SUMMARY
Parasitic infections are widespread throughout the tropics and sub-tropics, and infection with multiple parasite species is the norm rather than the exception. Despite the ubiquity of polyparasitism, its public health significance has been inadequately studied. Here we review available studies investigating the nutritional and pathological consequences of multiple infections with Plasmodium and helminth infection and in doing so, encourage a reassessment of the disease burden caused by polyparasitism. The available evidence is conspicuously sparse but is suggestive that multiple human parasite species may have an additive and/or multiplicative impact on nutrition and organ pathology. Existing studies, however, suffer from a number of methodological limitations, and adequately designed studies are clearly necessary. Current methods of estimating the potential global morbidity due to parasitic diseases underestimate the health impact of polyparasitism, and possible reasons for this are presented. As international strategies to control multiple parasite species are rolled-out, there are a number of options to investigate the complexity of polyparasitism, and it is hoped that that the parasitological resarch community will grasp the opportunity to understand better the health of polyparasitism in humans.
Keywords: Polyparasitism, helminths, malaria, morbidity, undernutrition, anaemia, disease burden
INTRODUCTION
There has been a general renaissance in the epidemiological investigation of polyparasitism, with a particular focus on multiple helminth species (Drake & Bundy, 2001) and more recently, on Plasmodium-helminth co-infection (Brooker et al., 2007,Hartgers & Yazdanbakhsh, 2006,Mwangi et al., 2006). Building on Buck’s seminal studies of polyparasitism (Buck et al., 1978), studies across multiple epidemiological settings have shown recently that polyparasitism is the norm rather than the exception and occurs at different frequencies than would be expected under assumptions of independence (Booth & Bundy, 1995,Booth et al., 1998b,Fleming et al., 2006,Howard et al., 2001,Howard et al., 2002,Keiser et al., 2002,Tchuem Tchuente et al., 2003). Interactions between parasites in humans can be synergistic or antagonistic. For example, studies have demonstrated a positive association between intensity and concurrent infection of helminth species, suggesting that individuals harbouring multiple helminth species also harbour the most the intense infections (Booth et al., 1998a,Brooker et al., 2000,Chamone et al., 1990,Faulkner et al., 2005,Ferreira et al., 1994,Haswell-Elkins et al., 1987,Holland et al., 1989,Kightlinger et al., 1995,Needham et al., 1998). It is conceivable therefore that polyparasitism may have a greater impact on morbidity than single species infections since morbidity is typically related to infection intensity for most parasite species. Multiple species infections may also increase susceptibility to other infections (Druilhe et al., 2005,Mwangi et al., 2006,Nacher, 2004). However, the health impacts of polyparasitism have not been studied sufficiently despite their potential significance for public health.
This paper provides a brief review of recent field studies on the nutritional and pathological consequences of polyparasitism in humans and in doing so, hopes to encourage a reassessment of the disease burden caused by polyparasitism. Our focus is on soil-transmitted helminth infections (STH: Ascaris lumbricoides, Trichuris trichiura and hookworm) schistosomiasis (Schistosoma haematobium, S. mansoni and S. japonicum) and Plasmodium spp. infections since these species are ubiquitous throughout much of the developing world and frequently result in common morbidities. Figure 1 presents a conceptual framework which summarizes these insults and highlights the complex and self-perpetuating relationships and potential mechanisms between polyparasitism and morbidity.
Figure 1. Conceptual framework.
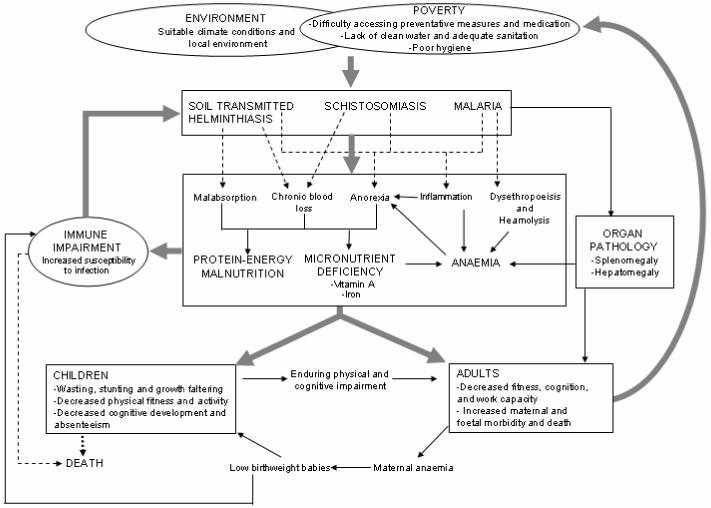
The negative cycle between polyparasitism, malnutrition and cognitive and physical development. Helminth and malaria infections impact upon host nutrition through a number of mechanisms, which may have additive or multiplicative impacts, especially in childhood. Helminth infections cause and/or aggravate malnutrition through worm-induced gastro-intestinal tract physiopathology and reduced food intake (Crompton & Nesheim, 2002), chronic blood-loss (Hotez et al., 2004) and intestinal inflammation (Stephenson et al., 2000b). Malaria may contribute to protein-energy malnutrition (PEM) through a number of mechanisms triggered by augmented levels of inflammatory cytokines, including anorexia and the induction of a catabolic response (Nyakeriga et al., 2004,Tracey & Cerami, 1992), and to reduced haemoglobin levels through increased rates of destruction and removal of RBCs and anaemia of inflammation. Sequestration of red blood cells in those with P.falciparum- and schistosome-associated splenomegaly can also contribute to anaemia (Friedman et al., 2005). Associations may also be due to the effect of undernourishment on the immune response, leading to an increased susceptibility to infection (Koski & Scott, 2001). PEM and anaemia associated with parasitic infection can result in decreased physical fitness, cognitive development and school achievement in children (Boivin & Giordani, 1993,Boivin et al., 1993,Bundy & Guyatt, 1996), and in decreased cognition and work capacity in adults (Guyatt, 2000,Haas & Brownlie, 2001); factors which are in turn associated with poor socio-economic status, in itself a risk factor for infection.
WHO IS AT RISK OF POLYPARASITISM?
An examination of age-infection profiles of different parasite species helps identify those individuals at greatest risk of polyparasitism. For most helminth species, intensity of infection rises dramatically with age, with the age of maximum intensity varying for each helminth species (Figure 2). Age-profiles of Plasmodium spp. suggest that blood stage infection is most prevalent in school-aged children, and it is among this age group that co-infections are most likely therefore to occur. Pregnant women (particularly primigravidae) are also at risk. However, adults remain at considerable risk of harbouring multiple STH species, although at reduced intensity, and are often neglected in the helminthological literature. Understanding the age patterns of infection can provide insight into who is most at risk of the health consequences of polyparasitism, but the underlying age-specific susceptibilities to morbidity are also important.
Figure 2. Age-associated prevalence and intensity profiles of STH, schistosome and malaria infections.
