Abstract
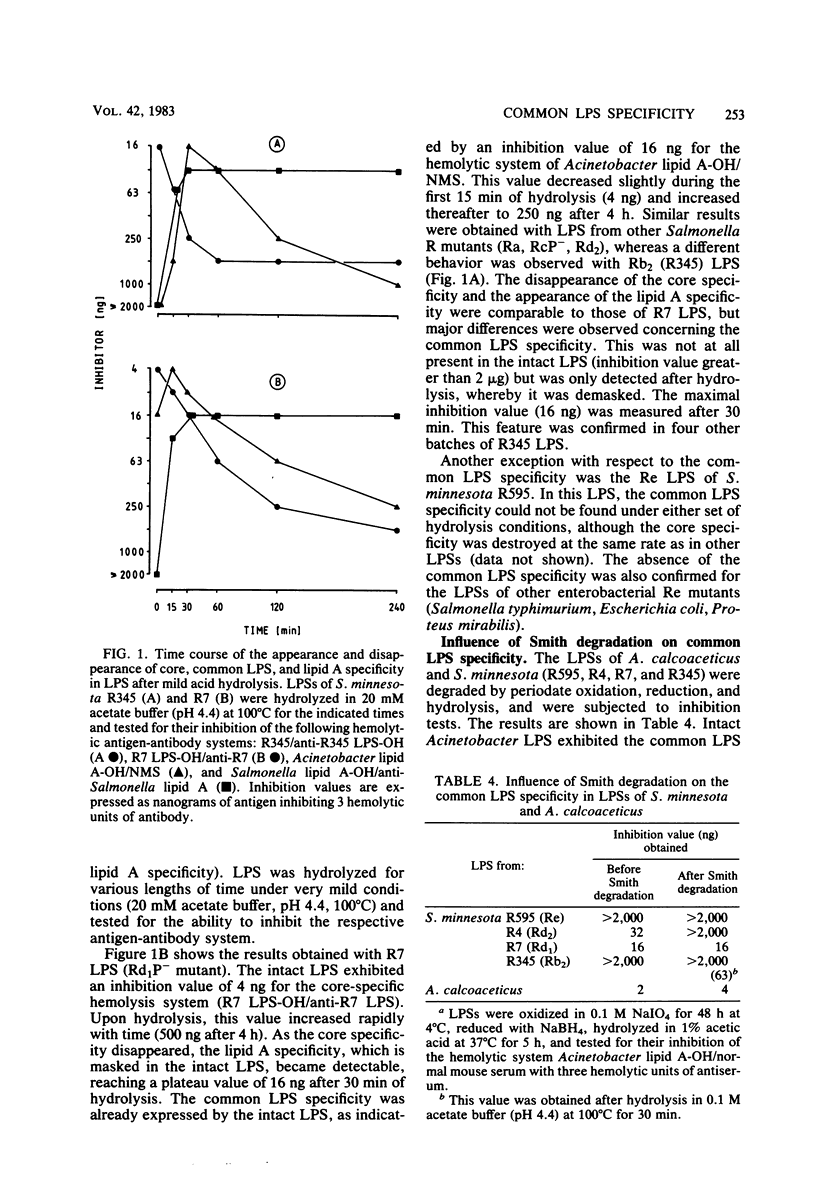
A new antigenic specificity, referred to here as common lipopolysaccharide (LPS) specificity, is described in the LPSs of gram-negative bacteria belonging to various families. The specificity is present in S- and R-form LPS but absent in Re mutants of different enterobacterial genera. By the use of purified LPS and monospecific antibodies obtained by immunoabsorption, the specificity is differentiated from the known core specificities of the genus Salmonella and the lipid A specificity by aid of the passive hemolysis and passive hemolysis inhibition test. In Salmonella minnesota R-form LPS, the specificity may be cryptic (R345, Rb2 mutant) or partly exposed in the intact molecule (R7, Rd1 mutant). The specificity is either demasked or completely exposed after mild acid hydrolysis for a short time, whereas it is destroyed after prolonged hydrolysis. Periodate oxidation, reduction, and hydrolysis under conditions that do not affect the ketosidic linkages of 2-keto-3-deoxyoctulosonic acid destroy the specificity in R4 (Rd2 mutant) LPS, but do not do so in R7 LPS. It is suggested that 2-keto-3-deoxyoctulosonic acid and a following neutral sugar are the compositional requirements for expressing the specificity.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brade H., Galanos C. A new lipopolysaccharide antigen identified in Acinetobacter calcoaceticus: occurrence of widespread natural antibody. J Med Microbiol. 1983 May;16(2):203–210. doi: 10.1099/00222615-16-2-203. [DOI] [PubMed] [Google Scholar]
- Brade H., Galanos C. Isolation, purification, and chemical analysis of the lipopolysaccharide and lipid A of Acinetobacter calcoaceticus NCTC 10305. Eur J Biochem. 1982 Feb;122(2):233–237. doi: 10.1111/j.1432-1033.1982.tb05871.x. [DOI] [PubMed] [Google Scholar]
- Brade H., Galanos C., Lüderitz O. Differential determination of the 3-Deoxy-D-mannooctulosonic acid residues in lipopolysaccharides of Salmonella minnesota rough mutants. Eur J Biochem. 1983 Mar 1;131(1):195–200. doi: 10.1111/j.1432-1033.1983.tb07249.x. [DOI] [PubMed] [Google Scholar]
- Brade H., Galanos C., Lüderitz O. Isolation of a 3-deoxy-D-mannooctulosonic acid disaccharide from Salmonella minnesota rough-form lipopolysaccharides. Eur J Biochem. 1983 Mar 1;131(1):201–203. doi: 10.1111/j.1432-1033.1983.tb07250.x. [DOI] [PubMed] [Google Scholar]
- Bruins S. C., Stumacher R., Johns M. A., McCabe W. R. Immunization with R mutants of Salmonella minnesota. II. Comparison of the protective effect of immunization with lipid A and the Re mutant. Infect Immun. 1977 Jul;17(1):16–20. doi: 10.1128/iai.17.1.16-20.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dröge W., Lehmann V., Lüderitz O., Westphal O. Structural investigations on the 2-keto-3-deoxyoctonate region of lipopolysaccharides. Eur J Biochem. 1970 May 1;14(1):175–184. doi: 10.1111/j.1432-1033.1970.tb00276.x. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O. Electrodialysis of lipopolysaccharides and their conversion to uniform salt forms. Eur J Biochem. 1975 Jun;54(2):603–610. doi: 10.1111/j.1432-1033.1975.tb04172.x. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. Preparation and properties of antisera against the lipid-A component of bacterial lipopolysaccharides. Eur J Biochem. 1971 Dec 22;24(1):116–122. doi: 10.1111/j.1432-1033.1971.tb19661.x. [DOI] [PubMed] [Google Scholar]
