Abstract
Endogenous mediators within the inflammatory milieu play a critical role in directing the scope, duration, and resolution of inflammation. High-molecular-weight extracellular matrix hyaluronan (HA) helps to maintain homeostasis. During inflammation, hyaluronan is broken down into fragments that induce chemokines and cytokines, thereby augmenting the inflammatory response. Tissue-derived adenosine, released during inflammation, inhibits inflammation via the anti-inflammatory A2 adenosine receptor (A2aR). We demonstrate that adenosine modulates HA-induced gene expression via the A2aR. A2aR stimulation inhibits HA fragment–induced pro-fibrotic genes TNF-α, keratinocyte chemoattractant (KC), macrophage inflammatory protein (MIP)-2, and MIP-1α while simultaneously synergizing with hyaluronan fragments to up-regulate the TH1 cytokine IL-12. Interestingly, A2aR stimulation mediates these affects via the novel cAMP-activated guanine nucleotide exchange factor EPAC. In addition, A2aR-null mice are more susceptible to bleomycin-induced lung injury, consistent with a role for endogenous adenosine in inhibiting the inflammation that may lead to fibrosis. Indeed, the bleomycin treated A2aR-null mice demonstrate increased lung inflammation, HA accumulation, and histologic damage. Overall, our data elucidate the opposing roles of tissue-derived HA fragments and adenosine in regulating noninfectious lung inflammation and support the pursuit of A2aR agonists as a means of pharmacologically inhibiting inflammation that may lead to fibrosis.
Keywords: adenosine, hyaluronan, chemokines, lung fibrosis
CLINICAL RELEVANCE
Endogenous mediators are critical in the scope, duration, and resolution of inflammation. We show that adenosine modulates hyaluronan-induced genes via the A2a receptor, and thus elucidate the roles of hyaluronan fragments and adenosine in lung inflammation.
It is becoming increasingly clear that the tissue microenvironment plays a critical role in regulating inflammation and tissue destruction. Chronic inflammation and tissue fibrosis lead not only to increased turnover of the extracellular matrix but also to an increase in inflammatory cells, mediators, and extracellular concentration of the purine nucleotide adenosine. Recently, fragments of the extracellular matrix component hyaluronan (HA) have been shown to play a central role in the development of lung inflammation and fibrosis in mice (1, 2). HA, normally a high-molecular-weight, extracellular matrix component, helps to maintain lung conformational integrity and water homeostasis (3, 4). During inflammation, the high-molecular-weight HA is degraded into lower-molecular-weight (LMW) fragments that stimulate macrophages and airway epithelial cells to produce mediators of tissue injury and repair such as TNF-α, IL-12, keratinocyte chemoattractant (KC), macrophage inflammatory protein (MIP)-1α, MIP-2, monokine induced by interferon-γ (MIG), IL-8, interferon-inducible protein (IP)-10, matrix metalloelastase (MME), plasminogen activator inhibitor (PAI)-1, tissue inhibitor of metalloprotease (TIMP)-1, and inducible nitric oxide synthase (iNOS) (5–8). Thus the extracellular matrix is not only the target of inflammation, but plays a central role in inflammatory cell activation.
Adenosine is an endogenous intracellular purine nucleotide that is normally at low concentrations extracellularly (9–11). During inflammation and tissue destruction, adenosine is released into the extracellular space, with local accumulations as high as 10 to 100 μM, and acts as a negative regulator of both inflammation and immune-mediated tissue destruction (9, 11). Tissue derived adenosine exerts its anti-inflammatory effects by engaging the high-affinity adenosine A2a receptor (A2aR) that is expressed on macrophages, dendritic cells, T cells, B cells, and epithelial cells. Selective engagement of the A2 adenosine receptor (A2aR) on human and murine macrophages by the A2aR agonist CGS-21680 (2-[p-(2-carbonyl-ethyl)-phenyl-ethylamino]-5′-N-ethylcarboxamido-adenosine) inhibits LPS-induced TNF-α expression (12). In addition, the critical role of the A2aR in regulating inflammation was demonstrated by the observation that A2aR-null mice exposed to normally harmless inflammatory stimuli develop overwhelming inflammation, tissue destruction, and death (13).
The A2aR has an extremely high affinity for adenosine and, when engaged, stimulatory G-proteins become activated, leading to increased adenylate cyclase activity and cyclic AMP (cAMP) production (14). Although the exact signal transduction pathways used by adenosine have not yet been fully elucidated, elevated levels of cAMP have been associated with activation of protein kinase A (PKA) and inhibition of NF-κB (15). Recently, a novel downstream target of cAMP, EPAC (exchange protein activated by cAMP), a cAMP-activated guanine nucleotide exchange factor for Rap GTPases, has been described (16). Currently two EPAC family members have been described (EPAC1 and EPAC2), but a role for EPAC in A2a receptor signaling has yet to be defined (16).
During lung inflammation, both LMW HA and adenosine accumulate in the extracellular milieu. Inasmuch as LMW HA plays a role in promoting lung inflammation and fibrosis and adenosine is a known negative regulator of inflammation, we proposed that adenosine acts as a key modulator of LMW HA–induced inflammation. In this article we demonstrate that A2aR engagement, but not A1- or A3-receptor engagement, profoundly modulates LMW HA–induced inflammatory gene expression in vitro and in vivo in the bleomycin model of lung injury. Furthermore, we demonstrate that adenosine modulates these effects not through the activation of PKA but rather by activating EPAC, which ultimately results in NF-κB inhibition.
MATERIALS AND METHODS
Cells
MH-S (mouse alveolar macrophage-like cell line) were purchased from American Type Cell Collection (Manassas, VA). Thioglycollate elicited peritoneal elicited cells (PEC) were obtained per our protocol (5).
Mice
The A2a−/− mice and littermate controls on a C57BL/6 background were a kind gift from Dr. Joel Linden (University of Virginia) (17).
Bleomycin Administration
Per our protocol that is approved by the Johns Hopkins Animal Welfare Review Committee, isoflurane-anesthetized mice aspirated 100 μl saline or saline + 0.375 U bleomycin sulfate (5). The animals were killed with sodium pentobarbital overdose (100 mg/kg, intraperitoneally; Ampro Pharmaceutical, Arcadia, CA). Bronchoalveolar lavage (BAL) was preformed as per our protocol (5). Histology: 0.5 ml 4% paraformaldehyde was instilled intratracheally into the right lung, the airway ligated, and the lung submerged in the same fixative. The tissue was embedded in paraffin, and histologic sections viewed after hematoxylin and eosin (H&E) and Masson trichrome staining.