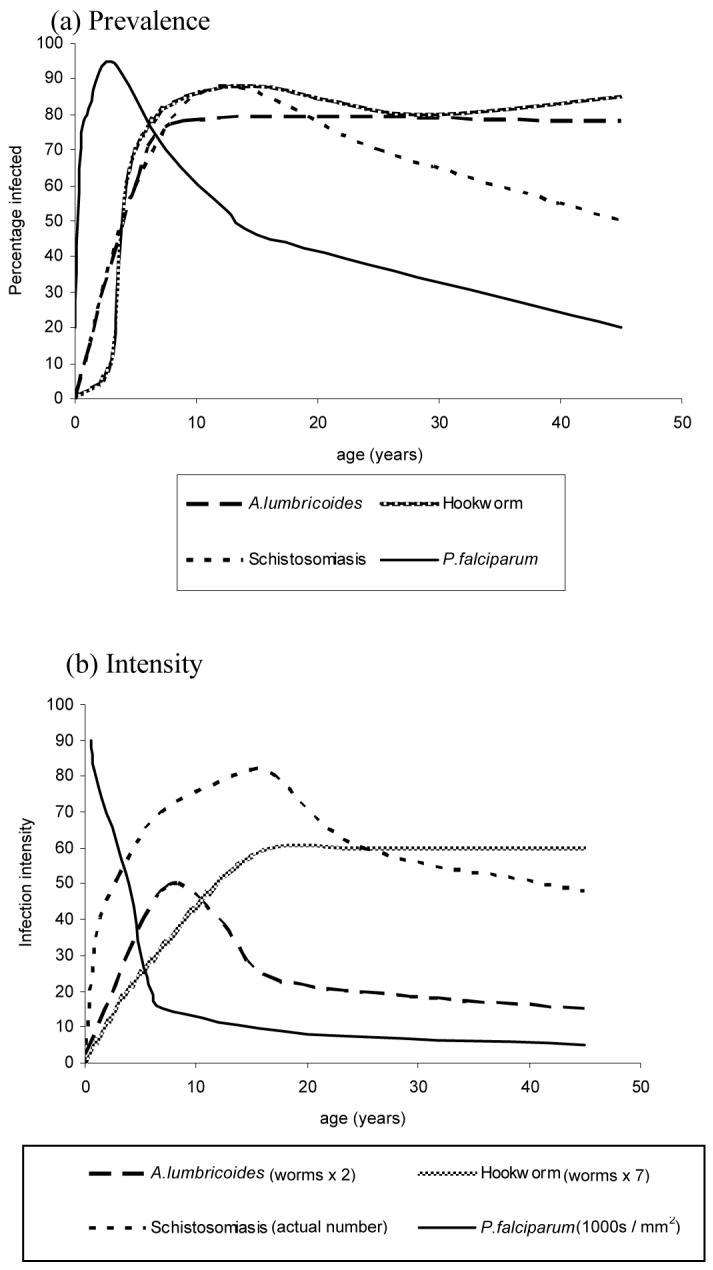
typical age profiles for (a) prevalence and (b) intensity of infection with A.lumbricoides, schistosomiasis, hookworm and P.falciparum malaria; adapted from Bundy (1995) and Anderson and May (2001)
IMPACT ON NUTRIENT DEFICIENCY
Undernutrition, whether characterised in terms of growth impairment or micronutrient malnutrition, is a pervasive problem in the developing world, contributing substantially to both child morbidity and mortality (WHO, 2002a), and to poor cognitive and motor performance (Walker et al., 2006). As important contributors to undernutrition, helminth and malaria infections impact upon host nutrition through a number of mechanisms, including anorexia, chronic blood loss and malabsorption. In light of the nutritional insults arising from single species infection, multiple species infections may have an additive or multiplicative impact on nutrition, especially in childhood.
Growth impairment
Chronic growth stunting is estimated to affect a third of children under five in developing countries (UNICEF, 2004). Infection with STH species contributes significantly to stunting and impaired growth in children living where infection is endemic (Stephenson et al., 2000b). Although the nutritional consequences of infection are generally most pronounced in those who harbour the heaviest infections, relatively light worm burdens may also contribute to growth deficits in nutritional vulnerable populations (Crompton & Nesheim, 2002,Stephenson et al., 2000b). Schistosome infection is also associated with malnutrition and stunting in children, particularly in nutritionally vulnerable populations (King et al., 2005,Mott, 2004). Helminth infections are considered to cause and/or aggravate malnutrition through two processes: worm-induced gastro-intestinal tract physiopathology and reduced food intake (Crompton & Nesheim, 2002). For example, A. lumbricoides infection causes physiological abnormalities in the small intestine (Tripathy et al., 1972), resulting in malabsorption, nutritional deficiency and growth failure (Hlaing, 1993). T. trichiura does not directly affect dietary nutrient absorption, but can result in chronic colitis and impaired growth (Bundy & Cooper, 1989). In contrast, the involvement of malaria in undernutrition remains unclear, although some role appears likely from observations made during malaria control programmes (Bradley-Moore et al., 1985,McGregor et al., 1956,Snow et al., 1997,ter Kuile et al., 2003). Malaria may contribute to malnutrition through a number of mechanisms triggered by augmented levels of inflammatory cytokines, including anorexia and the induction of a catabolic response (Nyakeriga et al., 2004,Tracey & Cerami, 1992). Intestinal inflammation is also suggested to be a mechanism contributing to poor growth in hookworm and schistosome infections (Stephenson et al., 2000b).
Studies investigating the nutritional consequences of multiple helminth species are noticeably lacking. An early study conducted in Tanzania identified significant correlation between malnutrition and multiple infection with A. lumbricoides, hookworm and S. haematobium (Meakins et al., 1981), although no appropriate adjustment for potentially confounding factors was undertaken. A more recent study of Tanzanian school children, which adjusted for age and sex but included no measures of socioeconomic status or nutritional intake, found no evidence that individuals with multiple species infections were at increased risk of morbidity compared to individuals with single species infections (Booth et al., 1998b). This may be attributable to an observed low prevalence of co-infection, with very few individuals harbouring heavier infections typically associated with increased morbidity. In Brazil, a cross-sectional study, which appropriately adjusted for calorific intake, socio-economic status and demographic factors, revealed that whilst no significant association existed for each species individually, children harbouring concomitant infection with A. lumbricoides and T. trichiura were at increased risk of stunting (Saldiva et al., 1999).
A major limitation of cross-sectional studies is that associations can operate in both directions and it is difficult to determine whether polyparasitism precedes or determines nutritional deficits (Koski & Scott, 2001). Importantly, chronic and acute undernutrition is a common cause of secondary immune deficiency (Ing et al., 2000,Koski et al., 1999,Schaible & Kaufman, 2007). For example, altered architecture of the gut mucosa can result in compromised immune defence at the epithelial barrier (Beisel, 1996,Koski et al., 1999), which could theoretically increase susceptibility to intestinal helminth infection. Similarly, in murine models of nematode infection protein malnutrition subdues the Th2 cytokine response important in control of helminth infection (Ing et al., 2000). Although there is some evidence from experimental studies in livestock and rodent models that well-nourished animals resist intestinal parasitism better than those less adequately fed, no evidence of a cause-effect relationship between malnutrition and intestinal helminth infections has emerged from epidemiological controlled intervention studies in humans (see (Koski & Scott, 2001) for a review).
Surprisingly, no identified studies have investigated directly the nutritional impacts of Plasmodium-helminth co-infections. The effect of malaria on nutritional status are generally greater among children under two years (Friedman et al., 2003,McGregor et al., 1956,Nyakeriga et al., 2004). As incidence of malaria is often still rising in children over two years, this is unlikely to simply reflect lower burden of malaria in older children and may reflect age-specific differences in immune responsiveness profiles or the fact that linear growth is maximal in younger children (Nyakeriga et al., 2004). Levels of helminth infections are generally low in young children, resulting in few Plasmodium-helminth co-infections, such that even if nutritional insults arsing from Plasmodium-helminth co-infection occur they are likely to be of less public health importance.
