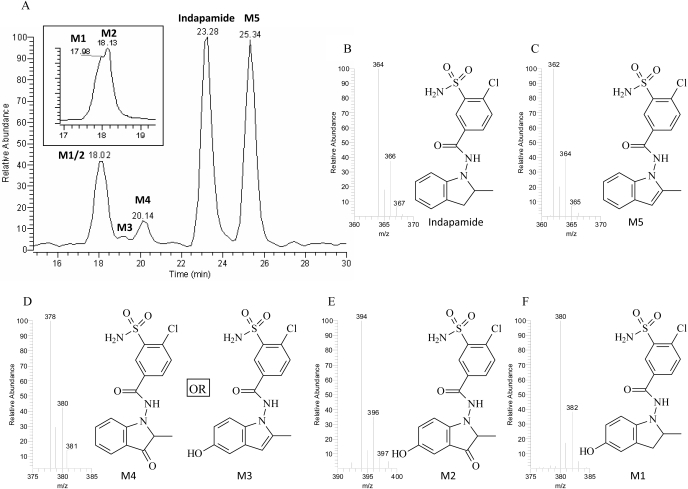
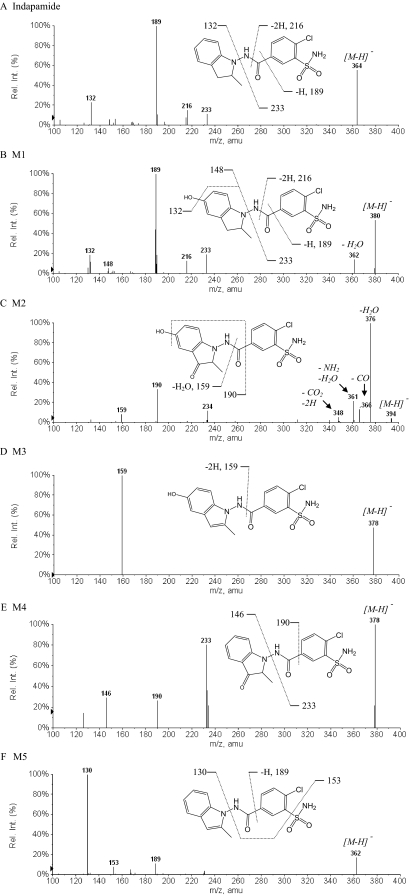
Metabolic Activation of Indapamide. To characterize the dehydrogenation activities of individual P450 enzymes, CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP2E1, and CYP3A4 were examined for their ability to produce dehydroindapamide. CYP3A4 exhibited the greatest dehydrogenation activity, followed by CYP2C19 and CYP2C8, which only had 37 and 21% of CYP3A4's activity, respectively. The other P450 enzymes catalyzed less than 20% of CYP3A4's dehydrogenase activity. Five NADPH-dependent metabolites were identified in incubations of indapamide with human liver microsomes or CYP3A4 (Fig. 2), as numbered consecutively according to their separation order from a reverse-phase C18 column (Fig. 3A). They all showed the prototypical chlorine isotope cluster (35Cl/37Cl) with the ratio of approximately 3:1 in intensity under negative mode of ionization (Fig. 3, B–F): indapamide ([M-H]– = 364/366), dehydroindapamide (M5, [M-H]– = 362/364), 5-hydroxyindapamide (M1, [M-H]– = 380/382), 3-oxyindapamide (M4, [M-H]– = 378/380), 3-oxy-5-hydroxyindapamide (M2, [M-H]– = 394/396), and 5-hydroxydehydroindapamide (M3, [M-H]– = 378/380). MS/MS of indapamide by CID of the ion m/z 364 formed four major product ions: m/z 132 (2-methylindoline moiety), m/z 233 (chlorosulfamoylbenzamide part), and m/z 216 (the cleavage of the amide bond), from which an additional loss of the carbonyl group formed the ion at m/z 189 (Fig. 4A). Inspection of the MS/MS spectrum of M5 (m/z 362) confirmed the dehydrogenation of the indoline ring of indapamide; specifically, the corresponding product ion at m/z 130 confirmed the formation of 2-methylindole. In addition, the product ion at m/z 189 corresponded to the chlorosulfamoylbenzene moiety (the remainder of the parent molecule). The ion at m/z 153 was assigned as an additional cleavage of the sulfamoyl group from the corresponding m/z 233 ion of indapamide (Fig. 4F). Assignment of M5 as dehydroindapamide was confirmed by comparison with the synthetic standard, which had the same retention time, mass spectrum, and tandem mass spectrum.
Fig. 2.
Proposed indapamide metabolic pathways catalyzed by human liver microsomes or CYP3A4 (M1–M5) in the presence of microsomal epoxide hydrolase (M6) or glutathione (M7). To simplify the figure, single regioisomers are shown for the epoxide, phenol, diol, and glutathione adduct structures. However, the sites of substitution on the phenyl or cyclohexadiene rings were not established. The putative epoxide reactive intermediate is shown with brackets. EH, microsomal epoxide hydrolase; GSH, glutathione.
Fig. 3.
Metabolic profiles of indapamide by human liver microsomes or CYP3A4 as determined by MS selected-ion scan (A), HPLC/UV (A, inset, at 250 nm) and LC/MS (B–F). Molecular ions in the negative scan mode [M-H]– for indapamide and its metabolites are as follows: indapamide, m/z 364 (B); M5, m/z 362 (C); M4 and M3, m/z 378 (D); M2, m/z 394 (E); and M1, m/z 380 (F). The isotopic clusters for the molecular ions and M + 2 ions, with approximate ratios of 3:1 (M/M + 2) are characteristic of the presence of chlorine. These clusters are observed with each metabolite.
Fig. 4.
LC/MS/MS product ion spectra of indapamide and its metabolites by human liver microsomes or CYP3A4, obtained by CID of the [M-H]– ions corresponding to m/z 364, indapamide (A); m/z 380, M1 (B); m/z 394, M2 (C); m/z 378, M3 (D); m/z 378, M4 (E); and m/z 362, M5 (F). The putative assignments of characteristic fragment ions are shown.
