Summary
Peptide histidine isoleucine (PHI) and vasoactive intestinal peptide (VIP) are neuropeptides synthesized from a common precursor, prepro-VIP, and share structural similarity and biological functions in many systems. Within the central nervous system and peripheral tissues, PHI and VIP have overlapping distribution. PHI-mediated functions are generally via activation of VIP receptors; however, the potency and affinity of PHI for VIP receptors are significantly lower than VIP. In addition, several studies suggest distinct PHI receptors that are independent of VIP receptors. PHI receptors have been cloned and characterized in fish, but their existence in mammals is still unknown. This study focuses on the functional role of PHI in the thalamus because of the localization of both PHI and VIP receptors in this brain region.
Using extracellular multiple-unit recording techniques, we found that PHI strongly attenuated the slow intrathalamic rhythmic activity. Using intracellular recording techniques, we found that PHI selectively depolarized thalamic relay neurons via an enhancement of the hyperpolarization-activated mixed cation current, Ih. Further, the actions of PHI were occluded by VIP and dopamine, indicating these modulators converge onto a common mechanism. In contrast to previous work, we found that PHI was more potent than VIP in producing excitatory actions on thalamic neurons. We next used the transgenic mice lacking a specific VIP receptor, VPAC2, to identify its possible role in PHI-mediated actions in the thalamus. PHI depolarized all relay neurons tested from wild-type mice (VPAC2+/+); however, in knockout mice (VPAC2-/-), PHI produced no change in membrane potential in all neurons tested. Our findings indicate that excitatory actions of PHI are mediated by VPAC2 receptors, not by its own PHI receptors and the excitatory actions of PHI clearly attenuates intrathalamic rhythmic activities, and likely influence information transfer through thalamocortical circuits.
Keywords: PHI, VIP, thalamus, vasoactive intestinal peptide, thalamocortical, electrophysiology, epilepsy
Introduction
Peptide histidine isoleucine (PHI) was originally isolated from porcine intestine (Tatemoto and Mutt, 1981) and later found in porcine brain (Tatemoto et al., 1983). Considering the VIP precursor prepro-VIP also contains PHI (Bodner et al., 1985;Itoh et al., 1983;Nishizawa et al., 1985), the distribution of PHI and VIP tends to be highly correlated in the nervous system and other systems including the gastrointestinal tract, pancreas, reproductive system, and respiratory systems (Baldino et al., 1989;Beinfeld et al., 1984;Christofides et al., 1984;Lundberg et al., 1984;Fahrenkrug et al., 1985;Mikkelsen and Fahrenkrug, 1994;Palle et al., 1989).
In the periphery, PHI is involved with many regulatory functions including vasodilation, secretion in the digestive tract, and smooth muscle activity in respiratory tract and genital tract (Gozes and Brenneman, 1989;Christofides et al., 1984;Palle et al., 1989;Anagnostides et al., 1983;Ghiglione et al., 1982;Bailey et al., 1990;Bataille et al., 1980). Furthermore, the activation of VIP-containing neurons induces co-release of PHI and VIP in the peripheral nervous system but the functional significance of co-release of these peptides is not fully understood (Lundberg et al., 1984;Fahrenkrug, 1987;Holst et al., 1987). PHI has been found to produce many actions in the central nervous system: PHI decreases the size of white matter lesions induced by intracerebral administration of a glutamate agonist indicating a neuroprotective function (Rangon et al., 2005), stimulates somatostatin release from neocortical neurons, and stimulates prolactin secretion from anterior pituitary cells (Tapia-Arancibia and Reichlin, 1985;Inoue et al., 1988). In addition, PHI is released from rat cortical slices in a calcium-dependent manner indicating a potential role in neurotransmission (Korchak et al., 1985). Despite the variety of actions, the electrophysiological functions of PHI are unknown.
The biological actions of PHI appear to be mediated via three different receptor subtypes: VIP-sensitive receptors (VPAC1, VPAC2) and an independent PHI receptor. PHI has been described as a weak agonist for both VPAC1 and VPAC2 receptors (Harmar et al., 1998;Palle et al., 1989;Inoue et al., 1988;Lundberg et al., 1984;Tapia-Arancibia and Reichlin, 1985;Lutz et al., 1993;Gourlet et al., 1998;Moriarty et al., 1984;Yuwiler et al., 1993). More recently, an independent PHI receptor has been cloned and characterized in fish; however, the existence of this receptor in mammals is still unknown (Tse et al., 2002).
In the thalamus, PHI and VIP are colocalized in thalamic reticular nucleus neurons (Baldino et al., 1989;Burgunder et al., 1999). VIP receptors are highly localized within primary thalamic nuclei such as ventrobasal nucleus and the dorsal lateral geniculate nucleus (Sheward et al., 1995;Usdin et al., 1994;Vertongen et al., 1997); however, the existence of PHI receptors in the thalamus is still unknown. Recent studies have demonstrated VIP-mediated changes in neuronal excitability in thalamic neurons (Lee and Cox, 2006;Lee and Cox, 2003;Sun et al., 2003): however, the actions of PHI within the thalamus remain unknown and serve as the focus of this study. We investigated the actions of PHI on intrathalamic rhythms and the actions of this peptide on the excitability of individual neurons. Our results indicate that PHI attenuates intrathalamic rhythms by selectively depolarizing thalamocortical relay neurons via VPAC2 receptor activation.
Methods
In most experiments, Sprague-Dawley rats (postnatal age 9 - 55 days) were used. In a subset of experiments, we identified receptors involved in PHI-mediated depolarization, and in these experiments VPAC2 receptor knockout (VPAC2-/-) and wild-type (VPAC2+/+) mice (postnatal age 12 - 16 days) were used. The mice were originally generated by Harmar et al. (2002). Procedures of mice genotyping used in these experiments were similar to those previously described (Lee and Cox, 2006).
Brain slicing procedures used in these experiments were similar to those previously described (Lee and Cox, 2006). Briefly, animals were deeply anesthetized with sodium pentobarbital (50 mg/kg) and decapitated. The brain was quickly removed and placed into cold, oxygenated slicing medium containing (in mM): 2.5 KCl, 10.0 MgCl2, 0.5 CaCl2, 1.25 NaH2PO4, 26.0 NaHCO3, 11.0 glucose, and 234.0 sucrose. Tissue slices (250 - 400 μm thickness) were cut in the horizontal plane using a vibrating tissue slicer, transferred to a holding chamber, and incubated at least 1 hour before recording. Individual slices were then transferred to a recording chamber, and continuously superfused with oxygenated physiological saline at 30°C. The physiological solution contained (in mM): 126.0 NaCl, 2.5 KCl, 2.0 MgCl2, 2.0 CaCl2, 1.25 NaH2PO4, 26.0 NaHCO3, and 10.0 glucose. This solution was gassed with 95% O2/5% CO2 to a final pH of 7.4.
Intracellular recordings, using the whole-cell configuration were obtained with the visual aid of a modified Nikon microscope equipped with differential interference contrast optics (Zeiss Instruments, Thornwood NY). A low power objective (4×) was used to identify various thalamic nuclei, and a high-power water immersion objective (40×) was used to visualize individual neurons. Recording pipettes were pulled from 1.5 mm outer diameter capillary tubing and had tip resistances of 3-6 MΩ when filled with the following intracellular solution (in mM): 117 K-gluconate, 13 KCl, 1.0 MgCl2, 0.07 CaCl2, 0.1 EGTA, 10.0 HEPES, 2.0 Na2-ATP, and 0.4 Na-GTP. The pH was adjusted to 7.3 using KOH and osmolarity was adjusted to 290-300 mosm with distilled H2O. An Axoclamp2B amplifier (Molecular Devices, Foster City, CA) was used in bridge mode for voltage recordings or switching single electrode voltage-clamp mode for current recordings. Voltage and current protocols were generated using pClamp software (Molecular Devices) and data were digitized and stored on computer. In current-clamp recordings, an active bridge circuit was continuously adjusted to balance the drop in potential produced by passing current through the recording electrode. The apparent input resistance of the neuron was calculated from the linear slope of the voltage-current relationship obtained by applying constant current pulses ranging from -100 to +40 pA (800 ms duration). During agonist application, changes in input resistance were determined by membrane response to single-intensity constant current hyperpolarizing pulses (5 - 40 pA, 500 ms, 0.2 Hz). For voltage-clamp recordings, an Axoclamp2B amplifier was used in discontinuous mode and the switching frequency ranged from 2.5 to 3.5 kHz with a gain of 150 to 800 pA/mV. The headstage was continually monitored to ensure that the current transients have completely decayed before voltage measurements. Voltage-clamp recordings were limited to neurons that had stable access resistances less than 20 MΩ. We corrected all voltage measurements for liquid junction potential error by adding 10mV.
Extracellular multiple unit recordings were obtained using sharpened tungsten microelectrodes (1-4 MΩ; Frederick Haer, Inc., Bowdoinham, ME). All data were digitized (1-2 kHz) and stored using Axotape software (Molecular Devices). Monopolar electrical stimulation was applied to either TRN or internal capsule using sharpened tungsten electrodes (200-600 kΩ, Frederick Haer).
Concentrated stock solutions of PHI (0.1 mM) were prepared in distilled water and diluted in physiological saline to a final concentration of 0.002 – 3.0 μM. Agonists were applied by injecting a bolus into the input line of the chamber over 60 seconds using a motorized syringe pump. Based on the rate of syringe pump and chamber perfusion, the final bath concentration of drugs was estimated to one-eighth of the concentration introduced in the flow line (Cox et al., 1995). Control injections of physiological saline did not alter intrathalamic activity during extracellular recording or membrane potential/input resistance during current-clamp recordings, suggesting that the temporary increase in flow rate during the bolus injections had no effect on the recordings. PHI was purchased from Calbiochem (San Diego, CA) and ZD7288 from Tocris (Ellisville, MO). All remaining compounds were purchased from Sigma (St. Louis, MO).
Analyses of intrathalamic rhythmic activities were similar to those described previously (Lee and Cox, 2003). Data are presented as mean ± standard deviation. Most statistical analyses consist of Mann-Whitney U test, and when appropriate, the Wilcoxon test for paired samples. In some noted instances, a paired students' t-test was used for testing statistical significance. The difference between the means was considered significant when p<0.05.