Micronutrient deficiencies
The high iron demands of infant growth and pregnancy means that anaemia is most common and severe among children and pregnant women. These are also the individuals most likely to harbour multiple parasite infections. Malaria, hookworm, schistosomiasis and a lesser extent T. trichiura are associated with iron loss and imbalances predisposing to anaemia among nutritionally vulnerable populations, with risk being closely correlated with infection intensity (Friedman et al., 2005,Hotez et al., 2004,Menendez et al., 2000,Stephenson et al., 2000a,Stephenson et al., 1985,Stoltzfus et al., 1997b). Malaria contributes to reduced haemoglobin levels though a number of mechanisms, principally by increasing rates of destruction and removal of red blood cells (RBCs) and through ‘anaemia of inflammation’, in which pro-inflammatory mediators disrupts RBC production and longevity and affect iron absorption and metabolism (McDevitt et al., 2004,Menendez et al., 2000). By contrast, hookworm primarily causes anaemia through the process of intestinal blood loss (Hotez et al., 2004). Several mechanisms have been proposed for schistosome-induced anaemia, including chronic blood-loss as a result of egg migration, sequestration of red blood cells in those with splenomegaly and anaemia of inflammation (Friedman et al., 2005). Although only a feature of very heavy T. trichiura infection, Trichuris dysentery syndrome (TDS) can also cause considerable blood-loss, thus contributing to iron loss and subsequent anaemia.(Stephenson et al., 2000a) Most studies in school-aged children have typically found that hookworm contributes more to anaemia than schistosomiasis or malaria (Stephenson et al., 1985,Stoltzfus et al., 1997c).
Given the distinct mechanisms by which P. falciparum, soil-transmitted helminths and schistosomes reduce haemoglobin levels, it is probable that these parasites would be additive in their ability to cause anaemia. However, although the impact of single species helminth infection on the risk of anaemia are well documented, few studies have to date investigated the effect of multiple helminth infection on anaemia. A recent study among Filipino school children showed that even low intensity concurrent infections were associated with an increased risk of anaemia relative to children with no infection, although significance was only marginal after adjusting for age, sex, socioeconomic status and anthropometric indices (Ezeamama et al., 2005). In particular, S. japonicum and hookworm (Necator americanus) were observed to play a significant and intensity-dependent role in the association between multiple infection and anaemia (Ezeamama et al., 2005). A similar study among rural Brazilian school children found the risk of anaemia among children infected with S. mansoni and two or three STH infections was significantly higher that those harbouring single STH species, although those with multiple STH infection in absence of S. mansoni were not at increased risk (Brito et al., 2006). This study also collected data on calorific intake, revealing infection status to only be important in those with inadequate iron intake, thus emphasising that the consequences of infection are greatest in nutritionally vulnerable populations. However, there is a limitation in analyses of this type since they were secondary analyses of previously collected data and substantially over-sampled those with schistosome infections. In particular, the Brazilian study did not include individuals singly infected with schistosomiasis; therefore, although those with schistosomiasis co-infection did have increased risk of anaemia it cannot be ruled out that this is simply a result of S. mansoni mono-infection. Another limitation in such cross-sectional studies, as highlighted above, is uncertainty in the directionality of causality.
It is conceivable that the combined presence of P.falciparum and helminth ssp. might interact to further increase the risk of anaemia. In particular, although malarial anaemia is more typically associated with the acute clinical state, there is evidence to suggest that asymptomatic malaria (typically observed in older children, who are also at higher risk of helminth infection) may contribute substantially to anaemia in endemic regions (Korenromp et al., 2004,Kurtzhals et al., 1999). A hospital-based study in Nigeria revealed that pregnant women infected with both malaria and STH infection had lower haemoglobin concentration than women with Plasmodium infection alone, although this result was not statistically significant (Egwunyenga et al., 2001). In Cameroon, school children infected exclusively with P. falciparum had greater levels of anaemia compared with children uninfected, those with co-infections, and those harboring only helminths (Nkuo-Akenji et al., 2006). Re-analysing available data from Kenya, Brooker et al. (2007) found no significant difference amongst mono- and co-infected pregnant women, but found statistical evidence of an additive impact of Plasmodium-hookworm co-infection on anaemia in pre-school and school-aged children. Although these initial results are suggestive of an additive impact of P. falciparum-helminth co-infection on anaemia in certain age groups, there is substantial potential for confounding by socio-economic, genetic and nutritional factors.
A. lumbricoides has been associated with vitamin A and C deficiency (Hlaing, 1993), possibly by inhibiting intestinal absorption (Mahalanabis et al., 1979,Tripathy et al., 1972). Vitamin A is essential for maintaining eye health, growth and immune function (Sommer & West, 1996), and its deficiency is estimated to contribute substantially to the worldwide burden of malaria and diarrhoeal diseases, poor birth outcomes and maternal and infant mortality (WHO, 2002a). In addition, there is clear evidence of an association between serum levels of vitamin A and anaemia (Hodges et al., 1978,Suharno et al., 1993); although the precise mechanism has yet to be confirmed, it has been suggested that vitamin A plays a role in the haemoglobin synthetic pathway (Bloem et al., 1990). It is therefore possible that A. lumbricoides-associated vitamin A deficiency may further increase the risk of anaemia in those co-infected with malaria and other helminth species. Few studies have addressed this hypothesis, but a cross-sectional survey of Bangladeshi school children found no evidence of such an interaction (Persson et al., 2000).
A major limitation of parasite-haematological studies is the use of a single iron status indicator, namely haemoglobin, which is often measured for practical reasons but is confounded by a number of factors including biological variation and other micronutrient deficiencies (Gibson, 2005). However, given the complexity of mechanisms through which mild and asymptomatic malaria infection are thought to contribute to anaemia, the validity of alternative iron status indicators such as serum ferritin, serum transferrin receptor and erythrocyte protoporphyrin in individuals with asymptomatic malaria remains uncertain (Asobayire et al., 2001,Das et al., 1997,Menendez et al., 2001,Odunukwe et al., 2000,Stoltzfus et al., 1997a,Verhoef et al., 2001). Stoltzfus et al (2002) suggest that both hookworm infection and asymptomatic malaria infection may modify the relationship of iron indicators with haemoglobin levels. If we wish to quantify better the impact of polyparasitism on anaemia and iron deficiency, we need to clarify our understanding of the reliability of iron status indicators for children living in areas endemic for multiple parasitic species.
IMPACT ON ORGAN PATHOLOGY
Childhood infection with S. mansoni and S. japonicum is commonly associated with hepatomegaly and splenomegaly, a result of eosinophilic inflammatory and granulomatous reactions against parasite eggs trapped in host tissues (Gryseels et al., 2006,Vennervald & Dunne, 2004). Aberrant immune response to repeated or chronic P. falciparum malarial infection is also an important cause of hepatomegaly and splenomegaly in the tropics (Grobusch & Kremsner, 2005). As well as acting as an antecedent of severe disease in young and middle-aged adults with long-standing intense schistosome infection (Gryseels et al., 2006), splenomegaly is associated with anaemia, possibly because sequestration of red blood cells (RBCs) in the spleen reduces the effective circulating mass of RBCs (Abdel-Salam et al., 1986,Kloos et al., 1997).
It is possible therefore that co-infections with Schistosoma and Plasmodium species may have synergistic effects on organ pathology. Early epidemiological studies suggest that chronic exposure to malaria and infection with schistosomes may interact in childhood hepatosplenomegaly (Fulford et al., 1991,Whittle et al., 1969). In a later study in Kenya, children with the highest levels of anti-P. falciparum immunoglobulin (Ig)G3 and highest S. mansoni egg counts were significantly more likely to present with splenomegaly(Booth et al., 2004b). After regular mollusciciding of the source of infection and praziquantel treatment for schistosomiasis, children living in areas with highest exposure to malaria showed the slowest rates of both splenomegaly and hepatomegaly regression, further suggesting that malaria co-infection exacerbates schistosomiasis-associated hepatosplenomegaly; although it should be noted that it was not possible to control for self-treatment for malaria during the follow-up period (Booth et al., 2004a). However, due to the seasonality of malaria transmission in the region, these studies had to rely on IgG3 responses to P. falciparum schizont antigen as a proxy for exposure to malaria infection as very few cohort members presented with blood-smear detectable parasitaemia. Another Kenyan study noted that hepatosplenic children had significantly higher levels of anti-S. mansoni and anti-P. falciparum antibodies when compared with non-hepatosplenic children, suggesting that past or chronic exposure to malaria may influence the development of hepatosplenomegaly in S. mansoni-infected children (Mwatha et al., 2003). Likewise, a cross-sectional study of children living in this region revealed that whilst children chronically exposed to malaria (again measured using Pfs-IgG3 levels) but without S. mansoni infection can have hepatosplenomegaly, even light S. mansoni infections can exacerbate in an intensity-dependent manner. Although the authors do note that poor model fit suggests some other environmental agent in the study area to be contributing to hepatosplenomegaly in these children, the results do suggest that concurrent chronic exposure to both infections can have an additive or synergistic effect on childhood morbidity (Wilson et al., 2007). However, the possibility that hepatosplenomegaly, or the genetic propensity to develop this condition, may in some way influence immune responses to infection cannot be excluded.