The synthesis of the dehydroindapamide standard was accomplished by the oxidation of indapamide with either MnO2 or 2,3-dichloro-5,6-dicyanobenzoquinone, and the product was confirmed by proton and 13C NMR. The 1H NMR and the assignment of the proton signals of dehydroindapamide showed that the signals for the single proton at C-2 and two protons at C-3 of indapamide were abolished, and instead a new singlet at 6.34 ppm appeared (Fig. 1A). These results are consistent with the formation of an indole product from an indoline. The strong correlation between the proton signal at 6.34 ppm and the carbon signal at 99.4 ppm observed in HMBC spectrum also clearly indicated that the saturated carbons of the parent compound are now aromatic carbons with a chemical shift consistent with indole compounds (Fig. 1B). The correlations and chemical shifts of all the other protons and carbons of the molecule were consistent with the proposed dehydroindapamide structure.
The MS/MS spectra and fragmentation patterns of M1 through M4 are illustrated in Fig. 4, B through E. The product ion spectrum of M1 showed a +16 amu fragment ion at m/z 148 (5-hydroxy-2-methylindoline moiety), the ion at m/z 362 for the loss of water. Additional ions were similar to indapamide, including ions at m/z 132, 216, and 233. The specific hydroxylation position of M1 was not established, but in vivo metabolism of indapamide previously showed that 5-hydroxylation is the major pathway (Kitao et al., 1982; Klunk et al., 1983), which supports the assigned structure, but without an authentic standard, the structure is still tentative. Other evidence comes from its additional metabolites, M2 and M3, which were tentatively assigned as the 3-oxygenation and dehydrogenation products of M1, respectively. M4 was postulated to be the 3-oxygenation product of indapamide, with typical MS/MS product ions at m/z 146 (3-oxy-2-methylindoline moiety) and m/z 233 (chlorosulfamoylbenzamide portion). It is possible that M3 could be a 3-oxy-2-methylindoline and M4 a 5-hydroxyindole, as illustrated in Fig. 3. Mass spectra of these two metabolites (Fig. 4) did not permit us to definitively assign them.
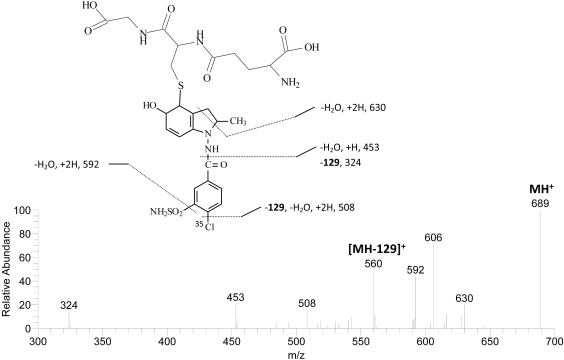
GSH Adduct from Indapamide Epoxidation. A well defined glutathione adduct (M7, MH+ = 689) was identified by LC/MS/MS neutral loss scan of 129 amu, supporting an epoxidation process at the C-5/C-6 position, with the fragmentation patterns shown in Fig. 5. Several characteristic product ions were observed, including the loss of 129 amu at m/z 560, corresponding to the loss of pyroglutamate residue of the GSH moiety (Baillie and Davis, 1993). The product ion at m/z 630 was attributed to the cleavage of the C-2 and C-3 carbons of the indoline ring of indapamide. This fragmentation pattern was also observed in the GSH adduct of indoline epoxidation (Sun et al., 2007). The cleavage of the amide bond of indapamide and loss of water formed the ion at m/z 453, from which an additional loss of 129 amu formed the ion at m/z 324. Moreover, the loss of chlorine and water and 129 amu formed the ion at m/z 508, and the cleavage of the sulfamoyl group and loss of water produced the ion at m/z 592. Epoxide formation was also confirmed by another metabolite (M6, [M-H]– = 398), which we opine is the diol, formed only in the presence of epoxide hydrolase (Fig. 2). However, the amounts of M6 were so small that it was not feasible to detect MS/MS fragments to confirm the structure.
Fig. 5.
LC/MS/MS product ion spectra of the glutathione adduct of a putative epoxide was obtained by CID of the MH+ ion at m/z 689. The assignments of characteristic fragment ions are shown.
Kinetics of Indapamide Dehydrogenation. The enzyme kinetics of indapamide dehydrogenation by CYP3A4 appeared to follow normal Michaelis-Menten kinetics (Table 1). The Km was calculated as 99.7 μM, approximately 25% lower than indoline (Sun et al., 2007). The Vmax was calculated as 20.4/min, and the total indapamide dehydrogenation efficiency (Vmax/Km) of CYP3A4 was determined as 204/min/mM, approximately 10-fold higher than that of indoline dehydrogenation by CYP3A4 (Sun et al., 2007).
TABLE 1.
Kinetic constants for dehydrogenation of indapamide and indoline by CYP3A4
| Compounds | Km | Vmax | Vmax/Km |
|---|---|---|---|
| μM | /min | /min/mM | |
| Indapamide | 99.7 | 20.4 | 204 |
| Indolinea | 135 | 2.9 | 21.5 |
Data from Sun et al. (2007).