Results
PHI attenuates intrathalamic rhythmic activity
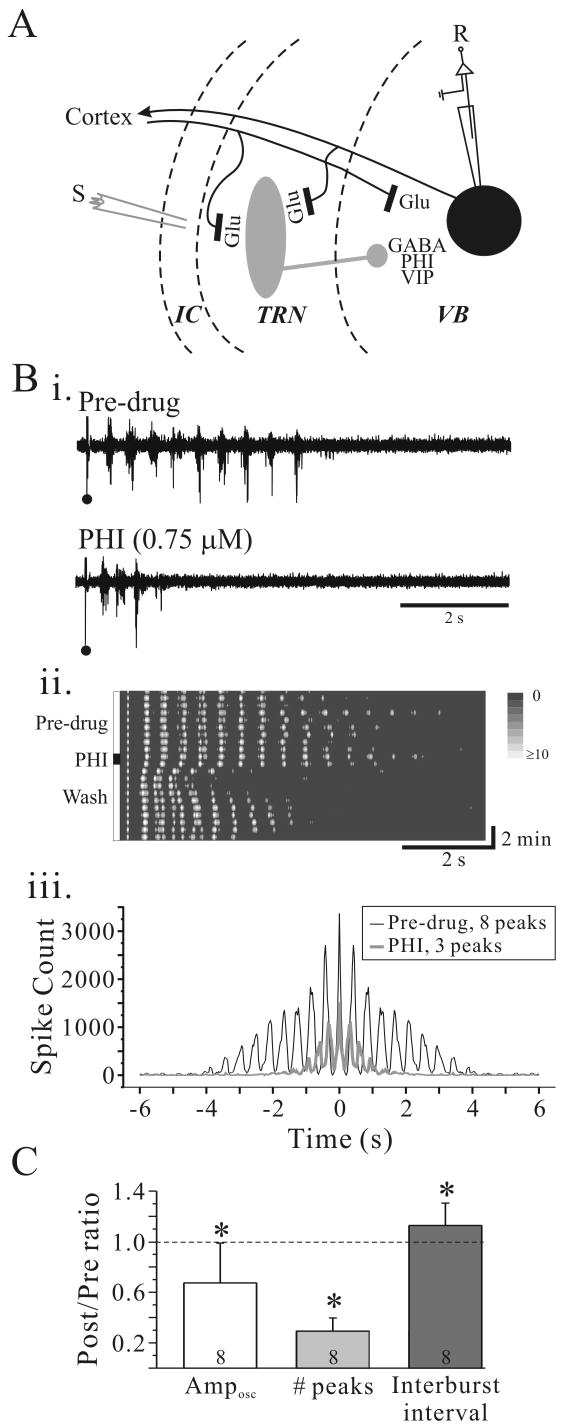
The reciprocal synaptic connectivity between TRN and the adjacent ventrobasal thalamic nucleus (VB) is preserved in the in vitro slice preparation (Fig. 1A). In the presence of the GABAA receptor antagonist, bicuculline methiodide (BMI, 10 μM), electrical stimulation within internal capsule evoked a stable rhythmic activity ranging from 2.1 to 2.9 Hz that could last many seconds (Fig. 1Bi, Pre-drug). Short bath application of PHI (0.75 μM, 60 seconds duration) suppressed the rhythmic activity in a reversible manner in 7 of 8 slices tested (Fig. 1Bi, ii). The autocorrelogram clearly indicates a highly synchronized rhythmic response that lasts approximately 4 seconds in control condition (Fig. 1Biii, black line). Following PHI application the number of cycles and the total number of spikes per trial were reduced (Fig. 1Biii, gray line). The interburst frequency was increased from 2.3 to 2.7 Hz following PHI application (Fig. 1Bi, iii). The population data regarding the effects of PHI on rhythmic activity are summarized in Fig. 1C. PHI significantly decreased the number of peaks (Wilcoxon test, p=0.01) and the overall number of action potential discharges (Amposc) (Wilcoxon test, p=0.03). The interburst frequency was significantly increased following PHI application (Wilcoxon test, p=0.04).
Figure 1.
PHI attenuates intrathalamic rhythmic activity. A. Simplified schematic illustrating thalamic circuitry with putative localization of PHI. Abbreviations: S, stimulus electrode; R, recording electrode; GABA, γ-aminobutyric acid; Glu, glutamate; PHI, peptide histidine isoleucine; VB, ventrobasal nucleus; TRN, thalamic reticular nucleus; IC, internal capsule. Bi. Extracellular multiple-unit recording from VB in a rat thalamic slice. In BMI (10 μM; Pre-drug), a single stimulus (•) in TRN evokes rhythmic discharge in VB. PHI (0.75 μM, 60 seconds) dramatically suppresses the rhythmic activity. Bii. Contour plot of experiment in Bi illustrates the time course of PHI effect on intrathalamic rhythmic activity. Prior to PHI application the rhythmic activity is very stable and lasts for many cycles. After PHI application, the rhythmic activity is dramatically attenuated, but returns near control levels within 5 minutes. Biii. Autocorrelogram of experiment in Bi illustrates a highly synchronized response that lasts nearly four seconds in control conditions (black trace). PHI (gray trace) reduces the numbers of peaks from 8 to 3. C. Summary of effects of PHI on oscillation amplitude (Amposc), number of peaks, and oscillation frequency. * p<0.05, **p<0.01.
PHI depolarizes thalamic relay neurons
The persistence of the rhythmic activity is dependent on the action potential discharge mode of TRN and thalamic relay neurons, and this discharge mode is voltage-dependent (Jahnsen and Llinás, 1984a;Steriade and Llinás, 1988;von Krosigk et al., 1993). We next tested whether PHI alters certain intrinsic properties of thalamic neurons such as resting membrane potential and apparent input resistance. Whole cell recordings were obtained from 68 VB relay neurons and 5 TRN neurons from young rats (postnatal age: 9–18 days). The average resting membrane potential of relay neurons (-67.8 ± 2.7 mV; n=68) was significantly depolarized compared to TRN neurons (-78.2 ± 2.5 mV; n=5; p<0.01, Mann-Whitney). Despite the difference in resting membrane potentials, the apparent input resistance did not significantly differ between the relay neurons (203.3 ± 92.9 MΩ; n=68) and the TRN neurons (173.8 ± 43.5 MΩ; n=5; p>0.2).
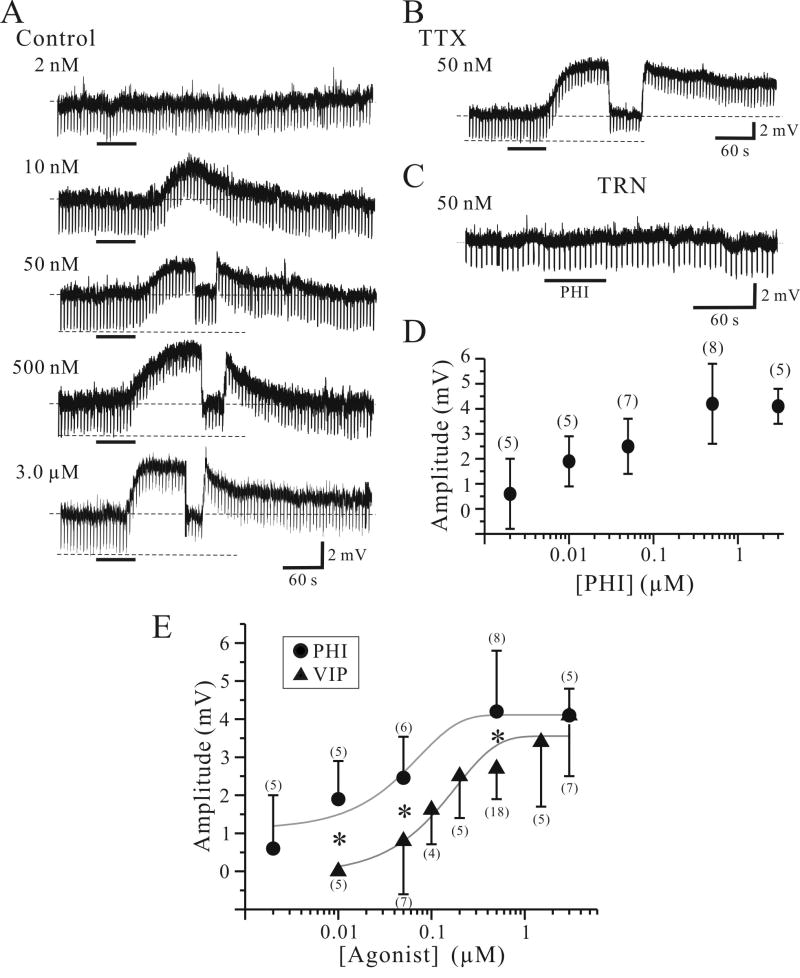
PHI produced a slow onset, long duration depolarization in VB relay neurons (Fig. 2A). The amplitude and occurrence of the PHI-mediated depolarizations were concentration-dependent (Fig. 2D, one-way ANOVA, p<0.01). At the lowest PHI concentration tested (2 nM), PHI depolarized only 1 of 5 neurons. At a higher concentration (10 nM), PHI depolarized 4 of 5 neurons with an average peak amplitude of 1.9 ± 1.1 mV (n=5). At higher concentrations (≥ 50 nM) produced depolarization in all relay neurons tested and produced peak depolarization of 2.5 ± 1.1 mV (n=7; 50 nM), 4.2 ± 1.6 mV (n=8; 500 nM), and 4.1 ± 0.7 mV (n=5; 3 μM).
Figure 2.
PHI selectively depolarizes relay neurons. A. Intracellular recording from thalamic relay neurons reveal that PHI produces a concentration-dependent depolarization. Lowest PHI concentration (2 nM) produced no membrane depolarization. In a different relay neuron, higher PHI concentration (500 nM) produced a larger depolarization (4.2 mV). The depolarization is briefly interrupted by current injection to test for alteration in apparent input resistance. The downward deflections are voltage responses to hyperpolarizing current steps (10 pA, 500 ms, 0.2 Hz), and in this neuron PHI decreased input resistance by 12 %. Dotted lines indicate resting membrane potentials ranging -68 mV to -72 mV. B. In TTX (1 μM), PHI (500 nM) produces a similar membrane depolarization (6.2 mV). Upper dotted line indicates -71 mV. C. PHI (500 nM) produces no detectable changes in membrane potential or input resistance in a TRN neuron. Dotted line indicates -74 mV. D. Summary of all PHI-mediated membrane depolarizations of VB neurons. Cell counts for each concentration are listed in parentheses. E. Summary of PHI-mediated (●) and VIP-mediated (▲) membrane depolarizations of VB neurons. Solid gray lines indicate the sigmoidal function of each agonoist-mediated depolarizations. Cell counts for each concentration are listed in parentheses. *, p<0.05.
