Abstract
In the present study we tested the responsiveness of human corneal epithelial cells (HCECs) and corneal fibroblasts to lipopolysaccharide (LPS), a TLR4 ligand. Purified P aeruginosa LPS was used to stimulate telomerase-immortalized HCECs (HUCL) and stromal fibroblast (THK) cell lines. Exposure of cells to LPS induced a time-dependent activation of NF-κB in THK but not in HUCL cells, as assessed by an increase in IκB-α phosphorylation and degradation. Concomitant with NF-κB activation, LPS-treated THK cells, but not HUCL cells, produced significantly more cytokines than control untreated cells. A cell surface biotinylation assay revealed that HUCL cells express TLR4 intracellularly whereas TLR5 is expressed on the cell surface. Furthermore, RT-PCR analysis revealed that HUCL and primary HCECs, in contrast to THK cells, do not express MD-2. Thus, our results demonstrate that the LPS unresponsiveness of HCECs might be due to deficient expression of MD2, an essential component for LPS-TLR4 signaling.
Keywords: corneal epithelial cells, keratocytes, toll-like receptors, proinflammatory, cytokines, innate response
Introduction
The Gram-negative bacterium P. aeruginosa is an opportunistic bacterial pathogen and a leading cause of bacterial keratitis with increased incidence among contact lens users with extended wearing. 1,2 If left untreated, infections could lead to perforation of the cornea resulting in permanent loss of vision and potential loss of the eye. 3,4 P. aeruginosa elaborates a multitude of factors including glycocalyx, lipopolysaccharide (LPS), endotoxin, and flagellin.5,6 These factors, notably LPS, may induce the release of multiple proinflammatory cytokines and chemokines from resident corneal cells and this initial inflammatory response plays a key role in containment of the infection. 6,7 LPS, a major component of the outer membrane of P. aeruginosa, is a virulence factor that can cause inflammatory responses in tissues, the consequences of which can be severe and even lethal.8 LPS activates monocytes and macrophages which produce several inflammatory cytokines including TNF-α, IL-1, IL-6, IL-8 and IL-12, leading to serious systemic disorders. 9,10 LPS also induces inflammatory responses in endothelial cells 11,12 and respiratory, gastric, and bladder epithelial cells.13–16 Other epithelium, such as intestinal epithelial 17,18 and well-differentiated human airway epithelial cells 19, are hyporesponsive to LPS. In contrast others have reported that intestinal epithelial cells recognize and respond to the internalized LPS.20,21 These studies also revealed that the inflammatory response induced by LPS in epithelial and other cell types is related to Toll-like receptor (TLR) 4.
The major advancement in understanding the molecular mechanism of TLR4 in transducing LPS-mediated signals was the discovery of myeloid differentiation (MD)-2.22 MD-2 is a glycoprotein of approximately 17–25 kDa, co-expressed with TLR4 at the surface of various cell types, principally those of myeloid and endothelial lineages.23,24 Shimazu et al found that when MD-2 was co-transfected into HEK293 cells, it bound to TLR4 and greatly enhanced the response of TLR4-transfected cells to LPS.22 In another study, a forward genetic screen of CD14-transfected CHO cells revealed that MD-2 is essential for LPS responses and MD-2 can be supplied as a soluble receptor component to a TLR4-positive cell. 25 In addition, MD-2 has been suggested to play a role in the trafficking of TLR4 to the cell surface, at least in murine cells.26 Many mucosal epithelial cells such as those in the intestine 27 and in the airway19,28 have a limited response to LPS, and low or absent expression of MD-2 was suggested as the underlying mechanism for these cells.
TLRs recognize repetitive patterns, thus they are termed pattern-recognizing receptors (PRRs). These patterns are present on diverse microbes including Gram-positive and Gram-negative bacteria and viruses and are key components of innate immunity. 29,30 An early study revealed that HCECs expressed TLR4 and its co-receptor CD14 and responded to LPS challenge to produce proinflammatory cytokines within 24 h. 31 Recently, Ueta et al reported that the incubation of human corneal epithelial cells with LPS did not lead to the activation of NF-κB or the secretion of inflammation-associated molecules such as IL-6, IL-8, and human beta-defensin-2. 32 However, the application of LPS to abraded corneal epithelium in vivo resulted in the secretion of proinflammatory cytokines that mediate recruitment of neutrophils to the corneal stroma, thus inducing stromal edema and structural changes in corneal architecture in a MyD88-dependent manner. 33 In contrast to the other TLRs, TLR4 is unique as its downstream signaling can occur via two independent pathways 34. The first pathway depends on the MyD88 signal adaptor protein which is critical for the production of several proinflammatory cytokines, e.g., IL-6 and TNF-α, and in the recognition of Gram-negative bacteria. 35–37 In contrast, the MyD88-independent pathway, which depends on the Toll IL-1 receptor (TIR) domain-containing adaptor-inducing IFN-β (TRIF) signal adaptor protein, is mainly involved in production of type I IFN. 38
To date, although corneal fibroblasts (keratocytes) have been implicated in playing a role in keratitis 4, the putative involvement of keratocytes in innate immune responses as part of corneal host defense has not been characterized. Thus, we hypothesize that in addition to epithelial cells, other resident corneal cells such as stromal keratocytes, might be involved in recognizing and formulating an innate response for the cornea to LPS challenge. We tested this hypothesis in-vitro, using primary and immortalized human corneal epithelial and stromal keratocyte cell lines, by assessing LPS- mediated signal transduction and the production of proinflammatory cytokines/chemokines.
Results
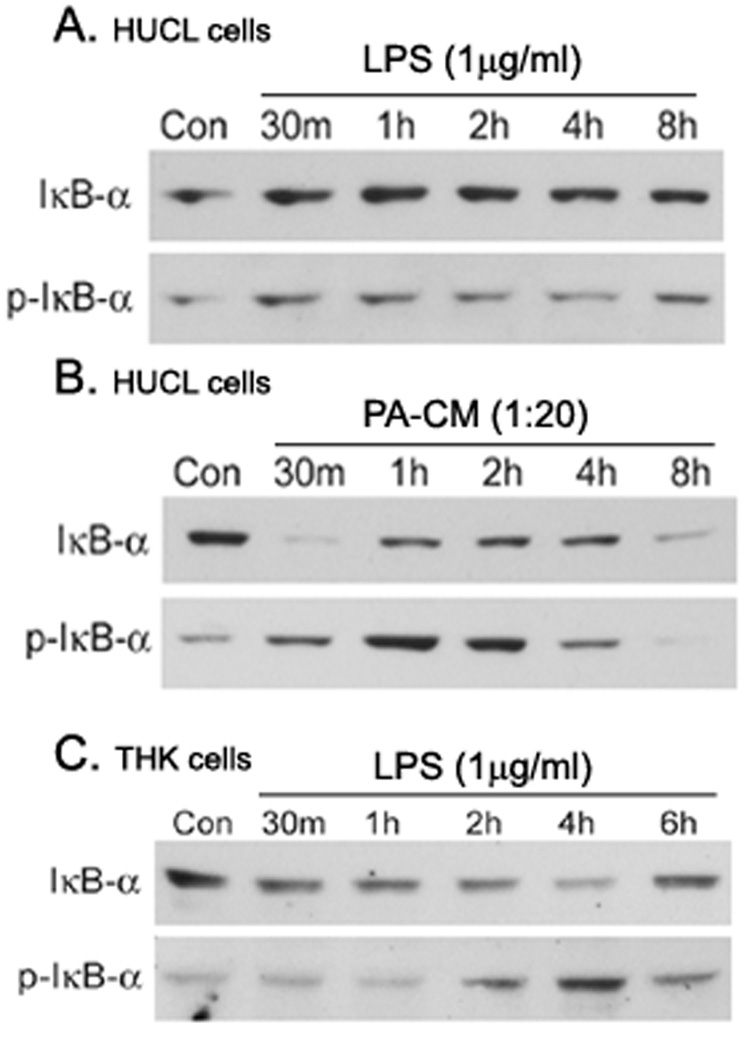
In epithelial cells, the transcription factor NF-κB plays a central role in regulating genes that govern the onset of mucosal inflammatory responses. The primary consequences of TLR activation is NF-κB activation and cytokine secretion in epithelial cells. To investigate LPS-triggered NF-κB activation, we first determined the dose response by treating HUCL cells with different concentration of LPS ranging from 1 ng to 10 µg/ml. We observed that LPS did not induce NF-κB as assessed by IκB-α phosphorylation in HUCL cells up to 10 µg/ml, whereas 1 µg/ml LPS significantly induced NF-κB activation in THK cells (data not shown). A time-course study (Figure 1) showed that LPS (1 µg/ml) in KBM had no apparent effect on the levels of phospho-IκB-α and IκB-α in HUCL cells for up to 8 h (Figure 1A). However, M9-conditioned medium derived from PAO1 culture (1:20 dilution) induced IκB-α phosphorylation and degradation in a time-dependent manner, suggesting that HCECs are responsive to PAO1 exoproducts (Figure 1A). In contrast, in THK cells LPS stimulated rapid IκB-α phosphorylation and IκB-α degradation that was maximal at 15 min post-stimulation (Figure 1B). Thus, LPS induced NF-κB activation in THK, but not in HUCL cells.