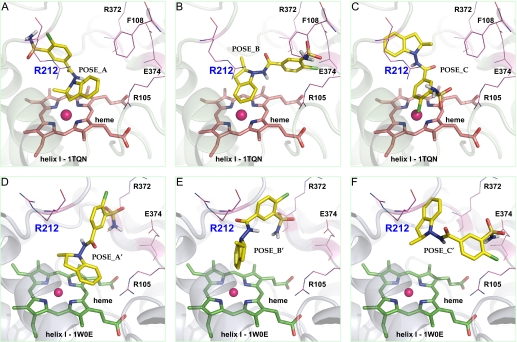
Molecular Docking Using AutoDock. Two thousand docking conformations from AutoDock were clustered at RMSD 2.0 Å for analysis. Three clusters with the lowest binding energies (within 1 kcal/mol between them, that is, no significant docking energy difference) for each template were considered as energetically favored poses. Figure 6, A through C, illustrates one conformation from each of these three clusters of indapamide in the active site of 1TQN, and Fig. 6, D through F, illustrates one conformation from each these three clusters in 1W0E. Conformation POS_A showed the potential dehydrogenation reaction with the indoline C-3 of indapamide nearest to the heme, and conformation POS_B is a possible indapamide aromatic hydroxylation and/or epoxidation position with C-4 and C-5 of the indoline ring closest to the heme iron. The conformation POS_C corresponded to a favored binding orientation but would not result in enzymatic turnover because it predicts oxidation of the chlorine. These poses were picked to evaluate the important active site residues of CYP3A4 that could affect the metabolism of indoline-containing drugs. We found that Arg212 residue in F-G loop (between helix F and F′) should play a key role for indapamide dehydrogenation and also would be expected to contribute to other metabolic pathways. The nitrogen atoms (either terminal or central) of the Arg212 guanidinium moiety are within the hydrogen bonding distance of the indoline nitrogen of indapamide, approximately 3.4 Å, 3.2 Å, and 3.3 Å for these three clusters, indicating that Arg212 could potentially be important for substrate binding. In addition, active site residues Phe108 and Arg105 within the B-C loop, together with Glu374 in β-sheet 1, have little effect on POS_A and POS_C but could have hydrophobic and hydrogen bonding interactions on POS_B with the chlorosulfamoylbenzene ring of indapamide, and thus juxtapose the indoline aromatic ring near the heme iron.
Fig. 6.
Molecular models of indapamide in the active site of CYP3A4 predicted by AutoDock. Conformations generated by Autodock are clustered at RMSD = 2.0 according to their docking energies. The lowest energy docking clusters (POSE_A, POSE_B, and POSE_C for template 1TQN; POSE_A', POSE_B', and POSE_C' for template 1W0E) were selected and illustrated using PyMOL: conformation POSE_A and POSE_A' showing the dehydrogenation orientation at the indoline C-3 position (A and D), conformation POSE_B and POSE_B' showing indoline aromatic hydroxylation or epoxidation orientation with the C-4 and C-5 atoms closest to the heme iron (B, E), and the metabolic inactive state with either the chlorosulfamoylbenzene ring closest to the heme iron (C) or the whole molecule binding away from heme iron (∼7Å; F). Active site residues Arg105, Phe108, Arg212, Arg372, and Glu374 are also illustrated. CYP3A4 is shown in a ribbon format, iron as a sphere, heme (pink for 1TQN and green for 1W0E) and indapamide (yellow) in color-coded sticks: nitrogen = blue, oxygen = red, chlorine = green, and sulfur = gold.
However, it has been suggested that the side chain of Arg212 could reorient after substrate binding to other directions (Yano et al., 2004). Therefore, indapamide was docked into 1W0E, in which the side chain of Arg212 is oriented out of the active site. Conformation POS_A′ shows another potential dehydrogenation reaction with the C-3 of indapamide nearest to the heme, and POS_B′ is another possible indapamide hydroxylation and/or epoxidation reaction with the C-4 and C-5 of the indoline ring closest to the heme. Conformation POS_C′ shows another metabolic “inactive” state because indapamide is bound too far from the heme for enzymatic turnover. These conformations suggest that Arg212 is not required to orient indapamide to bring about the dehydrogenation and hydroxylation/epoxidation reactions. However, Arg372 seems to form hydrogen bonds with the sulfonamide of indapamide in POS_A′ and POS_B′, and Glu374 seems to form hydrogen bonds with the sulfonamide in POS_C′.
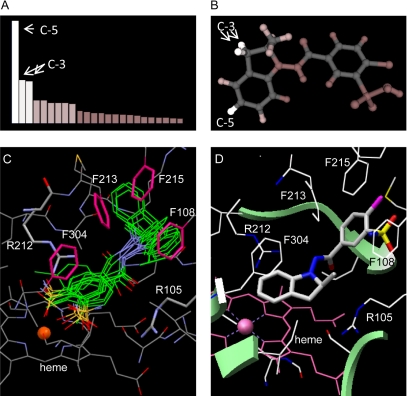
Metabolism Prediction Using MetaSite. MetaSite predicted the sites of indapamide metabolism from the lability of indapamide hydrogens, coupled with molecular orientation in the active site of CYP3A4 (Cruciani et al., 2005). The C-5 hydroxylated phenol metabolite, M1, was predicted to be the most likely oxygenation site of CYP3A4 (Fig. 7A). However, summation of the probabilities for the two C-3 indoline hydrogens would essentially equal the likelihood of C-5 position oxidation, thus predicting dehydroindapamide to be a major product (Fig. 7B). In contrast, other potential sites, such as the C-2 indoline hydrogen and the methyl hydrogens, had lower probabilities.
Fig. 7.
Predictions of indapamide metabolism by CYP3A4 with MetaSite (A and B), and molecular docking of indapamide in the active site of CYP3A4 by GOLD (C) and DOCK (D). The highest ranked hydrogen atoms (C-5 and C-3) by MetaSite that would most probably be metabolized are illustrated in A and B. Ten conformations of indapamide with the highest ranked fitness scores, as calculated by Goldscore function in GOLD, are illustrated by SILVER in overlapped green lines (C), which show the rotational trends of both the indoline ring and the chlorosulfamoylbenzamide moieties when docked in the active site of CYP3A4. The close distance between the two aromatic rings of indapamide and the phenylalanine cluster (Phe213, Phe215, and Phe108), as well as the Phe304 residue in the I-helix, indicate the domination of hydrophobic interactions. Phenylalanine rings are shown as pink sticks (C). The guanidinium moiety of Arg212 and Arg105 (in light blue sticks) are within hydrogen bonding distance of the benzamide oxygen (C). The highest ranked conformation of indapamide by DOCK is illustrated in B by Chimera using the anchor-and-grow algorithm in DOCK, in which the anchor is rigidly oriented, spatially clustered, and prioritized by its scoring function before the remaining flexible portion of indapamide is built. The conformation shows that the C-5 position of the indapamide indoline ring is the closest position to the heme iron (D). The heme is shown in pink with the iron as a sphere, and indapamide as color-coded sticks: nitrogen = blue, oxygen = red, chlorine = purple, and sulfur = yellow.