The PHI-mediated membrane depolarization was associated with a decrease in apparent input resistance (Fig. 2A). During the peak of the PHI-mediated depolarization, the membrane potential was manually repolarized to pre-drug levels to test for voltage independent changes in apparent input resistance (e.g. Fig. 2A, ≥50 nM). In relay neurons, PHI (500 nM) produced a decrease in the input resistance that averaged 24 ± 12 % (n=6; p<0.05, Wilcoxon). The PHI-mediated depolarization (500 nM) persisted in the presence of TTX in all relay neurons tested (Fig. 2B, 4.7 ± 1.6 mV, n=6). The amplitudes of the PHI-mediated depolarizations in control solutions and TTX did not significantly differ (p>0.5, Wilcoxon), suggesting the actions of PHI arise from postsynaptic mechanisms. In contrast to thalamic relay neurons, PHI (500 nM) produced no detectable changes in membrane potential or apparent input resistance in all TRN neurons tested (Fig. 2C, n=5).
In addition to its own specific PHI receptor (Tse et al., 2002), PHI may act as a weak agonist on VIP receptors (Harmar et al., 1998;Gourlet et al., 1998;Palle et al., 1989;Inoue et al., 1988;Lundberg et al., 1984;Moriarty et al., 1984;Tapia-Arancibia and Reichlin, 1985;Yuwiler et al., 1993;Lutz et al., 1993). We next compared our current results of PHI-mediated depolarizations with our previously published results regarding VIP-mediated depolarizations of thalamic relay neurons (Lee and Cox, 2003). Our results indicate that the amplitudes of PHI-mediated depolarizations in relay neurons are significantly greater than VIP-mediated depolarizations at most concentrations tested (10-500 nM, Fig. 2E, p<0.05, Mann-Whitney). At 3 μM concentration, the amplitudes of the depolarizations did not differ between two groups (p>0.5, Mann-Whitney). The maximum response produced by VIP and PHI did not significantly differ, however the agonist concentration required to produce the half-maximum response was 0.048 μM and 0.13 μM for PHI and VIP, respectively.
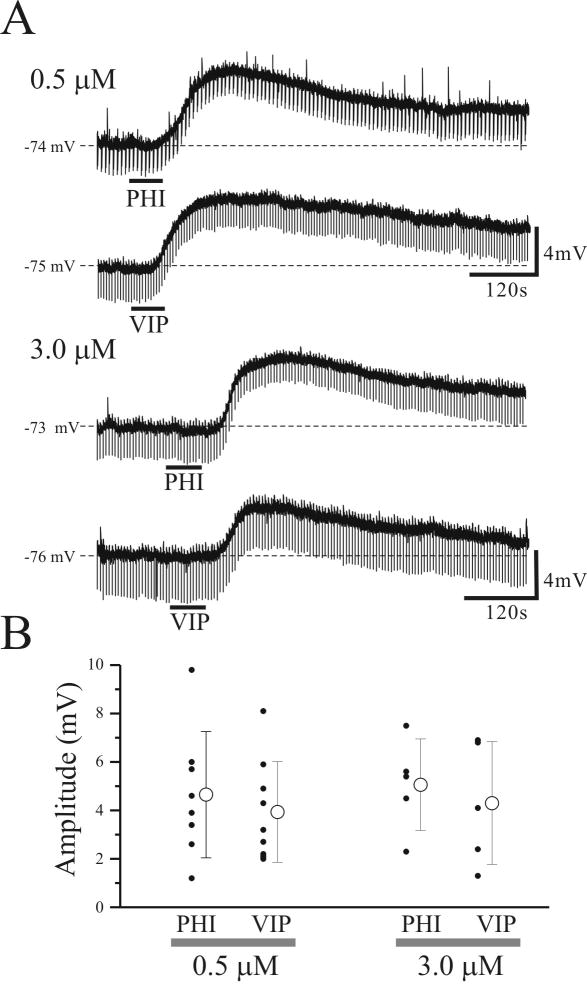
Next, we examined whether the relative contributions of PHI and VIP in depolarizing thalamocortical relay neurons was developmentally regulated. Whole cell recordings were obtained from 27 VB relay neurons from adult rats (postnatal age: 40-55 days). The average resting membrane potential of these neurons (-71.1 ± 3.5 mV; n=27) significantly differed from relay neurons of young rats (postnatal age: 9 – 18 days; -67.8 ± 2.7 mV; n=68, p<0.01) and apparent input resistance also significantly differed between the two age groups (adult: 66.4 ± 37.0 MΩ; n=27; young: 203.3 ± 92.9 MΩ; n=68, p<0.01). In addition, the amplitude and voltage-dependancy of Ih also siginificantly differed between adult and young rats (see supplementary Fig. 1); however, PHI (3 μM) and VIP (3 μM) produced similar membrane depolarizations in VB relay neurons from adult rats to those from young animals (Figs. 2,3), suggesting no developmental differences in the excitatory actions of these peptides. In adult animals, the average amplitude of PHI- and VIP-mediated depolarizations did not significantly differ (0.5 μM and 3.0 μM; Fig. 3B; p>0.5), suggesting that PHI specific receptors are not present in adult rats.
Figure 3.
PHI and VIP depolarize relay neurons from adult rats (postnatal age: 40-55 days). A. Intracellular recording from thalamic relay neurons reveal that PHI (≥0.5μM) and VIP (≥0.5μM) produce a similar membrane depolarization in all VB relay neurons tested (n=27). B. Summary of all PHI- and VIP- mediated membrane depolarizations of VB neurons. Open circle symbol in the right indicates the mean ± SD of membrane depolarizations evoked by PHI and VIP.
PHI enhances Ih in relay neurons
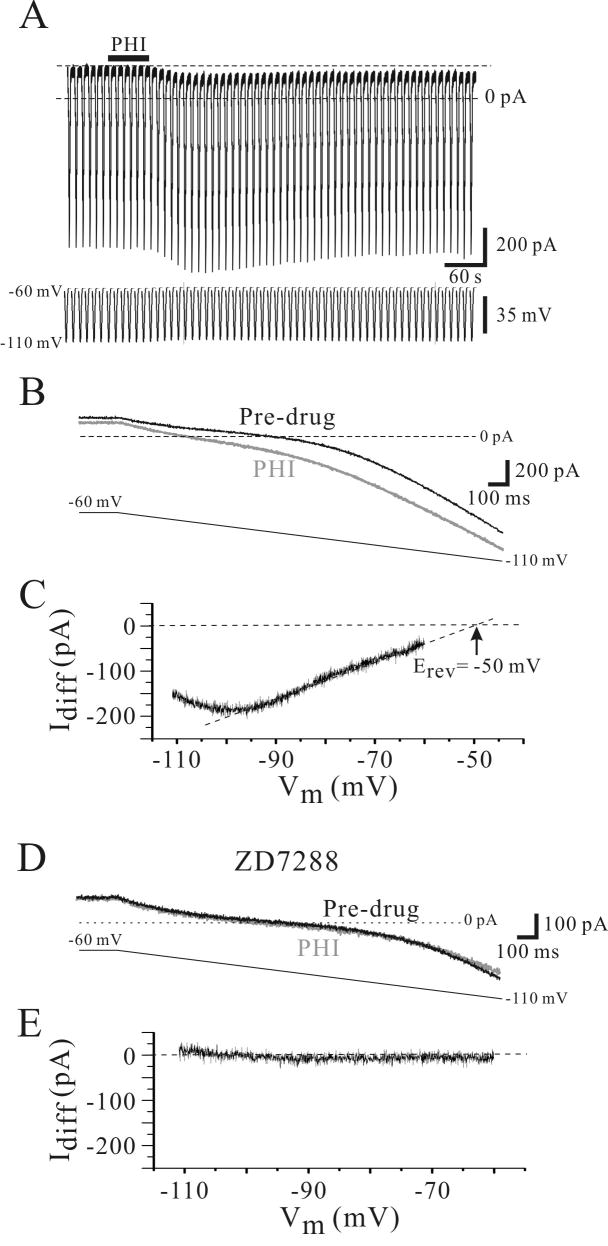
Voltage-clamp recordings were used to characterize conductance changes produced by PHI in relay neurons. Consistent with our current clamp recordings above, PHI (0.5 μM) produced an inward current that lasted many minutes (Fig. 4A). In the presence of TTX (1.0 μM), PHI produced an inward current that averaged 45 ± 23 pA (n=8). Slow voltage command ramps (-60 mV to -120 mV, 2 seconds duration, 0.1 Hz) were used to determine conductance changes produced by PHI (Fig. 4B). To quantify changes in the “resting” conductance of the neuron, we analyzed the initial portion of the current response near resting membrane potential (-60 to -80 mV). The resting conductance of the neurons prior to PHI application averaged 6.0 ± 2.0 nS (n=7). Following PHI (0.5 μM) application, the conductance was significantly increased to 9.1 ± 3.9 nS (n = 7; p<0.01, Wilcoxon). To access the voltage dependence of the conductance altered by PHI, the difference of the current responses before and after PHI application was calculated (Idiff, Fig. 4C). This PHI-mediated conductance (Idiff), was usually linear over the voltage range of -60 mV to -100 mV. Extrapolating the linear fit of Idiff indicated that the PHI-sensitive current had a reversal potential of -44 ± 5 mV (n=7, Fig. 4C).
Figure 4.
PHI enhances Ih in a VB neuron. A. In voltage clamp recordings from a VB neuron, PHI (500 nM) produces an inward current associated with increase in membrane conductance. Slow ramped voltage commands (-60 to -110 mV, 4 s duration) are used to measure conductance before and after agonist application. B. Expanded traces of the membrane response to the ramped voltage command reveal not only the inward current, but the conductance increase by PHI (gray trace). Each trace consists of an average of 3 subsequent responses prior to and at the peak of the PHI-mediated inward current. C. The difference between the PHI (B, gray trace) and Pre-drug (B, black trace) is indicative of the PHI-sensitive current (Idiff). Extrapolation of the linear portion of this current (dotted line) indicates that the PHI-mediated current has a reversal potential (Erev) of -50 mV. D. The Ih blocker ZD7288 attenuates the PHI receptor-mediated inward current in the same neuron. In TTX (1.0 μM) and ZD7288 (100 μM), PHI does not produce an inward current or alter membrane conductance. E. The difference between the PHI and pre-drug clearly indicates the lack of PHI effect in ZD7288.