Chemicals and Reagents
Purified human umbilical cord LMW HA (MW, 200 kD; ICN Biomedicals, Inc., Costa Mesa, CA) is free of protein and other glycosaminoglycans. Polymixin B was purchased from Calbiochem Novabiochem (San Diego, CA). Adenosine receptor agonists CGS21680 (CGS), 5′-(N-ethylcarboxamido)-adenosine (NECA), 2-chloro-N6-cyclopentyladenosine (CCPA), and N6-(3-iodobenzyl)adenosine-5′-N-methyluronamide (IB-MECA) were purchased from Sigma (St. Louis, MO). CGS was prepared in PBS to a stock concentration of 2 mM. NECA, CCPA, and IB-MECA were resuspended in DMSO to stock concentrations of 40 mM, 40 mM, and 15 mM, respectively. ZM-241385 (10 μM) and EPAC activator (8-(4-Chlorophenylthio)-2′-O-methyladenosine-3′,5′-cyclic monophosphate sodium salt) were from Tocris Bioscience (Ellisville, MO). EPAC siRNA was made per Dharmacon algorithm. Stock solutions were tested for LPS with Limulus assay.
Adenosine Agonist/Antagonist Assays
Cells, plated at a density of 1.5 × 106 cells/ml in 12-well plates, were pretreated with the appropriate adenosine agonist in serum-free RPMI 10 hours before stimulation with HA. Cells received the A2aR ZM (10 μM) 30 minutes before adenosine agonists. After pretreatment, cells were stimulated for 18 hours with 250 μg/ml HA. Supernatants were collected and assayed for TNF-α, MIP-2, KC, IP-10, MIP-1α, and IL-12 via enzyme-linked immunosorbent assay (ELISA) (R&D Systems, Minneapolis, MN).
Western Blots and ELISA
Western blots and ELISA were performed as previously described (5). Antibodies and kits were purchased from R&D Systems. Colorimetric changes were measured by ELISA plate reader with Microplate Manager III (Bio-Rad, Hercules, CA) software.
Northern Analysis and RT-PCR of mRNA Production
Total cellular RNA was isolated via TriReagent and Northern analysis were performed as previously described (5). RT-PCR was performed using TaqMan and commercially available primers for murine IL-12 p40, p35, 18S, and TNF-α (Applied Biosystems, Foster City, CA). Bands were quantified with a phosphoimager (Molecular Dynamics, Sunnyvale, CA).
Transient Transfections
Cells were transfected with 0.25 μg of the pNiFty NF-κB luciferase reporter plasmid (Invivogen, San Diego, CA) using FuGENE 6 transfection reagent (Roche, Branchburg, NJ) according to the manufacturer's protocol. Luciferase was assayed with Promega Luciferase Assay System using a FB12 luminometer (Zylux, Huntsville, AL) as previously described (18). Measured firefly luciferase was normalized to the constitutively produced renilla luciferase and reported as fold induction over unstimulated transfected cells.
Statistics
Differences between groups were analyzed using ANOVA with Fisher's PLSD test for pairwise comparisons (Graphpad). A P value < 0.05 was considered significant.
RESULTS
A2aR Stimulation Modulates LMW HA–Induced Inflammatory Gene Expression
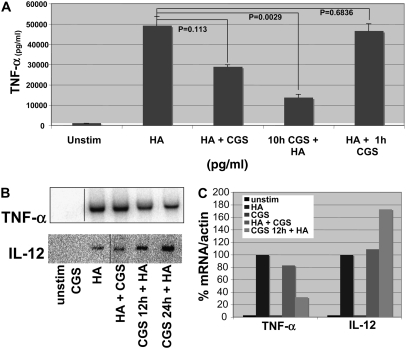
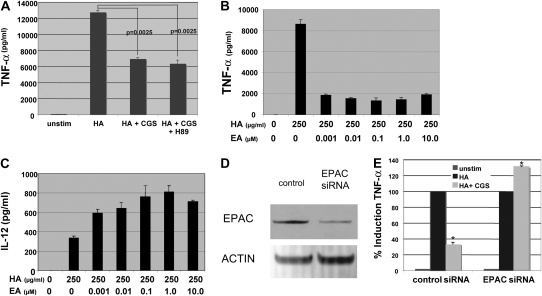
Fragments of the extracellular matrix component HA, produced at sites of tissue inflammation, play a central role in the activation of the innate immune system in the development of lung inflammation and fibrosis in an animal model (1, 8). Similarly, adenosine has been shown to be a crucial negative regulator of inflammation and tissue protector from immune destruction (9, 11). We propose that the extracellular matrix is not only the target of inflammation but plays a central role in inflammatory cell activation, and furthermore that adenosine is a key modulator of HA-induced inflammatory gene expression, especially TNF-α and IL-12, as they are potently induced by LMW HA fragments (7, 8, 19). Thus to assess the effect of A2aR stimulation on LMW HA–induced inflammatory chemokine gene expression, we stimulated the alveolar macrophage cell line MH-S with LMW HA and the A2aR-specific agonist CGS for 18 hours and analyzed cell supernatants for specific chemokine and cytokine expression by ELISA (Figure 1A). We demonstrated that A2aR stimulation with CGS markedly inhibits LMW HA-induced TNF-α by 41% (P = 0.0113) when given simultaneously with LMW HA. Interestingly, when cells were pre-stimulated with CGS for 10 hours before LMW HA for 18 hours, A2aR engagement resulted in a 71% (P = 0.0029) decrease in LMW HA–induced TNF-α expression. However, when the A2aR is engaged after LMW HA stimulation, there was no significant inhibition of LMW HA–induced TNF-α expression (5%, P = 0.6836). The dose of CGS chosen for these and subsequent experiments was based on dose–response curves (data not shown) and is consistent with previously published reports examining the effect of CGS on macrophages (16, 20). The specificity of CGS for the A2aR at this dose is further confirmed by the lack of affect of CGS on macrophages derived from A2aR-null mice (see below).
Figure 1.
A2 adenosine receptor (A2aR) stimulation modulates low-molecular-weight (LMW) hyaluronan (HA)–induced inflammatory gene expression. (A) MH-S macrophages were stimulated with LMW HA (250μg/ml) + CGS (3μM) simultaneously or pre-stimulated with CGS (3μM) for 10 hours before LMW HA, and enzyme-linked immunosorbent assay (ELISA) for TNF-α on cell cultured media was performed after 18 hours of stimulation. These data represent the average of at least three individual experiments. (B) Northern blot analysis of mRNA harvested from thioglycollate elicited peritoneal macrophages pretreated with CGS for 10 hours before stimulation of LMW HA for 6 hours. (C) Graphic representation of mRNA normalized to actin. These data are representative of at least three individual experiments.
To confirm that the A2aR modulated LMW HA–induced genes in primary cells, thioglycollate elicited peritoneal macrophages (PEC) were pretreated with the A2aR-specific agonist CGS for 10 hours before stimulation with LMW HA for 6 hours. Total RNA was isolated and Northern blot analysis performed. Unstimulated PEC and cells treated with the A2aR-specific agonist CGS alone had no TNF-α or IL-12 mRNA expression. However, LMW HA induced significant up-regulation of TNF-α mRNA, which was blocked by CGS (Figures 1B and 1C). The inhibition of LMW HA–induced TNF-α mRNA expression by CGS was also greater with pre-stimulation of the cells with CSG for 12 hours before stimulation with LMW HA. Pretreatment of LMW HA–stimulated PEC with CGS also resulted in a marked increase in HA-induced IL-12 mRNA production (Figures 1B and 1C). Thus, A2aR stimulation modulates LMW HA–induced mRNA expression of chemokines in primary macrophages. The fact that IL-12 expression increases with A2aR stimulation rules out the possibility that CGS is inducing cell death or nonspecifically inhibiting cell function.