Figure 1. LPS stimulated IκB-α phosphorylation and degradation in THK, but not HUCL cells.
HUCL (A) or THK (B) cells were stimulated with phenol-extracted LPS (1 µg/ml) for the indicated times. As a positive control, HUCL cells were also treated with 5% supplemented P. aeruginosa-conditioned medium (PA-CM) in KBM or KBM alone (Con). Total protein was extracted, and 20 µg of protein were subjected to SDS-PAGE followed by phospho-IκB-α (p-IκB-α) and IκB-α immunoblotting using a chemiluminescence technique. The results are representative of two independent experiments.
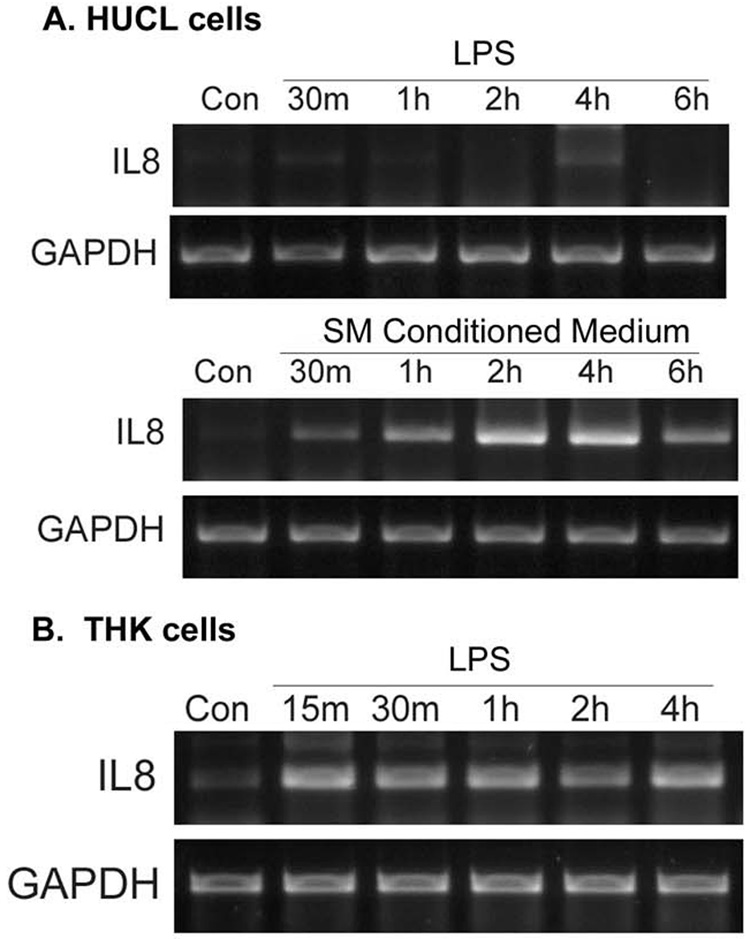
To assess the biological relevance of induced NF-κB activation, we measured the effect of LPS on proinflammatory cytokine expression and production (secretion) in HCECs and keratocytes. The effect of LPS on IL-8 mRNA expression was determined by RT-PCR. IL-8 mRNA was not detectable in untreated or 1 µg/ml LPS-challenged HUCL cells (Figure 2A). However, a PCR product of the expected size (347 bp) was observed in HUCL cells 30 min post-stimulation with P. aeruginosa conditioned medium; the band intensity increased, peaked at 2 h, and was still detectable at 6 h (Figure 2B). Similar to HUCL cells, IL-8 mRNA in unstimulated THK cells was barely detectable. However, incubation with LPS resulted in an increase in the expression of IL-8 mRNA in THK cells in a time-dependent manner.
Figure 2. LPS induced IL-8 mRNA expression in THK, but not HUCL cells.
(A) HUCL were incubated with 1 µg/ml LPS for the indicated times; as a control, cells were also treated with 5% SMCM. (B) THK cells were treated with 1 µg/ml LPS for the indicated times. Total RNA was extracted, reverse transcripted, and amplified using IL-8 primers with GAPDH as control. PCR products were separated and stained as described in Materials and Methods. Results are representative of three independent experiments.