Considering the reversal potential of PHI-mediated current in relay neurons (-44 ± 5 mV) is similar to the reported reversal potential of the hyperpolarization-activated mixed cation conductance, Ih in thalamic neurons (Zhu et al., 1999;McCormick and Pape, 1990b;Lee and Cox, 2006;Lee and Cox, 2003), we speculated that PHI may alter Ih in these neurons. We next tested the sensitivity of the PHI-mediated current to the selective Ih antagonist, ZD7288 (N-ethyl-1,6-dihydro-1,2-dimethyl-6-(methylimino)-N-phenyl-4-pyrimidin amine). In control conditions (TTX, 1.0 μM), PHI produced an inward current that averaged 45 ± 30 pA (n=5). Following the addition of ZD7288 (100 μM), the subsequent application of PHI produced no apparent change in the holding current (4 ± 3 pA, n=5; p<0.05, paired t-test). The blockade of the inward current was also reflected in the conductance measurements as well. In TTX alone, PHI produced an increase in membrane conductance that averaged 47 ± 20% (n=5). In ZD7288 (100 μM) and TTX, PHI did not alter the membrane conductance (Fig. 4E, 5 ± 5%, n=5). Our data indicate that PHI produces an increase in Ih.
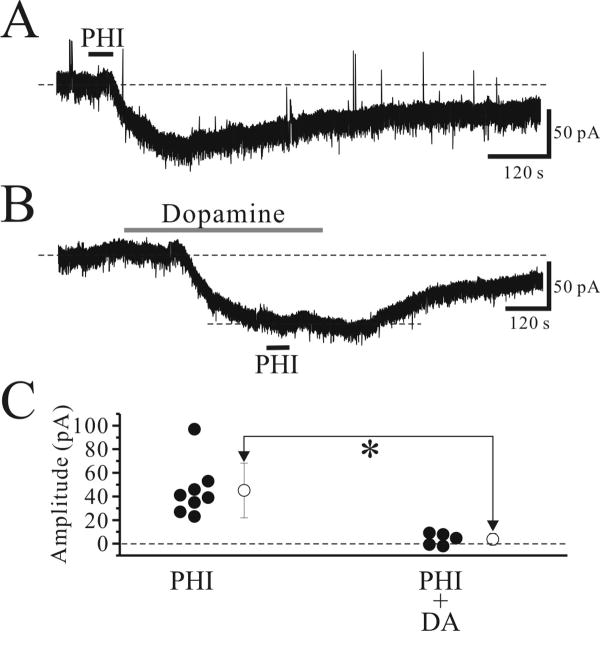
Next, we tested the possible convergence of PHI-mediated actions with actions of neurotransmitters that have been shown to alter Ih. Our previous studies showed that dopamine depolarizes thalamocortical relay neurons via activation of Ih (Govindaiah and Cox, 2005). In TTX (0.5 μM), dopamine (50 μM) also produced a robust inward current that averaged 88 ± 16 pA (Fig. 5B, n=5). We next examined if the PHI-mediated current may be occluded by prior exposure to dopamine. In the presence of TTX (0.5 μM), PHI (0.5 μM) produced average inward currents of 45 ± 23 pA (Fig. 5A, n=8), when applied alone. Bath application of dopamine (50 μM) produced inward current and subsequent application of PHI in the presence of dopamine produced a significant smaller inward current (Fig. 5B,C; -4 ± 5 pA, n=5; p<0.05, Mann-Whitney U test).
Figure 5.
Excitatory actions of PHI are occluded by dopamine. A. In a voltage clamp recording of relay neurons (Vhold=-60mV), PHI (0.5 μM) produces an inward current. B. In a different relay neuron, DA (50 μM) produces an inward current that reaches a steady state, and at this point, subsequent PHI application produces a significantly smaller inward current. C. Summary of PHI-mediated inward currents in the presence and absence of DA. Open circle symbol in the right indicate the mean ± standard deviation of inward currents evoked by PHI. *, p<0.05.
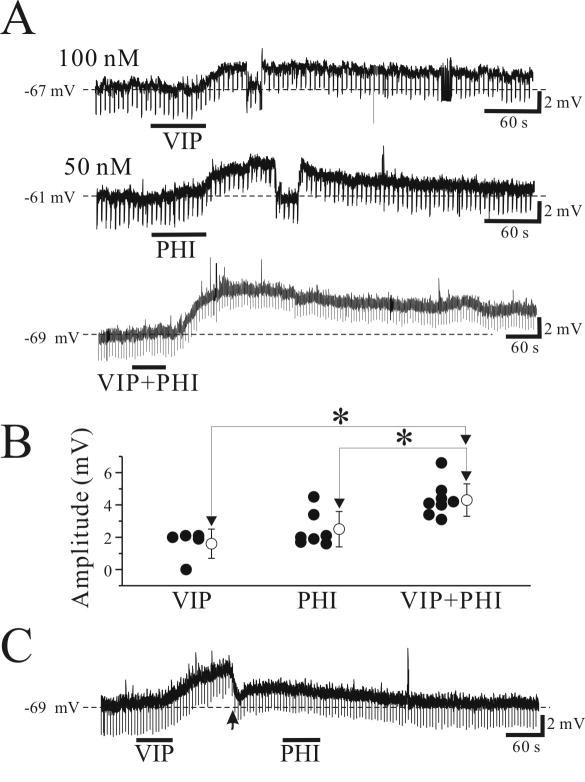
Next, we examined whether co-application of VIP and PHI at submaximal concentrations produces additive or synergistic effects on depolarizing actions on thalamocortical neurons considering both peptides share the same ionic mechanisms. In this experiment, we carefully choose agonist concentrations required to produce a half-maximum response. PHI (0.05 μM) and VIP (0.1 μM) produced average membrane depolarizations of 1.6 ± 0.9 mV (n=5) and 2.5 ± 1.0 mV (n=7), respectively, when applied separately (Fig. 6A). In other cells, combined application of PHI (0.05 μM) and VIP (0.1 μM) produced a significantly larger membrane depolarization than those of each individual agonist-induced depolarizations (4.3 ± 1.1 mV, n=8, p<0.05; Fig. 6A,B) close to the sum of each agonist-induced membrane depolarizations (4.1 mV), suggesting additive effects, not synergistic effects. Finally, we tested whether PHI and VIP share identical pathways involved in membrane depolarization. We tested if VIP could occlude the membrane depolarization produced by PHI. As illustrated in Figure 6C, VIP (1.0 μM) depolarized all relay neurons tested (n=5, 3.4±0.8 mV). At the peak of VIP effect, the membrane potential was manually adjusted to resting levels, and PHI (0.5 μM) was applied. Under these condition, PHI produced no obvious depolarization in all cells tested (n=5; Fig. 6C). These data indicate that PHI may share the same ionic mechanisms as other neurotransmitters that have been shown to alter Ih.
Figure 6.
Additive effects of submaximal responses of VIP and PHI in thalamic relay neurons. A. PHI (0.05 μM) and VIP (0.1 μM) produced small membrane depolarizations when applied separately. In another cell, combined application of PHI (0.05 μM) and VIP (0.1 μM) produced a significantly larger depolarization than the individual agonists, and is similar to that the sum of each agonists-induced membrane depolarizations. B. Population data illustrating individual and combined agonist applications. Open circle symbol in the right indicates the mean ± standard deviation of membrane depolarizations evoked by agonists. *, p<0.05. C. High VIP concentration (1 μM) occludes PHI-mediated membrane depolarizations. At the peak of VIP-mediated depolarization (arrowhead), the membrane potential was manually adjusted to resting levels, and PHI (0.5 μM) was applied. Under these conditions, PHI produced no changes in membrane potential.
PHI depolarizes VB and dLGN relay neurons via VPAC2 receptors
The described biological actions of PHI can be mediated through three different receptor subtypes: PHI, VPAC1, and VPAC2 receptors (Harmar et al., 1998;Gourlet et al., 1998;Palle et al., 1989;Inoue et al., 1988;Lundberg et al., 1984;Moriarty et al., 1984;Yuwiler et al., 1993;Tapia-Arancibia and Reichlin, 1985;Tse et al., 2002). With regards to VPAC receptors, VPAC2 receptors are highly localized in the thalamus whereas VPAC1 receptors are rarely found in the thalamus (Usdin et al., 1994;Sheward et al., 1995;Vertongen et al., 1997;Vaudry et al., 2000;Burgunder et al., 1999). We next tested if VPAC2 receptors or PHI receptors mediate the excitatory actions of PHI in relay neurons. To test this we used the VPAC2 receptor knockout (VPAC2-/-) and wild-type (VPAC2+/+) mice because of the lack of commercially available potent selective antagonists. Whole-cell recordings were obtained from VB relay neurons in slices from VPAC2+/+ and VPAC2-/- mice. The average resting membrane potential (VPAC2+/+: -65.3 ± 2.3 mV, n=7; VPAC2-/-: -67.1 ± 2.1 mV, n=8) and apparent input resistance (VPAC2+/+: 200.4 ± 55.3 MΩ; VPAC2-/-, n=7: 225.4 ± 42.8 MΩ, n=8) of the neurons from VPAC2+/+ and VPAC2-/- mice did not differ significantly (p>0.1, Mann-Whitney U test).
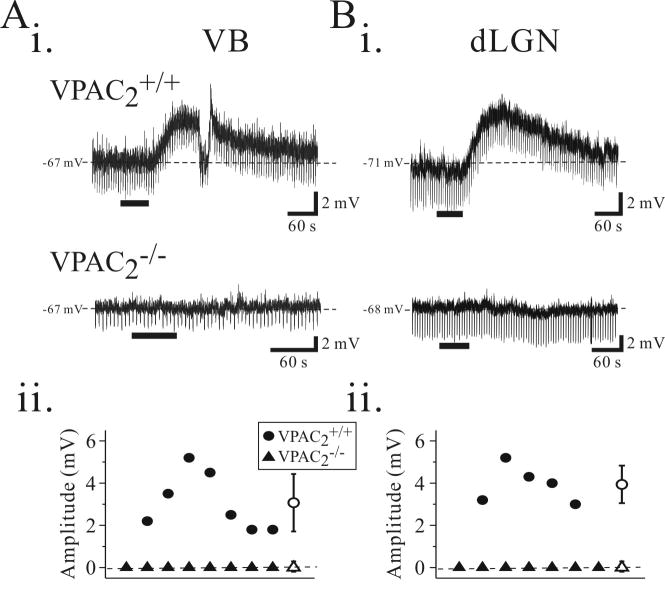
Similar to what we observed in rat relay neurons, PHI (0.5 μM) depolarized all relay neurons tested from VPAC2+/+ mice with an average amplitude of 3.1 ± 1.4 mV (n=7, Fig. 7, VB, VPAC2+/+). In a subset of neurons in which the membrane potential was manually repolarized to pre-drug levels during the peak of the PHI-mediated depolarization, the input resistance was decreased by 49 ± 11 % (n=4). In contrast, in slices from VPAC2-/- animals, PHI (0.5 μM) did not alter the membrane potential in all 8 VB neurons tested (Fig. 7A, VPAC2-/-). The resting membrane potential prior to PHI application in the VB relay neurons from VPAC2-/- mice averaged -66.6 ± 2.1 mV (n=8) and was not significantly altered following agonist application (-66.7 ± 2.1mV, n=8; p>0.5, Wilcoxon test).