S. haematobium is not usually associated with hepatosplenomegaly. It has been postulated however that it may play a role in the causality of organ pathology (Friis et al., 1996). For example, in a study among schoolchildren in Zimbabwe although neither S. mansoni nor S. haematobium were associated with spleen size, S. mansoni had an effect on liver size only in the presence, but not in the absence, of S. haematobium co-infection.(Friis et al., 1996) In contrast, in a study in western Sudan, children with S. haematobium appeared to be protected from malarial splenomegaly (Friis et al., 2000). The precise mechanisms of the impact of such co-infection on organ pathology remains, however, speculative since no relevant studies have been undertaken to date.
QUANTIFYING THE DISEASE BURDEN OF POLYPARASITISM
Clearly polyparasitism is widespread throughout the tropics and sub-tropics, although accurately estimating the magnitude of the problem remains a major epidemiological challenge. Recent analysis combining climate-based risk maps and demographic surfaces suggest that in sub-Saharan Africa alone, between 25 and 45 million school-aged children live in areas of co-endemicity of P. falciparum and different STH species (Brooker et al., 2007). This type of analysis is obviously crude since it involves estimating only co-distribution and not co-infection. It nonetheless is based on objective and quantitative assessment. The potential burden of polyparasitism with other species and in different regions of the world remains unknown.
A recent approach to assessing the global burden of disease has been adopted by the Global Burden of Disease (GBD) study, which employs the metric of disability-adjusted life years (DALYs) (Mathers et al., 2006,Murray & Lopez, 1994,, 1996). This is a summary measure of both mortality and morbidity, which combines in a single indicator years of life lost due to premature death and years of life lived with a disability, using a disability weighting scheme in which normal functioning has a weight of 0 and 1 corresponds to death. Globally, malaria is estimated to cause 46 million DALYs, STH species 2.9 million, and schistosomiasis 1.7 million (WHO, 2004); although several researchers have questioned the accuracy of disease burden estimates due to chronic parasitic infections (Engels & Savioli, 2006,Hotez et al., 2006,King et al., 2005). The estimation of DALYs adopts a ‘one death, one disease approach’, in which mortality and loss of health is disaggregated into specific diseases as defined by the diagnostic categories of the International Classification of Diseases (ICD). Owning to the estimation methods employed, disability states in DALYs cannot take into account co-morbid conditions, and there is no provision to simultaneously consider all conditions occurring within the same individual or population (Gold et al., 2002). For example, GBD analysis separates specific causes of disease from their associated morbidities, such as anaemia and undernutrition (Mathers et al., 2006). The current disease burden framework is therefore unable to assess the health impact of polyparasitism and this is a particular weakness of current DALY estimates. We suggest therefore that the public health impact of polyparasitism is currently underestimated.
There are a number of reasons why the burden of polyparasitism may not be adequately captured by the current disease burden calculations. First, as our review highlights, there are few direct estimates of the nutritional and pathological sequelae of polyparasitism. It is therefore both surprising and unsatisfactory that the health consequences of polyparasitism have to date received so little research effort. Second, because of the interacting complexity of contributing factors (Figure 1), it is difficult to disentangle adequately the effects of single and multiple species infections. Though plausible mechanisms exist, confounding by socio-economic, demographic and immunological variables in observed associations remains a major challenge in research studies. Third, there is a lack of systematic and comparable information on the impact of polyparasitisim among different age groups and in different epidemiological and nutritional settings. Available data indicate that the impact of polyparasitism may be substantially greater in certain populations than in others. Finally, much of the morbidity of parasitic infections remains insidious and indirect, for example, societal consequences of cognitive impairment arising from helminth infections and malaria (Drake & Bundy, 2001,Kihara et al., 2006). Such non-health consequences of polyparasitism may have greater public health relevance than the direct nutritional and pathological sequelae in individuals.
A FUTURE RESEARCH AGENDA
While it has been convenient for researchers to investigate the epidemiology and health consequences of parasite species in isolation, the majority of infections in the complex, and invariably messy, reality of the developing world typically involves multiple infections. However, despite considerable recent advances in our epidemiological understanding of polyparasitism, the present review highlights that our scientific understanding of the public health significance of polyparasitism is currently inadequate. Available evidence is typically based on retrospective, secondary analyses of previously collected data, which therefore lack sufficient statistical power and are invariably subject to confounding due to socio-economic or dietary intake factors. Purposively designed studies of the health impact of polyparasitism are clearly necessary.
The clearest evidence of the health impacts of polyparasitism can be provided by well-designed intervention studies. Because the most intense helminth infections occur at school age (Bundy & Medley, 1992,Bundy, 1988), and infection can have adverse consequences for health and development (Hotez et al., 2006), school age children are a natural target for such studies. The most robust evidence can be provided by factorial, randomised controlled trials, whereby different interventions are randomised between study populations. However, given that the public health case for deworming is already well established, withholding treatment from control groups raises ethical issues. The recently launched studies of intermittent preventative treatment (IPT) for malaria in school-aged children (in which curative treatment doses of sulphadoxine-pyrimethamine (SP) are delivered to children every term during the school year, regardless of infection status) can potentially be combined with deworming to provide an alternative platform to examine the consequences of co-infection on child health (Brooker et al., 2007). Another opportunity would be therefore to assess whether combined deworming and malaria control has a greater health impact than programmes providing deworming alone.
A limitation of such intervention studies is that given the possibly irreparable damage of undernutrition on children’s cognitive and physical development, there is a risk that they underestimate the impact of polyparasitism. Additional insight can be ascertained from the application of attributable-fraction methods (Booth, 1998). For example, chronic morbidity such as bladder lesions and hepatosplenomegaly may take weeks or months to resolve after treatment, during which time some individuals are inevitably being reinfected. In this scenario, detailed cross-sectional surveys in different epidemiological settings and estimation of attributable fractions may prove informative. Given the problems of proving directionality in such study designs, adequate control for socioeconomic, nutritional and genetic confounding is essential.
But why should we study the health impact of polyparasitism, given that we know already that helminths and Plasmodium spp. contribute to malnutrition and ill health, and that cost-effective interventions already exist? We argue that in a world of limited resources for disease control programmes, determining the health impact of polyparasitism is vital to the rational design of control programmes (for a discussion of the issues see review by Brooker et al., 2007). In particular, it is important to prioritise the most cost-effective interventions in areas where interventions are most needed and will produce the greatest benefits. For example, in order to reduce rates of maternal anaemia (Shulman et al., 1999) and malaria morbidity (Newman et al., 2003,Rogerson et al., 2000) IPT with SP is currently recommended for control of malaria in pregnancy (WHO, 2002b). In hookworm or schistosomiasis endemic areas, it will be important to co-administer anthelmintics (Brooker et al., 2007). We suggest that different interventions are likely to have health impacts and cost-effectiveness balances that may vary under different conditions of polyparasitism and this represents an important public health reason to investigate further the consequences of polyparasitism.