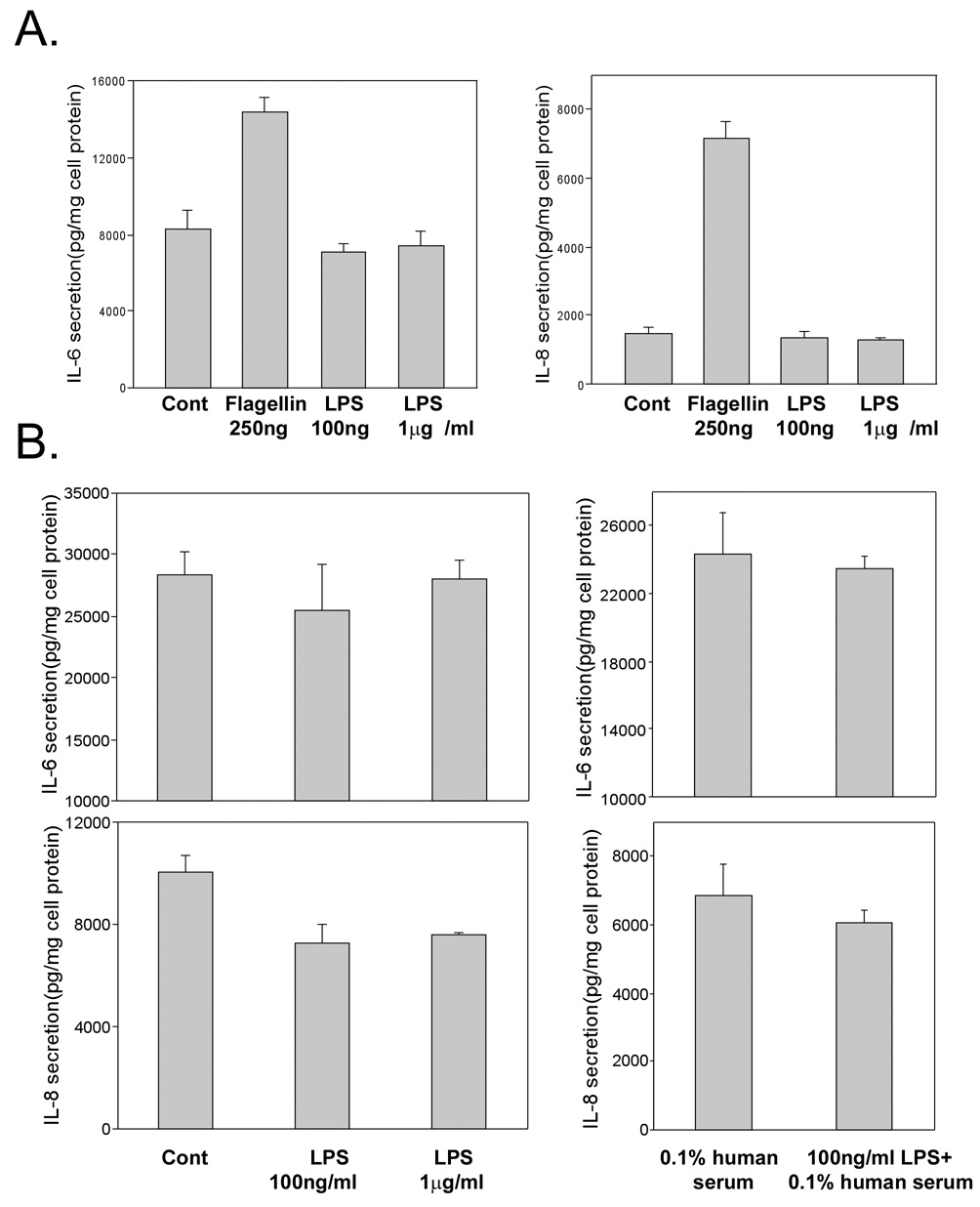
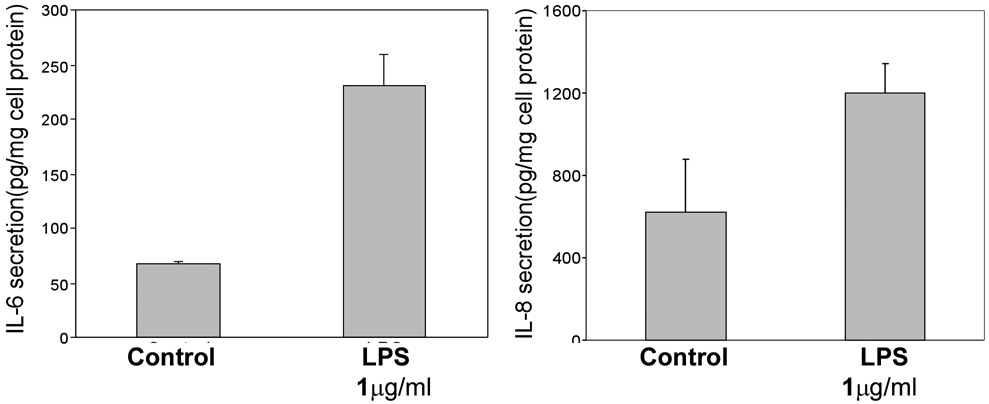
The effect of LPS on IL-6 and IL-8 secretion by HUCL cells was assessed by ELISA. Consistent with a lack of NF-κB activation, there was no induced accumulation of IL-6 and IL-8 in HUCL cells treated with either 100 ng/ml or 1 µg/ml LPS (Figure 3A). These cells however, responded to a challenge of 250 ng/ml flagellin that served as a positive control by producing significantly more IL-8 (5.8 fold) and IL-6 (2.6 fold) as compared to the control untreated cells (p<0.01). To examine whether CD14 or LPS binding protein (LBP) is responsible for the unresponsiveness of HUCL cells to LPS challenge, human serum that provides soluble CD14 and/or LBP 31,39 was added to the culture. No significant increase in IL-6 or IL-8 production was observed in LPS-challenged HUCL cells with or without human serum. THK cells, on the other hand, produced significantly more IL-6 and IL-8 in response to the LPS challenge even without the addition of serum. 100 ng/ml LPS was as effective as 1 µg/ml LPS in inducing the secretion of the cytokines (Figure 4). Taken together, LPS differentially stimulates resident corneal cells by inducing IL-6 and IL-8 production in THK, but not in HUCL cells.
Figure 3. LPS failed to induce IL-6 and IL-8 secretion in HUCL cells.
(A) HUCL cells were stimulated with phenol-extracted LPS (1 µg/ml and 100 ng/ml) or medium alone (control) for 8 h; as a positive control, cells were also treated with 250 ng/ml purified flagellin for 8h. (B) HUCL cells were treated with 1 µg/ml phenol-extracted LPS with or without 0.1% human serum for 24 h. The effects of LPS and flagellin on IL-6 and -8 secretion were measured in cell culture supernatants by ELISA. Data are representative of triplicate experiments and are expressed as the mean ± SD. Statistically significant differences in secreted IL-6 and IL-8 in LPS-treated cells were determined by ANOVA with probabilities shown both for overall significance and pairwise comparison (*P<0.01).
Figure 4. LPS stimulated IL-6 and IL-8 secretion in THK cells.
THK cells were treated with phenol-extracted LPS (1 µg/ml) for 8 h, and with medium alone at the same time point as control. The effects of LPS on IL-6 and -8 secretion were measured in THK culture supernatants by ELISA. Data are representative of triplicate experiments and are expressed as the mean ± SD. Statistically significant differences in secreted IL-6 and IL-8 in LPS-treated cells were determined by ANOVA with pair-wise comparison (*P<0.01).