Figure 7.
PHI-mediated depolarizations are dependent on VPAC2 receptor activation in the mouse thalamus. Ai. Intracellular recording from a VB neuron in a slice from a wildtype mouse (VPAC2+/+) reveals that PHI (500 nM) evokes a long-lasting, membrane depolarization associated with a decrease in input resistance (top trace). In a slice from a knock-out (VPAC2-/-) animal, PHI does not alter membrane potential (bottom trace). Aii. Population data illustrating membrane depolarizations produced by PHI in VB neurons from VPAC2+/+ (n = 7, solid circles) and VPAC2-/- (n = 8, solid triangles) animals. Bi. Intracellular recordings from VPAC2+/+ dLGN relay neurons reveals that PHI (500 nM) evokes membrane depolarizations (top trace). In dLGN neurons from VPAC2-/- animal, PHI do not alter membrane potentials (bottom trace). Bii. Population data illustrating membrane depolarizations produced in dLGN neurons from VPAC2+/+ (n = 5) and VPAC2-/- (n = 7) slices. Open circle symbol in the right indicates the mean ± standard deviation of membrane depolarizations evoked in VPAC2+/+ mice.
We next examined whether the effects of PHI on relay neurons can be generalized to other thalamic nuclei, so we also tested the effect of PHI on relay neurons in the dorsal lateral geniculate nucleus (dLGN). The average resting membrane potential (VPAC2+/+: -70.4 ± 0.5 mV, n=5; VPAC2-/-: -70.3 ± 2.1 mV, n=7) and apparent input resistance (VPAC2+/+: 262.4 ± 96.0 MΩ, n=5; VPAC2-/-: 225.9 ± 44.5 MΩ, n=7) of dLGN relay neurons from VPAC2+/+ and VPAC2-/- animals did not differ significantly (p>0.5, Mann-Whitney U test). PHI (0.5 μM) consistently depolarized all dLGN relay neurons tested from VPAC2+/+ mice with an average amplitude of 4.0 ± 0.9 mV (n=5, Fig. 7B, VPAC2+/+). In slices from VPAC2-/- animals, PHI (0.5 μM) did not alter the membrane potential in any dLGN relay neurons tested (n=7, Fig. 7B, VPAC2-/-). The resting membrane potential prior to PHI application in dLGN relay neurons from VPAC2-/- mice averaged -71.1 ± 2.3 mV (n=7) and was not significantly altered following PHI application (-71.0 ± 2.4 mV, n=7; p>0.5, Wilcoxon test). The excitatory actions of PHI on thalamic relay neurons are mediated via activation of VPAC2 receptors.
Discussion
Our study demonstrates that PHI selectively excites thalamic relay neurons via the activation of VPAC2 receptors and not via PHI-specific receptors. In contrast to previous studies, our results suggest that PHI appears to be a more potent agonist than VIP for VPAC2 receptors in thalamocortical neurons. In addition, the PHI-mediated depolarization of thalamocortical neurons results from an enhancement of Ih, and ultimately the resulting depolarization decreases the probability of burst discharge of thalamic relay neurons, thereby attenuating the intrathalamic rhythmic activity. Our working hypothesis is that PHI and/or VIP may be released from TRN neurons during each cycle of rhythmic activity, their excitatory actions summating leading to a switching of thalamic neurons from burst- to tonic-firing mode and thereby attenuating the rhythmic activity.
Our results demonstrate that PHI (≥ 10 nM) selectively depolarizes thalamic relay neurons and did not affect TRN neurons. These results are similar to our previous study with VIP (Lee and Cox, 2003). Such overlap is in accordance with previous reports indicating PHI and VIP exert similar effects in peripheral tissues and the central nervous system (Bataille et al., 1980;Lundberg et al., 1984;Moriarty et al., 1984;Tapia-Arancibia and Reichlin, 1985;Yuwiler et al., 1993). Although several studies demonstrate that VIP depolarizes thalamic relay neurons and brainstem neurons and increases the excitability of hippocampal, neocortical, and locus coeruleus neurons (Haas and Gahwiler, 1992;Kohlmeier and Reiner, 1999;Murphy et al., 1993;Pawelzik et al., 1992;Wang and Aghajanian, 1989), electrophysiological actions of PHI have not been studied. In these experiments, we found the first evidence that PHI depolarizes neurons.
Our data clearly indicate PHI depolarizes all relay neurons from VPAC2+/+ mice, but produced no change in neurons from VPAC2-/- mice, indicating PHI-mediated depolarizations are mediated by VPAC2 receptors, not by its own PHI receptors. There are numerous evidences that PHI shares common receptors with VIP (Palle et al., 1989;Tapia-Arancibia and Reichlin, 1985;Inoue et al., 1988;Yuwiler et al., 1993;Ishihara et al., 1992;Bataille et al., 1980;Suzuki et al., 1991;Huang and Rorstad, 1990). However, several studies also suggest the potential existence of PHI specific receptors. Such a hypothesis is supported by study demonstrating that PHI-mediated biological actions are opposite to VIP-mediated actions (Kimura et al., 1987). Second, specific VIP antagonists did not attenuate PHI-mediated responses (Nurko et al., 1989). Recently, the first PHI specific receptor has been isolated and characterized in goldfish (Tse et al., 2002), but to date, these receptors have not been identified in mammals.
Is PHI a more potent agonist than VIP?
In contrast with previous studies, our results suggest that PHI appears to be a more potent agonist than VIP for VPAC2 receptors of thalamocortical neurons. Higher sensitivity of relay neurons to PHI in in vitro slices compared with VIP could result from three possibilities. First, PHI may have a higher affinity for VPAC2 receptors in rat VB than VIP. Binding studies using radiolabeled PHI and VIP suggest the presence of VIP receptors with greater affinities for PHI compared with VIP found in liver, muscle cells, and neuroblastoma cells, but such studies have not been carried out on thalamic tissue (Paul et al., 1987;Murthy et al., 1993;Lelievre et al., 1998). Second, PHI could have easier access to the receptors than VIP. This could occur if PHI-specific peptidases exist at a low concentration in the thalamus. Dipeptidyl-peptidase IV, a highly specialized peptidase removing dipeptides only from peptides with N-terminal penultimate proline or alanine selectively degrades PHI, not VIP (Mentlein et al., 1993). This peptidase has been localized in the brain, however, the detailed distribution of this peptidase within the brain is unknown (Mentzel et al., 1996). Third, VIP-specific peptidases may exist at a relatively higher concentration in the thalamus. Neutral endopeptidase 24.11 (NEP) and angiotensin I-converting enzyme (ACE) are membrane-bound, extracellularly oriented peptidases (i.e., ectoenzymes) which degrade several neuropeptides including VIP (Skidgel and Erdos, 2004;Konkoy and Davis, 1996;Turner and Tanzawa, 1997;Duggan et al., 1994;Suzuki et al., 1996;Woie et al., 1987). Indeed, VIP-mediated responses were enhanced by antagonists for NEP, phosphoramidon and thiorphan (Suzuki et al., 1996;Shimozato and Kincade, 1997). Both ectoenzymes are localized in many brain regions including the thalamus (Marcel et al., 1990;Back and Gorenstein, 1989;Mendelsohn et al., 1984). In the thalamus, NEP is localized in primary sensory nuclei of the thalamus including the VB and dLGN (Back and Gorenstein, 1989). ACE is localized in the thalamus, however, the detailed distribution of this peptidase in the thalamus is unknown (Mendelsohn et al., 1984). The potential actions of both peptidases on PHI are unknown. We speculate the VIP-mediated responses would be enhanced by antagonists for ACE and/or NEP. To test this possibility, we examined whether VIP-mediated responses increase by a cocktail of antagonists for ACE and NEP (i.e., captopril, ACE antagonist; phosphoramidon and thiorphan, NEP antagonists); however, this did not enhance the VIP-mediated responses (data not shown). Lastly, the difference in molecular weight of PHI (3011.4) and VIP (3325.9) may cause the difference of their access to the receptors.
In contrast, several studies demonstrated VIP produces several actions including alterations in intrinsic properties of neurons and changes in synaptic transmission in the central nervous system (Gozes and Brenneman, 1989;Kohlmeier and Reiner, 1999;Murphy et al., 1993;Wang et al., 1997;Lee and Cox, 2003). The biological actions of PHI are mediated through three receptor subtypes; VPAC1, VPAC2, PHI receptors. PHI binds to VPAC1 and VPAC2 receptor with a lower affinity than VIP and has been known as a weak agonist for VPAC1 and VPAC2 receptors (Harmar et al., 1998;Gourlet et al., 1998;Palle et al., 1989;Inoue et al., 1988;Lundberg et al., 1984;Moriarty et al., 1984;Tapia-Arancibia and Reichlin, 1985;Yuwiler et al., 1993;Lutz et al., 1993). Messenger RNAs (mRNAs) for VPAC1 and VPAC2 receptors are differentially distributed in the central nervous system. VPAC1 receptor mRNA is most abundant in the cerebral cortex and hippocampus (Ishihara et al., 1992), whereas VPAC2 receptor mRNA is found in the thalamus and suprachiasmatic nucleus (Sheward et al., 1995;Usdin et al., 1994;Usdin et al., 1994). In addition to VIP receptors, PHI receptors have been cloned and characterized in fish (Tse et al., 2002), but the existence of PHI receptors in mammals is still unknown. Our data clearly indicate that PHI acts on VPAC2 receptors, and not distinct PHI receptors in thalamocortical relay neurons. In our recordings from both young and adult animals suggest that thalamocortical relay neurons do not have PHI receptors in either age group; however, we cannot exclude the possibility that PHI receptors may be present in neuron types (i.e., TRN neurons, local interneurons).