CONCLUSIONS
The health consequences of polyparasitism in humans are poorly understood at present. Unravelling the complexity of the processes involved in the messy reality of human polyparasitism is extremely challengingly and considerable effort is required to improve our basic knowledge. This will necessitate the systematic investigation of the relative impact of single and multiple species infection, and a very considerable research effort evaluating integrated disease control programmes. Such effort will not be straightforward however and will require careful study design, especially in our rapidly changing world in the face of widespread up-scaling of available interventions. As such interventions reduce the intensity of parasite infections it is likely that the health consequences of multiple low-grade grade infections will become more important, especially among vulnerable populations. We hope that the parasitological resarch community will grasp the current opportunty to investigate the complexity of human polyparasitism further.
ACKNOWLEDGEMENTS
RP is supported by a Medical Research Council DTA-funded studentship and SB is supported by a Wellcome Trust Career Development Fellowship (081673).
REFERENCES
- Abdel-Salam E, Abdel Khalik A, Abdel-Meguid A, Barakat W, Mahmoud AA. Association of HLA class I antigens (A1, B5, B8 and CW2) with disease manifestations and infection in human schistosomiasis mansoni in Egypt. Tissue Antigens. 1986;27:142–146. doi: 10.1111/j.1399-0039.1986.tb01513.x. [DOI] [PubMed] [Google Scholar]
- Anderson RM, May RM. Infectious Diseases of Humans: dynamics and control. Oxford University Press; Oxford: 1991. [Google Scholar]
- Asobayire FS, Adou P, Davidsson L, Cook JD, Hurrell RF. Prevalence of iron deficiency with and without concurrent anemia in population groups with high prevalences of malaria and other infections: a study in Cote d’Ivore. American Journal of Clinical Nutrition. 2001;74:776–782. doi: 10.1093/ajcn/74.6.776. [DOI] [PubMed] [Google Scholar]
- Beisel WR. Nutrition in pediatric HIV infection: Setting the research agenda. Nutrition and immune function: Overview. Journal of Nutrition. 1996;126 doi: 10.1093/jn/126.suppl_10.2611S. [DOI] [PubMed] [Google Scholar]
- Bloem MW, Wedel M, Van Agtmaal EJ, Speek AJ, Soawakontha S, Schreurs WHP. Vitamin A intervention: short term effects of a single oral massive dose on iron metabolism. American Journal of Clinical Nutrition. 1990;51:76–79. doi: 10.1093/ajcn/51.1.76. [DOI] [PubMed] [Google Scholar]
- Booth M. The application of attributable risk analysis in helminth epidemiology. Parasitology Today. 1998;14:497–500. doi: 10.1016/s0169-4758(98)01349-0. [DOI] [PubMed] [Google Scholar]
- Booth M, Bundy DA. Estimating the number of multiple-species geohelminth infections in human communities. Parasitology. 1995;111(Pt 5):645–653. doi: 10.1017/s0031182000077131. [DOI] [PubMed] [Google Scholar]
- Booth M, Bundy DA, Albonico M, Chwaya HM, Alawi KS, Savioli L. Associations among multiple geohelminth species infections in schoolchildren from Pemba Island. Parasitology. 1998a;116(Pt 1):85–93. doi: 10.1017/s003118209700190x. [DOI] [PubMed] [Google Scholar]
- Booth M, Mayombana C, Kilima P. The population biology and epidemiology of schistosome and geohelminth infections among schoolchildren in Tanzania. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1998b;92:491–495. doi: 10.1016/s0035-9203(98)90886-0. [DOI] [PubMed] [Google Scholar]
- Booth M, Vennervald BJ, Butterworth AE, Kariuki HC, Amaganga C, Kimani G, Mwatha JK, Otedo A, Ouma JH, Dunne DW. Exposure to malaria affects the regression of hepatosplenomegaly after treatment for Schistosoma mansoni infection in Kenyan children. BMC Medicine. 2004a;2:36. doi: 10.1186/1741-7015-2-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth M, Vennervald BJ, Kenty L, Butterworth AE, Kariuki HC, Kadzo H, Ireri E, Amaganga C, Kimani G, Mwatha JK, Otedo A, Ouma JH, Muchiri E, Dunne DW. Micro-geographical variation in exposure to Schistosoma mansoni and malaria, and exacerbation of splenomegaly in Kenyan school-aged children. BMC Infectious Diseases. 2004b;4:13. doi: 10.1186/1471-2334-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley-Moore AM, Greenwood BM, Bradley AK, Kirkwood BR, Gilles HM. Malaria chemoprophylaxis with chloroquine in young Nigerian children. III. Its effect on nutrition. Annals of Tropical Medicine and Parasitology. 1985;79:575–584. doi: 10.1080/00034983.1985.11811964. [DOI] [PubMed] [Google Scholar]
- Brito LL, Barreto ML, R DECRS, Assis AM, Reis MG, Parraga IM, Blanton RE. Moderate- and Low-Intensity Co-Infections by Intestinal Helminths and Schistosoma Mansoni, Dietary Iron Intake, and Anemia in Brazilian Children. American Journal of Tropical Medicine & Hygiene. 2006;75:939–944. [PubMed] [Google Scholar]
- Brooker S, Akhwale WS, Pullan R, Estambale B, Clarke S, Hotez PJ. Epidemiology of Plasmodium-Helminth coinfeciton in Africa: potential impact on anaemia and prospects for combining control. American Journal of Tropical Medicine & Hygiene. 2007 submitted for publication. [PMC free article] [PubMed] [Google Scholar]
- Brooker S, Miguel EA, Moulin S, Luoba AI, Bundy DA, Kremer M. Epidemiology of single and multiple species of helminth infections among school children in Busia District, Kenya. East African Medicine Journal. 2000;77:157–161. doi: 10.4314/eamj.v77i3.46613. [DOI] [PubMed] [Google Scholar]
- Buck AA, Anderson RI, Macrae AA. Epidemiology of poly-parasitism. I. Occurrence, frequency and distribution of multiple infections in rural communities in Chad, Peru, Afghanistan, and Zaire. Tropenmedizin und Parasitologie. 1978;29:61–70. [PubMed] [Google Scholar]
- Bundy DA. Epidemiology and Transmission of Intestinal Helminths. In: Farthing M, Keusch GT, Wakelin D, editors. Enteric Infection 2, Intestinal Helminths. Chapman & Hall Medical; London: 1995. [Google Scholar]
- Bundy DA, Medley GF. Immuno-epidemiology of human geohelminthiasis: ecological and immunological determinants of worm burden. Parasitology. 1992;104(Suppl):S105–119. doi: 10.1017/s0031182000075284. [DOI] [PubMed] [Google Scholar]
- Bundy DAP. Population ecology of intestinal helminth infections in human communities. Philosphical Transactions of the Royal Society (Series B) 1988;321:405–420. doi: 10.1098/rstb.1988.0100. [DOI] [PubMed] [Google Scholar]
- Bundy DAP, Cooper ES. Trichuris and trichuriasis in humans. Advances in Parasitology. 1989;28:107–173. doi: 10.1016/s0065-308x(08)60332-2. [DOI] [PubMed] [Google Scholar]
- Canning D. Priority setting and the ‘neglected’ tropical diseases. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2006;100:499–504. doi: 10.1016/j.trstmh.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Chamone M, Marques CA, Atuncar GS, Pereira AL, Pereira LH. Are there interactions between schistosomes and intestinal nematodes? Transactions of the Royal Society of Tropical Medicine and Hygiene. 1990;84:557–558. doi: 10.1016/0035-9203(90)90039-h. [DOI] [PubMed] [Google Scholar]