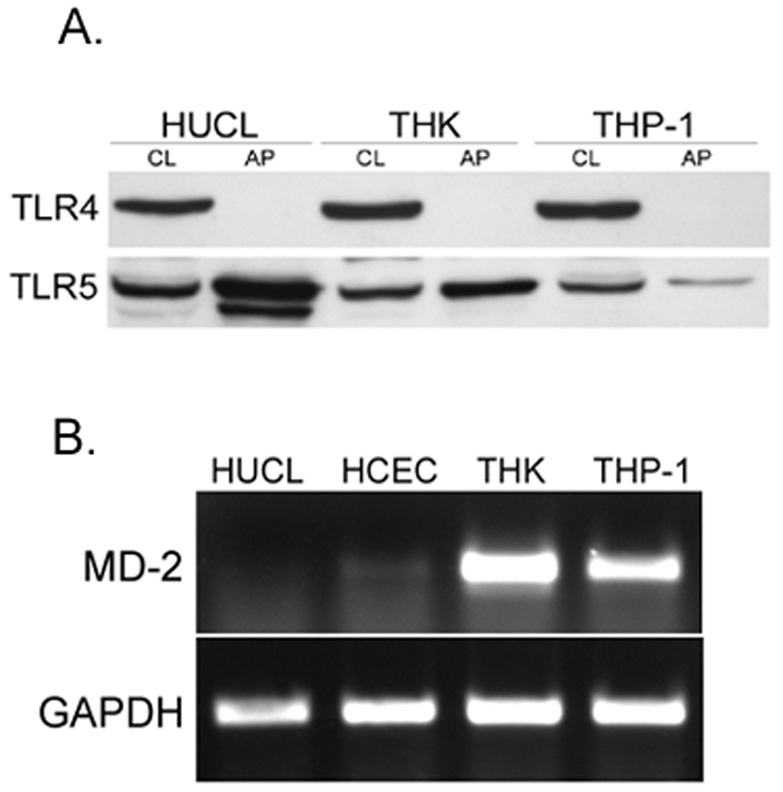
Recently, Ueta et al reported the intracellular localization of TLR4 in HCECs. To confirm TLR4 localization in HCECs, HUCL cells were biotinylated with plasma membrane-impermeable NHS-LC-biotin, followed by avidin precipitation of biotinylated proteins and Western blotting with TLR antibodies. While HUCL cells expressed abundant TLR4, there was no surface biotinylated TLR4 detected. Under identical conditions, TLR5 was found to be labeled at the cell surface (Figure 5A).
Figure 5. Lack of TLR4 cell surface expression and MD-2 expression in HCECs.
(A) HUCL monolayers were labeled with NHS-LC-biotin. Biotinlyated proteins were either directly analyzed by Western blotting (C) or precipitated from the cell lysate by streptavidin-conjugated agarose and analyzed using Western blotting (M) with TLR4- or TLR5-specific antibodies. The figure is representative of three independent experiments. (B) HUCL, THK and THP-1 cells were extracted and the obtained RNA was reverse transcripted and amplified using human MD2 primers with GAPDH as control. PCR products were separated and stained. Results are representative of three independent experiments.
To understand the underlying mechanism of the hyporesponsiveness of HCECs to LPS, we assessed the expression of TLR4 co-receptors known to be required for LPS-TLR interaction. While CD14 was expressed in both epithelial and stromal fibroblasts (data not shown), MD-2 was found to be abundantly expressed in THK cells as well as in THP-1 cells (a human monocytic leukemia cell line, sensitive to LPS), but not in HCECs, including both the HUCL cell line and primary HCECs (Figure 5B). Little or no RT-PCR amplicons of MD-2 were detected in either HUCL cells or primary HCECs.
Discussion
In this study, we showed that P. aeruginosa LPS stimulates the activation of the NF-κB signaling pathway and pro-inflammatory cytokine and chemokine production in human corneal stromal fibroblast cells, but fails to activate an inflammatory response in HCECs. We demonstrated that HCECs express abundant TLR4 intracellularly. However, there was no detectable increase in LPS-induced NF-κB activation or production of proinflammatory cytokines in these cells exposed to LPS for up to 24 h. This finding is consistent with that reported by Ueta et al. 32 We further demonstrated that the unresponsiveness of HCECs to LPS challenge is likely due to the lack of MD-2 expression in HCECs. Human corneal stromal fibroblast cells, on the other hand, expressed TLR4 and MD-2 and responded to LPS challenge by secreting proinflammatory cytokines. Thus, the cornea is able to recognize LPS once it has penetrated into the stroma, and mounts an innate immune response to the invading pathogens by producing proinflammatory cytokines that recruit PMN into the site of infection.
Bacterial endotoxins (LPS) are among the most potent inducers of innate immune responses and are a major virulence factor of Gram-negative bacteria including P. aeruginosa. The structural analysis of LPS divides the molecule into a hydrophobic lipid A region, which replaces phospholipids in the outer membrane, a central core oligosaccharide region, and a repeating polysaccharide portion referred to as O antigen or O polysaccharide. 40 The O-antigen portion of the P. aeruginosa LPS is responsible for conferring serogroup specificity, which is defined by antibodies specific to the different variants of this antigen. Recent studies revealed that the inflammatory response to LPS in cells is mediated by its interaction with TLR4. 41,42 Molecular interaction studies showed that TLR4 is essential for the recognition of the lipid A portion of bacterial LPS,43 and mediates both effective host resistance to infection as well as some of the pathology associated with LPS-induced shock. 44 Therefore, it should be noted that the Pseudomonas LPS used in this study is unlikely to influence TLR4 signaling regardless of its serotype. A previous study by Pier et al showed that P. aeruginosa LPS induces less inflammation and lower overall host responses compared with that induced by enterobacterial (E. coli) LPS. 45 However, more recent studies showed a high degree of variability in the P. aeruginosa lipid A structure that can be synthesized depending on the strain and growth conditions, and have also shown that TLR4-mediated responses are highly dependent on the level of acylation of lipid A .46
A maximally sensitive response to LPS requires the sequential participation of several extracellular and cell-surface LPS-binding proteins, including LBP, CD14, MD-2, and TLR4. Briefly, LBP binds to Gram-negative bacteria or aggregates of LPS, thus decreasing the binding energy of LPS monomers. The LPS molecule is shuttled to CD14, which transfers the LPS to MD-2. The binding of lipid A to MD-2 causes the rearrangement of TLR4, leading to the association of its intracellular TIR domains and the recruitment of adapter proteins. Therefore, MD-2 plays a pivotal role in LPS sensing, bridging the recognition of endotoxins initiated by LBP and CD14 in response to the activation of TLR4 to pro-inflammatory cytokine and chemokine production. 18,41,42,47 The responsiveness of epithelial cells to LPS appears to be tissue-specific; respiratory, gastric and bladder epithelial cells have been shown to be activated by LPS, whereas intestinal and oral epithelial cells fail to recognize and respond to LPS. 18,48,49 The reason for endotoxin hyporesponsiveness of intestinal epithelial cells is low levels of TLR4 and/or MD-2.18
In the cornea, an early study showed that HCECs express TLR4 and CD14 and respond to LPS stimulation through the production of IL-6, IL-1α, IL-8 and TNF-α. 31 However, a recent study by Ueta et al revealed that HCECs are unresponsive to LPS challenge. 32 Our results are consistent with that reported by Ueta et al. 32 We also confirmed intracellular localization of TLR4 in HCECs. The intracellular expression of TLR4 was suggested to be an underlying mechanism for the immunosilent environment at the ocular mucosal epithelium. 32 Similarly, TLR4 was also found intracellularly in pulmonary and intestinal epithelial cells, but intracellular TLR4 in these cells is capable of recognizing internalized LPS. Since we showed that HCECs respond to flagellin through TLR5 50, which shares the MyD88-dependent signaling pathways with TLR430, the unresponsiveness of HCECs to TLR4 ligand is not due to defects in intracellular signal transduction, but more likely due to the lack of molecules required for proper LPS-TLR4 interaction. CD14 and MD-2 are critical co-receptors for TLR4 signaling.51 MD-2 and LPS form complexes that induce TLR4-expressing epithelial cells to secrete IL-6,, IL-8, andMD-2. Thus, the LPS-MD-2 complex plays a crucial role in the LPS response by activating epithelial cells in the inflammatory microenvironment. 19,52–54 Our results have shown that there was little or no MD-2 mRNA detected in HCECs, in both HUCL cell lines as well as in primary cultured cells. In line with our study, the low level of MD2 expression in intestinal epithelial cells was attributed to the hyporesponsiveness of these cells to LPS. 18 Although the role of MD2 in LPS internalization has not been reported, the lack of MD-2 expression may also be responsible for the failure of HCECs to internalize LPS in vitro and in vivo. 32,55 A more recent study also showed that human conjunctival epithelial cells are unresponsive to LPS due to the lack of MD2 expression 56. Thus, we conclude that the lack of MD-2 is a major factor which is responsible for the LPS signaling defect in HCECs, and for the tolerance of the cornea towards the constant exposure of bacteria and non-threatening amounts of bacterial endotoxins.