Functional significance of PHI effects on intrathalamic oscillation
Intrathalamic rhythmic activities are closely related to animals' behavioral states, as well as certain pathophysiological conditions such as generalized absence epilepsy (for review, see (Domich et al., 1986;Williams, 1953;Steriade et al., 1993;Steriade and Llinás, 1988)). The reciprocal synaptic connection between TRN and relay nuclei provides the anatomical organization for intrathalamic oscillations. Another key factor for rhythmic activity involves intrinsic membrane properties of thalamic neurons that will ultimately determine the firing mode of these cells: burst or tonic-firing mode. Certain neuromodulators, such as acetylcholine (ACh), norepinephine (NE), or serotonin (5HT), and dopamine (DA) which arise from brainstem nuclei, alter the firing modes of thalamic neuron and thereby attenuate these rhythms (Lee and McCormick, 1996;Lee and McCormick, 1997;Govindaiah and Cox, 2003). In addition to these brainstem-derived compound, another class of modulators, namely the neuropeptides that are intrinsic to the thalamic circuitry such as cholecystokinin (CCK), somatostatin (SS), and VIP have also been found to alter the firing mode of thalamic neurons and attenuate intrathalamic rhythmic activity (Cox et al., 1997;Sun et al., 2002;Lee and Cox, 2003). Our data suggest that a different endogenous modulator, PHI can strongly suppress intrathalamic oscillation. PHI is contained within TRN neurons whose output is restricted within the thalamus (Burgunder et al., 1999), and thus may serve as an endogenous modulator of thalamic circuit. Thus understanding the variety of modulators that may regulate the firing mode of these cells will ultimately provide a better understanding of the conditions that give rise to the initiation or cessation of the intrathalamic rhythmic activities.
The fundamental mechanisms required to maintain the intrathalamic rhythmic activity are well understood (Steriade and Deschênes, 1984;Steriade and Llinás, 1988;Steriade et al., 1993;von Krosigk et al., 1993;Huguenard and Prince, 1994;Bal et al., 1995a;Bal et al., 1995b;Bal et al., 2000). While there are a number of key characteristics required for the rhythmic activity, a critical aspect is the firing mode of thalamic neurons. Thalamic neurons discharge action potentials in two basic modes: tonic and burst firing (Steriade and Deschênes, 1984;Jahnsen and Llinás, 1984b;Jahnsen and Llinás, 1984a). The occurrence of either of these firing modes is dependent on voltage-dependent intrinsic properties of these neurons, and ultimately depends on the activation of low threshold, voltage dependant Ca2+ current, IT. At relatively depolarized membrane potentials, IT is inactivated and the cell responds in tonic mode in which the output of the cell is linearly related to the strength of depolarization. In contrast, at relatively hyperpolarized membrane potentials, channels underlying IT are deinactivated, and with activation of IT, the neuron fires a short duration (100-200 ms), high frequency (>200Hz) discharge of action potentials. In order to maintain the rhythmic activity, cells in relay nuclei as well as TRN must discharge in burst mode (Steriade and Deschênes, 1984;Bal et al., 1995a;Bal et al., 1995b). Therefore, modulators that alter the firing mode of these neurons, attenuate the rhythmic activity.
Several neuromodulators depolarize thalamic neurons, predisposing the neurons to a tonic mode and thereby terminating the intrathalamic rhythmic activity. Two obvious candidates are the resting K current, Kleak, and Ih. Numerous neuromodulators, including ACh, glutamate (via metabotropic receptors), NE, 5HT, CCK and histamine depolarize either relay neurons or TRN/PGN (perigeniculate nucleus) neurons by decreasing the resting leak potassium current, shifting the discharge mode of these neurons into a tonic mode, and ultimately terminating the rhythmic activity (Lee and McCormick, 1996;McCormick and Williamson, 1991;Cox et al., 1997). In addition, NE, 5HT, histamine, DA and VIP have been found to enhance Ih leading to membrane depolarization of varying amplitudes, and ultimately terminating the intrathalamic oscillation (McCormick and Pape, 1990a;McCormick and Williamson, 1991;Lee and McCormick, 1996;Lee and Cox, 2003;Govindaiah and Cox, 2003). Our results indicate that PHI enhances Ih, and thus may share a common effector mechanism as described for NE and 5HT.
Although various neuromodulators may share common effector mechanisms, an obvious difference amongst them is their origin. Many of these compounds studied thus far, ACh, NE, 5HT, DA, and histamine, originate from brainstem regions, and thus their activity is closely associated with levels of arousal and sleep-wake states (Steriade et al., 1993;McCormick and Bal, 1997). The neuropeptides studied thus far, PHI, VIP, CCK, SS, and neuropeptide Y, are endogenous to the intrathalamic circuit, and therefore the release of these compounds is closely correlated to intrathalamic circuit activity. Considering high-frequency spike discharges may be optimal to release peptide (Bartfai et al., 1988), we speculate that the influence of PHI and VIP might be most prominent during this rhythmic activity or during other conditions in which TRN neurons discharge at high frequencies. To date studies directly testing this have been limited. Ideally, antagonists for VIP receptors should enhance intrathalamic oscillations if endogenous anti-oscillatory effects of these peptides actually occur. The lack of commercially available potent, selective antagonists has prevented direct testing of this hypothesis. However, we utilized transgenic mice that lack VPAC2 receptors to test this hypothesis (Lee and Cox, 2006, Figure 1). If endogenous anti-oscillatory effects of these peptides actually occur, we would predict prolonged intrathalamic oscillations in slices from VPAC2-/- mice, however no significant differences in slow intrathalamic oscillations between VPAC2+/+ and VPAC2-/- mice were observed. We speculate that compensatory mechanisms may occur in VPAC2-/- mice or endogenous anti-oscillatory effects of these peptides may not result from peptide release from TRN neurons. We have emphasized the notion that VIP and PHI are endogenous to the intrathalamic circuit because they are localized within TRN neurons; however, VIP and PHI are also localized in brainstem regions that innervate the thalamus. These brain regions are excluded from in vitro brain slices used in this study. Future studies using intact brains are required to test if synaptically released VIP-related peptides from these regions by a high frequency stimulus train can attenuate the intrathalamic rhythms.
Our working hypothesis is that high frequency burst activity of TRN which is required for intrathalamic rhythmic activity may be optimal for peptide release and therefore produce the release of PHI and/or VIP. Synaptically released PHI and/or VIP activate VPAC2 receptors on relay neurons, enhancing Ih, and thereby depolarize the relay neurons. This depolarization biases the cells to tonic discharge mode and may contribute to the termination of rhythmic activity thereby regulating the duration of rhythmic activity. We speculate that PHI and VIP acts as intrinsic modulators of intrathalamic oscillations that could regulate the duration of the rhythmic activities. We predict that overall decreases in PHI and VIP release could lead to a prolonged durations of intrathalamic rhythmic activities, similar to that during absence seizure activity. The role of neuropeptides in the thalamus may not only influence rhythmic activities, but considering their long-lasting actions, these compounds may also play an important role in regulating the overall information transfer through thalamocortical circuits.
Supplementary Material
Ih.increases during development. A. To test whether potential changes in Ih during development occur, membrane currents are evoked by voltage commands (-50 mV to -120 mV, 10 mV increments) from an initial Vhold of -50 mV. In TTX (PN11, PN17, PN55), increasing amplitude of hyperpolarizing voltage commands evokes a larger long latency inward current. B. Summary of the amplitude of Ih in relay neurons from PN11 (n=6), PN17 (n=5), and PN55(n=5). The data were quantified by calculating the difference in steady state current near the end of the voltage command (Iss in A) and instantaneous current (Iins in A). There is a clear difference in putative Ih in the range of -80 mV to -120 mV C. Summary of the voltage dependence of Ih in relay neurons from PN11 (n=6), PN17 (n=5), and PN55 slices (n=5)..