- Crompton DW, Nesheim MC. Nutritional impact of intestinal helminthiasis during the human life cycle. Annual Review of Nutrition. 2002;22:35–59. doi: 10.1146/annurev.nutr.22.120501.134539. [DOI] [PubMed] [Google Scholar]
- Das BS, Thurnham DI, Das DB. Influence of malaria on markers of iron status in children: implications for interpreting iron status in malaria-endemic communities. British Journal of Nutrition. 1997;78:751–760. doi: 10.1079/bjn19970192. [DOI] [PubMed] [Google Scholar]
- Drake LJ, Bundy DA. Multiple helminth infections in children: impact and control. Parasitology. 2001;122(Suppl):S73–81. doi: 10.1017/s0031182000017662. [DOI] [PubMed] [Google Scholar]
- Druilhe P, Tall A, Sokhna C. Worms can worsen malaria: towards a new means to roll back malaria? Trends in Parasitology. 2005;21:359–362. doi: 10.1016/j.pt.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Egwunyenga AO, Ajayi JA, Nmorsi OP, Duhlinska-Popova DD. Plasmodium/intestinal helminth co-infections among pregnant Nigerian women. Memórias do Instituto Oswaldo Cruz. 2001;96:1055–1059. doi: 10.1590/s0074-02762001000800005. [DOI] [PubMed] [Google Scholar]
- Engels D, Savioli L. Reconsidering the underestimated burden caused by neglected tropical diseases. Trends in Parasitology. 2006;22:363–366. doi: 10.1016/j.pt.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Ezeamama AE, Friedman JF, Olveda RM, Acosta LP, Kurtis JD, Mor V, Mcgarvey ST. Functional significance of low-intensity polyparasite helminth infections in anemia. Journal of Infectious Disease. 2005;192:2160–2170. doi: 10.1086/498219. [DOI] [PubMed] [Google Scholar]
- Faulkner H, Turner J, Behnke J, Kamgno J, Rowlinson MC, Bradley JE, Boussinesq M. Associations between filarial and gastrointestinal nematodes. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2005;99:301–312. doi: 10.1016/j.trstmh.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Ferreira CS, Ferreira MU, Nogueira MR. The prevalence of infection by intestinal parasites in an urban slum in Sao Paulo, Brazil. Journal of Tropical Medicine and Hygiene. 1994;97:121–127. [PubMed] [Google Scholar]
- Fleming FM, Brooker S, Geiger SM, Caldas IR, Correa-Oliveira R, Hotez PJ, Bethony JM. Synergistic associations between hookworm and other helminth species in a rural community in Brazil. Tropical Medicine & International Health. 2006;11:56–64. doi: 10.1111/j.1365-3156.2005.01541.x. [DOI] [PubMed] [Google Scholar]
- Friedman JF, Kanzaria HK, Mcgarvey ST. Human schistosomiasis and anemia: the relationship and potential mechanisms. Trends in Parasitology. 2005;21:386–392. doi: 10.1016/j.pt.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Friedman JF, Phillips-Howard PA, Hawley WA, Terlouw DJ, Kolczak MS, Barber M, Okello N, Vulule JM, Duggan C, Nahlen BL, Ter Kuile FO. Impact of permethrin-treated bed nets on growth, nutritional status, and body composition of primary school children in Western Kenya. American Journal of Tropical Medicine & Hygiene. 2003;68:78–85. [PubMed] [Google Scholar]
- Friis H, El Karib SA, Sulaiman SM, Rahama A, Magnussen P, Mascie-Taylor CG. Does Schistosoma haematobium co-infection reduce the risk of malaria-induced splenomegaly? Transactions of the Royal Society of Tropical Medicine and Hygiene. 2000;94:535–536. doi: 10.1016/s0035-9203(00)90079-8. [DOI] [PubMed] [Google Scholar]
- Friis H, Ndhlovu I, Kaondera K, Franke D, Vennervald BJ, Christensen N, Doehring E. Ultrasonographic assessment of Schistosoma mansoni and S. haematobium morbidity in Zimbabwean schoolchildren. American Journal of Tropical Medicine & Hygiene. 1996;55:290–294. doi: 10.4269/ajtmh.1996.55.290. [DOI] [PubMed] [Google Scholar]
- Gibson RS. Principles of Nutritional Assessment. Oxford University Press; New York: 2005. Assessment of iron status. [Google Scholar]
- Gold MR, Stevenson D, Fryback DG. HALYS and QALYS and DALYS, Oh My: similarities and differences in summary measures of population Health. Annual Review of Public Health. 2002;23:115–134. doi: 10.1146/annurev.publhealth.23.100901.140513. [DOI] [PubMed] [Google Scholar]
- Grobusch MP, Kremsner PG. Uncomplicated malaria. Current Topics in Microbiology and Immunology. 2005;295:83–104. [PubMed] [Google Scholar]
- Gryseels B, Polman K, Clerinx J, Kestens L. Human schistosomiasis. Lancet. 2006;368:1106–1118. doi: 10.1016/S0140-6736(06)69440-3. [DOI] [PubMed] [Google Scholar]
- Hartgers FC, Yazdanbakhsh M. Co-infection of helminths and malaria: modulation of the immune responses to malaria. Parasite Immunology. 2006;28:497–506. doi: 10.1111/j.1365-3024.2006.00901.x. [DOI] [PubMed] [Google Scholar]
- Haswell-Elkins MR, Elkins DB, Anderson RM. Evidence for predisposition in humans to infection with Ascaris, hookworm, Enterobius and Trichuris in a South Indian fishing community. Parasitology. 1987;95(Pt 2):323–337. doi: 10.1017/s0031182000057772. [DOI] [PubMed] [Google Scholar]
- Hlaing T. Ascariasis and childhood malnutrition. Parasitology. 1993;107(Suppl):S125–136. doi: 10.1017/s0031182000075557. [DOI] [PubMed] [Google Scholar]
- Hodges RE, Sauberlich HE, Canham JEEA. Hematopoietic studies in Vitamin A deficiency. American Journal of Clinical Nutrition. 1978;31:876–885. doi: 10.1093/ajcn/31.5.876. [DOI] [PubMed] [Google Scholar]
- Holland CV, Asaolu SO, Crompton DW, Stoddart RC, Macdonald R, Torimiro SE. The epidemiology of Ascaris lumbricoides and other soil-transmitted helminths in primary school children from Ile-Ife, Nigeria. Parasitology. 1989;99(Pt 2):275–285. doi: 10.1017/s003118200005873x. [DOI] [PubMed] [Google Scholar]
- Hotez PJ, Brooker S, Bethony JM, Bottazzi ME, Loukas A, Xiao S. Hookworm infection. New England Journal of Medicine. 2004;351:799–807. doi: 10.1056/NEJMra032492. [DOI] [PubMed] [Google Scholar]
- Hotez PJ, Bundy DAP, Beegle K, Brooker S, Drake L, De Silva N, Montresor A, Engels D, Jukes M, Chitsulo L, Chow J, Laxminarayan R, Michaud C, Bethony J, Oliveira R, Xiao SH, Fenwick A, Savioli L. Helminth Infections: soil-transmitted helminth infections and schistosomiasis New York. In: Jamison DT, Breman JG, Measham AR, Alleyne G, Claeson M, Evans DB, Jha P, Mills A, Musgrove P, editors. Disease Control Priorities in Developing Countries. Oxford University Press; New York: 2006. pp. 467–497. [Google Scholar]
- Howard SC, Donnell CA, Chan MS. Methods for estimation of associations between multiple species parasite infections. Parasitology. 2001;122:233–251. doi: 10.1017/s0031182001007272. [DOI] [PubMed] [Google Scholar]
- Howard SC, Donnelly CA, Kabatereine NB, Ratard RC, Brooker S. Spatial and intensity-dependent variations in associations between multiple species helminth infections. Acta Tropica. 2002;83:141–149. doi: 10.1016/s0001-706x(02)00093-1. [DOI] [PubMed] [Google Scholar]