While HCECs are unresponsive to LPS, the underlying stromal fibroblasts, keratocytes, are rapidly activated by LPS. Unlike epithelia, stromal cells are not constantly exposed to bacteria or their products. When tissues such as the cornea are exposed to bacteria, the barrier function of epithelium would prevent bacteria from direct interaction with TLRs which are usually located in the inner cell layers of stratified epithelium 50 or on the basolateral side of simple epithelium57. Because of the barrier function and the unique localization of TLRs, the released endotoxin may also exhibit minimal effects on epithelial cells. However, once the barrier is breached and bacteria invade the epithelial layer, the pattern recognizing receptors, such as TLR5 58,59, recognize the pathogens in the epithelial layer and initiate corneal innate immune responses by producing antimicrobial molecules such as hBD2 60,61 and recruiting neutrophils via IL-8. Many pathogens may be eliminated upon initial contact by this mechanism 62. However, this initial defense may be overwhelmed by the pathogens, leading to the penetration of bacteria or released endotoxin into the stromal layer 55. In other mucosal epithelial cells (urinary, intestinal, and pulmonary), flagellin and TLR5 is the major responsive unit, not LPS-TLR4. We believe that the unresponsiveness of epithelial cells is a way to avoid unwanted inflammation as these epithelial linings, unlike immune cells, are constantly exposed to LPS, though not to flagellin. This exception occurs because flagellin is a protein which is usually not detected in the fluids that cover the epithelial lining in the body.
The presence of bacterial products such as flagellin and/or LPS in the stroma should act as a sign of stromal infection and a rapid response on the part of the resident cells is necessary for clearing the pathogens. The expression of functional TLR4, including its coreceptors CD14 and MD-2, and TLR5 (Yu et al, unpublished result) in keratocytes should allow the cells to rapidly recognize infection, and the release of proinflammatory cytokines and chemokines that recruit more neutrophils and monocytes to the infected stroma. It is interesting to note that corneal stromal fibroblasts stimulated with LPS secrete higher levels of cytokines than flagellin-stimulated HCECs when normalized with cellular proteins: over 2 times more IL-6 and approximately 15 times more IL-8 is secreted by THK cells. IL-8 is the major chemoattractant in the cornea for PMN infiltration. 63,64. Thus, the increased level of IL-8 released from keratocytes may attract a large number of PMN to the stroma that clear the pathogen and may also cause severe local inflammation, leading to the development of keratitis.
In summary, the data presented in this study demonstrate thatHCECs failed to respond to LPS. The unresponsiveness of HCECs is likely due to the lack of MD-2 expression, which is an important component of LPS-TLR4 signaling. Since the cornea is constantly exposed to environmental stimuli including LPS, these findings suggest that the human corneal epithelium possesses a regulatory mechanism, similar to that observed in intestinal epithelial cells, for the inhibition of TLR4-mediated innate immunity. However, the breakdown of the epithelial barrier during infection or trauma leads to a direct interaction of bacterial products with submucosal cells, i.e stromal keratocytes. Therefore these cells participate in innate immune responses by sensing and responding to bacterial products, such as LPS, that have penetrated into the subepithelial compartment of the cornea. Thus, targeting TLR4-mediated signaling pathways in keratocytes may permit the development of novel, specific therapies that could promote innate defense and prevent some of the destructive consequences of ocular Gram-negative bacterial infections.
Materials and Methods
Purification and preparation of P. aeruginosa LPS and conditioned medium
Commercially available LPS derived from P. aeruginosa (Catalog no. L9143, Sigma-Aldrich, St. Louis, MO) was resuspended in 1 ml of endotoxin-free water containing 0.2% triethylamine (TEA) and extracted with water-saturated phenol as previously described 65. Purified LPS was re-suspended in keratinocyte basic medium (KBM, Biowittaker Inc., Walkersville, MD). One hundred percent recovery was assumed. This was referred to as “phenol re-extracted LPS”.
P. aeruginosa (PAO1 strain) was cultured in supplemented M9 medium for 72 h and the culture media was centrifuged to remove bacteria and sterilized by filtration through a 0.2 µm filter.66 The conditioned media was stored at 4°C and used at 5% concentration (i.e 5 % of saturated P. aeruginosa growth medium and 95% PBS). The flagellin of P. aeruginosa was purified as previously described 50.
Cell culture and stimulation
Human telomerase-immortalized corneal epithelial (HUCL) cells,67 kindly provided by Dr. Rheinwald and Dr. Gipson, were cultured in defined keratinocyte-SFM (Invitogen Life Technologies, Carlsbad, CA) at 37°C in a humidified incubator with 95% room air, 5%CO2. HUCL cells were cultured in KBM overnight before experimentation (growth factor starvation). Human corneal stromal fibroblast cells (THK) were provided by Dr. James Jester 68 and were cultured in DMEM/F12 medium (Invitrogen-Life Technologies, Carlsbad, CA) with 10% fetal bovine serum (Mediatech Inc., Herndon, VA) at 37°C in a humidified incubator with 95% room air, 5%CO2. Before treatment, THK cells were washed with PBS two times and cultured in serum-free DMEM/F12 medium overnight for serum starvation. Both epithelial and stromal fibroblasts were treated with phenol re-extracted LPS in KBM or serum-free DMEM/F12 media for various times and then processed for Western blotting and cytokine measurement.
Primary HCECs were isolated from human donor corneas obtained from the Michigan Eye Bank. The epithelial sheet was separated from underlying stroma after overnight dispase treatment at 4°C. The dissected epithelial sheet was trypsinized, and cells were then collected by centrifugation. Primary HCECs were grown in defined keratinocyte SFM in a humidified 5% CO2 incubator at 37°C and then used at passage 3.
RT-PCR
RNA was isolated with extraction reagent (TRIzol; Invitrogen), and 2 µg of the total RNA was reverse-transcribed for cDNA synthesis using MLV-RT (SuperScript; Invitrogen). cDNA was amplified by PCR with specific primers for human IL-6 (sense: CTCCTTCTCCACAAGCGCCTTC, anti-sense: GCGCAGAATGAGATGAGTTGTC, product: 583bp), IL-8 (sense: GCAGTTTTGCCAAGGAGTGCT, anti-sense: GCATCTGGCAACCCTACAACA, product: 347bp), MD-2 (sense: TATTGGGTCTGCAACTCAT and anti-sense: CTCCCAGAAATAGCTTCAAC, product: 358 bp) and Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (sense: CACCACCAACTGCTTAGCAC and antisense CCCTGTTGCTGTAGCCAAAT, product: 515 bp). IL-6, IL-8 and MD-2 were amplified at 26, 32 and 28 cycles, respectively, with annealing temperature 58°C (45 sec) and extension temperature 72°C (1 min). GAPDH was amplified 20 cycles under the same conditions. The PCR products (5 µL) were subjected to electrophoresis on 2% agarose gels containing ethidium bromide. Staining was captured by digital camera (Gel logic 100 system; Eastman Kodak, Rochester, NY).
