Abstract
Multiple endocrine neoplasia type 1 (MEN1) is a dominantly inherited tumor syndrome that results from the mutation of the MEN1 gene that encodes protein menin. Stable overexpression of MEN1 has been shown to partially suppress the RAS-mediated morphological changes of NH3 fibroblast cells. Little is known about the molecular mechanisms by which menin decreases the oncogenic effects on cell morphology and other phenotypes. Here we showed that ectopic expression of menin in pretumor beta cells increases islet cell adhesion and reduces cell migration. Our further studies revealed that menin interacts with the scaffold protein, IQGAP1, reduces GTP-Rac1 interaction with IQGAP1 but increases E-cadherin/ß-catenin interaction with IQGAP1. Consistent with an essential role for menin in regulating ß cell adhesion in vivo, accumulations of β-catenin and E-cadherin are reduced at cell junctions in the islets from Men1-excised mice. Together, these results define a novel menin-IQGAP1 pathway that controls cell migration and cell-cell adhesion in endocrine cells.
Keywords: menin, IQGAP1, intercellular adhesion, migration
Introduction
Multiple endocrine neoplasia type 1 (MEN1) is a dominantly inherited tumor syndrome that results from the mutation of Men1 (Chandrasekharappa et al., 1997), which encodes menin protein. Menin has been found to interact with mixed lineage leukemia (MLL) protein (Hughes et al., 2004), which is a histone H3 lysine 4 (H3K4) methyltransferase (Milne et al., 2002). Multiple lines of evidence indicate that one of menin’s biochemical functions is to promote MLL-dependent H3K4 methylation, leading to enhanced transcription of target genes such as Hoxa9 and cyclin dependent kinase inhibitors, p18 and p27 (Chen et al., 2006; Milne et al., 2005; Yan et al., 2006a; Yokoyama et al., 2005). However, it is not clear why the attenuated menin expression specifically causes a unique and restricted pattern of endocrine tumors in both humans and mice despite the ubiquitous expression of menin in almost all tissues (Guru et al., 1999).
Men1-/- mice die in mid-gestation (between embryonic day 11.5 to 13.5), while Men1+/- mice develop endocrine tumors later in their life with a spectrum similar to that in MEN1 patients (Crabtree et al., 2001). Heterogeneous expression of Men1 suggests that attenuation, rather than complete abrogation of Men1 function, facilitates a tumorigenic signaling pathway in endocrine cells (Crabtree et al., 2003). Although conditional alleles have been generated to allow tissue-specific homozygous deletion of Men1 (Schnepp et al., 2006), Men1+/- mice, presumably retaining a lower level of menin expression, were used to gain an unbiased assessment of the functional in vivo interaction between menin with p18 and p27 proteins (Bai et al., 2007).
An alternate approach to evaluate Men1-mutation phenotype is to use neoplastic cells transformed by oncogenes. Ras is an oncogene originally identified in rat sarcoma virus. Transforming Ras genes cause tumor progression, often accompanied by somatic mutations in tumor suppressor genes (Jiang et al., 2004). Through this approach, Kim and colleagues first demonstrated that stable overexpression of Men1 partially suppressed the Ras-mediated tumor phenotype in NH3 fibroblast cells (Kim et al., 1999). However, little is known about the molecular mechanisms by which menin decreases the oncogenic effects upon cell morphology.
To address menin’s role in endocrine cells, we sought to test menin’s tumor suppressing function by ectopic expression of menin in βHC9 pretumor beta cells. This cell line was generated from individual hyperplastic pancreatic islets (a tumor precursor stage) of transgenic mice expressing the SV40 large T antigen under control of the rat insulin promoter (Radvanyi et al., 1993). Using this cell model, we found that ectopic menin expression increased cell adhesion and promoted normal endocrine cell morphology. Menin interacted with a scaffold protein, IQGAP1, and attenuated GTP-Rac1 binding to IQGAP1 but enhanced binding of E-cadherin/ß-catenin to IQGAP1. Immunostaining showed that E-cadherin and β-catenin were reduced at cell junctions in the islets of Men1-excised mice. These results define a novel menin-IQGAP1 pathway that links the cytoskeleton to cell adhesion and migration in endocrine cells.
Results
Menin enhances aggregation of islet cells
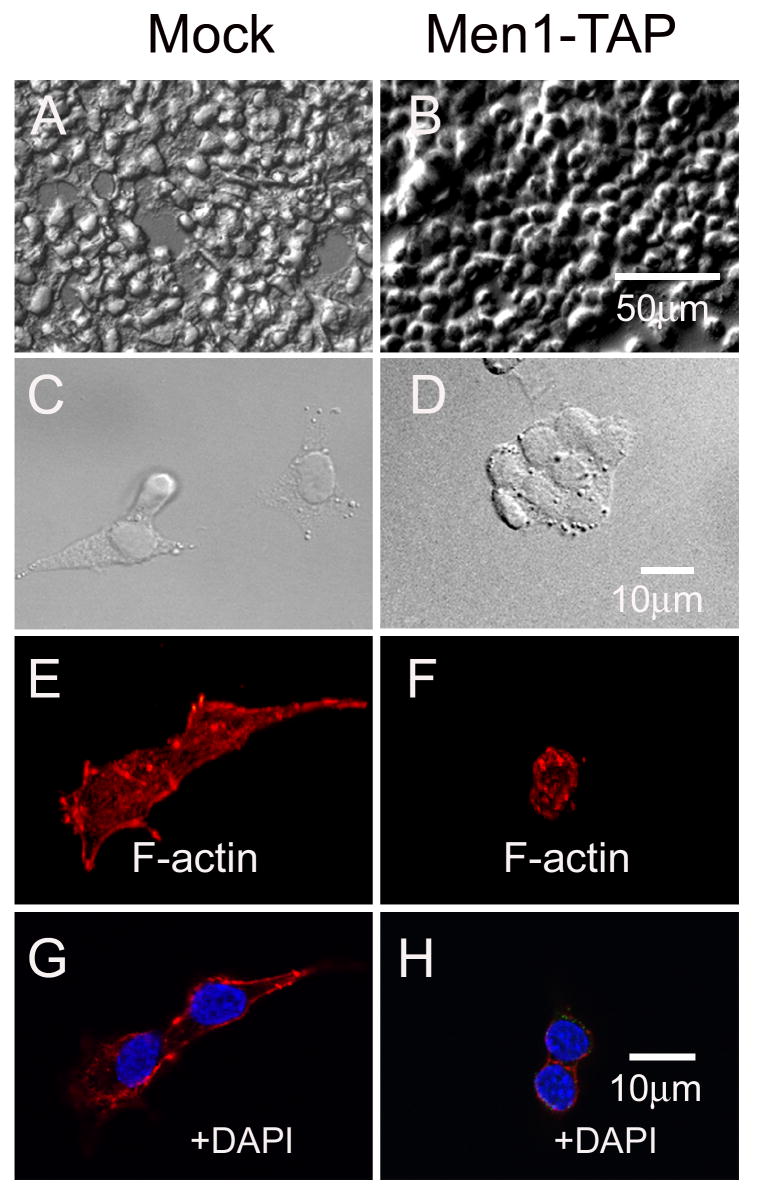
To investigate the function of menin in islet ß cells, we ectopically expressed menin in an insulin-secreting ß cell line, ßHC9. More specifically, ßHC9 cells were infected with either control recombinant retroviruses or retroviruses expressing menin tagged with the TAP (tandem affinity purification) epitopes, SBP (streptavidin binding peptide) and CBP (calmodulin binding peptide) (supplemental data, Fig. S1A). The resulting pooled and menin-expressing retrovirus-infected cells (Men1-TAP) showed approximately 2-3 fold higher menin expression, with the size of the tagged menin slightly bigger than the endogenous menin, as compared with the vector-infected control cells (Mock) based on immunoblotting (Fig. S1C). High density Men1-TAP cells but not control cells formed multilayers of cell clusters and thus more closely mimicked normal islet morphology (Fig. 1A, B). At a low density, control cells were well spread and separated from each other, and readily formed lamellipodia (Fig. 1C). In contrast, low-density Men1-TAP cells were relatively round and formed compact clusters, but not lamellipodia (Fig.1D). The polarized appearance that distinguished individual control cells from Men1-TAP cells were defined by cell shape index, i.e. 2.5±0.5 vs 1±0.1 (n=10; ±S.D.) (see Fig.1 legend).
Fig. 1.