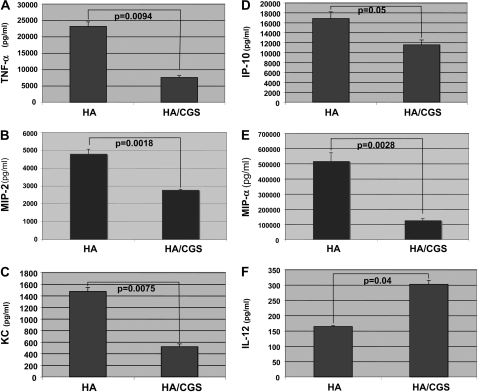
Interestingly, TNF-α has been associated with lung fibrosis, and IL-12 (by promoting IFN-γ production) has been associated with nonfibrotic resolution of lung inflammation (21). Indeed, A2aR activation seems to be antifibrotic by both inhibiting HA-induced TNF-α and augmenting HA-induced IL-12 expression. Thus, we also examined the affect of A2aR stimulation on the production of the profibrotic chemokines MIP-2, KC, IP-10, and MIP-1α. As determined at the protein level by ELISA, CGS inhibited TNF-α, MIP-2, KC, IP-10, and MIP-1α (66%, 42%, 80%, 32%, and 77%, respectively) while simultaneously augmenting IL-12 by 60% (Figure 2). Thus, A2aR stimulation differentially modulates LMW HA–induced inflammation.
Figure 2.
Adenosine modulates LMW HA–induced inflammatory gene expression via the A2aR. MH-S macrophages were stimulated with CGS (3μM) for 10 hours before LMW HA (250μg/ml) for 18 hours. Cell cultured supernatants were collected and ELISAs performed for (A) TNF-α, (B) MIP-2, (C) KC, (D) IP-10, (E) MIP-1α, and (F) IL-12. These data represent the average of at least three individual experiments.
Adenosine Modulates LMW HA–Induced Gene Expression Specifically via the A2aR
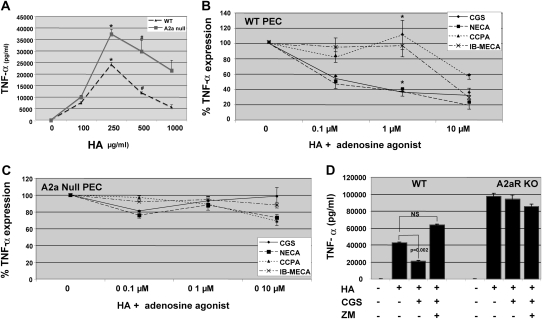
To further determine the role of A2aR activation in modulating LMW HA–induced gene expression, we activated PEC from wild-type (WT) and A2aR-null mice. As seen in Figure 3A, PECs from A2aR-null mice produce more TNF-α in response to LMW HA stimulation than HA-stimulated PECs derived from WT mice. These findings suggest that adenosine in the culture medium, acting via the A2aR, negatively regulates PEC activation in vitro.
Figure 3.
Adenosine modulates LMW HA–induced gene expression specifically via the A2aR. Thioglycollate elicited peritoneal macrophages were isolated from wild-type (WT) and littermate control A2aR-null mice. (A) Dose response of PEC stimulated with LMW HA fragments for 18 hours, after which cultured supernatants were collected and TNF-α ELISA performed. *P = 0.027; #P = 0.04. (B and C) WT or A2aR-null PEC were pre-stimulated with CGS (A2a-specific agonist), NECA (nonselective adenosine receptor agonist), CCPA (A1-specific agonist), and IB-MECA (A3-specific agonist) for 12 hours before LMW HA (250 μg/ml) for 18 hours. Cultured cell supernatants were collected and ELISA for TNF-α performed. Only statistical difference between samples was for WT PEC CGS and NECA v. CCPA and IB-MECA at 1μm dose agonist, * P < 0.05. (D) WT or A2aR-null PEC were pre-stimulated with CGS (3μM) ± the A2aR-specific antagonist ZM for 12 hours before the addition of LMW HA. TNF-α ELISA was performed on cultured cell supernatants after 18 hours. These data represent the average of at least three individual experiments.
To demonstrate that the inhibition of LMW HA–induced TNF-α by endogenous adenosine is specific to the A2a receptor and not due to the stimulation of A1- or A3-adenosine receptors, we performed dose–response curves with adenosine receptor–specific agonists. Using PEC from WT mice, we compared the effect of CGS (A2aR-specific agonist) on LMW HA–fragment induced TNF-α expression in PEC with that of the nonselective adenosine receptor agonist NECA, the A1-specific agonist CCPA and the A3-specific agonist IB-MECA. As demonstrated in Figure 3B, only CGS (A2a-specific agonist) and NECA (nonspecific agonist) inhibited LMW HA–induced TNF-α expression in a dose-dependent fashion. Although LMW HA–induced TNF-α was inhibited at high doses of CCPA (A1-specific agonist) and IB-MECA (A3-specific agonist), this appeared to be nonspecific due to the high doses of the agonists. To further show that the A2aR is primarily responsible for the modification of LMW HA–induced chemokine expression, we performed experiments with PEC from A2aR-null mice. In the A2aR-null PECs, there was little inhibition of HA-induced TNF-α by any of the agonists (Figure 3C).
As both CGS (A2aR-specific agonist) and NECA (nonspecific adenosine agonist) inhibit LMW HA–induced TNF-α expression in WT PEC, we postulated that the A2aR antagonist ZM241385 would block the effects of these agonists. Both CGS and NECA inhibit LMW HA–induced TNF-α expression in WT PEC (CGS 51%, P = 0.002), and the inhibition is reversed by the A2aR antagonist ZM241385 (Figure 3D). There is no effect of CGS or ZM on HA-induced gene expression in the A2aR-null mice. Thus, adenosine inhibits LMW HA–induced gene expression specifically via A2aR stimulation.