Molecular Docking Using GOLD. Ten conformations of indapamide with the highest ranked fitness scores were calculated by GoldScore functions within the GOLD program, and in sum predicted the rotation of both the indoline ring and the chlorosulfamoylbenzamide moiety of indapamide in the active site of CYP3A4 (Fig. 7C). Compared with GoldScore, ChemScore showed similar conformational clusters but placed the molecule approximately 1.0 Å farther from the heme. The GOLD program predicted an inactive state of indapamide with the chlorosulfamoylbenzene ring closest to the heme iron, but this orientation was different from the inactive state predicted by AutoDock. In particular, the indoline portion moved from the F-F′ loop clockwise to the phenylalanine cluster and the B-C loop side of CYP3A4. The crystal structure of CYP3A4 showed that several phenylalanine residues, including Phe108, Phe213, Phe215, Phe241, and Phe304, appeared to build the “roof” of the active site. The GOLD program predicted that the Phe213 and Phe215 residues in the F-G loop, the Phe108 in the B-C loop, and the Phe304 in the I helix positioned indapamide through aromatic hydrophobic interactions with the indoline and chlorosulfamoylbenzene rings. The guanidinium moieties of Arg212 in the F-G loop and Arg105 in the B-C loop appeared to be significantly more distal from the indoline nitrogen of indapamide than they were in the AutoDock prediction. However, the Arg105 residue now formed a hydrogen bond with the carbonyl connector of indapamide (Fig. 7C).
Molecular Docking Using DOCK. The best ranked conformation (Fig. 7D) of indapamide from the DOCK program, using the anchor-and-grow algorithm, showed the C-5 position of the indapamide indoline ring to be the preferred oxidation site. In DOCK, the anchor (the indoline moiety) was chosen first and rigidly oriented, spatially clustered, and prioritized by the scoring function before the remaining flexible portion of indapamide was built. The phenylalanine cluster of CYP3A4 seemed to interact with indapamide's aromatic rings, much like the AutoDock prediction. However, the Arg212 and Arg105 residues were further away from the indoline nitrogen than in the AutoDock prediction.
Incubation of Indapamide with CYP3A4R212A. To elucidate the role of Arg212 in CYP3A4-medidated dehydrogenation of indapamide, a mutant was constructed where Arg212 was mutated to the nonpolar residue alanine. The ability of CYP3A4R212A to produce dehydroindapamide was compared with that of the CYP3A4 native enzyme in a reconstituted system. The mutant enzyme dehydrogenated indapamide with an activity that was 66% of the rate of the recombinant wild-type enzyme. No additional metabolites were observed with the mutant enzyme. The lower activity for dehydrogenation could mean that the Arg212 residue aided dehydrogenation selectivity, or it was possible that the native CYP3A4 enzyme in the reconstituted assay conditions could have had lower overall activities than the enzyme activity of CYP3A4 expressed in the baculovirus-directed system. Therefore, we determined CYP3A4 activity in the reconstituted system for its prototypical substrate, testosterone, to produce 6β-hydroxytestosterone using the assay conditions published previously (Kassahun et al., 2005). The native CYP3A4 enzyme produced 6β-hydroxytestosterone at a rate of 9.7 nmol/min/nmol P450, and CYP3A4R212A catalyzed the formation of this metabolite with a rate of 5.4 nmol/min/nmol P450, which was approximately 56% of the native enzyme. Therefore, we concluded that the loss of the Arg212 residue's interactions with indapamide did not selectively decrease dehydrogenation activity, but rather the mutant possessed about half of the native enzyme's activity for its standard substrate and a comparable 66% of the dehydrogenation rate for indapamide.
Discussion
More than a century ago, chemists developed methods to synthesize indoles (Baeyer and Emmerling, 1869) and indolines (Brunner, 1900; Braun and Sobecki, 1911). Since then, the indole moiety has been used as the core feature in many drugs. The indole moiety is also the core of multiple natural products such as the therapeutic indole alkaloids, tryptophan, serotonin, and melatonin. However, indoline was only used as an intermediate for synthesis until indapamide was discovered and became a successful antihypertensive drug in the 1980s. In recent years, indoline-containing compounds have been widely used in various therapeutic areas. There are many examples: J30, an indoline-sulfonamide has potent anticancer activity through the disruption of microtubules (Liou et al., 2007); an indoline analog of razaxaban (BMS-561389), made by replacing its aniline group with indoline, is a potent factor Xa inhibitor (Varnes et al., 2007). The indoline form of arylaminoprolylthiazolidine compounds are potent antidiabetic agents through a DPP-IV inhibition mechanism (Sakashita et al., 2007). N,N-Dimethyl-3-arylpropan-1-amine indolines act as dual-acting norepinephrine and serotonin reuptake inhibitors (Mahaney et al., 2006). Indoline-derived amide-ketoacids are potent HIV-integrase inhibitors (Walker et al., 2006). SB-206553 is a selective 5-HT2C/2B receptor antagonist (Kennett et al., 1996); KMD-3213 is a potent α1A-adrenoceptor-selective antagonist (Shibata et al., 1995). Indoline forms of idazoxan, produced by replacing its dioxan ring with indolines, are potent α2-antagonists and α1-agonists (Fagan et al., 1988). Many other indoline therapeutic compounds were discussed previously (Sun et al., 2007), including SB-242084 (a selective 5-HT2C receptor antagonist), DW2282 (an anticancer agent), 1-(1-indolinyl)-2-propylamine (a 5-HT2C receptor agonist), indoline derivatives of DX-9065a (a potent factor Xa inhibitor), SB-224289 (a selective 5-HT1B receptor inverse agonist), 5-amino-1-(3,5-dimethylphenyl)indoline (a selective cyclooxygenase-1 inhibitor), 1-hexylindolinelactam-V (a protein kinase C selective activator), and indoline methotrexate (an antirheumatic agent).