Menin overexpression induces βHC9 cells to become compact and clustered. Two types of βHC9-derived cell lines are shown: mock (pMX-puro empty vector) and Men1-TAP (overexpressing menin-TAP). Differential interference contrast images of cells are shown at low (A, B) and high (C, D) magnification. Texas Red-phalloidin staining was used to assess the organization of F-actin. Eleven confocal Z-stacks were taken and projected into one image (E, F), and a single confocal section stained for F-actin and for nuclei with DAPI is also shown (G-H). The morphological polarity of a cell was defined by a seminquantitative index of cell shape as described by Donnadieu and coworkers (Donnadieu et al., 1992). Briefly, the cell was traced as an ellipse using Photoshop software. Two lines were drawn: one along the longest axis of the ellipse; the other perpendicular to the first and bisecting it. The cell shape index was determined by the ratio of the long axis to the short axis.
To test the possibility that the epitope tags fused with menin, rather than ectopic menin expression, contributed to the morphological changes, we generated an additional stable cell line by infecting ßHC9 cells with retroviruses expressing untagged menin (Fig. S1A). The resulting cell line (Men1), showed a higher level of menin expression (Fig. S1C, lane 3), and a more compact, clustered phenotype as compared to control, vector-infected cells (Fig. S1B).
Menin expression changes actin distribution
The morphological changes described above were likely to reflect changes in cytoskeletal organization, particularly of actin filaments. Although stress fibers were rarely seen in both cell types, more F-actin patches were found in the cytoplasm of mock-infected control cells than Men1-TAP cells. The other conspicuous difference in actin organization between the two cell types was the accumulations of F-actin surrounding the cell membrane of Men1-TAP versus at the leading edge of control cells (Fig.1E-H). The distribution difference was reflected by a ~50% loss of β-actin in menin overexpressing cells, as determined by immunobloting (Fig.2D) and quantitative real-time PCR (data not shown). These results suggest that menin expression promotes actin assembly at cell membrane and negatively regulates steady state levels of β-actin in cytoplasm, possibly due to delocalization of IQGAP1 (see below).
Fig. 2.
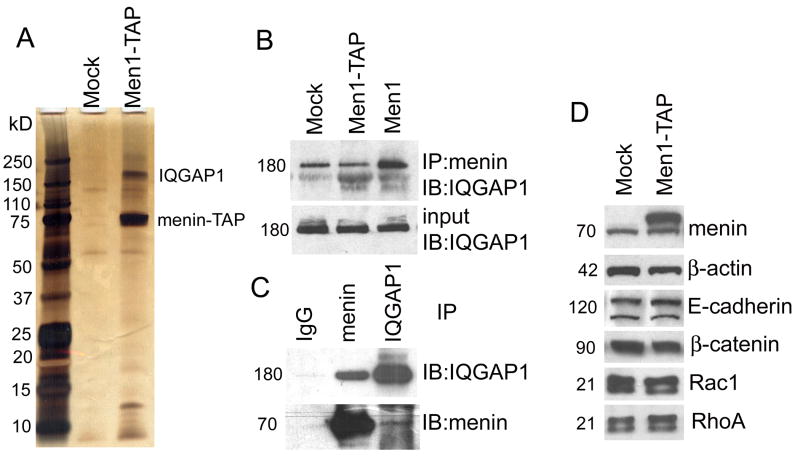
IQGAP1 is a major cytoplasmic binding partner of menin. Soluble extracts from mock cells and Men1-TAP cells were used for TAP purification. (A) The purified products were separated on SDS-PAGE and identified with silver staining. The two most prominent bands in lane 3 were excised from the gel, and their tryptic peptides were identified as menin and IQGAP1 by mass spectrometry (nanoLC/nanospray/MS/MS). (B) and (C) Physical interactions between menin and IQGAP1 were verified by in vitro reciprocal immunoprecipitation. (D) Comparative expression levels of proteins marked by the indicated antibodies in mock and Men1-TAP cells.
Menin interacts with IQGAP1
During our investigation into the role of menin in controlling cell morphology, we tried to identify menin interacting proteins in β cells. To that end, we subjected the mock cells and Men1-TAP cells to sequential affinity purifications, using streptavidin and calmodulin-conjugated beads. One major band at ~180 kD was reproducibly identified during multiple rounds of purification in Men1-TAP cells, but not in the mock cells, indicating specific association with the tagged menin (Fig. 2A). Mass spectrometry identified this band as IQGAP1, a scaffold protein that interacts with a variety of proteins, including Rac1, Cdc42, E-cadherin, β–catenin and F-actin to regulate cell motility and adhesion (Fukata et al., 1999; Lambert et al., 2002; Mataraza et al., 2007).
Physical interaction between menin and IQGAP1 was confirmed by a co-immunoprecipitation (IP) assay. An anti-menin antibody was able to pull down IQGAP1 in both mock-infected and menin overexpressing cells (Fig. 2B), and more IQGAP1 was present in anti-menin IPs obtained from Men1 cells than from control cells (Fig. 2B). The latter result suggested that IQGAP1 interacted with both endogenous and ectopically expressed menin. We noticed, however, that the amount of IQGAP1 in IPs obtained from mock and Men1-TAP cells were nearly identical, despite the large presence of menin in the Men1-TAP cells (Fig.2B, 2D). This is likely due to the fact that the immunoprecipitating anti-menin antibody (BL342) was generated using a synthetic peptide corresponding to the C-terminus of human menin and thus may have had a lower affinity for menin modified at its C-terminus with the TAP epitope tag than for unmodified menin. Reciprocal co-IP experiments demonstrated that anti-IQGAP1 could pull down menin (Fig. 2C), albeit less effectively than anti-menin pulled down IQGAP1 (Fig 2B). This result suggests that either a small fraction of menin binds IQGAP1 or the affinity between menin and IQGAP1 is negatively affected by the anti-IQGAP1 antibody. In either case, these collective results indicate that menin and IQGAP1 interact in β cells.
Menin increases IQGAP1 accumulation at the plasma membrane
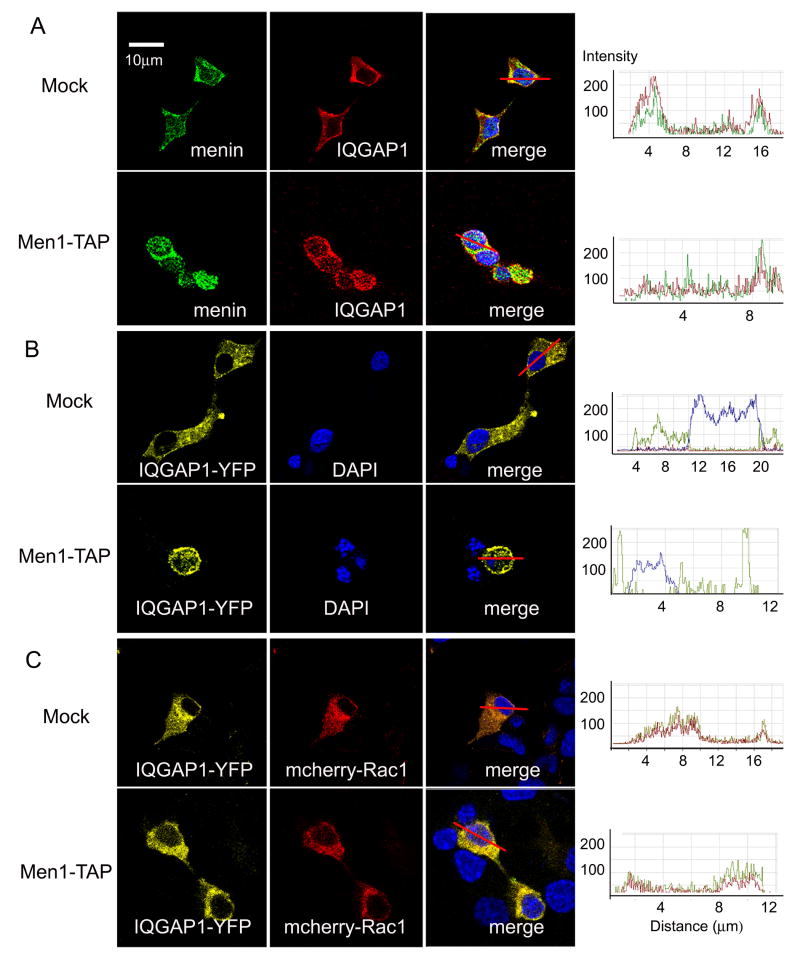
Consistent with our biochemical evidence for an IQGAP1-menin association in cells (Fig. 2A-C), we found extensive immunofluorescent co-localization of IQGAP1 with a non-nuclear pool of menin in our β cell lines. However, co-localization around the plasma membrane appeared more extensive in Men1-TAP cultures than in controls, especially at cell-cell junctions (Fig. 3A). The impression that menin overexpression enhanced IQGAP1 localization at the cell surface was further supported by transient transfection studies of IQGAP1-YFP. Cortical enrichment of the fluorescent fusion protein was much more evident in the Men1-TAP cells than in control cells (Fig.3B). Interestingly, co-expression of mCherry-Rac1 with IQGAP1-YFP prevented the overexpressed menin in Men1-TAP cells from targeting IQGAP1 to the cortex (Fig. 3C). As expected, overexpression of mCherry-Rac1 increased the level of Rac1-GTP, an active form to bind IQGAP1 (Fig. S3). These results suggest that the intracellular distribution of IQGAP1 is controlled by both menin and Rac1, but that Rac1 is dominant.