- Greisman S. E., DuBuy J. B., Woodward C. L. Experimental gram-negative bacterial sepsis: reevaluation of the ability of rough mutant antisera to protect mice. Proc Soc Exp Biol Med. 1978 Jul;158(3):482–490. doi: 10.3181/00379727-158-40231. [DOI] [PubMed] [Google Scholar]
- Hase S., Rietschel E. T. Isolation and analysis of the lipid A backbone. Lipid A structure of lipopolysaccharides from various bacterial groups. Eur J Biochem. 1976 Mar 16;63(1):101–107. doi: 10.1111/j.1432-1033.1976.tb10212.x. [DOI] [PubMed] [Google Scholar]
- Lüderitz O., Galanos C., Risse H. J., Ruschmann E., Schlecht S., Schmidt G., Schulte-Holthausen H., Wheat R., Westphal O., Schlosshardt J. Structural relationship of Salmonella O and R antigens. Ann N Y Acad Sci. 1966 Jun 30;133(2):349–374. doi: 10.1111/j.1749-6632.1966.tb52376.x. [DOI] [PubMed] [Google Scholar]
- Michael J. G., Mallah I. Immune response to parental and rough mutant strains of Salmonella minnesota. Infect Immun. 1981 Sep;33(3):784–787. doi: 10.1128/iai.33.3.784-787.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullan N. A., Newsome P. M., Cunnington P. G., Palmer G. H., Wilson M. E. Protection against gram-negative infections with antiserum to lipid A from Salmonella minnesota R595. Infect Immun. 1974 Dec;10(6):1195–1201. doi: 10.1128/iai.10.6.1195-1201.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NETER E. Bacterial hemagglutination and hemolysis. Bacteriol Rev. 1956 Sep;20(3):166–188. doi: 10.1128/br.20.3.166-188.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng A. K., Chen C. L., Chang C. M., Nowotny A. Relationship of structure to function in bacterial endotoxins: serologically cross-reactive components and their effect on protection of mice against some gram-negative infections. J Gen Microbiol. 1976 May;94(1):107–116. doi: 10.1099/00221287-94-1-107. [DOI] [PubMed] [Google Scholar]
- Prehm P., Stirm S., Jann B., Jann K. Cell-wall lipopolysaccharide from Escherichia coli B. Eur J Biochem. 1975 Aug 1;56(1):41–55. doi: 10.1111/j.1432-1033.1975.tb02205.x. [DOI] [PubMed] [Google Scholar]
- Young L. S., Stevens P., Ingram J. Functional role of antibody against "core" glycolipid of Enterobacteriaceae. J Clin Invest. 1975 Oct;56(4):850–861. doi: 10.1172/JCI108164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler E. J., Douglas H., Sherman J. E., Davis C. E., Braude A. I. Treatment of E. coli and klebsiella bacteremia in agranulocytic animals with antiserum to a UDP-gal epimerase-deficient mutant. J Immunol. 1973 Aug;111(2):433–438. [PubMed] [Google Scholar]


