Abstract
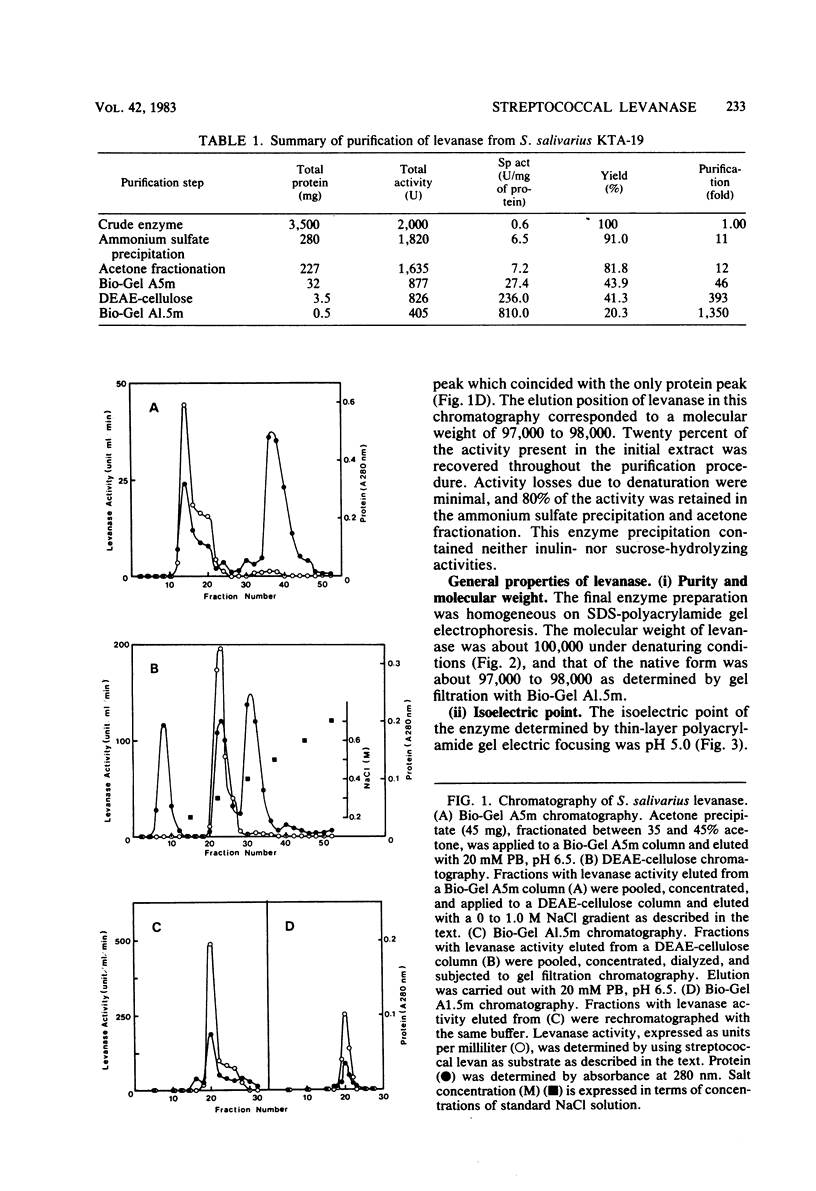
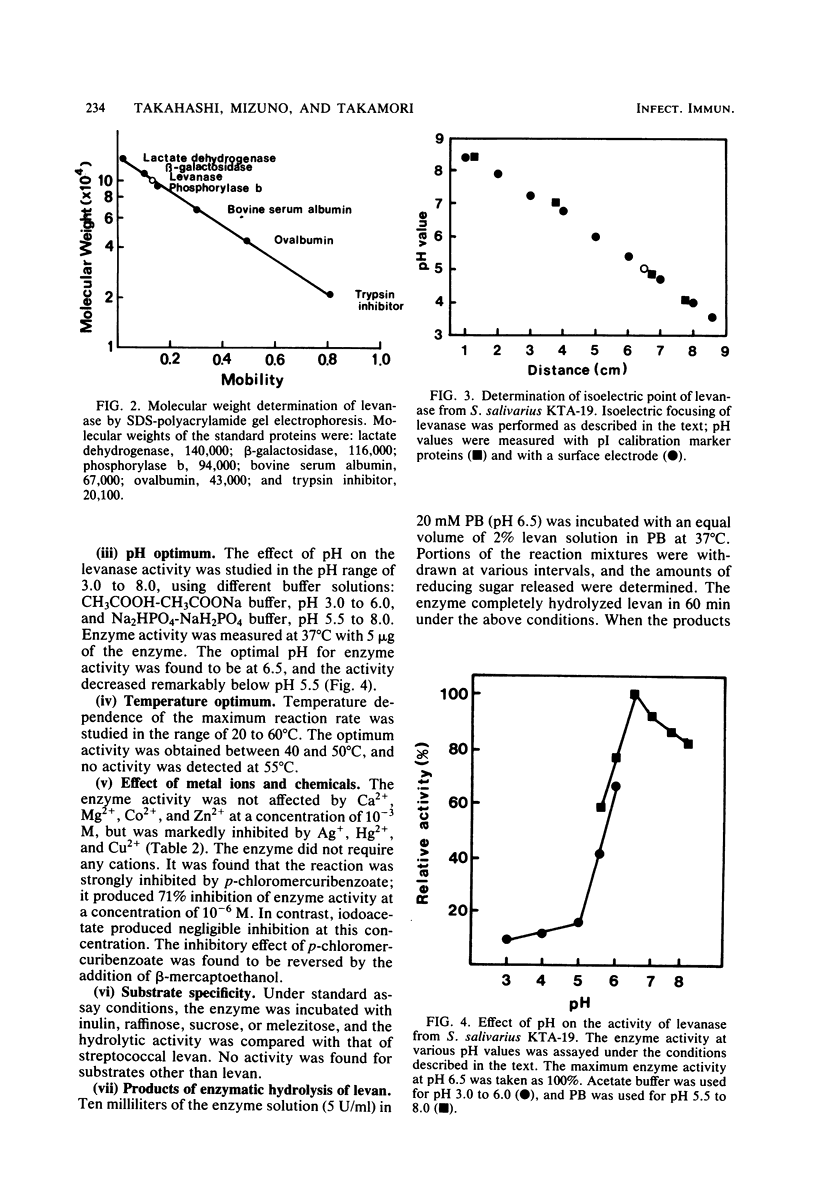
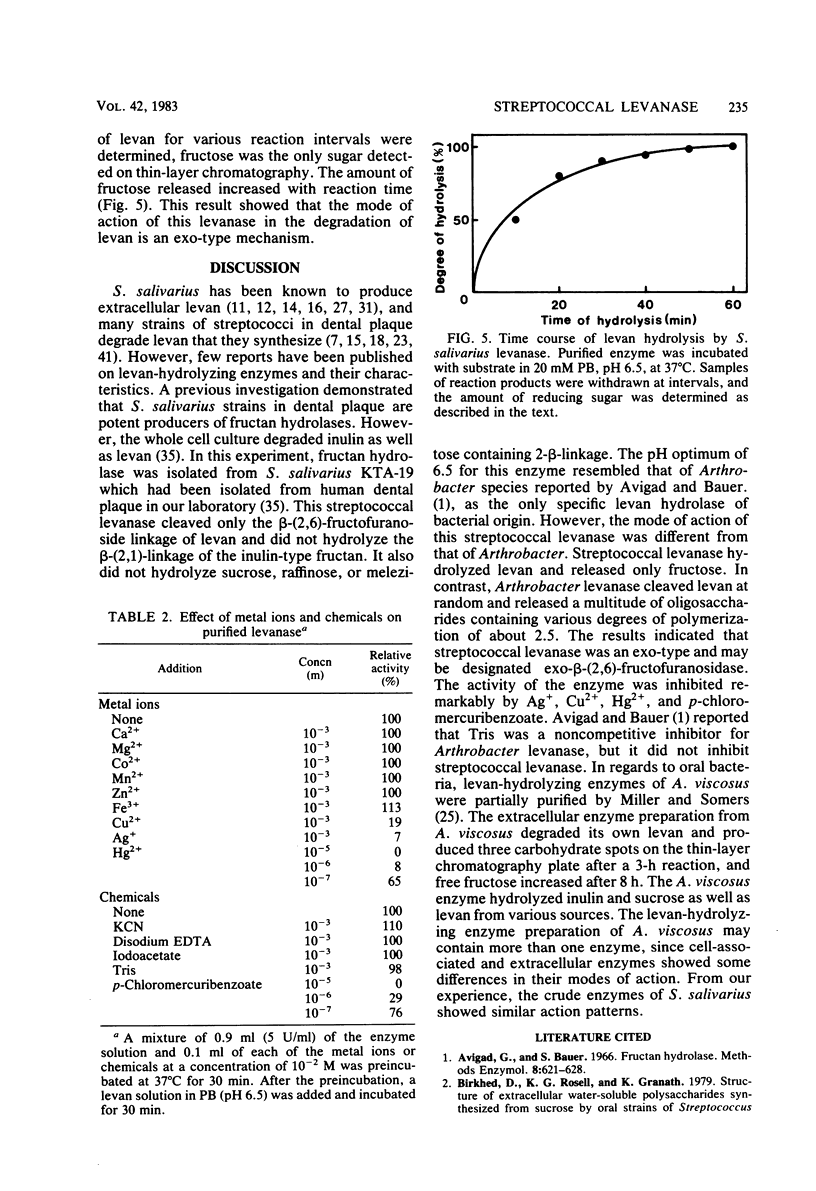
Fructan-hydrolyzing enzyme from Streptococcus salivarius KTA-19 isolated from human dental plaque was investigated. The enzyme was purified by ammonium sulfate precipitation, acetone fractionation, and column chromatography on Bio-Gel and DEAE-cellulose. The purified enzyme showed a single band on sodium dodecyl sulfate-polyacrylamide gel electrophoresis and isoelectric focusing. Its molecular weight was 100,000 as determined by sodium dodecyl sulfate polyacrylamide gel electrophoresis. The enzyme exhibited an optimum pH of 6.5 and decreased its activity from pH 6.0 and especially below pH 5.5. The optimum temperature was 40 to 50 degrees C, and enzyme activity was reduced by 90% at 55 degrees C. Enzyme activity was markedly inhibited by Hg2+, Ag+, Cu2+, and p-chloromercuribenzoate at a concentration of 10(-3) M, but not by other metal ions or chemical effectors. Fructose was the only by-product of the enzyme action on levan. These results indicated that the levanase of S. salivarius KTA-19 is an exo-beta-(2,6)-fructofuranosidase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carlsson J. A levansucrase from Streptococcus mutans. Caries Res. 1970;4(2):97–113. doi: 10.1159/000259632. [DOI] [PubMed] [Google Scholar]
- Ceska M. Biosynthesis of levan and a new method for the assay of levansucrase activity. Biochem J. 1971 Nov;125(1):209–211. doi: 10.1042/bj1250209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigan A. J., Robyt J. F. Nature of the fructan of Streptococcus mutans OMZ 176. Infect Immun. 1979 Oct;26(1):387–389. doi: 10.1128/iai.26.1.387-389.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley P., Wood J. M., Saxton C. A., Leach S. A. The polymerisation of dietary sugars by dental plaque. Caries Res. 1967;1(2):112–129. doi: 10.1159/000259506. [DOI] [PubMed] [Google Scholar]