Transcriptional and Post-Transcriptional Regulation of HA-Induced Gene Expression by A2aR Stimulation
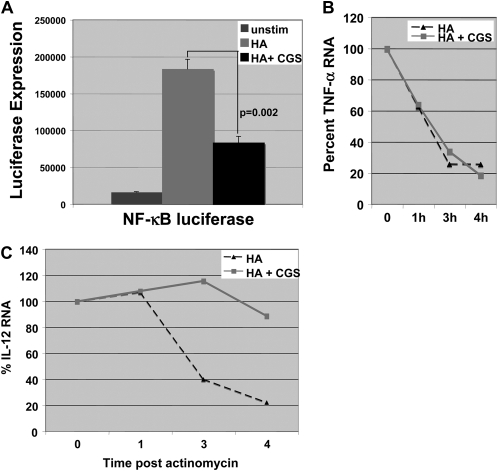
Previously we and others have demonstrated that LMW HA–induced TNF-α gene expression is mediated by activating NF-κB (22). Likewise, it has been shown that A2aR stimulation can inhibit NF-κB activation in monocytes and endothelial cells (15). Thus, we hypothesized that A2aR stimulation modulated HA-induced gene expression in part by inhibiting NF-κB. We transfected MH-S cells with an NF-κB–driven luciferase reporter construct overnight, then pre-stimulated the transfected cells with CGS for 12 hours before stimulation with LMW HA for 18 hours. Cytoplasmic extracts were isolated and luciferase activity was measured. Figure 4A demonstrates that LMW HA–induced NF-κB–driven luciferase expression is significantly inhibited by that A2aR stimulation with CGS by 55% (P = 0.002). Thus, the effect of A2aR stimulation on the inhibition of LMW HA–induced inflammatory gene expression by A2aR stimulation appears to be in part due to the inhibition of the NF-κB pathway.
Figure 4.
Adenosine A2aR engagement inhibits LMW HA–induced NF-κB expression. (A) MH-S macrophages were transfected with an NF-κB–driven luciferase reporter construct overnight, and transfected cells were pre-stimulated with CGS (3μM) for 12 hours before stimulation with LMW HA (250 μg/ml) for 18 hours. Cytoplasmic extracts were isolated and luciferase activity was measured. (B and C) MH-S macrophages were pre-stimulated with CGS (3μM) for 12 hours before stimulation with LMW HA (250 μg/ml) for 2 hours before the addition of actinomycin. RT-PCR for TNF-α or IL-12 p40 was performed on RNA isolated at time 0, 1 hour, 3 hours, and 4 hours after actinomycin. These data are representative of at least three individual experiments.
We also demonstrate that A2aR stimulation synergizes with HA to induce IL-12, a gene often reported to be transcriptionally regulated by NF-κB (23). However, IL-12 has also been demonstrated to be regulated post-transcriptionally (24, 25). Specifically, p38 mitogen-activated protein kinase (MAPK) activation inhibits IL-12 expression by promoting IL-12 mRNA degradation (25). In addition, the A2aR agonist CGS has been reported to inhibit p38 activation (24). Thus, we wanted to determine the potential role of A2aR stimulation on the stability of LMW HA–induced mRNA. MH-S cells were pre-stimulated with CGS (3μM) for 10 hours before the addition of LMW HA for 2 hours. After 2 hours of stimulation with HA, actinomycin was added to the cells to inhibit further mRNA transcription. RNA was then harvested at 1, 3, and 4 hours after actinomycin, subjected to RT-PCR for IL-12 p40 or TNF-α, and normalized to 18S. CGS had no effect on the decay of TNF-α mRNA (Figure 4B), but markedly enhanced the stability of IL-12 mRNA (Figure 4C). Thus CGS has both transcriptional and post-transcriptional effects on LMW HA–induced TNF-α and IL-12, respectively, leading to the ultimate differential regulation of these genes. Specifically, CGS inhibits HA-induced TNF-α by inhibiting NF-κB–regulated TNF-α transcription, but has no effect on TNF-α mRNA stability. Furthermore, although CGS may inhibit LMW HA–induced NF-κB–mediated IL-12 transcription, this effect is offset by the marked stabilization of IL-12 mRNA leading to the overall effect of increased IL-12 gene product.
A2aR-Induced Modulation of LMW HA–Induced TNF-α Is Mediated by EPAC
Although the exact signal transduction pathways used by adenosine have yet to be fully elucidated, elevated levels of cAMP have been associated with activation of protein kinase A (PKA) and inhibition of NF-κB (15). To investigate the role of PKA in mediating CGS-induced inhibition of LMW HA–induced TNF-α, we stimulated WT PEC with LMW HA + CGS + the PKA inhibitor H89 for 18 hours and measured TNF-α protein expression by ELISA. As seen in Figure 5A, the PKA inhibitor H89 did not reverse the inhibition of LMW HA–induced TNF-α by CGS. It is known that dibutyryl-cAMP inhibits LPS-induced TNF-α in a PKA-dependent pathway (26, 27). As a positive control to ensure that this dose of H89 significantly inhibited the PKA regulation of this pathway, we confirmed that H89 blocked dibutyryl-cAMP inhibition of LPS-induced TNF-α by 47% (data not shown). Thus, the ability of A2aR activation to inhibit LMW HA-induced gene expression is not mediated through PKA.
Figure 5.
Adenosine A2aR engagement modulates LMW HA–induced gene expression via a PKA-independent EPAC-dependent pathway. (A) TNF-α ELISA of cell cultured supernatants from WT PEC stimulated with LMW HA (250 μg/ml) + CGS (3μM) + the PKA inhibitor H89 (1μM) for 18 hours. EPAC activator (EA) directly inhibits LMW HA–induced protein expression by ELISA of TNF-α (B) and induces IL-12 (C) in MH-S macrophages pre-stimulated with EA for 12 hours before stimulation of LMW HA. There are statistical differences between HA v. HA + EA (all doses) for both TNF-α and IL-12, P < 0.002. (D) MH-S macrophages were transfected with EPAC siRNA for 24 hours and Western analysis was performed for EPAC protein. (E) MH-S macrophages were transfected with the control or EPAC siRNA for 24 hours, stimulated with CGS (μM) for 12 hours, and then stimulated with LMW HA for 18 hours (*P = 0.0007 for HA + CGS control siRNA v. EPAC siRNA). Cell conditioned media was collected and TNF-α protein expression was measured by ELISA. These data represent the average of at least three individual experiments.
Recently, a novel downstream target of cAMP, EPAC, has been described (16, 28). We wanted to determine if EPAC might play a role in regulating LMW HA–induced gene expression. First, we evaluated the ability of 8-CPT-2′-O-Me-cAMP, an EPAC activator (EA), to inhibit HA-induced gene expression. As evident in Figures 5B and 5C, direct EPAC activation (LMW HA + EA) alone inhibited LMW HA–induced TNF-α. Specifically, direct EPAC activation mirrored the inhibition seen with CGS. Likewise, similar to CGS stimulation, direct EPAC activation enhanced IL-12 expression. Thus, upon LMW HA stimulation, the direct activation of EPAC mimicked the gene expression profile seen with A2aR engagement. Once again, the doses of EPAC activator employed are consistent with previously published findings using this agonist with macrophages (16, 20).
Next, we wanted to further assess the ability of A2aR stimulation to modulate LMW HA–induced gene expression via EPAC. We hypothesized that if EPAC were necessary for A2aR modulation of LMW HA gene expression, then CGS would not inhibit TNF-α production in macrophages in which EPAC was knocked down. To test this hypothesis, MH-S macrophages were transfected overnight with either control siRNA or EPAC siRNA. Compared with the control transfection, in the EPAC siRNA–transfected cells there was significant knockdown of EPAC protein expression as determined by Western blot analysis (Figure 5D). Cells transfected with the control or EPAC siRNA were stimulated with CGS for 12 hours and then stimulated with LMW HA for 18 hours. TNF-α protein expression was then measured by ELISA. Figure 5E demonstrates that CGS was no longer able to inhibit LMW HA–induced TNF-α expression in the cells transfected with the EPAC siRNA but still inhibited LMW HA-induced TNF-α by greater than 60% in cells transfected with the control siRNA (P = 0.0007). Taken together, our data suggest that A2aR stimulation modulates LMW HA–induced gene expression in part by activating EPAC.