Fig. 3.
Menin co-localizes with IQGAP1 in β cell lines. The right panel showed quantitative analyses of fluorescence intensity across co-localized areas using Zeiss LSM510 software, underscoring the fluorescence distribution in the membrane and cytoplasm (i.e. peak’s breadth). (A) Distinct enrichments of menin at the cell-cell contacts in mock and Men1-TAP cells. (B) Distinct accumulations of overexpressed IQGAP1-YFP on the cell surface and in the cytoplasm between mock and Men1-TAP cell. (C) Overexpressed mCherry-Rac1 delocalizing subcellular distributions of IQGAP1-YFP in Men1-TAP cells.
Menin increases accumulation of E-cadherin/β-catenin at cell-cell contact sites
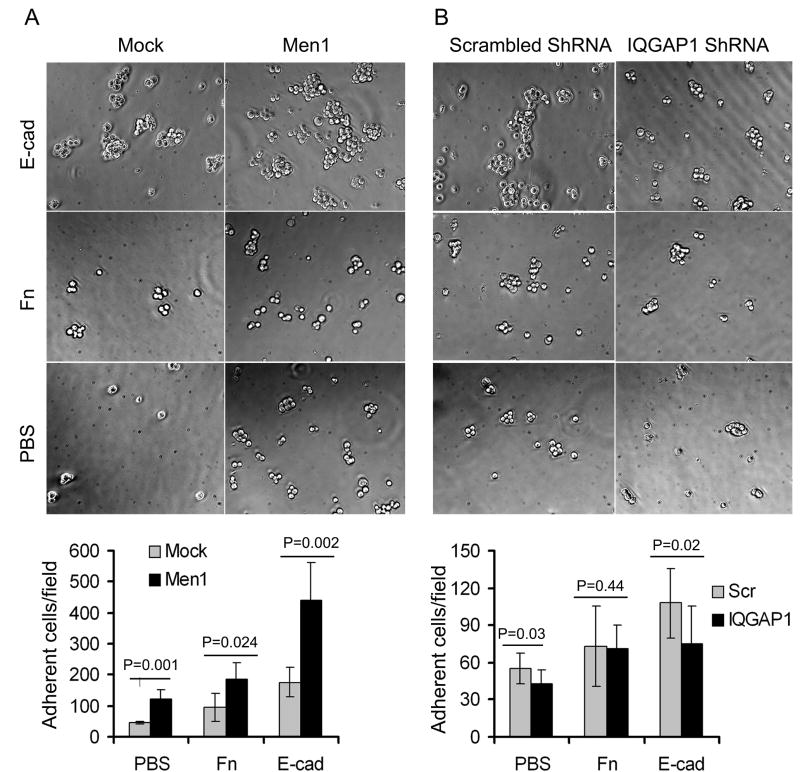
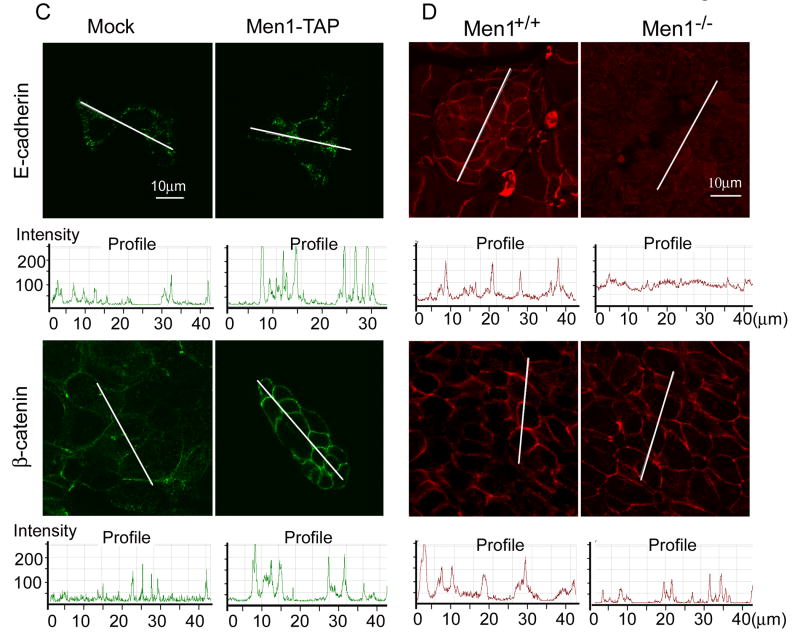
Having established the intracellular distributions of menin and IQGAP1, we next investigated the biological significance of menin-induced IQGAP1 accumulation at intercellular adhesion sites. As determined by cell adhesion assays (Price et al., 2004), cells expressing menin (Men1) had strong adhesion, particularly to Fc-E-cadherin (Fig. 4A). By immunofluorescence, E-cadherin was 2-3 times more concentrated at sites of cell-cell contact in menin-expressing cells than anywhere along the surfaces of mock-infected control cells (Fig. 4C). The distribution of β-catenin was similar to E-cadherin staining in βHC9-derived cells. Menin-expressing cells displayed broader bands of β-catenin staining, reflecting regions of cellular contact (Fig. 4C). In mock cells, however, β-catenin was frequently restricted to a thin line where adjacent mock cells made contact, and showed a 2-3 fold of lower staining intensity than in menin-expressing cells (Fig. 4C). Immunoblots of cell lysates revealed comparable levels of E-cadherin and β-catenin in the mock and Men1-TAP cell lines (Fig. 2D). Together, these results suggest that menin-expression significantly increased intercellular adhesion via E-cadherin pathway.
Fig. 4.