- DISCHE Z. New color reactions for determination of sugars in polysaccharides. Methods Biochem Anal. 1955;2:313–358. doi: 10.1002/9780470110188.ch11. [DOI] [PubMed] [Google Scholar]
- DaCosta T., Gibbons R. J. Hydrolysis of levan by human plaque streptococci. Arch Oral Biol. 1968 Jun;13(6):609–617. doi: 10.1016/0003-9969(68)90139-8. [DOI] [PubMed] [Google Scholar]
- Dahlqvist A., Krasse B., Olsson I., Gardell S. Extracellular polysaccharides formed by caries-inducing streptococci. Monosaccharide composition of the substrate soluble component. Helv Odontol Acta. 1967 Apr;11(1):15–21. [PubMed] [Google Scholar]
- Ebisu S., Kato K., Kotani S., Misaki A. Structural differences in fructans elaborated by streptococcus mutans and Strep. salivarius. J Biochem. 1975 Nov;78(5):879–887. doi: 10.1093/oxfordjournals.jbchem.a130993. [DOI] [PubMed] [Google Scholar]
- Ehrlich J., Stivala S. S., Bahary W. S., Garg S. K., Long L. W., Newbrun E. Levans: I. Fractionation, solution viscosity, and chemical analysis of levan produced by Streptococcus salivarius. J Dent Res. 1975 Mar-Apr;54(2):290–297. [PubMed] [Google Scholar]
- Garszczynski S. M., Edwards J. R. Synthesis of a broth levan by a cell-bound levansucrase from Streptococcus salivarius (SS2). Arch Oral Biol. 1973 Feb;18(2):239–251. doi: 10.1016/0003-9969(73)90144-1. [DOI] [PubMed] [Google Scholar]
- Gold W., Preston F. B., Lache M. C., Blechman H. Production of levan and dextran in plaque in vivo. J Dent Res. 1974 Mar-Apr;53(2):442–446. doi: 10.1177/00220345740530024401. [DOI] [PubMed] [Google Scholar]
- Hancock R. A., Marshall K., Weigel H. Structure of the levan elaborated by Streptococcus salivarius strain 51: an application of chemical-ionisation mass-spectrometry. Carbohydr Res. 1976 Jul;49:351–360. doi: 10.1016/s0008-6215(00)83152-3. [DOI] [PubMed] [Google Scholar]