- Ing R, Su Z, Scott ME, Koski KG. Suppressed T helper 2 immunity and prolonged survival of a nematode parasite in protein-malnourished mice. Proceedings of the National Academy of Sciences USA. 2000;97:7078–7083. doi: 10.1073/pnas.97.13.7078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keiser J, N’goran EK, Singer BH, Lengeler C, Tanner M, Utzinger J. Association between Schistosoma mansoni and hookworm infections among schoolchildren in Cote d’Ivoire. Acta Tropica. 2002;84:31–41. doi: 10.1016/s0001-706x(02)00135-3. [DOI] [PubMed] [Google Scholar]
- Kightlinger LK, Seed JR, Kightlinger MB. The epidemiology of Ascaris lumbricoides, Trichuris trichiura, and hookworm in children in the Ranomafana rainforest, Madagascar. Journal of Parasitology. 1995;81:159–169. [PubMed] [Google Scholar]
- Kihara M, Carter JA, Newton CR. The effect of Plasmodium falciparum on cognition: a systematic review. Tropical Medicine & International Health. 2006;11:386–397. doi: 10.1111/j.1365-3156.2006.01579.x. [DOI] [PubMed] [Google Scholar]
- King CH, Dickman K, Tisch DJ. Reassessment of the cost of chronic helmintic infection: a meta-analysis of disability-related outcomes in endemic schistosomiasis. Lancet. 2005;365:1561–1569. doi: 10.1016/S0140-6736(05)66457-4. [DOI] [PubMed] [Google Scholar]
- Kloos H, Fulford AJ, Butterworth AE, Sturrock RF, Ouma JH, Kariuki HC, Thiongo FW, Dalton PR, Klumpp RK. Spatial patterns of human water contact and Schistosoma mansoni transmission and infection in four rural areas in Machakos District, Kenya. Social Science and Medicine. 1997;44:949–968. doi: 10.1016/s0277-9536(96)00218-3. [DOI] [PubMed] [Google Scholar]
- Korenromp EL, Armstrong-Schellenberg JR, Williams BG, Nahlen BL, Snow RW. Impact of malaria control on childhood anaemia in Africa - a quantitative review. Tropical Medicine & International Health. 2004;9:1050–1065. doi: 10.1111/j.1365-3156.2004.01317.x. [DOI] [PubMed] [Google Scholar]
- Koski KG, Scott ME. Gastrointestinal nematodes, nutrition and immunity: breaking the negative spiral. Annual Review of Nutrition. 2001;21:297–321. doi: 10.1146/annurev.nutr.21.1.297. [DOI] [PubMed] [Google Scholar]
- Koski KG, Su Z, Scott ME. Energy deficits suppress both systemic and gut immunity during infection. Biochemical and Biophysics Research Communications. 1999;264:796–801. doi: 10.1006/bbrc.1999.1596. [DOI] [PubMed] [Google Scholar]
- Kurtzhals JA, Addae MM, Akanmori BD, Dunyo S, Koram KA, Appawu MA, Nkrumah FK, Hviid L. Anaemia caused by asymptomatic Plasmodium falciparum infection in semi-immune African schoolchildren. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1999;93:623–627. doi: 10.1016/s0035-9203(99)90073-1. [DOI] [PubMed] [Google Scholar]
- Mahalanabis D, Simpson TW, Chakraborty ML, Ganguli C, Bhattacharjee AK, Mukherjee KL. Malabsorption of water miscible vitamin A in children with giardiasis and ascariasis. American Journal of Clinical Nutrition. 1979;32:313–318. doi: 10.1093/ajcn/32.2.313. [DOI] [PubMed] [Google Scholar]
- Mathers CD, Lopez AD, Murray CJL. Global Burden of Disease and Risk Factors. Oxford University Press; New York: 2006. [Google Scholar]
- Mcgregor IA, Gilles HM, Walters JH, Davies AH, Pearson FA. Effects of heavy and repleted malarial infections on Gambian infants and children. Effects of erythrocyte parasitization. British Medical Journal. 1956;2:686–692. doi: 10.1136/bmj.2.4994.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meakins RH, Harland PS, Carswell F. A preliminary survey of malnutrition and helminthiasis among schoolchildren in one mountain and one lowland ujamaa village in Northern Tanzania. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1981;75:731–735. doi: 10.1016/0035-9203(81)90164-4. [DOI] [PubMed] [Google Scholar]
- Menendez C, Fleming AF, Alonso PL. Malaria-related Anaemia. Parasitology Today. 2000;16:469–476. doi: 10.1016/s0169-4758(00)01774-9. [DOI] [PubMed] [Google Scholar]
- Menendez C, Quinto LL, Kahigwa E, Alvarez L, Fernandez R, Gimenez N, Schellenberg D, Aponte JJ, Tanner M, Alonso PL. Effect of malaria on soluble transferrin receptor levels in Tanzanian infants. American Journal of Tropical Medicine & Hygiene. 2001;65:138–142. doi: 10.4269/ajtmh.2001.65.138. [DOI] [PubMed] [Google Scholar]
- Mott KE. Schistosomaisis. In: Murray CJL, Lopez AD, Mathers CD, editors. The global epidemiology of infectious diseases. WHO; Geneva: 2004. [Google Scholar]
- Murray CJ, Lopez AD. Quantifying disability: data, methods and results. Bulletin of the World Health Organisation. 1994;72:481–494. [PMC free article] [PubMed] [Google Scholar]
- Murray CJ, Lopez AD. Evidence-based health policy - lessons from the Global Burden of Disease Study. Science. 1996;274:740–743. doi: 10.1126/science.274.5288.740. [DOI] [PubMed] [Google Scholar]
- Mwangi TW, Bethony JM, Brooker S. Malaria and helminth interactions in humans: an epidemiological viewpoint. Annals of Tropical Medicine & Parasitology. 2006;100:551–570. doi: 10.1179/136485906X118468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwatha JK, Jones FM, Mohamed G, Naus CW, Riley EM, Butterworth AE, Kimani G, Kariuki CH, Ouma JH, Koech D, Dunne DW. Associations between anti-Schistosoma mansoni and anti-Plasmodium falciparum antibody responses and hepatosplenomegaly, in Kenyan schoolchildren. Journal of Infectious Disease. 2003;187:1337–1341. doi: 10.1086/368362. [DOI] [PubMed] [Google Scholar]
- Nacher M. Interactions between worm infections and malaria. Clinical Reviews in Allergy and Immunology. 2004;26:85–92. doi: 10.1007/s12016-004-0003-3. [DOI] [PubMed] [Google Scholar]
- Needham C, Kim HT, Hoa NV, Cong LD, Michael E, Drake L, Hall A, Bundy DA. Epidemiology of soil-transmitted nematode infections in Ha Nam Province, Vietnam. Tropical Medicine & International Health. 1998;3:904–912. doi: 10.1046/j.1365-3156.1998.00324.x. [DOI] [PubMed] [Google Scholar]
- Newman RD, Parise ME, Slutsker L, Nahlen B, Steketee RW. Safety, efficacy and determinants of effectiveness of antimalarial drugs during pregnancy: implications for prevention programmes in Plasmodium falciparum-endemic sub-Saharan Africa. Tropical Medicine & International Health. 2003;8:488–506. doi: 10.1046/j.1365-3156.2003.01066.x. [DOI] [PubMed] [Google Scholar]
- Nkuo-Akenji TK, Chi PC, Cho JF, Ndamukong KKJ, Sumbele I. Malaria and helminth co-infection in children living in a malaria endemic setting of Mount Cameroon and predictors of anaemia. Journal of Parasitology. 2006;92:1191–1195. doi: 10.1645/GE-895R.1. [DOI] [PubMed] [Google Scholar]
- Nyakeriga AM, Troye-Blomberg M, Chemtai AK, Marsh K, Williams TN. Malaria and nutritional status in children living on the coast of Kenya. American Journal of Clinical Nutrition. 2004;80:1604–1610. doi: 10.1093/ajcn/80.6.1604. [DOI] [PubMed] [Google Scholar]