A2aR-Null Mice Are More Susceptible to Bleomycin-Induced Lung Injury
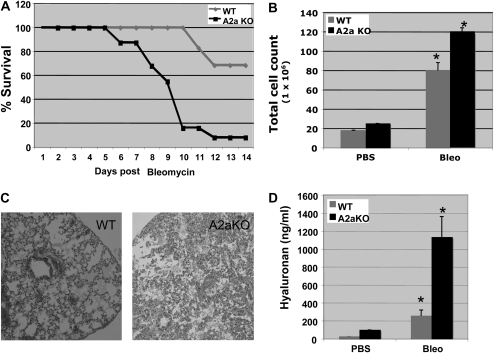
Pulmonary fibrosis is the end result of persistent dysregulated inflammation (29). Instillation of bleomycin into the lungs of mice results in acute inflammation, ultimately leading to fibrosis and death (30). Interestingly, bleomycin-induced lung injury is associated with an increase in TNF-α, IP-10, and KC and potentially ameliorated by IFN-γ (31, 32). Likewise, bleomycin injury results in excess accumulation of LMW HA fragments in injured lungs and appears to play a critical role in promoting persistent lung inflammation (1, 2). Since A2aR stimulation inhibits the production of LMW HA–induced pro-fibrotic cytokine/chemokine production, we wanted to determine the importance of this in vivo in the bleomycin-induced lung injury model. WT and A2aR-null mice were exposed to 0.375 U of intratracheal bleomycin and followed for 14 days. Figure 6A demonstrates the survival curves for the A2aR-null and WT animals. WT mice had a 100% survival rate up to Day 10, with 70% ultimate survival at 14 days. In contrast, A2aR-knockout mice started dying after Day 5, and by Day 14 the survival rate of these mice was only 10%. (The survival data shown is from a single experiment, but is representative of three experiments with a total of 24 WT and 26 A2aR-knockout mice.) Thus, in the absence of the A2aR, there is an earlier and higher mortality rate during the initial inflammatory stage of bleomycin injury.
Figure 6.
A2aR-null mice are more susceptible to bleomycin-induced lung injury. A2aR-null or WT mice were given 0.375 U of intratracheal bleomycin and followed for 14 days. (A) Survival curves. (B) Day 7 BAL for total cells count (*P = 0.027 for bleomycin-injured WT v. A2aR-null for total BAL cell count). (C) Day 7 hematoxylin and eosin stain of lung tissue. (D) Day 7 BAL HA concentration (*P = 0.002 for bleomycin-injured WT v. A2aR-null for total BAL HA content).
As there appeared to be increased early death in the A2aR-knockout mice, on Day 7 we obtained BAL for total cell count and HA concentration as well as histology. On Day 7 there was an increased total number of inflammatory cells and an increased percentage of neutrophils in the A2aR-null animals (Figure 6B; P = 0.027). On addition, as seen in the H&E stain from bleomycin-injured A2aR-null mice on Day 7, there was increased neutrophilia, exudates, and tissue damage (Figure 6C). Similarly, HA levels measured in Day 7 BAL samples revealed elevated levels of HA in the A2aR-null mice compared with WT controls (Figure 6D; P = 0.002). Thus, these data suggest that decreased survival in the A2aR-null mice is due to increased inflammation, injury, and HA accumulation in response to bleomycin injury.
DISCUSSION
As the tissue microenvironment plays a critical role in regulating inflammation, our studies sought to define the roles of the extracellular matrix component HA and adenosine in this process. Extracellular matrix degradation products are not only the result of inflammation but also active participants in the perpetuation of the inflammatory process (1, 2, 8). During inflammation, as the extracellular matrix is degraded and remodeled, HA breakdown products accumulate. Normally, in the course of an immune response, inflammation is self-limiting and the biologically active LMW HA fragments are removed as healing occurs. However, in states of ongoing inflammation and fibrosis, such as sarcoidosis, chronic bronchitis, and idiopathic pulmonary fibrosis, there is ongoing tissue destruction and remodeling leading to persistence of HA degradation products (4, 33, 41). Although the precise physiologic concentration of LMW HA is unknown, during inflammation, there is increased overall HA as reflected in the serum (patients with rheumatoid arthritis, 150 ng/ml; patients with liver failure, 390 ng/ml; healthy control subjects, 36 ng/ml) and BAL (patients with sarcoidosis, 430 μg/ml) (33–35). Similarly, during inflammation and tissue destruction, adenosine is released into the extracellular space and can act as a negative regulator of both inflammation and immune-mediated tissue destruction (9, 11). A number of the anti-inflammatory properties of adenosine have been shown to be mediated by the adenosine A2a receptor (9). In this article we demonstrate that engagement of the A2aR, not A1 or A3 receptors, significantly modulates LMW HA–induced inflammatory genes by inhibition of the pro-fibrotic TNF-α, MIP-2, MIP-1α, IP-10, and KC but synergistic enhancement of HA fragment–induced IL-12. Our data indicate that A2aR modulation of these genes is independent of PKA and is the result of A2aR-dependent activation of EPAC. Furthermore, in an in vivo model of noninfectious lung injury, the absence of the A2aR's anti-inflammatory influence leads to massive lung inflammation and death.
Adenosine is an endogenous intracellular purine nucleotide that is normally found at low concentrations extracellularly (9, 11). During inflammation and tissue destruction, adenosine is released into the extracellular space and acts as a negative regulator of both inflammation and immune-mediated tissue destruction (9, 11). The engagement of the high-affinity A2aR by adenosine activates stimulatory G-proteins that activate adenylate cyclase, leading to increased cAMP production (36). Elevated levels of cAMP have been associated with activation of protein kinase A (PKA) and inhibition of NF-κB (15). EPAC is a novel downstream target of cAMP that may mediate many of the inhibitory effects of cAMP that were initially attributed to PKA; however, to our knowledge a role for EPAC in A2aR signaling has not previously been described (16, 28). Our data indicate that A2aR stimulation can modulate LMW HA–induced gene expression even in the presence of a PKA inhibitor. Furthermore, we demonstrate the ability of a potent EPAC activator to simultaneously inhibit LMW HA–induced TNF-α production and synergize with LMW HA to induce IL-12. Thus we demonstrate that A2aR-mediated regulation of inflammation may occur via EPAC.