Cytokine ELISA
IL-6 and IL-8 secretion was determined by ELISA. HUCL cells or THK cells were plated at 4 × 105cells/well in 12-well plates. After growth factor/serum starvation, cells were treated with 100 ng/ml or 1µg/ml phenol re-extracted LPS for the indicated time; the supernatants were harvested for the measurement of IL-6 and IL-8 using ELISA which was performed according to the manufacturer's instructions (R& D Systems, Minneapolis, MN). The amount of IL-6 and IL-8 in culture media was normalized with the total amount of cellular protein lysed with RIPA buffer (150 mM NaCl, 100 mM Tris-HCl, pH 7.5, 1% deoxycholate, 0.1% SDS, 1% Triton X-100, 50 mM NaF, 100 mM sodium pyrophosphate, 3.5 mM sodium orthovanadate, proteinase inhibitor cocktails, and 0.1 mM PMSF). The protein concentration of cell lysate was determined with a Micro BCA kit (Pierce Biotechnology, Rockford. IL). Results were expressed as the mean pg of cytokine per mg cell lysate ± SE (n=3); P values were determined via ANOVA.
Western blot Analysis
LPS-treated HUCL and THK cells were lysed with RIPA buffer and protein concentration was determined with the Micro BCA kit (Pierce). IκB-α phosphorylation and degradation were detected with rabbit anti-IκB-α and anti-phospho-IκB-α which were purchased from Cell Signaling Technology (Beverly, MA) and developed with Supersignal reagents from Pierce.
Cell Surface Biotinylation
Cells grown in 100 mm dishes were rinsed 6 times with HBSS (Mediatech, Inc., Herndon, VA), supplemented with 0.1 mM CaCl2 and 1 mM MgCl2, and then incubated with freshly-prepared NHS-LC-Biotin (Pierce) diluted in the same solution (1mg/ml) for 5 minutes at room temperature. The reaction was quenched with 50 mM NH4Cl, and cells were washed with PBS and lysed with a solution containing 1% Triton X-100, 20 mM Tris (pH 8.0), 50 mM NaCl, 5 mM EDTA, and 0.2% BSA supplemented with protease inhibitors. Cell extract supernatant was incubated with immobilized streptavidin agarose (Pierce) for 16 hours at 4°C to bind biotinylated proteins. Proteins bound to the agarose slurry were solubilized with Laemmli buffer and analyzed by SDS-PAGE and Western blotting with purified rabbit antibodies against TLR4 and TLR5 from Santa Cruz Biotechnology (Santa Cruz, CA).
Acknowledgment
This work was supported by NIH Grants R01EY017960 and 10869 (F.S.Y.), Fight for Sight grant-in-aid (A.K.) and Midwest Eye-Banks (A.K.) and an unrestricted Grant from the Research to Prevent Blindness to the Department of Ophthalmology, Wayne State University School of Medicine. The authors thank Ms Jessica Yu (University of Michigan, Ann Arbor, MI) for critical reading of the manuscript.
Footnotes
Proprietary interest: none.
References
- 1.Robertson DM, Petroll WM, Jester JV, Cavanagh HD. Current concepts: contact lens related Pseudomonas keratitis. Cont Lens Anterior Eye. 2007;30:94–107. doi: 10.1016/j.clae.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Su DH, Chan TK, Lim L. Infectious keratitis associated with daily disposable contact lenses. Eye Contact Lens. 2003;29:185–186. doi: 10.1097/01.ICL.0000072832.48739.EE. [DOI] [PubMed] [Google Scholar]
- 3.Kumar A, Yin J, Zhang J, Yu FS. Modulation of corneal epithelial innate immune response to pseudomonas infection by flagellin pretreatment. Invest Ophthalmol Vis Sci. 2007;48:4664–4670. doi: 10.1167/iovs.07-0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hazlett LD. Corneal response to Pseudomonas aeruginosa infection. Prog Retin Eye Res. 2004;23:1–30. doi: 10.1016/j.preteyeres.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Kumar A, Hazlett LD, Yu FS. Flagellin suppresses the inflammatory response and enhances bacterial clearance in a murine model of Pseudomonas aeruginosa keratitis. Infect Immun. 2008;76:89–96. doi: 10.1128/IAI.01232-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lyczak JB, Cannon CL, Pier GB. Establishment of Pseudomonas aeruginosa infection: lessons from a versatile opportunist. Microbes Infect. 2000;2:1051–1060. doi: 10.1016/s1286-4579(00)01259-4. [DOI] [PubMed] [Google Scholar]
- 7.Kurpakus-Wheater M, Kernacki KA, Hazlett LD. Maintaining corneal integrity how the "window" stays clear. Prog Histochem Cytochem. 2001;36:185–259. [PubMed] [Google Scholar]
- 8.Cryz S, Pitt T, Furer E, Germanier R. Role of lipopolysaccharide in virulence of Pseudomonas aeruginosa. Infect Immun. 1984;44:508–513. doi: 10.1128/iai.44.2.508-513.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guha M, Mackman N. LPS induction of gene expression in human monocytes. Cell Signal. 2001;13:85–94. doi: 10.1016/s0898-6568(00)00149-2. [DOI] [PubMed] [Google Scholar]
- 10.Tannenbaum C, Koerner T, Jansen M, Hamilton T. Characterization of lipopolysaccharide-induced macrophage gene expression. J Immunol. 1988;140:3640–3645. [PubMed] [Google Scholar]
- 11.Zhang Y, Liou GI, Gulati AK, Akhtar RA. Expression of phosphatidylinositol 3-kinase during EGF-stimulated wound repair in rabbit corneal epithelium. Invest Ophthalmol Vis Sci. 1999;40:2819–2826. [PubMed] [Google Scholar]
- 12.Grandel U, Grimminger F. Endothelial responses to bacterial toxins in sepsis. Crit Rev Immunol. 2003;23:267–299. doi: 10.1615/critrevimmunol.v23.i4.20. [DOI] [PubMed] [Google Scholar]
- 13.Smith GV, Moran AP, Bajaj-Elliott M, Farthing MJ. Induction of cyclooxygenase 2 by Escherichia coli but not Helicobacter pylori lipopolysaccharide in gastric epithelial cells in vitro. Helicobacter. 2003;8:513–520. doi: 10.1046/j.1523-5378.2003.00170.x. [DOI] [PubMed] [Google Scholar]
- 14.Gon Y, Asai Y, Hashimoto S, et al. A20 inhibits toll-like receptor 2- and 4-mediated interleukin-8 synthesis in airway epithelial cells. Am J Respir Cell Mol Biol. 2004;31:330–336. doi: 10.1165/rcmb.2003-0438OC. [DOI] [PubMed] [Google Scholar]
- 15.Skerrett SJ, Liggitt HD, Hajjar AM, et al. Respiratory epithelial cells regulate lung inflammation in response to inhaled endotoxin. Am J Physiol Lung Cell Mol Physiol. 2004;287:L143–L152. doi: 10.1152/ajplung.00030.2004. [DOI] [PubMed] [Google Scholar]
- 16.Schilling JD, Martin SM, Hunstad DA, et al. CD14- and Toll-like receptor-dependent activation of bladder epithelial cells by lipopolysaccharide and type 1 piliated Escherichia coli. Infect Immun. 2003;71:1470–1480. doi: 10.1128/IAI.71.3.1470-1480.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naik S, Kelly EJ, Meijer L, Pettersson S, Sanderson IR. Absence of Toll-like receptor 4 explains endotoxin hyporesponsiveness in human intestinal epithelium. J Pediatr Gastroenterol Nutr. 2001;32:449–453. doi: 10.1097/00005176-200104000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Abreu MT, Vora P, Faure E, et al. Decreased expression of Toll-like receptor-4 and MD-2 correlates with intestinal epithelial cell protection against dysregulated proinflammatory gene expression in response to bacterial lipopolysaccharide. J Immunol. 2001;167:1609–1616. doi: 10.4049/jimmunol.167.3.1609. [DOI] [PubMed] [Google Scholar]