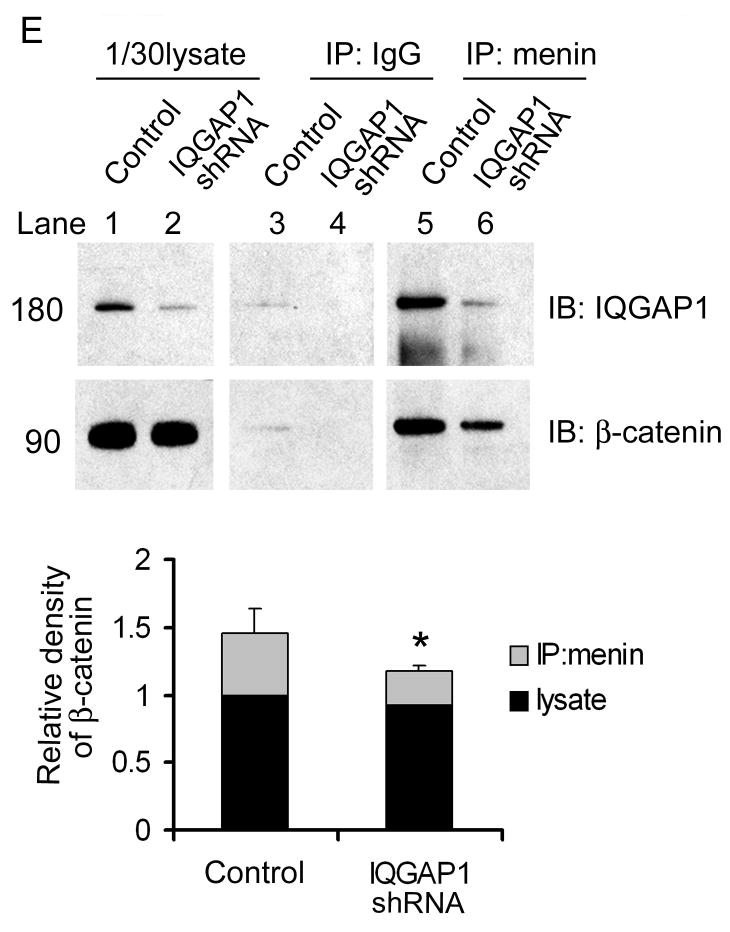
Menin promotes cell-cell adhesions. (A) Cells were detached with 1.5mM EDTA-PBS at 37 °C for either 5min (Mock) or 15min (Men1). The detached cells were diluted and added to wells coated with PBS, Fibronectin (Fn) or Fc-E-cadherin (E-cad). The number of adherent cells/microscopic field is presented (n=5; ± S.D). (B) IQGAP1 knockdown reduced cell adhesion to E-cadherin in menin overexpression cells. Men1 cells were infected with either IQGAP1 ShRNA or scramble ShRNA lentiviruses, followed by adhesion assays (n=8; ±S.D.). Two tailed t-test was used to determine the P value for the difference as shown in the figure. Intercellular junctions marked by E-cadherin and β-catenin in βHC9-derived cell lines (C) and paraffin-embedded islets from wild type or Men1+/- mice (D) were imaged by immunofluorescence. Quantitative analyses of fluorescence intensity across long linear paths that intersect with at least three cell-cell contacts were performed using Zeiss LSM510 software. (E) Detection of menin, IQGAP1 and β-catenin complex. IQGAP1 was knocked down with a pool of 5 lentiviral shRNA constructs against murine IQGAP1. The cell lysates from control or IQGAP1 ShRNA knockdown Men1 cells were immunoprecipitated with menin-specific antibodies, followed by Western blotting to determine the association of IQGAP1 and β-catenin with menin. The bottom histogram shows quantification of β-catenin in lysate and menin-IP from control and IQGAP1-deficient cells as in B, normalized by β-catenin level in control lysate. *, paired t-test, P<0.05.
To examine whether distributions of β-catenin and E-cadherin are altered in Men1-associated islet tumors, we immunostained enlarged islets from the Men1-excised mice and control islets from wild type mice (Schnepp et al., 2006). As shown in Fig. 4D, the staining intensity of E-cadherin surrounding the cell membrane was much higher in menin-expressing control islets. In the case of β-catenin, staining intensity was very similar in the control and Men1-excised mice, but β-catenin labeling was confined to a narrower band near the surface of Men1 knockout islet cells. These regions of β-catenin immunoreactivity ranged from 0.5-2 μm wide in Men1-deleted islet cells but were 2-4 μm wide in control islets. These results suggest that Men1-deletion leads to reduced distribution of E-cadherin and ß-catenin at the surface of pancreatic islet cells.
To demonstrate the association of menin and IQGAP1 with cadherin complex, Men1 cells and IQGAP1 shRNA-expressing cells were subjected to IP with anti-menin antibody, followed by Western blotting with either anti-ß-catenin or anti-IQGAP1 antibodies. IQGAP1 and β-catenin were simultaneously immunoprecipitated by menin-specific antibodies. At similar amount of β-catenin input, the levels of β-catenin were reduced by ~60% in IPs obtained from IQGAP1-knock down cells (Fig. 4E). Furthermore, IQGAP1-deficient cells reduced staining intensity of E-cadherin and β-catenin at the cell-cell contacts (Fig.S4), and displayed a weak cell adhesion (Fig. 4B). Thus, IQGAP1 is crucial to bridge the menin E-cadherin/β-catenin interactions in islet cells.
Menin expression inhibits β cells migration
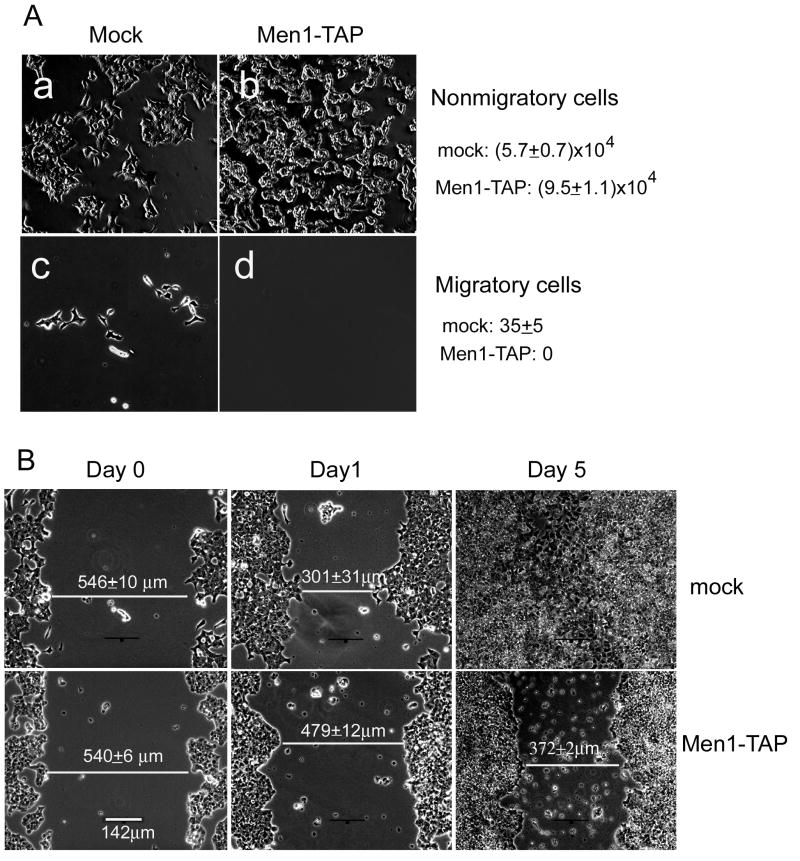
To assess the importance of the menin-IQGAP1 interaction in ß cell motility, we performed a modified Boyden chamber assay. After 24 hours of migration, virtually all of the Men1-TAP cells remained in the upper chamber, but a small number of mock cells migrated completely through the filter membrane (Fig. 5A). Because the total number of migratory and non-migratory mock cells was less than that of the seeded cells, we suspected that quite a number of mock cells might be trapped in the filter membrane. We therefore used an alternative approach, the scratch wound assay, to compare the motility of mock versus Men1-TAP cells. As can be seen in Figure 5B, the degree of wound closure achieved by mock cells within 24 hours of wounding was comparable to what was seen after 5 days by the Men1-TAP cells. The dramatic difference between the two cells lines reinforces the impression from the Boyden chamber migration assays that menin overexpression reduces cell motility.
Fig. 5.
Menin inhibits migration of ßHC9-derived cells. (A) An equal number of mock cells or Men1-TAP cells were seeded onto the upper chamber and 24 hours later. Cells that remained in the upper chamber and those moved to the lower surface of the filter membrane were photographed and counted. (B) Menin overexpression inhibited closure of artificial wounds made in confluent cellular monolayers. One of three assays was shown (n=3; ±S.D.).
To test the effect of Rac1 activity on menin inhibition of cell mobility, we transiently overexpressed constitutively active mutant, RFP-Rac1 (Q61L) in Men1-TAP cells. After 24 hours, Men1-TAP cells reduced the wound gap by 13 % (64 μm of 511 μm) relative to 53% (276 μm out of 519 μm) of mock control cells (Fig. S5). However, overexpression of active Rac1 (Q61L) increased the migration speed of Men1-TAP cell 2 fold from 64 μm to 122 μm (Fig. S5). Thus, active Rac1 can abrogate the migratory inhibition caused by Men1 expression in β cell.
Menin modulates IQGAP1 interactions with Rac1
IQGAP1 has been reported to promote cell motility via interactions with Rac1 and Cdc42 (Bensenor et al., 2007; Mataraza et al., 2007). To test whether menin affects the IQGAP1-Rac1 interaction, we performed affinity pull-down assays to examine associations of menin, IQGAP1 and Rac1 (Fig. S6A). We precoated Ni-NTA agarose with His-tagged IQGAP1 and then subsequently incubated the coated beads with menin and activated or inactive Rac1. As expected, menin and GTPγS-Rac1 bound independently to IQGAP1 (lanes 4 and 6). The affinity of GDP-Rac1 to IQGAP1 was very low, slightly higher than its background binding to naked Ni-NTA agarose beads (lane 5 vs lane 2). However, GTPγS-Rac1 binding to IQGAP1 was substantially reduced in the presence of menin (lane 7). Thus, activated Rac1 and menin may competitively bind to IQGAP1. This conclusion was fortified by studies of live cells. Mock cells and menin-overexpressing cells were subjected to IPs and immunoblotting. Anti-Rac1 pulled down IQGAP1 but 2.8 fold less effectively in cells that ectopically expressed menin (Fig. S6B, C).