Adenosine mediates a negative feedback loop altering LPS-stimulated TNF-α production, presumably via A2aR, as the A2aR-specific agonist CGS-21680 has been demonstrated to inhibit TNF-α expression by LPS-treated human monocytic leukemia cells (12). Increases in TNF-α production in response to LPS stimulation, and the subsequent inhibition of it with adenosine analogs, have also been observed in both human monocytes and murine peritoneal macrophages fragments (37, 38). In addition, the ability of the A2aR to act as a negative regulator of inflammation has been dramatically demonstrated by the observation that when normally harmless inflammation is induced in A2aR-null mice, they die of overzealous immune responses (13, 37, 38). Inasmuch as both LMW HA and adenosine are components of the inflammatory milieu, the ability of adenosine acting via the A2aR to modulate LMW HA–induced inflammation reveals an important regulatory component of the tissue microenvironment.
Surprisingly, A2aR activation did not uniformly inhibit all LMW HA–induced cytokines. LMW HA–induced IL-12 was augmented at both the protein and mRNA levels via a post-transcriptional mechanism. Although IL-12 is primarily considered to be a proinflammatory chemokine, it has been shown to exhibit anti-inflammatory properties as well (39, 40). The presence of IL-12 in the inflammatory milieu induces expression of the anti-inflammatory cytokine IL-10 (39, 40). IL-12 also plays a key role in balancing activation of Th-1 and Th-2 helper T cells by promoting naïve T cells to differentiate into IFN-γ–producing Th-1 helper cells (39, 40). Interestingly, although A2aR stimulation synergized with LMW HA to induce IL-12, in both human monocytes and murine macrophages LPS-induced IL-12 expression is decreased in response to A2aR stimulation (37, 38). Our data suggesting that the increase in HA induced IL-12 by CGS is mediated by increased stability of IL-12 mRNA implies that LPS and HA use different pathways. Indeed, it may be the result of different downstream activation specific to ligation of TLR-2 (HA) and TLR-4 (LPS). As CGS has been demonstrated to inhibit p38 MAPK activation and p38 MAPK activation increases IL-12 mRNA degradation, we speculate that the synergy between LMW HA and A2aR activation by CGS may be the result of CGS-mediated inhibition of p38 MAPK (24, 25).
The bleomycin model of lung injury in animals, which approximates the pathology seen in fibrotic lung disorders such as idiopathic pulmonary fibrosis, has been well established (30). Intratracheal administration of bleomycin causes an initial alveolitis followed by a fibroproliferative phase with dysregulated matrix remodeling and ultimately fibrosis (30). There is increased turnover, production, and accumulation of LMW HA fragments in bleomycin-injured lungs (41). The course of bleomycin-induced fibrosis has been ameliorated by various interventions such as treatment with antibodies against MIP-1α, TNF-α, or administration of exogenous IL-12 and IFN-γ, suggesting that shifting the chemokine profile away from the pro-fibrotic toward the anti-fibrotic chemokines may be important in this model of lung injury (31, 42). The role of LMW HA fragments in bleomycin-induced lung injury has been demonstrated by the increased mortality and accumulation of LMW HA in bleomycin-injured CD44-null mice (2). As the CD44 receptor is critical for the clearance of HA fragments from sites of injury, the resultant lack of clearance of HA leads to enhanced inflammation (2). In addition, lung-specific HA synthase-2 (HAS-2)–transgenic mice that overexpress HMW HA in their lungs are resistant to bleomycin injury (1). We demonstrate that degraded HA, in the form of LMW HA fragments, plays an important role in modulating the magnitude and quality of an immune response via interaction with the adenosine A2aR. Interestingly, we see significant inhibition of LMW HA–induced TNF-α expression with simultaneous and pre-incubation with A2aR agonists, but no significant inhibition of LMW HA–induced TNF-α expression if the A2aR agonist is given after HA stimulation (Figure 1A). These findings suggest that one mechanism by which LMW HA may be promoting inflammation is by inhibiting the anti-inflammatory effects of the A2aR. Likewise, in the total absence of A2aR function (A2aR-null mice) there is a significant increase in LMW HA–induced inflammatory gene expression (Figure 3A), suggesting that the A2aR may have a tonic inhibitory effect that keeps a damper on LMW HA–induced inflammation in macrophages. Thus, the degree of inflammation is not only dictated by the nature and extent of tissue injury/insult, but also by the balance between tissue-derived adenosine-A2aR modulation of LMW HA–induced inflammatory genes.
The role of adenosine in lung inflammation is emerging as being both important and complex. Adenosine deaminase (ADA)-null mice, which lack the enzyme that metabolizes adenosine, have massive accumulation of adenosine in their lungs, resulting in pulmonary inflammation and airways remodeling (43). If the adenosine deaminase is only partially knocked out or partially exogenously replaced, the mice accumulate adenosine slowly over time and develop a progressive lung fibrosis with increased myofibroblasts and collagen deposition (43, 44). In addition, complete adenosine deaminase replacement (with subsequent decrease in adenosine lung levels) after the development of the lung fibrosis leads to regression of the fibrosis (44). In these models it is unclear which of the adenosine receptors is the culprit; although adenosine deaminase/A1R double knockout mice have increased inflammation, the adenosine deaminase/A3R double knockout mice have less inflammation (45, 46). In addition, IL-13–transgenic mice have recently been shown to have high levels of lung adenosine and decreased adenosine deaminase function (47). These mice have increased A1, A2b, and A3 receptor expression but not A2aR in the setting of increased lung inflammation (47). These data are consistent with the previously described proinflammatory role of A2bR in adenosine-dependent lung inflammation via stimulating fibroblast proliferation, MMP-2 activity and collagen deposition (48, 49). Interestingly, where A2b receptor stimulation has been shown to have proinflammatory activity in airway epithelial cells, A2aR stimulation in lung epithelial cells have been shown to promote wound healing (50). In fact, we have data demonstrating that A2aR engagement inhibits LMW HA–induced IL-8 but not IP-10 in human airway epithelial cells (data not shown). Furthermore, numerous studies have demonstrated the anti-inflammatory, antifibrotic role of A2aR stimulation in preventing toxin-induced hepatic fibrosis, allergen- or tobacco-induced lung inflammation, and acute lung injury after hemorrhagic shock (51–53).
Our data elucidate the opposing roles of tissue-derived hyaluronan fragments and adenosine in regulating noninfectious lung inflammation, and support the pursuit of A2a receptor agonists as a means of pharmacologically inhibiting inflammation that may lead to fibrosis. Thus, we propose that adenosine released into the extracellular space during inflammation serves to negatively regulate acute inflammation via the A2aR. In the absence of the A2aR, there is uncontrolled inflammation, leading to death. On the other hand, persistent increased adenosine in the lung (for example, in the ADA-null mice) leads to the chronic stimulation of the A2b receptor, which in turn may promote fibrosis.
Acknowledgments
The authors thank David B. Jacoby for his advice and critical reading of the manuscript.
This work was funded by grants from FAMRI and the National Heart, Lung, and Blood Institute (HL073855-01 and HL086632) (to M.R.H.).