- 19.Jia HP, Kline JN, Penisten A, et al. Endotoxin responsiveness of human airway epithelia is limited by low expression of MD-2. Am J Physiol Lung Cell Mol Physiol. 2004;287:L428–L437. doi: 10.1152/ajplung.00377.2003. [DOI] [PubMed] [Google Scholar]
- 20.Hornef MW, Frisan T, Vandewalle A, Normark S, Richter-Dahlfors A. Toll-like receptor 4 resides in the Golgi apparatus and colocalizes with internalized lipopolysaccharide in intestinal epithelial cells. J Exp Med. 2002;195:559–570. doi: 10.1084/jem.20011788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hornef MW, Normark BH, Vandewalle A, Normark S. Intracellular Recognition of Lipopolysaccharide by Toll-like Receptor 4 in Intestinal Epithelial Cells. J. Exp. Med. 2003;198:1225–1235. doi: 10.1084/jem.20022194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shimazu R, Akashi S, Ogata H, et al. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll- like receptor 4. J Exp Med. 1999;189:1777–1782. doi: 10.1084/jem.189.11.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gruber A, Mancek M, Wagner H, Kirschning CJ, Jerala R. Structural model of MD-2 and functional role of its basic amino acid clusters involved in cellular lipopolysaccharide recognition. J Biol Chem. 2004;279:28475–28482. doi: 10.1074/jbc.M400993200. [DOI] [PubMed] [Google Scholar]
- 24.Miyake K, Nagai Y, Akashi S, et al. Essential role of MD-2 in B-cell responses to lipopolysaccharide and Toll-like receptor 4 distribution. J Endotoxin Res. 2002;8:449–452. doi: 10.1179/096805102125001055. [DOI] [PubMed] [Google Scholar]
- 25.Schromm AB, Lien E, Henneke P, et al. Molecular genetic analysis of an endotoxin nonresponder mutant cell line: a point mutation in a conserved region of MD-2 abolishes endotoxin-induced signaling. J Exp Med. 2001;194:79–88. doi: 10.1084/jem.194.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagai Y, Akashi S, Nagafuku M, et al. Essential role of MD-2 in LPS responsiveness and TLR4 distribution. Nat Immunol. 2002;3:667–672. doi: 10.1038/ni809. [DOI] [PubMed] [Google Scholar]
- 27.Lenoir C, Sapin C, Broquet AH, et al. MD-2 controls bacterial lipopolysaccharide hyporesponsiveness in human intestinal epithelial cells. Life Sci. 2008;82:519–528. doi: 10.1016/j.lfs.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 28.Ohnishi T, Muroi M, Tanamoto K. The lipopolysaccharide-recognition mechanism in cells expressing TLR4 and CD14 but lacking MD-2. FEMS Immunol Med Microbiol. 2007;51:84–91. doi: 10.1111/j.1574-695X.2007.00281.x. [DOI] [PubMed] [Google Scholar]
- 29.Ozinsky A, Underhill DM, Fontenot JD, et al. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between Toll-like receptors. PNAS. 2000;97:13766–13771. doi: 10.1073/pnas.250476497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 31.Song PI, Abraham TA, Park Y, et al. The expression of functional LPS receptor proteins CD14 and toll-like receptor 4 in human corneal cells. Invest Ophthalmol Vis Sci. 2001;42:2867–2877. [PubMed] [Google Scholar]
- 32.Ueta M, Nochi T, Jang MH, et al. Intracellularly expressed TLR2s and TLR4s ontribution to an immunosilent environment at the ocular mucosal epithelium. J Immunol. 2004;173:3337–3347. doi: 10.4049/jimmunol.173.5.3337. [DOI] [PubMed] [Google Scholar]
- 33.Johnson AC, Heinzel FP, Diaconu E, et al. Activation of Toll-Like Receptor (TLR)2, TLR4, and TLR9 in the Mammalian Cornea Induces MyD88-Dependent Corneal Inflammation. Invest Ophthalmol Vis Sci. 2005;46:589–595. doi: 10.1167/iovs.04-1077. [DOI] [PubMed] [Google Scholar]
- 34.Takeda K, Akira S. TLR signaling pathways. Semin Immunol. 2004;16:3–9. doi: 10.1016/j.smim.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 35.Wieland CW, Florquin S, Maris NA, et al. The MyD88-dependent, but not the MyD88-independent, pathway of TLR4 signaling is important in clearing nontypeable haemophilus influenzae from the mouse lung. J Immunol. 2005;175:6042–6049. doi: 10.4049/jimmunol.175.9.6042. [DOI] [PubMed] [Google Scholar]
- 36.Lovitch SB, Esparza TJ, Schweitzer G, Herzog J, Unanue ER. Activation of type B T cells after protein immunization reveals novel pathways of in vivo presentation of peptides. J Immunol. 2007;178:122–133. doi: 10.4049/jimmunol.178.1.122. [DOI] [PubMed] [Google Scholar]
- 37.Shen H, Tesar BM, Walker WE, Goldstein DR. Dual signaling of MyD88 and TRIF is critical for maximal TLR4-induced dendritic cell maturation. J Immunol. 2008;181:1849–1858. doi: 10.4049/jimmunol.181.3.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jeyaseelan S, Young SK, Fessler MB, et al. Toll/IL-1 receptor domain-containing adaptor inducing IFN-beta (TRIF)-mediated signaling contributes to innate immune responses in the lung during Escherichia coli pneumonia. J Immunol. 2007;178:3153–3160. doi: 10.4049/jimmunol.178.5.3153. [DOI] [PubMed] [Google Scholar]
- 39.Triantafilou M, Triantafilou K. Lipopolysaccharide recognition: CD14, TLRs and the LPS-activation cluster. Trends Immunol. 2002;23:301–304. doi: 10.1016/s1471-4906(02)02233-0. [DOI] [PubMed] [Google Scholar]
- 40.Rocchetta HL, Burrows LL, Lam JS. Genetics of O-antigen biosynthesis in Pseudomonas aeruginosa. Microbiol Mol Biol Rev. 1999;63:523–553. doi: 10.1128/mmbr.63.3.523-553.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.da Silva Correia J, Soldau K, Christen U, Tobias PS, Ulevitch RJ. Lipopolysaccharide is in close proximity to each of the proteins in its membrane receptor complex. transfer from CD14 to TLR4 and MD-2. J Biol Chem. 2001;276:21129–21135. doi: 10.1074/jbc.M009164200. [DOI] [PubMed] [Google Scholar]
- 42.Backhed F, Meijer L, Normark S, Richter-Dahlfors A. TLR4-dependent recognition of lipopolysaccharide by epithelial cells requires sCD14. Cell Microbiol. 2002;4:493–501. doi: 10.1046/j.1462-5822.2002.00208.x. [DOI] [PubMed] [Google Scholar]
- 43.Fang WF, Cho JH, He Q, et al. Lipid A fraction of LPS induces a discrete MAPK activation in acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2007;293:L336–L344. doi: 10.1152/ajplung.00011.2007. [DOI] [PubMed] [Google Scholar]