We initiated research on indoline dehydrogenation with studies on the metabolic fate of the simplest example, indoline (Sun et al., 2007). Our results documented the novel indoline dehydrogenation pathway to indole by several P450s, and CYP3A4 was found to be the most efficient P450 enzyme. Our recombinant P450 enzyme screen of indapamide in this study again found CYP3A4 to have the highest dehydrogenation activity. The synthetic dehydroindapamide standard was used to quantitatively measure indapamide turnover rate by CYP3A4, which was approximately 10-fold higher than that of indoline, and slightly enhanced affinity for CYP3A4 (Table 1). An explanation for the high efficiency with indapamide is that the electron-donating group (an amide) connected to the indoline nitrogen of indapamide facilitates electron abstraction from the indoline nitrogen. However, we have evaluated dehydrogenation with several indoline derivatives, such as SB-224289 and DW2282, which contain strong electron-withdrawing carbonyl groups on the nitrogen, and found similar dehydrogenated indole products (H. Sun and G. S. Yost, unpublished data). Therefore, the dehydrogenation occurred regardless of whether the indoline derivatives had electron-donating or -withdrawing groups connected to the nitrogen of the indoline ring; that is, changes in electron density on the nitrogen did not abolish dehydrogenation activity. We did not detect alcohol metabolites at the C-2 or C-3 position of indapamide or the other indoline-containing compounds. The inability to find oxygenated metabolites could be explained by facile dehydration to produce thermodynamically stable aromatic indoles. In contrast, hydroxyl radical rebound from the putative radical intermediate could be disfavored, facilitating second electron oxidation and subsequent dehydrogenation.
We suggest that the mechanism of indoline dehydrogenation is initiated by hydrogen atom abstraction at either C-2 or C-3 of indoline ring, but not nitrogen one-electron oxidation. As a result, the ubiquitous formation of indoles by indoline derivative substrates, including indapamide, led to our hypothesis that there must be specific residues within the active site of CYP3A4 that partition oxidative catalysis toward dehydrogenation rather than oxygenation and orient the indolines to favor C-2 or C-3 hydrogen abstraction. The recent availability of CYP3A4-crystallized structures provided the opportunity to explore the active site environmental parameters of CYP3A4 that direct indapamide dehydrogenation using molecular docking approaches.
Substrate positioning within the active site of P450s is an important factor for P450-catalyzed reactions. Substrate functional groups could interact with active site residues through either polar hydrogen bonding interactions (such as the Arg212 guanidinium moiety of CYP3A4 as H-bond donors and the indoline ring nitrogen of indapamide as H-bond acceptor; Fig. 6) or nonpolar van der Waals interactions (e.g., between the phenylalanine cluster at F-G and B-C loops of CYP3A4 and the aromatic rings of indapamide; Fig. 7). Both could determine the final positioning of substrates within the active site of CYP3A4 to regulate their catalytic selectivity. From our molecular docking studies using 1TQN, we hypothesized that residue Arg212 interacts with indapamide through hydrogen bonding and thereby positions the carbon hydrogens at C-2 and C-3 of indoline within close catalytic range of the ferryl oxygen for hydrogen abstraction and dehydrogenation (Fig. 6A). This conclusion, based on the 1TQN crystal structure, seemed rational because a previous study on the interaction between the substrate loperamide and CYP3A4 (Marechal et al., 2006) predicted hydrogen bonding between residue Arg212 and the amide carbonyl group of loperamide, hydrophobic interactions between the phenyl rings of loperamide and the phenylalanine cluster of CYP3A4, and hydrogen bonding between the carboxyl oxygen of Glu374 of CYP3A4 and the piperidine hydroxyl group of loperamide. It was suggested by yet another study that residue Arg212 initially hydrogen-bonded to the backbone Ile369 carbonyl oxygen before substrate binding, but after substrate binding, Arg212 altered its conformation to hydrogen bond with the substrate diltiazem (Lill et al., 2006). It has also been suggested that the side chain of Arg212 could reorient during substrate binding (Yano et al., 2004). However, molecular docking studies with crystal structure 1W0E, in which the Arg212 side chain is oriented out of the active site, predicted that indapamide would still bind with the C-3 of the indoline in close catalytic range to the ferryl oxygen to facilitate hydrogen abstraction and dehydrogenation (Fig. 6D). Thus, even though the models were based on two different crystal structures, they both predicted the same low energy-binding orientations that favored selective dehydrogenation of this indoline. To elucidate the role of Arg212, it was vital to prepare a mutant of Arg212 and conduct enzyme activity studies. The CYP3A4R212A mutant catalyzed dehydrogenation of indapamide with 66% of the native enzyme activity, but the reconstituted mutant also had only 56% of the wild-type activity for its standard substrate, testosterone. Therefore, we concluded that hydrogen bonding of indapamide with the Arg212 residue is not necessary for substrate orientation to facilitate the dehydrogenation of indapamide.
In a similar example, the X-ray crystal structure of CYP3A4 (ITQN) suggested that Arg212 may provide general base catalysis of peroxide cleavage, and replacement by a nonbasic residue may decrease or even eliminate such activity (Yano et al., 2004). However, an H2O2-supported oxidation of 7-benzyloxyquinoline by the CYP3A4R212A mutant showed no significant effect of this replacement on kcat (Kumar et al., 2006). In an earlier study, the CYP3A4R212 mutant showed ∼2-fold increased activity with progesterone but unaltered activity with testosterone compared with the wild-type enzyme (Harlow and Halpert, 1997). In addition, the mutation did not significantly alter the activation of progesterone or testosterone hydroxylation by α-naphthoflavone.