Originally Published in Press as DOI: 10.1165/rcmb.2008-0168OC on August 14, 2008
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, Prestwich GD, Mascarenhas MM, Garg HG, Quinn DA, et al. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med 2005;11:1173–1179. [DOI] [PubMed] [Google Scholar]
- 2.Teder P, Vandivier RW, Jiang D, Liang J, Cohn L, Pure E, Henson PM, Noble PW. Resolution of lung inflammation by CD44. Science 2002;296:155–158. [DOI] [PubMed] [Google Scholar]
- 3.Turley EA. Hyaluronan and cell locomotion. Cancer Metastasis Rev 1992;11:21–30. [DOI] [PubMed] [Google Scholar]
- 4.Laurent TC. Functions of hyaluronan. A Rheum Dis 1995;54:429–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horton MR, Olman MA, Bao C, White KE, Choi AM, Chin BY, Noble PW, Lowenstein CJ. Regulation of plasminogen activator inhibitor-1 and urokinase by hyaluronan fragments in mouse macrophages. Am J Physiol Lung Cell Mol Physiol 2000;279:L707–L715. [DOI] [PubMed] [Google Scholar]
- 6.Horton MR, Shapiro S, Bao C, Lowenstein CJ, Noble PW. Induction and regulation of macrophage metalloelastase by hyaluronan fragments in mouse macrophages. J Immunol 1999;162:4171–4176. [PubMed] [Google Scholar]
- 7.McKee C, Penno M, Cowman M, Burdick M, Strieter R, Bao C, Noble P. Hyaluronan (HA) fragments induce chemokine gene expression in alveolar macrophages: the role of HA size and CD44. J Clin Invest 1996;98:2403–2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scheibner KA, Lutz MA, Boodoo S, Fenton MJ, Powell JD, Horton MR. Hyaluronan fragments act as an endogenous danger signal by engaging TLR2. J Immunol 2006;177:1272–1281. [DOI] [PubMed] [Google Scholar]
- 9.Dubyak GR, el-Moatassim C. Signal transduction via p2-purinergic receptors for extracellular ATP and other nucleotides. Am J Physiol 1993;265:C577–C606. [DOI] [PubMed] [Google Scholar]
- 10.Traut TW. Physiological concentrations of purines and pyrimidines. Mol Cell Biochem 1994;140:1–22. [DOI] [PubMed] [Google Scholar]
- 11.Van Belle H, Goossens F, Wynants J. Formation and release of purine catabolites during hypoperfusion, anoxia, and ischemia. Am J Physiol 1987;252:H886–H893. [DOI] [PubMed] [Google Scholar]
- 12.Khoa ND, Montesinos MC, Reiss AB, Delano D, Awadallah N, Cronstein BN. Inflammatory cytokines regulate function and expression of adenosine a(2a) receptors in human monocytic Thp-1 cells. J Immunol 2001;167:4026–4032. [DOI] [PubMed] [Google Scholar]
- 13.Ohta A, Sitkovsky M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature 2001;414:916–920. [DOI] [PubMed] [Google Scholar]
- 14.Stiles GL. Adenosine receptors. J Biol Chem 1992;267:6451–6454. [PubMed] [Google Scholar]
- 15.Montminy M. Transcriptional regulation by cyclic amp. Annu Rev Biochem 1997;66:807–822. [DOI] [PubMed] [Google Scholar]
- 16.Aronoff DM, Canetti C, Serezani CH, Luo M, Peters-Golden M. Cutting edge. Macrophage inhibition by cyclic amp (cAMP): differential roles of protein kinase A and exchange protein directly activated by cAMP-1. J Immunol 2005;174:595–599. [DOI] [PubMed] [Google Scholar]
- 17.Day YJ, Marshall MA, Huang L, McDuffie MJ, Okusa MD, Linden J. Protection from ischemic liver injury by activation of A2a adenosine receptors during reperfusion: inhibition of chemokine induction. Am J Physiol Gastrointest Liver Physiol 2004;286:G285–G293. [DOI] [PubMed] [Google Scholar]
- 18.Horton MR, Burdick MD, Strieter RM, Bao C, Noble PW. Regulation of hyaluronan-induced chemokine gene expression by IL-10 and IFN-gamma in mouse macrophages. J Immunol 1998;160:3023–3030. [PubMed] [Google Scholar]
- 19.Hodge-Dufour J, Noble PW, Horton MR, Bao C, Wysoka M, Burdick MD, Strieter RM, Trinchieri G, Pure E. Induction of il-12 and chemokines by hyaluronan requires adhesion-dependent priming of resident but not elicited macrophages. J Immunol 1997;159:2492–2500. [PubMed] [Google Scholar]
- 20.Kreckler LM, Wan TC, Ge ZD, Auchampach JA. Adenosine inhibits tumor necrosis factor-alpha release from mouse peritoneal macrophages via A2a and A2b but not the A3 adenosine receptor. J Pharmacol Exp Ther 2006;317:172–180. [DOI] [PubMed] [Google Scholar]
- 21.Keane MP, Belperio JA, Burdick MD, Strieter RM. IL-12 attenuates bleomycin-induced pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 2001;281:L92–L97. [DOI] [PubMed] [Google Scholar]
- 22.Horton MR, Boodoo S, Powell JD. NF-kappa B activation mediates the cross-talk between extracellular matrix and interferon-gamma (IFN-gamma) leading to enhanced monokine induced by IFN-gamma (MIG) expression in macrophages. J Biol Chem 2002;277:43757–43762. [DOI] [PubMed] [Google Scholar]
- 23.Goriely S, Neurath MF, Goldman M. How microorganisms tip the balance between interleukin-12 family members. Nat Rev Immunol 2008;8:81–86. [DOI] [PubMed] [Google Scholar]
- 24.Fotheringham JA, Mayne MB, Grant JA, Geiger JD. Activation of adenosine receptors inhibits tumor necrosis factor-alpha release by decreasing TNF-alpha mRNA stability and p38 activity. Eur J Pharmacol 2004;497:87–95. [DOI] [PubMed] [Google Scholar]
- 25.Saito Y, Yanagawa Y, Kikuchi K, Iijima N, Iwabuchi K, Onoe K. Low-dose lipopolysaccharide modifies the production of IL-12 by dendritic cells in response to various cytokines. J Clin Exp Hematop 2006;46:31–36. [DOI] [PubMed] [Google Scholar]
- 26.Grandjean-Laquerriere A, Le Naour R, Gangloff SC, Guenounou M. Differential regulation of TNF-alpha, IL-6 and IL-10 in uvb-irradiated human keratinocytes via cyclic amp/protein kinase A pathway. Cytokine 2003;23:138–149. [DOI] [PubMed] [Google Scholar]
- 27.Woo MS, Jung SH, Hyun JW, Kim HS. Differential regulation of inducible nitric oxide synthase and cytokine gene expression by forskolin and dibutyryl-camp in lipopolysaccharide-stimulated murine bv2 microglial cells. Neurosci Lett 2004;356:187–190. [DOI] [PubMed] [Google Scholar]