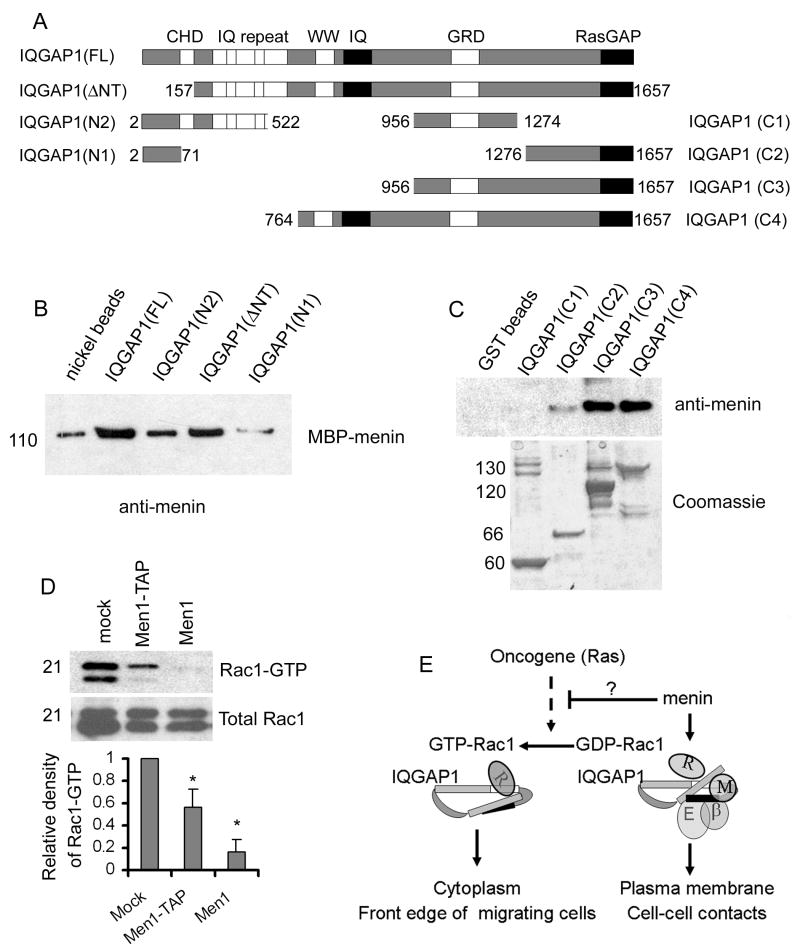
To determine regions in IQGAP1 responsible for menin binding, we tested menin binding to various recombinant fragments of IQGAP1. In these assays, menin showed a weak interaction with N-terminal fragment IQGAP1-N2 (aa 2-522) and strong binding to C-terminal fragments including IQGAP1-C3 (aa 956-1657). However, C3-splited shorter fragment C1(aa 956-1274) and C2 (aa 1276-1657) did not bind or displayed a weak interaction to menin (Fig. 6A-C). Together with a previous study showing direct interactions between C1, C2 and N2 (Grohmanova et al., 2004; Le Clainche et al., 2007), our data suggest that the binding of menin to IQGAP1 depends on the tertiary structure formed between the C-terminal (C3) and N-terminal (N2) domains. Menin-IQGAP1 structural binding may induce IQGAP1 C-terminal conformation affinity toward E-cadherin/β-catenin but against Rac1-GTP (Fig. 6E).
Fig.6.
Menin directly binds to IQGAP1. (A) A schematic diagram of functional domains present in full-length IQGAP1 and seven fragments used for binding assays. (B) Nickel–agarose beads were precoated with 2μg of his-tagged IQGAP1 (FL), IQGAP1 (ΔNT), IQGAP1 (522), IQGAP1 (71), or BSA as a negative control. The precoated beads were then incubated 0.2μg of menin. Chemiluminescent immunoblotting was used to detect any menin that may have bound to beads. (C) Precoated GST-fusion C-terminal proteins of IQGAP1 were incubated with menin (0.2μg). Top, anti-menin Western blot of bound menin. Bottom: Coomassie stained gel slices show input of the GST-fusion proteins. Comparison of Western blot and Coomassie staining indicated that all samples of pre-coated GST-fusions were saturated over menin-binding. (D) Menin overexpression reduces cellular levels of activated Rac1. PAK1-PBD agarose and Rhotekin RBD agarose were used to isolate GTP-Rac1 from whole cell lysates from each of the indicated cell lines. The collected complexes were then resolved by SDS-PAGE, and probed with an anti-Rac1 antibody. The amount of Rac1-GTP was quantified by densitometry and normalized by the total input Rac1 protein. Relative to mock cells, Rac1-GTP reduced 2 fold and 6 fold in Men1-TAP and Men1 cells respectively. *, paired t-test, P<0.05. (E) A model for menin-regulated IQGAP interaction with the Rac1 and E-cadherin/β-catenin complex. When membrane menin increases, menin inhibits Rac1 activation and binds the tertiary structure by folding the C-terminus (C1 and C2) and N-terminus (N2) of IQGAP1. Their bindings induce IQGAP1 conformation switch to E-cadherin/β-catenin binding (black box, RasGAP domain) at cell-cell contacts and enhance intercellular adhesion. When reduction of menin activity occurs in tumor cells by Men1 mutation, or increase of Rac1 activity by oncogene, such as Ras, Rac1-GTP interacts with IQGAP1 (blank box, GRD domain) and increases IQGAP1 accumulations in cytoplasm and at the front edge of migrating cells, thereby promoting cell mobility.
Menin expression reduces Rac1-GTP
Small GTPases of the Rho family, including Rac1, RhoA, and Cdc42, regulate multiple signaling pathways that affect cell shape and motility, transcription, and cell-cycle progression (Ridley, 2001). Thus, we sought to determine whether menin affects the activities of these small G proteins using assays to measure their binding to partners that selectively interact with GTP-bound forms of Rac1, RhoA or Cdc42 (Taylor and Shalloway, 1996). Prior to harvesting the cells for GTPase binding assays, the cells were serum starved and then stimulated with fresh serum. As shown in Figure 6D, menin overexpression caused a dramatic suppression of Rac1 activation by serum stimulation.
RhoA-GTP level was increased but not consistent in mock cells, suggesting that RhoA may play a complicate function in β cells (Fig.S6D). Neither Cdc42-GTP nor total Cdc42 were detected in these cell lines (Fig.S6D, S6E). Collectively, these results indicate that Rac1 is a major Rho family GTPase in the islet cell lines used for this study, and that menin potently prevents Rac1 activation. Inactivation of Rac1 is possibly another mechanism by which menin competitively inhibits Rac1-IQGAP1 interaction in living cells (Fig.6E).
Discussion
Menin mutations have been known for more than a decade to cause the MEN1 class of multiple endocrine tumors (Chandrasekharappa et al., 1997), but the oncogenic mechanisms have remained obscure. Until recently, most studies of menin have focused on its role in the nucleus, where it indirectly regulates histone H3 methylation and transcription of at least a few genes that are involved in cell cycle control (Milne et al., 2002; Milne et al., 2005; Schnepp et al., 2006). Although menin is primarily a nuclear protein, it is also detectable in the cytoplasm and on membranes (Guru et al., 1998), and its differential distribution between the nucleus and cytoplasm is cell cycle-dependent (Huang et al., 1999; Lopez-Egido et al., 2002). Because little is known about the function of cytoplasmic menin in β cells, we decided to search for cytoplasmic binding partners of menin. We found that a major cytoplasmic binding partner of menin is IQGAP1 (Fig. 2), a protein known to be intimately involved in controlling cellular motility and morphogenesis through regulation of actin assembly (Bensenor et al., 2007; Brandt et al., 2007; Le Clainche et al., 2007) and intercellular adhesion (Kuroda et al., 1998). We propose a menin-IQGAP1 pathway, which contrasts and complements the “menin-MLL pathway” in the nucleus (Chen et al., 2006; Hughes et al., 2004; Yan et al., 2006a; Yokoyama et al., 2005).