- Hardie J. M., Marsh P. D. Streptococci and the human oral flora. Soc Appl Bacteriol Symp Ser. 1978;7:157–206. [PubMed] [Google Scholar]
- Higuchi M., Iwami Y., Yamada T., Araya S. Levan synthesis and accumulation by human dental plaque. Arch Oral Biol. 1970 Jun;15(6):563–567. doi: 10.1016/0003-9969(70)90111-1. [DOI] [PubMed] [Google Scholar]
- Howell A., Jr, Jordan H. V. Production of an extracellular levan by Odontomyces viscosus. Arch Oral Biol. 1967 Apr;12(4):571–573. doi: 10.1016/0003-9969(67)90033-7. [DOI] [PubMed] [Google Scholar]
- Krichevsky M. I., Howell A., Jr, Lim S. Levan formation by Odontomyces viscosus. J Dent Res. 1969 Sep-Oct;48(5):938–942. doi: 10.1177/00220345690480055701. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lesher R. J., Gerencser V. F. Levan production by a strain of Rothia: activation of complement resulting in cytotoxicity for human gingival cells. J Dent Res. 1977 Sep;56(9):1097–1105. doi: 10.1177/00220345770560091401. [DOI] [PubMed] [Google Scholar]
- Manly R. S., Richardson D. T. Metabolism of levan by oral samples. J Dent Res. 1968 Nov-Dec;47(6):1080–1086. doi: 10.1177/00220345680470061301. [DOI] [PubMed] [Google Scholar]
- Miller C. H., Somers P. J. Degradation of levan by Actinomyces viscosus. Infect Immun. 1978 Oct;22(1):266–274. doi: 10.1128/iai.22.1.266-274.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. H., Warner T. N., Palenik C. J., Somers P. J. Levan formation by whole cells of Actinomyces viscosus ATCC 15987. J Dent Res. 1975 Jul-Aug;54(4):906–906. doi: 10.1177/00220345750540043701. [DOI] [PubMed] [Google Scholar]
- Pabst M. J., Cisar J. O., Trummel C. L. The cell wall-associated levansucrase of Actinomyces viscosus. Biochim Biophys Acta. 1979 Feb 9;566(2):274–282. doi: 10.1016/0005-2744(79)90031-7. [DOI] [PubMed] [Google Scholar]