- 28.Seino S, Shibasaki T. PKA-dependent and PKA-independent pathways for camp-regulated exocytosis. Physiol Rev 2005;85:1303–1342. [DOI] [PubMed] [Google Scholar]
- 29.Kuhn C. The pathogenesis of pulmonary fibrosis. Monogr Pathol 1993;78:1. [PubMed] [Google Scholar]
- 30.Bowden DH. Unraveling pulmonary fibrosis: the bleomycin model. Lab Invest 1984;50:487–488. [PubMed] [Google Scholar]
- 31.Smith RE, Strieter RM, Phan SH, Lukacs N, Kunkel SL. TNF and IL-6 mediate MIP-1alpha expression in bleomycin-induced lung injury. J Leukoc Biol 1998;64:528–536. [PubMed] [Google Scholar]
- 32.Smith RE, Strieter RM, Zhang K, Phan SH, Standiford TJ, Lukacs NW, Kunkel SL. A role for C–C chemokines in fibrotic lung disease. J Leukoc Biol 1995;57:782–787. [DOI] [PubMed] [Google Scholar]
- 33.Hallgren R, Eklund A, Engstrom-Laurent B, Schmekel B. Hyaluronate in bronchoalveolar lavage fluid: a new marker in sarcoidosis reflecting pulmonary disease. Br Med J (Clin Res Ed) 1985;290:1778–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manicourt DH, Poilvache P, Nzeusseu A, van Egeren A, Devogelaer JP, Lenz ME, Thonar EJ. Serum levels of hyaluronan, antigenic keratan sulfate, matrix metalloproteinase 3, and tissue inhibitor of metalloproteinases 1 change predictably in rheumatoid arthritis patients who have begun activity after a night of bed rest. Arthritis Rheum 1999;42:1861–1869. [DOI] [PubMed] [Google Scholar]
- 35.Shigemori M, Takei S, Imanaka H, Maeno N, Hokonohara M, Miyata K. Diagnostic significance of increased serum hyaluronic acid in juvenile rheumatoid arthritis. Pediatr Int 2002;44:394–399. [DOI] [PubMed] [Google Scholar]
- 36.Olah ME, Stiles GL. Adenosine receptors. Annu Rev Physiol 1992;54:211–225. [DOI] [PubMed] [Google Scholar]
- 37.Hasko G, Kuhel DG, Chen JF, Schwarzschild MA, Deitch EA, Mabley JG, Marton A, Szabo C. Adenosine inhibits IL-12 and TNF-[alpha] production via adenosine a2a receptor-dependent and independent mechanisms. FASEB J 2000;14:2065–2074. [DOI] [PubMed] [Google Scholar]
- 38.Link AA, Kino T, Worth JA, McGuire JL, Crane ML, Chrousos GP, Wilder RL, Elenkov IJ. Ligand-activation of the adenosine a2a receptors inhibits IL-12 production by human monocytes. J Immunol 2000;164:436–442. [DOI] [PubMed] [Google Scholar]
- 39.Chang HD, Radbruch A. The pro- and anti-inflammatory potential of interleukin-12. Ann N Y Acad Sci 2007;1109:40–46. [DOI] [PubMed] [Google Scholar]
- 40.Gately MK, Renzetti LM, Magram J, Stern AS, Adorini L, Gubler U, Presky DH. The interleukin-12/interleukin-12-receptor system: role in normal and pathologic immune responses. Annu Rev Immunol 1998;16:495–521. [DOI] [PubMed] [Google Scholar]
- 41.Nettelbladt O, Hallgren R. Hyaluronan (hyaluronic acid) in bronchoalveolar lavage fluid during the development of bleomycin-induced alveolitis in the rat. Am Rev Respir Dis 1989;140:1028–1032. [DOI] [PubMed] [Google Scholar]
- 42.Keane MP, Belperio JA, Arenberg DA, Burdick MD, Xu ZJ, Xue YY, Strieter RM. IFN-gamma-inducible protein-10 attenuates bleomycin-induced pulmonary fibrosis via inhibition of angiogenesis. J Immunol 1999;163:5686–5692. [PubMed] [Google Scholar]
- 43.Chunn JL, Mohsenin A, Young HW, Lee CG, Elias JA, Kellems RE, Blackburn MR. Partially adenosine deaminase-deficient mice develop pulmonary fibrosis in association with adenosine elevations. Am J Physiol Lung Cell Mol Physiol 2006;290:L579–L587. [DOI] [PubMed] [Google Scholar]
- 44.Chunn JL, Molina JG, Mi T, Xia Y, Kellems RE, Blackburn MR. Adenosine-dependent pulmonary fibrosis in adenosine deaminase-deficient mice. J Immunol 2005;175:1937–1946. [DOI] [PubMed] [Google Scholar]
- 45.Sun CX, Young HW, Molina JG, Volmer JB, Schnermann J, Blackburn MR. A protective role for the a1 adenosine receptor in adenosine-dependent pulmonary injury. J Clin Invest 2005;115:35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Young HW, Molina JG, Dimina D, Zhong H, Jacobson M, Chan LN, Chan TS, Lee JJ, Blackburn MR. A3 adenosine receptor signaling contributes to airway inflammation and mucus production in adenosine deaminase-deficient mice. J Immunol 2004;173:1380–1389. [DOI] [PubMed] [Google Scholar]
- 47.Blackburn MR, Lee CG, Young HW, Zhu Z, Chunn JL, Kang MJ, Banerjee SK, Elias JA. Adenosine mediates IL-13-induced inflammation and remodeling in the lung and interacts in an IL-13-adenosine amplification pathway. J Clin Invest 2003;112:332–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu X, Ostrom RS, Insel PA. Camp-elevating agents and adenylyl cyclase overexpression promote an antifibrotic phenotype in pulmonary fibroblasts. Am J Physiol Cell Physiol 2004;286:C1089–C1099. [DOI] [PubMed] [Google Scholar]
- 49.Sun CX, Zhong H, Mohsenin A, Morschl E, Chunn JL, Molina JG, Belardinelli L, Zeng D, Blackburn MR. Role of a2b adenosine receptor signaling in adenosine-dependent pulmonary inflammation and injury. J Clin Invest 2006;116:2173–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Allen-Gipson DS, Wong J, Spurzem JR, Sisson JH, Wyatt TA. Adenosine a2a receptors promote adenosine-stimulated wound healing in bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 2006;290:L849–L855. [DOI] [PubMed] [Google Scholar]
- 51.Bonneau O, Wyss D, Ferretti S, Blaydon C, Stevenson CS, Trifilieff A. Effect of adenosine a2a receptor activation in murine models of respiratory disorders. Am J Physiol Lung Cell Mol Physiol 2006;290:L1036–L1043. [DOI] [PubMed] [Google Scholar]
- 52.Chan ES, Montesinos MC, Fernandez P, Desai A, Delano DL, Yee H, Reiss AB, Pillinger MH, Chen JF, Schwarzschild MA, et al. Adenosine a(2a) receptors play a role in the pathogenesis of hepatic cirrhosis. Br J Pharmacol 2006;148:1144–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hasko G, Xu DZ, Lu Q, Nemeth ZH, Jabush J, Berezina TL, Zaets SB, Csoka B, Deitch EA. Adenosine a2a receptor activation reduces lung injury in trauma/hemorrhagic shock. Crit Care Med 2006;34:1119–1125. [DOI] [PubMed] [Google Scholar]