Functional interaction between menin and IQGAP1 in endocrine cells
Men1 deletion and mutation causes multiple endocrine tumorigenesis (Lakhani et al., 2007). Similarly, IQGAP1 is widely expressed in various tissues. IQGAP1-null mutant mice develop gastric hyperplasia, lung adenoma/adenocarcinoma, and testicular atrophy or mineralization relative to wild-type animals of the same genetic background (Li et al., 2000). These observations are consistent with the notion that menin and IQGAP proteins may functionally link to the same pathway in the endocrine cells.
IQGAP1 lies in the midst of a complex signal transduction network that regulates cortical microfilaments (MFs) organization, cell motility and cadherin-induced cell adhesion (Bashour et al., 1997; Kuroda et al., 1998). Several lines of evidence demonstrate that the interaction of IQGAP1 with menin contributes to the effects on cell adhesion and migration. First, menin increased β cell aggression, which was attenuated by IQGAP1 knockdown (Fig.4A-B). Second, increasing menin concentration leads to a concomitant augmentation of IQGAP1, E-cadherin and β-catenin on the cell surface (Fig.3B, 4C). In contrast, menin ablation from islets in vivo decreased the accumulations of E-cadherin and β-catenin at the cell-cell contacts (Fig. 4D). Third, menin forms supercomplex with IQGAP1 and β-catenin in β-cells (Fig. 4D). Fourth, menin and Rac1 control the subcellular distributions of IQGAP1 in β cells (Fig.3B, C). They competitively form complex with IQGAP1 in vitro and in cultured cells (Fig. 3, Fig.6, Fig. S6). This result is in agreement with the previous discovery that reduction of Rac1-GTP level by TPA stimulation could decrease the Rac1-IQGAP1 complexes and increase the IQGAP1-β-catenin complexes (Fukata et al., 2001).
Regulation of cell motility and cell adhesion through menin-IQGAP1 pathway
Menin overexpression led to decreased cellular motility and increased cell-cell adhesion caused by recruitment of IQGAP1, E-cadherin and β-catenin to intercellular junctions. Paralleling these effects of menin overexpression were a reduction in the cytoplasmic levels of activated Rac1 (Fig. 6D), a small G protein whose GTP-bound state and localization at membranes are positively regulated by IQGAP1, and which in turn regulates functional properties of IQGAP1 (Fukata et al., 2002; Hart et al., 1996; Mataraza et al., 2003; Watanabe et al., 2004). On the other hand, association of IQGAP1 with activated Rac1 was attenuated by menin structurally binding to IQGAP1 terminus (Fig 6E). These results implicate menin as an important regulator of IQGAP1 and Rac1, and by extension, the adhesion and motility of endocrine cells.
A previous model of intercellular adhesion stipulates specific regulatory roles for activated Rac1 and IQGAP1 (Noritake et al., 2005). The data on which the model is based on the binding of IQGAP1 to β-catenin abrogates binding of the β-catenin/E-cadherin complex to α-catenin and thus, leads to decreased adhesion. This IQGAP1-mediated inhibition of adhesion can be relieved, however, by binding of Rac1-GTP to IQGAP1, which displaces IQGAP1 from β-catenin (Kuroda et al., 1998). Other studies, however, show that activated Rac1 and Cdc42 interact with IQGAP1 and promote cell mobility and polarization (Mataraza et al., 2003; Watanabe et al., 2005; Watanabe et al., 2004). The result presented here indicated that strong adhesion in our β cell lines was correlated with low, basal levels of Rac1-GTP, whereas weak adhesion was correlated with high Rac1-GTP levels and high cell mobility. These results strike us as different mechanisms to modulate IQGAP1 polarity in cell type-specific behavior.
Interplay between menin and IQGAP1 regulates IQGAP1 polarity function
Given that IQGAP1 functions in both cell adhesion and mobility, it would be asked what mechanism modulates the two activities in the same cell. IQGAP1 is both a downstream effector and an upstream activator of Cdc42, where active Cdc42 antagonizes IQGAP1 dissociation of the cell-cell contacts (Fukata et al., 1999; Lambert et al., 2002). Recently Cdc42 has been shown to inhibit IQGAP1’s role in polarized secretion in β cells or perhaps migration (Rittmeyer et al., 2008). Our results suggest that, at least in the Cdc42-deficient βHC9 cells, menin can replace the active Cdc42 and compete with Rac1 in a complex with IQGAP1, to increase IQGAP1-cadherin association at cell junctions (Fig. 6E). In contrast, activation of Rac1 could increase IQGAP1-Rac1 activity in the cytoplasm and inhibit IQGAP1 association with the E-cadherin-β-catenin complex. Thus, menin and Rac1 competitive interactions with IQGAP1 drive IQGAP1 polarity functions at the cell-cell contacts vs. its polarity functions at the leading edge of migrating cells (Fig. 6E).
Since menin represses Ras-mediated transformation in NIH 3T3 cells (Kim et al., 1999), and Rac1 is required for Ras-induced development of lung cancer in mice (Kissil et al., 2007), the results of this study may provide a new insight into how menin represses the oncogenic activity of Ras. On the other hand, our model may explain the loose association among islet cells in Men1 insulinoma (Bertolino et al., 2003). In epithelial cells, E-cadherin plays a key role in cell-cell adhesion, and loss of E-cadherin is a hallmark of tumor progression, cancer cell invasion and metastasis (Andl et al., 2006). Further work is required to biochemically decipher how menin inhibits Rac1 activation (Fig.6E).
Materials and Methods
Constructs and cell lines
To generate pMX-Men1-TAP construct, the human Men1 cDNA was subcloned in frame into Bam-HI-Hind III restriction sites of a pCTAP vector (Stratagene), and the resulting menin-TAP fragment was excised from this construct and cloned into the Not1 and BamH1 sites of retroviral plasmid, pMX-puro (Fig. S1A). pMX-Men1 was constructed by directly inserting human Men1 cDNA fragment into pMX-puro at Not1 and BamH1 sites. All the retroviruses were packaged to infect βHC9 cells as previously described (Chen et al., 2006). To stably overexpress wild-type menin, βMX1 cells were reinfected by pcDNA3-Men1 (La et al., 2004). A pLKO.1 lentiviral shRNA set targeting mouse IQGAP1 (RMM4534-NM_016721, OpenBiosystem) was used to knockdown IQGAP1. All stable cell lines were created by antibiotic selection (puromycin for pMX-plasmids or G418 for pcDNA3.1-plasmids) (Fig. S1). The cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) (Mediatech, Inc, Herndon, VA) in the presence of 10% fetal bovine serum, 5% iron-supplemented calf serum (cosmic calf serum) (Hyclone), and 1% penicillin-streptomycin (Invitrogen).
The mCherry-Rac1 vector was produced from an eCFP-Rac1 vector obtained from the lab of Dr. Klaus Hahn. The eCFP coding region was excised from the eCFP-Rac1 vector and the mCherry coding region from an mCherry-C1 vector by NheI and BsrGI digestion (Shaner et al., 2004). β cell lines were transiently transfected using Lipofectamine™ 2000 Reagent (Invitrogen).
Fluorescence microscopy
Cells were fixed and stained with indicated antibodies as previously described (Yan et al., 2006b). The primary antibodies used in the study were purchased from companies as described in supplementary data. Texas-Red Phalloidin (Invitrogen) was used to stain F-actin. The fluorescence was examined using a confocal laser scanning microscope (LSM510, Carl Zeiss, Oberkochene, Germany), and a series of images from 1-μm YZ sections were collected. Images were stacked using the LSM510 software and processed by Adobe Photoshop. Genotyping, excision of the floxed Men1 locus and islet slide staining of mice were performed as describe earlier (Schnepp et al., 2006).
Purification of the tagged-menin complex and Mass Spectrometry analysis of the purified proteins
Mock and Men1-TAP cells (1.5×108) were separately lysed in cell lysis buffer [250mM NaCL, 1mM MgCL2, 1mM CaCL2, 50mM Tris-HCL at pH 8.0, 0.5% NP-40, 5% glycerol, 2x EDTA-complete protease inhibitor cocktail (Roche)]. The lysates were quickly frozen in dry ice/ethanol slurry for 10 min, followed by incubation for 20 mins in ice-water. Two steps of purification were performed as recommended by the manufacturer (Stratagene). For analysis of protein complexes, a 1/15 volume of sample was separated in NuPAGE 4-12% Bis-Tris gel and visualized by silver stain (Silver staining kit, Invitrogen). The other 1/15 volume was immunoblotted against menin antibody (Fig. S2). The remaining sample was fractionated in the same SDS gel and stained with colloidal Coommassie blue staining. Bands of interest were excised and analyzed by mass spectrometry.