Acknowledgments
We thank Dr. Anthony Harmar, University of Edinburgh for providing the VPAC2 receptor knock out animals. This research was supported by the National Institutes of Health (EY014024).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anagnostides AA, Manolas K, Christofides ND, Yiangou Y, Welbourn RB, Bloom SR, Chadwick VS. Peptide histidine isoleucine (PHI). A secretagogue in porcine intestine. Dig Dis Sci. 1983;28:893–896. doi: 10.1007/BF01317039. [DOI] [PubMed] [Google Scholar]
- Bailey CJ, Wilkes LC, Conlon JM, Armstrong PH, Buchanan KD. Effects of gastric inhibitory polypeptide, vasoactive intestinal polypeptide and peptide histidine isoleucine on the secretion of hormones by isolated mouse pancreatic islets. J Endocrinol. 1990;125:375–379. doi: 10.1677/joe.0.1250375. [DOI] [PubMed] [Google Scholar]
- Bal T, Debay D, Destexhe A. Cortical feedback controls the frequency and synchrony of oscillations in the visual thalamus. J Neurosci. 2000;20:7478–7488. doi: 10.1523/JNEUROSCI.20-19-07478.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bal T, von Krosigk M, McCormick DA. Role of the ferret perigeniculate nucleus in the generation of synchronized oscillations in vitro. J Physiol (Lond) 1995a;483:665–685. doi: 10.1113/jphysiol.1995.sp020613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bal T, von Krosigk M, McCormick DA. Synaptic and membrane mechanisms underlying synchronized oscillations in the ferret lateral geniculate nucleus in vitro. J Physiol (Lond) 1995b;483:641–663. doi: 10.1113/jphysiol.1995.sp020612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldino FJ, Fitzpatrick-McElligott S, Gozes I, Card JP. Localization of VIP and PHI-27 messenger RNA in rat thalamic and cortical neurons. J Mol Neurosci. 1989;1:199–207. [PubMed] [Google Scholar]
- Bartfai T, Iverfeldt K, Fisone G, Serfözö P. Regulation of the release of coexisting neurotransmitters. Ann Rev Pharmacol Toxicol. 1988;28:285–310. doi: 10.1146/annurev.pa.28.040188.001441. [DOI] [PubMed] [Google Scholar]
- Bataille D, Gespach C, Laburthe M, Amiranoff B, Tatemoto K, Vauclin N, Mutt V, Rosselin G. Porcine peptide having N-terminal histidine and C-terminal isoleucine amide (PHI): vasoactive intestinal peptide (VIP) and secretin-like effects in different tissues from the rat. FEBS Lett. 1980;114:240–242. doi: 10.1016/0014-5793(80)81124-0. [DOI] [PubMed] [Google Scholar]
- Beinfeld MC, Korchak DM, Roth BL, O'Donohue TL. The distribution and chromatographic characterization of PHI (peptide histidine isoleucine amide)-27-like peptides in rat and porcine brain. J Neurosci. 1984;4:2681–2688. doi: 10.1523/JNEUROSCI.04-11-02681.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodner M, Fridkin M, Gozes I. Coding sequences for vasoactive intestinal peptide and PHM-27 peptide are located on two adjacent exons in the human genome. Proc Natl Acad Sci U S A. 1985;82:3548–3551. doi: 10.1073/pnas.82.11.3548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgunder JM, Heyberger B, Lauterburg T. Thalamic reticular nucleus parcellation delineated by VIP and TRH gene expression in the rat. J Chem Neuroanat. 1999;17:147–152. doi: 10.1016/s0891-0618(99)00033-2. [DOI] [PubMed] [Google Scholar]
- Christofides ND, Polak JM, Bloom SR. Studies on the distribution of PHI in mammals. Peptides. 1984;5:261–266. doi: 10.1016/0196-9781(84)90216-x. [DOI] [PubMed] [Google Scholar]
- Cox CL, Huguenard JR, Prince DA. Cholecystokinin depolarizes rat thalamic reticular neurons by suppressing a K+ conductance. J Neurophysiol. 1995;74:990–1000. doi: 10.1152/jn.1995.74.3.990. [DOI] [PubMed] [Google Scholar]
- Cox CL, Huguenard JR, Prince DA. Peptidergic modulation of intrathalamic circuit activity in vitro: Actions of cholecystokinin. J Neurosci. 1997;17:70–82. doi: 10.1523/JNEUROSCI.17-01-00070.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domich L, Oakson G, Steriade M. Thalamic burst patterns in the naturally sleeping cat: a comparison between cortically-projecting and reticularis neurones. J Physiol (Lond) 1986;379:429–449. doi: 10.1113/jphysiol.1986.sp016262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahrenkrug J. Co-existence and co-secretion of the structurally related peptides VIP and PHI. Scand J Clin Lab Invest Suppl. 1987;186:43–50. [PubMed] [Google Scholar]
- Fahrenkrug J, Bek T, Lundberg JM, Hokfelt T. VIP and PHI in cat neurons: co-localization but variable tissue content possible due to differential processing. Regul Pept. 1985;12:21–34. doi: 10.1016/0167-0115(85)90183-1. [DOI] [PubMed] [Google Scholar]
- Ghiglione M, Christofides ND, Yiangou Y, Uttenthal LO, Bloom SR. PHI stimulates intestinal fluid secretion. Neuropeptides. 1982;3:79–82. doi: 10.1016/0143-4179(82)90002-6. [DOI] [PubMed] [Google Scholar]
- Gourlet P, Vandermeers A, Van Rampelbergh J, De Neef P, Cnudde J, Waelbroeck M, Robberecht P. Analogues of VIP, helodermin, and PACAP discriminate between rat and human VIP1 and VIP2 receptors. Ann N Y Acad Sci. 1998;865:247–252. doi: 10.1111/j.1749-6632.1998.tb11184.x. [DOI] [PubMed] [Google Scholar]
- Govindaiah, Cox CL. Excitatory actions of dopamine on rat thalamic neurons. Soc Neurosci Abst. 2003:699–15. [Google Scholar]
- Gozes I, Brenneman DE. VIP: Molecular biology and neurobiological function. Mol Neurobiol. 1989;3:201–236. doi: 10.1007/BF02740606. [DOI] [PubMed] [Google Scholar]
- Haas HL, Gahwiler BH. Vasoactive intestinal polypeptide modulates neuronal excitability in hippocampal slices of the rat. Neurosci. 1992;47:273–277. doi: 10.1016/0306-4522(92)90243-u. [DOI] [PubMed] [Google Scholar]
- Harmar AJ, Arimura A, Gozes I, Journot L, Laburthe M, Pisegna JR, Rawlings SR, Robberecht P, Said SI, Sreedharan SP, Wank SA, Waschek JA. International Union of Pharmacology. XVIII. Nomenclature of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase- activating polypeptide. Pharmacol Rev. 1998;50:265–270. [PMC free article] [PubMed] [Google Scholar]
- Holst JJ, Fahrenkrug J, Knuhtsen S, Jensen SL, Nielsen OV, Lundberg JM, Hokfelt T. VIP and PHI in the pig pancreas: coexistence, corelease, and cooperative effects. Am J Physiol. 1987;252:G182–G189. doi: 10.1152/ajpgi.1987.252.2.G182. [DOI] [PubMed] [Google Scholar]
- Huang M, Rorstad OP. PHI preferentially binds to VIP receptors in normal rat tissues. Peptides. 1990;11:1015–1020. doi: 10.1016/0196-9781(90)90026-2. [DOI] [PubMed] [Google Scholar]
- Huguenard JR, Prince DA. Intrathalamic rhythmicity studies in vitro: Nominal t-current modulation causes robust antioscillatory effects. J Neurosci. 1994;14:5485–5502. doi: 10.1523/JNEUROSCI.14-09-05485.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T, Kato Y, Koshiyama H, Yanaihara N, Imura H. Interaction between vasoactive intestinal polypeptide (VIP) and peptide histidine isoleucine (PHI) in stimulating the secretion of prolactin from rat anterior pituitary cells in vitro. Neurosci Lett. 1988;85:363–369. doi: 10.1016/0304-3940(88)90593-9. [DOI] [PubMed] [Google Scholar]
- Ishihara T, Shigemoto R, Mori K, Takahashi K, Nagata S. Functional expression and tissue distribution of a novel receptor for vasoactive intestinal polypeptide. Neuron. 1992;8:811–819. doi: 10.1016/0896-6273(92)90101-i. [DOI] [PubMed] [Google Scholar]
- Itoh N, Obata K, Yanaihara N, Okamoto H. Human preprovasoactive intestinal polypeptide contains a novel PHI-27-like peptide, PHM-27. Nature. 1983;304:547–549. doi: 10.1038/304547a0. [DOI] [PubMed] [Google Scholar]
- Jahnsen H, Llinás R. Electrophysiological properties of guinea-pig thalamic neurones: an in vitro study. J Physiol (Lond) 1984a;349:205–226. doi: 10.1113/jphysiol.1984.sp015153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahnsen H, Llinás R. Ionic basis for the electroresponsiveness and oscillatory properties of guinea-pig thalamic neurones in vitro. J Physiol (Lond) 1984b;349:227–247. doi: 10.1113/jphysiol.1984.sp015154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura F, Mitsugi N, Arita J, Akema T, Yoshida K. Effects of preoptic injections of gastrin, cholecystokinin, secretin, vasoactive intestinal peptide and PHI on the secretion of luteinizing hormone and prolactin in ovariectomized estrogen-primed rats. Brain Res. 1987;410:315–322. doi: 10.1016/0006-8993(87)90330-1. [DOI] [PubMed] [Google Scholar]
- Kohlmeier KA, Reiner PB. Vasoactive intestinal polypeptide excites medial pontine reticular formation neurons in the brainstem rapid eye movement sleep-induction zone. J Neurosci. 1999;19:4073–4081. doi: 10.1523/JNEUROSCI.19-10-04073.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korchak DM, Gysling K, Beinfeld MC. The subcellular distribution of peptide histidine isoleucine amide-27-like peptides in rat brain and their release from rat cerebral cortical slices in vitro. J Neurochem. 1985;44:255–259. doi: 10.1111/j.1471-4159.1985.tb07138.x. [DOI] [PubMed] [Google Scholar]
- Lee KH, McCormick DA. Abolition of spindle oscillations by serotonin and norepinephrine in the ferret lateral geniculate and perigeniculate nuclei in vitro. Neuron. 1996;17:309–320. doi: 10.1016/s0896-6273(00)80162-2. [DOI] [PubMed] [Google Scholar]
- Lee KH, McCormick DA. Modulation of spindle oscillations by acetylcholine, cholecystokinin and 1S,3R-ACPD in the ferret lateral geniculate and perigeniculate nuclei in vitro. Neurosci. 1997;77:335–350. doi: 10.1016/s0306-4522(96)00481-2. [DOI] [PubMed] [Google Scholar]
- Lee SH, Cox CL. Vasoactive intestinal peptide selectively depolarizes thalamic relay neurons and attenuates intrathalamic rhythmic activity. J Neurophysiol. 2003;90:1224–1234. doi: 10.1152/jn.00280.2003. [DOI] [PubMed] [Google Scholar]
- Lee SH, Cox CL. Excitatory actions of vasoactive intestinal peptide on mouse thalamocortical neurons are mediated by VPAC2 receptors. J Neurophysiol. 2006;96:858–871. doi: 10.1152/jn.01115.2005. [DOI] [PubMed] [Google Scholar]
- Lelievre V, Pineau N, Du J, Wen CH, Nguyen T, Janet T, Muller JM, Waschek JA. Differential effects of peptide histidine isoleucine (PHI) and related peptides on stimulation and suppression of neuroblastoma cell proliferation. A novel VIP-independent action of PHI via MAP kinase. J Biol Chem. 1998;273:19685–19690. doi: 10.1074/jbc.273.31.19685. [DOI] [PubMed] [Google Scholar]