- Pabst M. J. Levan and levansucrase of Actinomyces viscosus. Infect Immun. 1977 Feb;15(2):518–526. doi: 10.1128/iai.15.2.518-526.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMOGYI M. Notes on sugar determination. J Biol Chem. 1952 Mar;195(1):19–23. [PubMed] [Google Scholar]
- SNYDER M. L., HACKEDORN H. M., MARTIN D. O., JOHNSTON D. D. The synthesis of mucinous polysaccharides from sucrose by oral bacteria. J Dent Res. 1955 Jun;34(3):368–379. doi: 10.1177/00220345550340031101. [DOI] [PubMed] [Google Scholar]
- Sund M. L., Linder L. Cell-surface bound beta-fructofuranosidase (invertase) of the oral bacterium Streptococcus mitis. Arch Oral Biol. 1980;25(8-9):573–578. doi: 10.1016/0003-9969(80)90070-9. [DOI] [PubMed] [Google Scholar]
- Vesterberg O. Isoelectric focusing of proteins in polyacrylamide gels. Biochim Biophys Acta. 1972 Jan 26;257(1):11–19. doi: 10.1016/0005-2795(72)90248-6. [DOI] [PubMed] [Google Scholar]
- Warner T. N., Miller C. H. Cell-associated levan of Actinomyces viscosus. Infect Immun. 1978 Feb;19(2):711–719. doi: 10.1128/iai.19.2.711-719.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wood J. M. The amount, distribution and metabolism of soluble polysaccharides in human dental plaque. Arch Oral Biol. 1967 Jul;12(7):849–858. doi: 10.1016/0003-9969(67)90107-0. [DOI] [PubMed] [Google Scholar]
- van Houte J., Jansen H. M. Levan degradation by streptococci isolated from human dental plaque. Arch Oral Biol. 1968 Jul;13(7):827–830. doi: 10.1016/0003-9969(68)90102-7. [DOI] [PubMed] [Google Scholar]
- van der Hoeven J. S., Vogels G. D., Bekkers M. F. A levansucrase from Actinomyces viscosus. Caries Res. 1976;10(1):33–48. doi: 10.1159/000260187. [DOI] [PubMed] [Google Scholar]