Adhesion assays
Adhesion assays were performed essentially as described previously (Price et al., 2004) with certain modifications. Cells were detached with 1.5mM-EDTA in PBS (without calcium and magnesium) and diluted in serum-free medium. Cells (2×105 /well) were seeded to 96-well plates that had been precoated with either PBS, fibronectin (10μg/ml) or goat anti human IgG Fc (20 μg/ml) /Fc-E-cadherin (2μg/ml). After 20 minutes of incubation at room temperature and three washes, the adherent cells were counted from quadruplet wells.
Recombinant binding assay
All affinity purification were essentially performed as described previously (Grohmanova et al., 2004; Le Clainche et al., 2007). Briefly, GST-tagged IQGAP1 C-terminal fragments were expressed in Escherichia coli (DH5α) and induced with 0.1mM isopropyl 1-thio-β-D-galactopyranoside (IPTG) at 28 °C for 16 hrs. The cells were harvested in lysis buffer (10mM Tris-HCL, pH 8.0, 150mM NaCL, 0.5mM EDTA, 2mM MgCL2, 2mM CaCl2, 0.1% NP40), sonicated and centrifuged at 13,000×g for 20 min. The supernatants were purified on Glutathione Sepharose 4B (GE Healthcare). For menin binding assay, the purified GST-fusion proteins were incubated with 0.2 μg of menin for 1 hr at 4 °C.
IP, affinity assays, small G protein affinity binding assays and migration assays are described in supplemental methods
Supplementary Material
Acknowledgments
The parent clonal βHC9 insulin-secreting cells (Radvanyi et al., 1993) were obtained from the cell repository of the Diabetes Research Center at the University of Pennsylvania, with permission of Dr. F.M. Matschinsky. We thank Drs. Faming Zhang for his gifts of purified MBP-tagged menin and non-tagged menin, Martin A. Schwartz for mutant human Rac1 vectors, and Ruth Kroschewski for the IQGAP1 C-terminal constructs (C1-C4). All confocal images and quantitative analyses of fluorescent intensity were conducted at the Biomedical Imaging Core Facility at the University of Pennsylvania. We thank Peter Blessington, Alicia Nelson, Hai Shen, Mercy Gohil and Elena Blagoi for their technical assistance. A special thanks to Drs. Claudia Andl and Margaret Chou for their stimulating discussions. This work was supported in part from NIH grants (R01-CA-100912 and R01-CA-113962 to XH, and R01-NS051746 to GSB), and a grant from the American Diabetes Association (7-07-RA-60 to XH).
References
- Andl CD, Fargnoli BB, Okawa T, Bowser M, Takaoka M, Nakagawa H, et al. Coordinated functions of E-cadherin and transforming growth factor beta receptor II in vitro and in vivo. Cancer Res. 2006;66:9878–85. doi: 10.1158/0008-5472.CAN-05-4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai F, Pei XH, Nishikawa T, Smith MD, Xiong Y. p18Ink4c, but not p27Kip1, collaborates with Men1 to suppress neuroendocrine organ tumors. Mol Cell Biol. 2007;27:1495–504. doi: 10.1128/MCB.01764-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashour AM, Fullerton AT, Hart MJ, Bloom GS. IQGAP1, a Rac- and Cdc42-binding protein, directly binds and cross-links microfilaments. J Cell Biol. 1997;137:1555–66. doi: 10.1083/jcb.137.7.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensenor LB, Kan HM, Wang N, Wallrabe H, Davidson LA, Cai Y, et al. IQGAP1 regulates cell motility by linking growth factor signaling to actin assembly. J Cell Sci. 2007;120:658–69. doi: 10.1242/jcs.03376. [DOI] [PubMed] [Google Scholar]
- Bertolino P, Tong WM, Herrera PL, Casse H, Zhang CX, Wang ZQ. Pancreatic beta-cell-specific ablation of the multiple endocrine neoplasia type 1 (MEN1) gene causes full penetrance of insulinoma development in mice. Cancer Res. 2003;63:4836–41. [PubMed] [Google Scholar]
- Brandt DT, Marion S, Griffiths G, Watanabe T, Kaibuchi K, Grosse R. Dia1 and IQGAP1 interact in cell migration and phagocytic cup formation. J Cell Biol. 2007;178:193–200. doi: 10.1083/jcb.200612071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekharappa SC, Guru SC, Manickam P, Olufemi SE, Collins FS, Emmert-Buck MR, et al. Positional cloning of the gene for multiple endocrine neoplasia-type 1. Science. 1997;276:404–7. doi: 10.1126/science.276.5311.404. [DOI] [PubMed] [Google Scholar]
- Chen YX, Yan J, Keeshan K, Tubbs AT, Wang H, Silva A, et al. The tumor suppressor menin regulates hematopoiesis and myeloid transformation by influencing Hox gene expression. Proc Natl Acad Sci U S A. 2006;103:1018–23. doi: 10.1073/pnas.0510347103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree JS, Scacheri PC, Ward JM, Garrett-Beal L, Emmert-Buck MR, Edgemon KA, et al. A mouse model of multiple endocrine neoplasia, type 1, develops multiple endocrine tumors. Proc Natl Acad Sci U S A. 2001;98:1118–23. doi: 10.1073/pnas.98.3.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree JS, Scacheri PC, Ward JM, McNally SR, Swain GP, Montagna C, et al. Of mice and MEN1: Insulinomas in a conditional mouse knockout. Mol Cell Biol. 2003;23:6075–85. doi: 10.1128/MCB.23.17.6075-6085.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnadieu E, Cefai D, Tan YP, Paresys G, Bismuth G, Trautmann A. Imaging early steps of human T cell activation by antigen-presenting cells. J Immunol. 1992;148:2643–53. [PubMed] [Google Scholar]
- Fukata M, Kuroda S, Nakagawa M, Kawajiri A, Itoh N, Shoji I, et al. Cdc42 and Rac1 regulate the interaction of IQGAP1 with beta-catenin. J Biol Chem. 1999;274:26044–50. doi: 10.1074/jbc.274.37.26044. [DOI] [PubMed] [Google Scholar]
- Fukata M, Nakagawa M, Itoh N, Kawajiri A, Yamaga M, Kuroda S, et al. Involvement of IQGAP1, an effector of Rac1 and Cdc42 GTPases, in cell-cell dissociation during cell scattering. Mol Cell Biol. 2001;21:2165–83. doi: 10.1128/MCB.21.6.2165-2183.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukata M, Watanabe T, Noritake J, Nakagawa M, Yamaga M, Kuroda S, et al. Rac1 and Cdc42 capture microtubules through IQGAP1 and CLIP-170. Cell. 2002;109:873–85. doi: 10.1016/s0092-8674(02)00800-0. [DOI] [PubMed] [Google Scholar]
- Grohmanova K, Schlaepfer D, Hess D, Gutierrez P, Beck M, Kroschewski R. Phosphorylation of IQGAP1 modulates its binding to Cdc42, revealing a new type of rho-GTPase regulator. J Biol Chem. 2004;279:48495–504. doi: 10.1074/jbc.M408113200. [DOI] [PubMed] [Google Scholar]
- Guru SC, Crabtree JS, Brown KD, Dunn KJ, Manickam P, Prasad NB, et al. Isolation, genomic organization, and expression analysis of Men1, the murine homolog of the MEN1 gene. Mamm Genome. 1999;10:592–6. doi: 10.1007/s003359901051. [DOI] [PubMed] [Google Scholar]
- Guru SC, Goldsmith PK, Burns AL, Marx SJ, Spiegel AM, Collins FS, et al. Menin, the product of the MEN1 gene, is a nuclear protein. Proc Natl Acad Sci U S A. 1998;95:1630–4. doi: 10.1073/pnas.95.4.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart MJ, Callow MG, Souza B, Polakis P. IQGAP1, a calmodulin-binding protein with a rasGAP-related domain, is a potential effector for cdc42Hs. Embo J. 1996;15:2997–3005. [PMC free article] [PubMed] [Google Scholar]
- Huang SC, Zhuang Z, Weil RJ, Pack S, Wang C, Krutzsch HC, et al. Nuclear/cytoplasmic localization of the multiple endocrine neoplasia type 1 gene product, menin. Lab Invest. 1999;79:301–10. [PubMed] [Google Scholar]