In addition to the lowest energy-binding orientation that predicts indapamide dehydrogenation as discussed above, the molecular docking studies also found two other distinct energetically favored binding modes of indapamide in the active site of CYP3A4: one for the catalysis of the C-4/C-5 positions of indoline aromatic ring (Fig. 6, B and E; also predicted by DOCK, Fig. 7D), and the other as substrate “idle state” or noncatalysis mode (Fig. 6, C and F; also predicted by GOLD, Fig. 7C). These results from docking studies were in accordance with those predicted by MetaSite, a GRID molecular interaction field-based methodology, which showed the hydrogens at C-3 and C-5 positions are major active ones for reactions (Fig. 7, A and B). The C-5 position reaction could be either hydroxylation or epoxidation as confirmed by in vitro biotransformation results characterized by LC/MS and MS/MS. In addition, 5-hydroxyindapamide was also found to be a major metabolite in clinical studies (Kitao et al., 1982; Klunk et al., 1983). It is interesting to note that 5-hydroxyindoles were found to be bioactivated to form quinone imines (Yan et al., 2007), but we did not find a similar mechanism for 5-hydroxydehydroindapamide. We did find evidence for an epoxidation reaction, probably at the C-5/C-6 position, and the epoxide was trapped with GSH (Fig. 5) or cleaved by epoxide hydrolase. However, there were no reported side effects of indapamide associated with these reactive metabolites (epoxide or quinone imine). The paucity of adverse consequences is probably because the reactive intermediates are produced in negligible amounts from the low clinical doses of indapamide, or the electrophiles may be trapped efficiently by conjugation.
The quantitative models are always challenging, but they generally provide greater detail in mimicking biological systems (Di Ventura et al., 2006). In AutoDock, a two-factor clustering strategy based on both binding energy and RMSD space parameters provides a semiquantitative prediction if an appropriate cut-off threshold is chosen. When indapamide is placed into the active site of CYP3A4, AutoDock predicted approximately one third of indapamide was transformed to dehydroindapamide, another one third did not turnover, and the rest produced 5-hydroxylation and ring epoxidation metabolites. However, this study accentuates the limitations of using rigid crystal structures to model dynamic enzymes because the models are highly dependent on the accuracy of static crystal structures. Technically, higher resolution X-ray crystallization structures in various substrate binding conditions will be more reliable and could enhance in silico prediction (de Groot, 2006). In the interim, traditional laboratory techniques are crucial to validating these in silico techniques.
The comparisons of several diverse types of molecular modeling techniques presented in this article have shown reasonable predictive concordance. Other in silico modeling tools that have been used to study P450-catalyzed metabolism of xenobiotics include the ligand-based 3D-QSAR models and pharmacophore models. It is more and more important to predict drug metabolism and identify potential drug-drug interactions at an early stage of drug discovery (de Groot, 2006). These molecular docking and modeling procedures offer a powerful means to identify and structurally analyze potential problems during lead generation and will significantly shorten drug discovery timelines, in particular, early lead generation timelines in today's high-throughput drug screening discovery. For example, numerous series of compounds could have similar pharmacological potency for the same drug target, and ADME scientists must decide which one(s) have optimum ADME properties to generate lead compounds; in silico predictions and screening of lead structures could certainly prioritize these projects and thus increase overall productivity in the drug discovery process.
In conclusion, this study clarified the metabolic pathways of indapamide dehydrogenation and oxygenation by CYP3A4 in vitro and inferred several putative active site residues in silico. The results provide logical enzyme structures and substrate binding orientations to support the selectivity of indapamide dehydrogenation by CYP3A4. The combined use of molecular models and biochemical experiments should facilitate the process of hit profiling and hit-to-lead optimization and allow pharmaceutical scientists to realize the long-term goal of successful drug discovery and development.
Acknowledgments
We thank Drs. Eric Johnson (Scripps Research Institute) and Mike Wester (Pfizer) for guidance on the use of the docking software; Drs. Dennis Scott and Theodore Liston (Pfizer) for comments on the manuscript; and Diane Lanza (University of Utah) and Lili Yao (Pfizer) for technical support.
This work was supported in part by the National Institutes of Health National Heart, Lung, and Blood Institute [Grants HL13645, HL60143]; and the National Institutes of Health National Institute of General Medical Sciences [Grants GM074249, GM054995].
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
doi:10.1124/dmd.108.022707.
ABBREVIATIONS: indapamide, 4-chloro-N-(2-methyl-1-indolinyl)-3-sulfamoylbenzamide; P450, cytochrome P450; CCDC, Cambridge Crystallographic Data Center; GOLD, genetic optimization for ligand docking; ADME, absorption, distribution, metabolism and excretion; DDQ, 2,3-dichloro-5,6-dicyanobenzoquinone; LC/MS, liquid chromatography/mass spectrometry; DMSO, dimethyl sulfoxide; HMBC, heteronuclear multiple bond correlation; GSH, glutathione; HPLC, high-performance liquid chromatography; LS/MS/MS, liquid chromatography/tandem mass spectrometry; CID, collision-induced dissociation; amu, atomic mass unit; LGA, Lamarckian genetic algorithm; RMSD, root mean square deviation; J30, N-[1-(4-methoxybenzenesulfonyl)-2,3-dihydro-1H-indol-7-yl]-isonicotinamide; SB-206553, 5-methyl-1-(3-pyridylcarbamoyl)-1,2,3,5-tetrahydropyrrolo[2,3-f]indole; KMD-3213, 1-(3-hydroxypropyl)-5-[2-[2-[2-(2,2,2-trifluoroethoxy)phenoxy]-ethylamino]propyl]indoline-7-carboxamide; DW2282, (S)-(+)-4-phenyl-1-[N-(4-aminobenzoyl) indoline-5-sulfonyl]-4,5-dihydro-2-imidazolone]hydrochloride; BMS-561389, 1-(3′-aminobenzisoxazol-5′-yl)-3-trifluoromethyl-N-[2-fluoro-4-[(2′-dimethylaminomethyl)imidazol-1-yl]phenyl]-1H-pyrazole-5-carboxyamide; SB-242084, 6-chloro-5-methyl-1-[[2-(2-methylpyrid-3-yloxy)pyrid-5-yl]carbamoyl]indoline; SB-224289, 1′-methyl-5-[[2′-methyl-4′-(5-methyl-1,2,4-oxadiazol-3-yl)biphenyl-4-yl]carbonyl]-2,3,6,7-tetrahydrospiro[furo[2,3-f]indole-3,4′-piperidine].