- Odunukwe NN, Salako LA, Okany C, Ibrahim MM. Serum ferritin and other haematological measurements in apparently healthy adults with malaria parasitaemia in Lagos, Nigeria. Tropical Medicine & International Health. 2000;5:582–586. doi: 10.1046/j.1365-3156.2000.00601.x. [DOI] [PubMed] [Google Scholar]
- Persson V, Ahmed F, Gebre-Medhin M, Greiner T. Relationships between vitamin A, iron status and helminthiasis in Bangladeshi school children. Public Health Nutrition. 2000;3:83–89. doi: 10.1017/s1368980000000100. [DOI] [PubMed] [Google Scholar]
- Robertson LJ, Crompton DWT, Sanjur D, Nesheim MC. Haemoglobin concentrations and concomitant infections of hookworm and Trichuris trichiura in Panamanian primary schoolchildren. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1992;86:654–656. doi: 10.1016/0035-9203(92)90176-d. [DOI] [PubMed] [Google Scholar]
- Rogerson SJ, Chaluluka E, Kanjala M, Mkundika P, Mhango C, Molyneux ME. Intermittent sulfadoxine-pyrimethamine in pregnancy: effectiveness against malaria morbidity in Blantyre, Malawi, in 1997-99. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2000;94:549–553. doi: 10.1016/s0035-9203(00)90083-x. [DOI] [PubMed] [Google Scholar]
- Saldiva SR, Silveira AS, Philippi ST, Torres DM, Mangini AC, Dias RM, Da Silva RM, Buratini MN, Massad E. Ascaris-Trichuris association and malnutrition in Brazilian children. Paediatric and Perinatal Epidemiology. 1999;13:89–98. doi: 10.1046/j.1365-3016.1999.00145.x. [DOI] [PubMed] [Google Scholar]
- Schaible UE, Kaufman SHE. Malnutrition and Infection: Complex mechanisms and global impacts. Public Library of Science Medicine. 2007;4:806–812. doi: 10.1371/journal.pmed.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman CE, Dorman EK, Cutts F, Kawuondo K, Bulmer JN, Peshu N, Marsh K. Intermittent sulphadoxine-pyrimethamine to prevent severe anaemia secondary to malaria in pregnancy: a randomised placebo-controlled trial. Lancet. 1999;353:632–636. doi: 10.1016/s0140-6736(98)07318-8. [DOI] [PubMed] [Google Scholar]
- Snow RW, Molyneux CS, Njeru EK, et al. Omumbo J, Nevill CG, Muniu E, Marsh K. The effects of malaria control on nutritional status in infancy. Acta Tropica. 1997;65:1–10. doi: 10.1016/s0001-706x(96)00601-8. [DOI] [PubMed] [Google Scholar]
- Sommer A, West KPJ. Vitamin A deficiency: health, survival and vision. Oxford University Press; New York: 1996. [Google Scholar]
- Stephenson LS, Holland CV, Cooper ES. The public health significance of Trichuris trichiura. Parasitology. 2000a;121(Suppl):S73–95. doi: 10.1017/s0031182000006867. [DOI] [PubMed] [Google Scholar]
- Stephenson LS, Latham MC, Kurz KM, Kinoti SN, Oduori ML, Crompton DW. Relationships of Schistosoma hematobium, hookworm and malarial infections and metrifonate treatment to hemoglobin level in Kenyan school children. American Journal of Tropical Medicine & Hygiene. 1985;34:519–528. doi: 10.4269/ajtmh.1985.34.519. [DOI] [PubMed] [Google Scholar]
- Stephenson LS, Latham MC, Ottesen EA. Malnutrition and parasitic helminth infections. Parasitology. 2000b;121(Suppl):S23–38. doi: 10.1017/s0031182000006491. [DOI] [PubMed] [Google Scholar]
- Stoltzfus RJ, Chwaya HM, Albonico M, Schulze KJ, Savioli L, Tielsch JM. Serum ferritin, erythrocyte protoporphyrin and hemoglobin are valid indicators of iron status of school children in a malaria-holoendemic population. Journal of Nutrition. 1997a;127:293–298. doi: 10.1093/jn/127.2.293. [DOI] [PubMed] [Google Scholar]
- Stoltzfus RJ, Chwaya HM, Montresor A, Albonico M, Savioli L, Tielsch JM. Malaria, hookworms and recent fever are related to anemia and iron status indicators in 0- to 5-y old Zanzibari children and these relationships change with age. Journal of Nutrition. 2000;130:1724–1733. doi: 10.1093/jn/130.7.1724. [DOI] [PubMed] [Google Scholar]
- Stoltzfus RJ, Chwaya HM, Tielsch JM, Schulze KJ, Albonico M, Savioli L. Epidemiology of iron deficiency anemia in Zanzibari schoolchildren: the importance of hookworms. American Journal of Clinical Nutrition. 1997b;65:153–159. doi: 10.1093/ajcn/65.1.153. [DOI] [PubMed] [Google Scholar]
- Suharno D, West CE, Muhilal, Karyadi D, Hautvast GAJ. Supplementation with vitamin A and iron for nutritional anaemia in pregnant women in West Java, Indonesia. Lancet. 1993;342:1325–1328. doi: 10.1016/0140-6736(93)92246-p. [DOI] [PubMed] [Google Scholar]
- Tchuem Tchuente LA, Behnke JM, Gilbert FS, Southgate VR, Vercruysse J. Polyparasitism with Schistosoma haematobium and soil-transmitted helminth infections among school children in Loum, Cameroon. Tropical Medicine & International Health. 2003;8:975–986. doi: 10.1046/j.1360-2276.2003.01120.x. [DOI] [PubMed] [Google Scholar]
- Ter Kuile F, Terlouw DJ, Kariuki S, et al. Impact of permethrin-treated bed nets on malaria, anemia, and growth in infants in an area of intense perennial malaria transmission in western Kenya. American Journal of Tropical Medicine & Hygiene. 2003;68:68–77. [PubMed] [Google Scholar]
- Tracey KJ, Cerami A. Tumor necrosis factor in the malnutrition (cachexia) of infection and cancer. American Journal of Tropical Medicine & Hygiene. 1992;47:2–7. doi: 10.4269/ajtmh.1992.47.2. [DOI] [PubMed] [Google Scholar]
- Tripathy K, Duque E, Bolanos O, Lotero H, Mayoral LG. Malabsorption syndrome in ascariasis. American Journal of Clinical Nutrition. 1972;25:1276–1281. doi: 10.1093/ajcn/25.11.1276. [DOI] [PubMed] [Google Scholar]
- Unicef . The State of the World’s Children 2005: childhood under threat. UNICEF; New York: 2004. [Google Scholar]
- Vennervald BJ, Dunne DW. Morbidity in schistosomiasis: an update. Current Opinions in Infectious Disease. 2004;17:439–447. doi: 10.1097/00001432-200410000-00009. [DOI] [PubMed] [Google Scholar]
- Verhoef H, West CE, Ndeto P, Burema Y, Kok FJ. Serum transferrin receptor concentration indicates increased erythropoiesis in Kenyan children with aymptomatic malaria. American Journal of Clinical Nutrition. 2001;74:767–775. doi: 10.1093/ajcn/74.6.767. [DOI] [PubMed] [Google Scholar]
- Walker SP, Wachs TD, Gardner JM, Lozoff B, Wasserman GA, Pollitt E, Carter JA. Child development: risk factors for adverse outcomes in developing countries. Lancet. 2006;369:145–157. doi: 10.1016/S0140-6736(07)60076-2. [DOI] [PubMed] [Google Scholar]
- WHO . The world health report 2002 - Reducing Risks, Promoting Healthy Life. World Health Organisation; Geneva: 2002a. Quantifying selected major risks to health. [Google Scholar]
- WHO . Report of the WHO informal consultation on the use of Praziquantel during pregnancy/lactation and albendazole/mebendazole in children under 24 months. World Health Organisation; Geneva: 2002b. [Google Scholar]
- WHO . The global epidemiology of infectious diseases. World Health Organisation; Geneva: 2004. [Google Scholar]