- Hughes CM, Rozenblatt-Rosen O, Milne TA, Copeland TD, Levine SS, Lee JC, et al. Menin associates with a trithorax family histone methyltransferase complex and with the hoxc8 locus. Mol Cell. 2004;13:587–97. doi: 10.1016/s1097-2765(04)00081-4. [DOI] [PubMed] [Google Scholar]
- Jiang K, Sun J, Cheng J, Djeu JY, Wei S, Sebti S. Akt mediates Ras downregulation of RhoB, a suppressor of transformation, invasion, and metastasis. Mol Cell Biol. 2004;24:5565–76. doi: 10.1128/MCB.24.12.5565-5576.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YS, Burns AL, Goldsmith PK, Heppner C, Park SY, Chandrasekharappa SC, et al. Stable overexpression of MEN1 suppresses tumorigenicity of RAS. Oncogene. 1999;18:5936–42. doi: 10.1038/sj.onc.1203005. [DOI] [PubMed] [Google Scholar]
- Kissil JL, Walmsley MJ, Hanlon L, Haigis KM, Bender Kim CF, Sweet-Cordero A, et al. Requirement for Rac1 in a K-ras induced lung cancer in the mouse. Cancer Res. 2007;67:8089–94. doi: 10.1158/0008-5472.CAN-07-2300. [DOI] [PubMed] [Google Scholar]
- Kuroda S, Fukata M, Nakagawa M, Fujii K, Nakamura T, Ookubo T, et al. Role of IQGAP1, a target of the small GTPases Cdc42 and Rac1, in regulation of E-cadherin-mediated cell-cell adhesion. Science. 1998;281:832–5. doi: 10.1126/science.281.5378.832. [DOI] [PubMed] [Google Scholar]
- La P, Schnepp RW, P CD, S AC, Hua X. Tumor suppressor menin regulates expression of insulin-like growth factor binding protein 2. Endocrinology. 2004;145:3443–50. doi: 10.1210/en.2004-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakhani VT, You YN, Wells SA. The multiple endocrine neoplasia syndromes. Annu Rev Med. 2007;58:253–65. doi: 10.1146/annurev.med.58.100305.115303. [DOI] [PubMed] [Google Scholar]
- Lambert M, Choquet D, Mege RM. Dynamics of ligand-induced, Rac1-dependent anchoring of cadherins to the actin cytoskeleton. J Cell Biol. 2002;157:469–79. doi: 10.1083/jcb.200107104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Clainche C, Schlaepfer D, Ferrari A, Klingauf M, Grohmanova K, Veligodskiy A, et al. IQGAP1 stimulates actin assembly through the N-WASP-Arp2/3 pathway. J Biol Chem. 2007;282:426–35. doi: 10.1074/jbc.M607711200. [DOI] [PubMed] [Google Scholar]
- Li S, Wang Q, Chakladar A, Bronson RT, Bernards A. Gastric hyperplasia in mice lacking the putative Cdc42 effector IQGAP1. Mol Cell Biol. 2000;20:697–701. doi: 10.1128/mcb.20.2.697-701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Egido J, Cunningham J, Berg M, Oberg K, Bongcam-Rudloff E, Gobl A. Menin’s interaction with glial fibrillary acidic protein and vimentin suggests a role for the intermediate filament network in regulating menin activity. Exp Cell Res. 2002;278:175–83. doi: 10.1006/excr.2002.5575. [DOI] [PubMed] [Google Scholar]
- Mataraza JM, Briggs MW, Li Z, Entwistle A, Ridley AJ, Sacks DB. IQGAP1 promotes cell motility and invasion. J Biol Chem. 2003;278:41237–45. doi: 10.1074/jbc.M304838200. [DOI] [PubMed] [Google Scholar]
- Mataraza JM, Li Z, Jeong HW, Brown MD, Sacks DB. Multiple proteins mediate IQGAP1-stimulated cell migration. Cell Signal. 2007;19:1857–65. doi: 10.1016/j.cellsig.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateer SC, McDaniel AE, Nicolas V, Habermacher GM, Lin MJ, Cromer DA, et al. The mechanism for regulation of the F-actin binding activity of IQGAP1 by calcium/calmodulin. J Biol Chem. 2002;277:12324–33. doi: 10.1074/jbc.M109535200. [DOI] [PubMed] [Google Scholar]
- Milne TA, Briggs SD, Brock HW, Martin ME, Gibbs D, Allis CD, et al. MLL targets SET domain methyltransferase activity to Hox gene promoters. Mol Cell. 2002;10:1107–17. doi: 10.1016/s1097-2765(02)00741-4. [DOI] [PubMed] [Google Scholar]
- Milne TA, Hughes CM, Lloyd R, Yang Z, Rozenblatt-Rosen O, Dou Y, et al. Menin and MLL cooperatively regulate expression of cyclin-dependent kinase inhibitors. Proc Natl Acad Sci U S A. 2005;102:749–54. doi: 10.1073/pnas.0408836102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noritake J, Watanabe T, Sato K, Wang S, Kaibuchi K. IQGAP1: a key regulator of adhesion and migration. J Cell Sci. 2005;118:2085–92. doi: 10.1242/jcs.02379. [DOI] [PubMed] [Google Scholar]
- Price LS, Hajdo-Milasinovic A, Zhao J, Zwartkruis FJ, Collard JG, Bos JL. Rap1 regulates E-cadherin-mediated cell-cell adhesion. J Biol Chem. 2004;279:35127–32. doi: 10.1074/jbc.M404917200. [DOI] [PubMed] [Google Scholar]
- Radvanyi F, Christgau S, Baekkeskov S, Jolicoeur C, Hanahan D. Pancreatic beta cells cultured from individual preneoplastic foci in a multistage tumorigenesis pathway: a potentially general technique for isolating physiologically representative cell lines. Mol Cell Biol. 1993;13:4223–32. doi: 10.1128/mcb.13.7.4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ. Rho family proteins: coordinating cell responses. Trends Cell Biol. 2001;11:471–7. doi: 10.1016/s0962-8924(01)02153-5. [DOI] [PubMed] [Google Scholar]
- Rittmeyer EN, Daniel S, Hsu SC, Osman MA. A dual role for IQGAP1 in regulating exocytosis. J Cell Sci. 2008;121:391–403. doi: 10.1242/jcs.016881. [DOI] [PubMed] [Google Scholar]
- Schnepp RW, Chen YX, Wang H, Cash T, Silva A, Diehl JA, et al. Mutation of tumor suppressor gene Men1 acutely enhances proliferation of pancreatic islet cells. Cancer Res. 2006;66:5707–15. doi: 10.1158/0008-5472.CAN-05-4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaner NC, Campbell RE, Steinbach PA, Giepmans BN, Palmer AE, Tsien RY. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat Biotechnol. 2004;22:1567–72. doi: 10.1038/nbt1037. [DOI] [PubMed] [Google Scholar]
- Taylor SJ, Shalloway D. Cell cycle-dependent activation of Ras. Curr Biol. 1996;6:1621–7. doi: 10.1016/s0960-9822(02)70785-9. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Noritake J, Kaibuchi K. Roles of IQGAP1 in cell polarization and migration. Novartis Found Symp. 2005;269:92–101. discussion 101-5, 223-30. [PubMed] [Google Scholar]
- Watanabe T, Wang S, Noritake J, Sato K, Fukata M, Takefuji M, et al. Interaction with IQGAP1 links APC to Rac1, Cdc42, and actin filaments during cell polarization and migration. Dev Cell. 2004;7:871–83. doi: 10.1016/j.devcel.2004.10.017. [DOI] [PubMed] [Google Scholar]
- Yan J, Chen YX, Desmond A, Silva A, Yang Y, Wang H, et al. Cdx4 and menin co-regulate hoxa9 expression in hematopoietic cells. PLoS ONE. 2006a;1:e47. doi: 10.1371/journal.pone.0000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Xu L, Crawford G, Wang Z, Burgess SM. The Forkhead Transcription Factor FoxI1 Remains Bound to Condensed Mitotic Chromosomes and Stably Remodels Chromatin Structure. Mol Cell Biol. 2006b;26:155–68. doi: 10.1128/MCB.26.1.155-168.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama A, Somervaille TC, Smith KS, Rozenblatt-Rosen O, Meyerson M, Cleary ML. The Menin Tumor Suppressor Protein Is an Essential Oncogenic Cofactor for MLL-Associated Leukemogenesis. Cell. 2005;123:207–18. doi: 10.1016/j.cell.2005.09.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.