References
- Baeyer A and Emmerling A (1869) Synthese des Indole. Ber 2 679–682. [Google Scholar]
- Baillie TA and Davis MR (1993) Mass spectrometry in the analysis of glutathione conjugates. Biol Mass Spectrom 22 319–325. [DOI] [PubMed] [Google Scholar]
- Braun J and Sobecki W (1911) Darstellung und Aufspaltung des Dihydro-indols. Ber 44 2158–2161. [Google Scholar]
- Brunner K (1900) Synthese von Indolinbasen. Monatshefte fuer Chemie 21 156–183. [Google Scholar]
- Cruciani G, Carosati E, De Boeck B, Ethirajulu K, Mackie C, Howe T, and Vianello R (2005) MetaSite: understanding metabolism in human cytochromes from the perspective of the chemist. J Med Chem 48 6970–6979. [DOI] [PubMed] [Google Scholar]
- de Groot MJ (2006) Designing better drugs: predicting cytochrome P450 metabolism. Drug Discov Today 11 601–606. [DOI] [PubMed] [Google Scholar]
- Di Ventura B, Lemerle C, Michalodimitrakis K, and Serrano L (2006) From in vivo to in silico biology and back. Nature 443 527–533. [DOI] [PubMed] [Google Scholar]
- Domanski TL, Liu J, Harlow GR, and Halpert JR (1998) Analysis of four residues within substrate recognition site 4 of human cytochrome P450 3A4: role in steroid hydroxylase activity and alpha-naphthoflavone stimulation. Arch Biochem Biophys 350 223–232. [DOI] [PubMed] [Google Scholar]
- Fagan GP, Chapleo CB, Lane AC, Myers M, Roach AG, Smith CF, Stillings MR, and Welbourn AP (1988) Indoline analogues of idazoxan: potent alpha 2-antagonists and alpha 1-agonists. J Med Chem 31 944–948. [DOI] [PubMed] [Google Scholar]
- Harlow GR and Halpert JR (1997) Alanine-scanning mutagenesis of a putative substrate recognition site in human cytochrome P450 3A4. Role of residues 210 and 211 in flavonoid activation and substrate specificity. J Biol Chem 272 5396–5402. [DOI] [PubMed] [Google Scholar]
- Jorgensen WL (2004) The many roles of computation in drug discovery. Science 303 1813–1818. [DOI] [PubMed] [Google Scholar]
- Kassahun K, Skordos K, McIntosh I, Slaughter D, Doss GA, Baillie TA, and Yost GS (2005) Zafirlukast metabolism by cytochrome P450 3A4 produces an electrophilic alpha,beta-unsaturated iminium species that results in the selective mechanism-based inactivation of the enzyme. Chem Res Toxicol 18 1427–1437. [DOI] [PubMed] [Google Scholar]
- Kennett GA, Wood MD, Bright F, Cilia J, Piper DC, Gager T, Thomas D, Baxter GS, Forbes IT, Ham P, et al. (1996) In vitro and in vivo profile of SB 206553, a potent 5-HT2C/5-HT2B receptor antagonist with anxiolytic-like properties. Br J Pharmacol 117 427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitao K, Sakata T, Ueshima Y, Muro H, Fujitani M, and Murauchi H (1982) Phase I clinical study of a new non-thiazide antihypertensive drug indapamide—biological fate and diuretic action after a single oral dose in normal volunteers. Yakuri To Tiryo 10 2619–2631. [Google Scholar]
- Klunk LJ, Ringel S, and Neiss ES (1983) The disposition of 14C-indapamide in man. J Clin Pharmacol 23 377–384. [DOI] [PubMed] [Google Scholar]
- Kumar S, Liu H, and Halpert JR (2006) Engineering of cytochrome P450 3A4 for enhanced peroxide-mediated substrate oxidation using directed evolution and site-directed mutagenesis. Drug Metab Dispos 34 1958–1965. [DOI] [PubMed] [Google Scholar]
- Lill MA, Dobler M, and Vedani A (2006) Prediction of small-molecule binding to cytochrome P450 3A4: flexible docking combined with multidimensional QSAR. ChemMedChem 1 73–81. [DOI] [PubMed] [Google Scholar]
- Liou JP, Hsu KS, Kuo CC, Chang CY, and Chang JY (2007) A novel oral indoline-sulfonamide agent, N-[1-(4-methoxybenzenesulfonyl)-2,3-dihydro-1H-indol-7-yl]-isonicotinamide (J30), exhibits potent activity against human cancer cells in vitro and in vivo through the disruption of microtubule. J Pharmacol Exp Ther 323 398–405. [DOI] [PubMed] [Google Scholar]
- Mahaney PE, Vu AT, McComas CC, Zhang P, Nogle LM, Watts WL, Sarkahian A, Leventhal L, Sullivan NR, Uveges AJ, et al. (2006) Synthesis and activity of a new class of dual acting norepinephrine and serotonin reuptake inhibitors: 3-(1H-indol-1-yl)-3-arylpropan-1-amines. Bioorg Med Chem 14 8455–8466. [DOI] [PubMed] [Google Scholar]
- Marechal JD, Yu J, Brown S, Kapelioukh I, Rankin EM, Wolf CR, Roberts GC, Paine MJ, and Sutcliffe MJ (2006) In silico and in vitro screening for inhibition of cytochrome P450 CYP3A4 by comedications commonly used by patients with cancer. Drug Metab Dispos 34 534–538. [DOI] [PubMed] [Google Scholar]