- 44.Pier GB. Pseudomonas aeruginosa lipopolysaccharide: a major virulence factor, initiator of inflammation and target for effective immunity. Int J Med Microbiol. 2007;297:277–295. doi: 10.1016/j.ijmm.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pier GB, Markham RB, Eardley D. Correlation of the biologic responses of C3H/HEJ mice to endotoxin with the chemical and structural properties of the lipopolysaccharides from Pseudomonas aeruginosa and Escherichia coli. J Immunol. 1981;127:184–191. [PubMed] [Google Scholar]
- 46.Jerala R. Structural biology of the LPS recognition. Int J Med Microbiol. 2007;297:353–363. doi: 10.1016/j.ijmm.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 47.Faure E, Equils O, Sieling PA, et al. Bacterial lipopolysaccharide activates NF-kappaB through toll-like receptor 4 (TLR-4) in cultured human dermal endothelial cells. Differential expression of TLR-4 and TLR-2 in endothelial cells. J Biol Chem. 2000;275:11058–11063. doi: 10.1074/jbc.275.15.11058. [DOI] [PubMed] [Google Scholar]
- 48.Uehara A, Sugawara S, Takada H. Priming of human oral epithelial cells by interferon-gamma to secrete cytokines in response to lipopolysaccharides, lipoteichoic acids and peptidoglycans. J Med Microbiol. 2002;51:626–634. doi: 10.1099/0022-1317-51-8-626. [DOI] [PubMed] [Google Scholar]
- 49.Uehara A, Sugawara S, Tamai R, Takada H. Contrasting responses of human gingival and colonic epithelial cells to lipopolysaccharides, lipoteichoic acids and peptidoglycans in the presence of soluble CD14. Med Microbiol Immunol (Berl) 2001;189:185–192. doi: 10.1007/s004300100063. [DOI] [PubMed] [Google Scholar]
- 50.Zhang J, Xu K, Ambati B, Yu FS. Toll-like receptor 5-mediated corneal epithelial inflammatory responses to Pseudomonas aeruginosa flagellin. Invest Ophthalmol Vis Sci. 2003;44:4247–4254. doi: 10.1167/iovs.03-0219. [DOI] [PubMed] [Google Scholar]
- 51.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 52.Visintin A, Latz E, Monks BG, Espevik T, Golenbock DT. Lysines 128 and 132 Enable Lipopolysaccharide Binding to MD-2, Leading to Toll-like Receptor-4 Aggregation and Signal Transduction. J. Biol. Chem. 2003;278:48313–48320. doi: 10.1074/jbc.M306802200. [DOI] [PubMed] [Google Scholar]
- 53.Akashi S, Saitoh S-i, Wakabayashi Y, et al. Lipopolysaccharide Interaction with Cell Surface Toll-like Receptor 4-MD-2: Higher Affinity than That with MD-2 or CD14. J. Exp. Med. 2003;198:1035–1042. doi: 10.1084/jem.20031076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gioannini TL, Teghanemt A, Zhang D, et al. Isolation of an endotoxin-MD-2 complex that produces Toll-like receptor 4-dependent cell activation at picomolar concentrations. PNAS. 2004;101:4186–4191. doi: 10.1073/pnas.0306906101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schultz CL, Buret AG, Olson ME, et al. Lipopolysaccharide entry in the damaged cornea and specific uptake by polymorphonuclear neutrophils. Infect Immun. 2000;68:1731–1734. doi: 10.1128/iai.68.3.1731-1734.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Talreja J, Dileepan K, Puri S, et al. Human conjunctival epithelial cells lack lipopolysaccharide responsiveness due to deficient expression of MD2 but respond after interferon-gamma priming or soluble MD2 supplementation. Inflammation. 2005;29:170–181. doi: 10.1007/s10753-006-9014-y. [DOI] [PubMed] [Google Scholar]
- 57.Gewirtz AT, Navas TA, Lyons S, Godowski PJ, Madara JL. Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J Immunol. 2001;167:1882–1885. doi: 10.4049/jimmunol.167.4.1882. [DOI] [PubMed] [Google Scholar]
- 58.Kumar A, Zhang J, Yu FS. Innate immune response of corneal epithelial cells to Staphylococcus aureus infection: role of peptidoglycan in stimulating proinflammatory cytokine secretion. Invest Ophthalmol Vis Sci. 2004;45:3513–3522. doi: 10.1167/iovs.04-0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang J, Li H, Wang J, et al. Role of EGFR transactivation in preventing apoptosis in Pseudomonas aeruginosa-infected human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2004;45:2569–2576. doi: 10.1167/iovs.03-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kumar A, Zhang J, Yu FS. Toll-like receptor 2-mediated expression of beta-defensin-2 in human corneal epithelial cells. Microbes Infect. 2006;8:380–389. doi: 10.1016/j.micinf.2005.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McDermott AM, Redfern RL, Zhang B, et al. Defensin expression by the cornea: multiple signalling pathways mediate IL-1beta stimulation of hBD-2 expression by human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2003;44:1859–1865. doi: 10.1167/iovs.02-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Darling KE, Evans TJ. Effects of nitric oxide on Pseudomonas aeruginosa infection of epithelial cells from a human respiratory cell line derived from a patient with cystic fibrosis. Infect Immun. 2003;71:2341–2349. doi: 10.1128/IAI.71.5.2341-2349.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yan XT, Tumpey TM, Kunkel SL, Oakes JE, Lausch RN. Role of MIP-2 in neutrophil migration and tissue injury in the herpes simplex virus-1-infected cornea. Invest Ophthalmol Vis Sci. 1998;39:1854–1862. [PubMed] [Google Scholar]
- 64.Kernacki KA, Barrett RP, Hobden JA, Hazlett LD. Macrophage inflammatory protein-2 is a mediator of polymorphonuclear neutrophil influx in ocular bacterial infection. J Immunol. 2000;164:1037–1045. doi: 10.4049/jimmunol.164.2.1037. [DOI] [PubMed] [Google Scholar]
- 65.Hirschfeld M, Ma Y, Weis JH, Vogel SN, Weis JJ. Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine toll-like receptor 2. J Immunol. 2000;165:618–622. doi: 10.4049/jimmunol.165.2.618. [DOI] [PubMed] [Google Scholar]
- 66.Leidal KG, Munson KL, Denning GM. Small molecular weight secretory factors from Pseudomonas aeruginosa have opposite effects on IL-8 and RANTES expression by human airway epithelial cells. Am J Respir Cell Mol Biol. 2001;25:186–195. doi: 10.1165/ajrcmb.25.2.4273. [DOI] [PubMed] [Google Scholar]
- 67.Rheinwald JG, Hahn WC, Ramsey MR, et al. A two-stage, p16(INK4A)- and p53-dependent keratinocyte senescence mechanism that limits replicative potential independent of telomere status. Mol Cell Biol. 2002;22:5157–5172. doi: 10.1128/MCB.22.14.5157-5172.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jester JV, Huang J, Fisher S, et al. Myofibroblast differentiation of normal human keratocytes and hTERT, extended-life human corneal fibroblasts. Invest Ophthalmol Vis Sci. 2003;44:1850–1858. doi: 10.1167/iovs.02-0973. [DOI] [PubMed] [Google Scholar]