- Lundberg JM, Fahrenkrug J, Larsson O, Anggard A. Corelease of vasoactive intestinal polypeptide and peptide histidine isoleucine in relation to atropine-resistant vasodilation in cat submandibular salivary gland. Neurosci Lett. 1984;52:37–42. doi: 10.1016/0304-3940(84)90347-1. [DOI] [PubMed] [Google Scholar]
- Lutz EM, Sheward WJ, West KM, Morrow JA, Fink G, Harmar AJ. The VIP2 receptor: molecular characterisation of a cDNA encoding a novel receptor for vasoactive intestinal peptide. FEBS Lett. 1993;334:3–8. doi: 10.1016/0014-5793(93)81668-p. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Bal T. Sleep and arousal: Thalamocortical mechanisms. Annual Review of Neuroscience. 1997;20:185–215. doi: 10.1146/annurev.neuro.20.1.185. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Pape HC. Noradrenergic and serotonergic modulation of a hyperpolarization-activated cation current in thalamic relay neurons. J Physiol (Lond) 1990a;431:319–342. doi: 10.1113/jphysiol.1990.sp018332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Pape HC. Properties of a hyperpolarization-activated cation current and its role in rhythmic oscillation in thalamic relay neurons. J Physiol (Lond) 1990b;431:291–318. doi: 10.1113/jphysiol.1990.sp018331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Williamson A. Modulation of neuronal firing mode in cat and guinea pig LGNd by histamine: possible cellular mechanisms of histaminergic control of arousal. J Neurosci. 1991;11:3188–3199. doi: 10.1523/JNEUROSCI.11-10-03188.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentlein R, Gallwitz B, Schmidt WE. Dipeptidyl-peptidase IV hydrolyses gastric inhibitory polypeptide, glucagon-like peptide-1(7-36)amide, peptide histidine methionine and is responsible for their degradation in human serum. Eur J Biochem. 1993;214:829–835. doi: 10.1111/j.1432-1033.1993.tb17986.x. [DOI] [PubMed] [Google Scholar]
- Mentzel S, Dijkman HB, van Son JP, Koene RA, Assmann KJ. Organ distribution of aminopeptidase A and dipeptidyl peptidase IV in normal mice. J Histochem Cytochem. 1996;44:445–461. doi: 10.1177/44.5.8627002. [DOI] [PubMed] [Google Scholar]
- Mikkelsen JD, Fahrenkrug J. Concentrations and distribution of vasoactive intestinal peptide (VIP), peptide histidine isoleucine (PHI) and peptide histidine valine (PHV) in the cerebral cortex and the suprachiasmatic nucleus of the mouse. Brain Res. 1994;656:95–107. doi: 10.1016/0006-8993(94)91370-6. [DOI] [PubMed] [Google Scholar]
- Moriarty KJ, Hegarty JE, Tatemoto K, Mutt V, Christofides ND, Bloom SR, Wood JR. Effect of peptide histidine isoleucine on water and electrolyte transport in the human jejunum. Gut. 1984;25:624–628. doi: 10.1136/gut.25.6.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy PC, Grieve KL, Sillito AM. Effects of vasoactive intestinal polypeptide on the response properties of cells in area 17 of the cat visual cortex. J Neurophysiol. 1993;69:1465–1474. doi: 10.1152/jn.1993.69.5.1465. [DOI] [PubMed] [Google Scholar]
- Murthy KS, Zhang KM, Jin JG, Grider JR, Makhlouf GM. VIP-mediated G protein-coupled Ca2+ influx activates a constitutive NOS in dispersed gastric muscle cells. Am J Physiol. 1993;265:G660–G671. doi: 10.1152/ajpgi.1993.265.4.G660. [DOI] [PubMed] [Google Scholar]
- Nishizawa M, Hayakawa Y, Yanaihara N, Okamoto H. Nucleotide sequence divergence and functional constraint in VIP precursor mRNA evolution between human and rat. FEBS Lett. 1985;183:55–59. doi: 10.1016/0014-5793(85)80953-4. [DOI] [PubMed] [Google Scholar]
- Nurko S, Dunn BM, Rattan S. Peptide histidine isoleucine and vasoactive intestinal polypeptide cause relaxation of opossum internal anal sphincter via two distinct receptors. Gastroenterology. 1989;96:403–413. doi: 10.1016/0016-5085(89)91564-3. [DOI] [PubMed] [Google Scholar]
- Palle C, Ottesen B, Jorgensen J, Fahrenkrug J. Peptide histidine methionine and vasoactive intestinal peptide: occurrence and relaxant effect in the human female reproductive tract. Biol Reprod. 1989;41:1103–1111. doi: 10.1095/biolreprod41.6.1103. [DOI] [PubMed] [Google Scholar]
- Paul S, Chou J, Kubota E. High affinity peptide histidine isoleucine-preferring receptors in rat liver. Life Sci. 1987;41:2373–2380. doi: 10.1016/0024-3205(87)90661-8. [DOI] [PubMed] [Google Scholar]
- Pawelzik H, Dodt HU, Zieglgansberger W. Actions of vasoactive intestinal peptide (VIP) on neocortical neurons of the rat in vitro. Neurosci. 1992;147:167–170. doi: 10.1016/0304-3940(92)90586-v. [DOI] [PubMed] [Google Scholar]
- Rangon CM, Goursaud S, Medja F, Lelievre V, Mounien L, Husson I, Brabet P, Jegou S, Janet T, Gressens P. VPAC2 receptors mediate vasoactive intestinal peptide-induced neuroprotection against neonatal excitotoxic brain lesions in mice. J Pharmacol Exp Ther. 2005;314:745–752. doi: 10.1124/jpet.105.086405. [DOI] [PubMed] [Google Scholar]
- Sheward WJ, Lutz EM, Harmar AJ. The distribution of vasoactive intestinal peptide2 receptor messenger RNA in the rat brain and pituitary gland as assessed by in situ hybridization. Neurosci. 1995;67:409–418. doi: 10.1016/0306-4522(95)00048-n. [DOI] [PubMed] [Google Scholar]
- Steriade M, Deschênes M. The thalamus as a neuronal oscillator. Brain Res Rev. 1984;8:1–63. doi: 10.1016/0165-0173(84)90017-1. [DOI] [PubMed] [Google Scholar]
- Steriade M, Llinás R. The functional states of the thalamus and the associated neuronal interplay. Physiol Rev. 1988;68:649–742. doi: 10.1152/physrev.1988.68.3.649. [DOI] [PubMed] [Google Scholar]
- Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262:679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- Sun QQ, Huguenard JR, Prince DA. Somatostatin inhibits thalamic network oscillations in vitro: Actions on the GABAergic neurons of the reticular nucleus. J Neurosci. 2002;22:5374–5386. doi: 10.1523/JNEUROSCI.22-13-05374.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun QQ, Prince DA, Huguenard JR. Vasoactive intestinal polypeptide and pituitary adenylate cyclase-activating polypeptide activate hyperpolarization-activated cationic current and depolarize thalamocortical neurons in vitro. J Neurosci. 2003;23:2751–2758. doi: 10.1523/JNEUROSCI.23-07-02751.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y, Satoh S, Lederis K, Rorstad O. Cerebral vascular effects of peptide histidine methionine in canine and bovine species. Pharmacology. 1991;42:241–245. doi: 10.1159/000138803. [DOI] [PubMed] [Google Scholar]
- Tapia-Arancibia L, Reichlin S. Vasoactive intestinal peptide and PHI stimulate somatostatin release from rat cerebral cortical and diencephalic cells in dispersed cell culture. Brain Res. 1985;336:67–72. doi: 10.1016/0006-8993(85)90416-0. [DOI] [PubMed] [Google Scholar]
- Tatemoto K, Carlquist M, McDonald TJ, Mutt V. Isolation of a brain peptide identical to the intestinal PHI (peptide HI) FEBS Lett. 1983;153:248–252. doi: 10.1016/0014-5793(83)80617-6. [DOI] [PubMed] [Google Scholar]
- Tatemoto K, Mutt V. Isolation and characterization of the intestinal peptide porcine PHI (PHI-27), a new member of the glucagon--secretin family. Proc Natl Acad Sci U S A. 1981;78:6603–6607. doi: 10.1073/pnas.78.11.6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse DL, Pang RT, Wong AO, Chan SM, Vaudry H, Chow BK. Identification of a potential receptor for both peptide histidine isoleucine and peptide histidine valine. Endocrinology. 2002;143:1327–1336. doi: 10.1210/endo.143.4.8714. [DOI] [PubMed] [Google Scholar]
- Usdin TB, Bonner TI, Mezey E. Two receptors for vasoactive intestinal polypeptide with similar specificity and complementary distributions. Endocrinology. 1994;135:2662–2680. doi: 10.1210/endo.135.6.7988457. [DOI] [PubMed] [Google Scholar]
- Vaudry D, Gonzalez BJ, Basille M, Yon L, Fournier A, Vaudry H. Pituitary adenylate cyclase-activating polypeptide and its receptors: from structure to functions. Pharmacol Rev. 2000;52:269–324. [PubMed] [Google Scholar]
- Vertongen P, Schiffmann SN, Gourlet P, Robberecht P. Autoradiographic visualization of the receptor subclasses for vasoactive intestinal polypeptide (VIP) in rat brain. Peptides. 1997;18:1547–1554. doi: 10.1016/s0196-9781(97)00229-5. [DOI] [PubMed] [Google Scholar]
- von Krosigk M, Bal T, McCormick DA. Cellular mechanisms of a synchronized oscillation in the thalamus. Science. 1993;261:361–364. doi: 10.1126/science.8392750. [DOI] [PubMed] [Google Scholar]
- Wang HL, Li A, Wu T. Vasoactive intestinal polypeptide enhances the GABAergic synaptic transmission in cultured hippocampal neurons. Brain Res. 1997;746:294–300. doi: 10.1016/s0006-8993(96)00772-x. [DOI] [PubMed] [Google Scholar]
- Wang YY, Aghajanian GK. Excitation of locus coeruleus neurons by vasoactive intestinal peptide: evidence for a G-protein-mediated inward current. Brain Res. 1989;500:107–118. doi: 10.1016/0006-8993(89)90304-1. [DOI] [PubMed] [Google Scholar]
- Williams D. A study of thalamic and cortical rhythms in petit mal. Brain. 1953;76:50–69. doi: 10.1093/brain/76.1.50. [DOI] [PubMed] [Google Scholar]
- Yuwiler A, Brammer GL, Bennett BL. Commonalities in vasoactive intestinal peptide and peptide N-terminal histidine C-terminal isoleucine stimulation of N-acetyltransferase activity in the rat pineal. J Pineal Res. 1993;15:81–87. doi: 10.1111/j.1600-079x.1993.tb00513.x. [DOI] [PubMed] [Google Scholar]
- Zhu JJ, Uhlrich DJ, Lytton WW. Properties of a hyperpolarization-activated cation current in interneurons in the rat lateral geniculate nucleus. Neurosci. 1999;92:445–457. doi: 10.1016/s0306-4522(98)00759-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Ih.increases during development. A. To test whether potential changes in Ih during development occur, membrane currents are evoked by voltage commands (-50 mV to -120 mV, 10 mV increments) from an initial Vhold of -50 mV. In TTX (PN11, PN17, PN55), increasing amplitude of hyperpolarizing voltage commands evokes a larger long latency inward current. B. Summary of the amplitude of Ih in relay neurons from PN11 (n=6), PN17 (n=5), and PN55(n=5). The data were quantified by calculating the difference in steady state current near the end of the voltage command (Iss in A) and instantaneous current (Iins in A). There is a clear difference in putative Ih in the range of -80 mV to -120 mV C. Summary of the voltage dependence of Ih in relay neurons from PN11 (n=6), PN17 (n=5), and PN55 slices (n=5)..