- Morris GM, Goodsell DS, Halliday RS, Huey R, Hart WE, Belew RK, and Olson AJ (1998) Automated docking using a lamarckian genetic algorithm and empirical binding free energy function. J Comput Chem 19 1639–1662. [Google Scholar]
- Patel A, MacMahon S, Chalmers J, Neal B, Woodward M, Billot L, Harrap S, Poulter N, Marre M, Cooper M, et al. (2007) Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet 370 829–840. [DOI] [PubMed] [Google Scholar]
- Regal KA, Laws GM, Yuan C, Yost GS, and Skiles GL (2001) Detection and characterization of DNA adducts of 3-methylindole. Chem Res Toxicol 14 1014–1024. [DOI] [PubMed] [Google Scholar]
- Rummey C, Nordhoff S, Thiemann M, and Metz G (2006) In silico fragment-based discovery of DPP-IV S1 pocket binders. Bioorg Med Chem Lett 16 1405–1409. [DOI] [PubMed] [Google Scholar]
- Sakashita H, Akahoshi F, Yoshida T, Kitajima H, Hayashi Y, Ishii S, Takashina Y, Tsutsumiuchi R, and Ono S (2007) Lead optimization of [(S)-gamma-(arylamino)prolyl]thiazolidine focused on gamma-substituent: indoline compounds as potent DPP-IV inhibitors. Bioorg Med Chem 15 641–655. [DOI] [PubMed] [Google Scholar]
- Schames JR, Henchman RH, Siegel JS, Sotriffer CA, Ni H, and McCammon JA (2004) Discovery of a novel binding trench in HIV integrase. J Med Chem 47 1879–1881. [DOI] [PubMed] [Google Scholar]
- Schapira M, Raaka BM, Das S, Fan L, Totrov M, Zhou Z, Wilson SR, Abagyan R, and Samuels HH (2003) Discovery of diverse thyroid hormone receptor antagonists by high-throughput docking. Proc Natl Acad Sci U S A 100 7354–7359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnecke V and Boström J (2006) Computational chemistry-driven decision making in lead generation. Drug Discov Today 11 43–50. [DOI] [PubMed] [Google Scholar]
- Shibata K, Foglar R, Horie K, Obika K, Sakamoto A, Ogawa S, and Tsujimoto G (1995) KMD-3213, a novel, potent, alpha 1a-adrenoceptor-selective antagonist: characterization using recombinant human alpha 1-adrenoceptors and native tissues. Mol Pharmacol 48 250–258. [PubMed] [Google Scholar]
- Smith AS, van de Waterbeemd H, and Walker DK (2006) Pharmacokinetics and Metabolism in Drug Design, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany.
- Song H, Wang R, Wang S, and Lin J (2005) A low-molecular-weight compound discovered through virtual database screening inhibits Stat3 function in breast cancer cells. Proc Natl Acad Sci U S A 102 4700–4705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Ehlhardt WJ, Kulanthaivel P, Lanza DL, Reilly CA, and Yost GS (2007) Dehydrogenation of indoline by cytochrome P450 enzymes: a novel “aromatase” process. J Pharmacol Exp Ther 322 843–851. [DOI] [PubMed] [Google Scholar]
- Sun H and Yost GS (2008) Metabolic activation of a novel 3-substituted indole-containing TNF-alpha inhibitor: dehydrogenation and inactivation of CYP3A4. Chem Res Toxicol 21 374–385. [DOI] [PubMed] [Google Scholar]
- Tamura A, Sato T, and Fujii T (1990) Antioxidant activity of indapamide and its metabolite. Chem Pharm Bull (Tokyo) 38 255–257. [DOI] [PubMed] [Google Scholar]
- Varnes JG, Wacker DA, Jacobson IC, Quan ML, Ellis CD, Rossi KA, He MY, Luettgen JM, Knabb RM, Bai S, et al. (2007) Design, structure-activity relationship, and pharmacokinetic profile of pyrazole-based indoline factor Xa inhibitors. Bioorg Med Chem Lett 17 6481–6488. [DOI] [PubMed] [Google Scholar]
- Walker MA, Johnson T, Ma Z, Zhang Y, Banville J, Remillard R, Plamondon S, Pendri A, Wong H, Smith D, et al. (2006) Exploration of the diketoacid integrase inhibitor chemotype leading to the discovery of the anilide-ketoacids chemotype. Bioorg Med Chem Lett 16 5818–5821. [DOI] [PubMed] [Google Scholar]
- Williams PA, Cosme J, Vinkovic DM, Ward A, Angove HC, Day PJ, Vonrhein C, Tickle IJ, and Jhoti H (2004) Crystal structures of human cytochrome P450 3A4 bound to metyrapone and progesterone. Science 305 683–686. [DOI] [PubMed] [Google Scholar]
- Yan Z, Easterwood LM, Maher N, Torres R, Huebert N, and Yost GS (2007) Metabolism and bioactivation of 3-methylindole by human liver microsomes. Chem Res Toxicol 20 140–148. [DOI] [PubMed] [Google Scholar]
- Yano JK, Wester MR, Schoch GA, Griffin KJ, Stout CD, and Johnson EF (2004) The structure of human microsomal cytochrome P450 3A4 determined by X-ray crystallography to 2.05-A resolution. J Biol Chem 279 38091–38094. [DOI] [PubMed] [Google Scholar]
- Zhao L and Brinton RD (2005) Structure-based virtual screening for plant-based ERbeta-selective ligands as potential preventative therapy against age-related neurodegenerative diseases. J Med Chem 48 3463–3466. [DOI] [PubMed] [Google Scholar]