Abstract
Mucus hypersecretion with elevated MUC5B mucin production is a pathologic feature in many airway diseases associated with oxidative stress. In the present work, we evaluated MUC5B expression in airways and in primary cultures of normal human bronchial epithelial (NHBE) cells, as well as the mechanisms involved in its regulation. We found that oxidative stress generated by cigarette smoke or reactive oxygen species (ROS) induces MUC5B up-regulation in airway epithelium from smokers and in NHBE cells, respectively. We have previously shown that ROS-induced MUC5AC expression in NHBE cells is dependent on hyaluronan depolymerization and epidermal growth factor receptor (EGFR)/mitogen-activated protein kinase (MAPK) activation. Since hyaluronan fragments can activate MAPK through the hyaluronan receptor CD44, and CD44 heterodimerizes with EGFR, we tested whether ROS and/or hyaluronan fragments induce MUC5B mRNA and protein expression through CD44/EGFR. We found that ROS promotes CD44/EGFR interaction, EGFR/MAPK activation, and MUC5B up-regulation that are prevented by blocking CD44 and/or EGFR. These results were mimicked by hyaluronan fragments. In summary, our results show that oxidative stress in vivo (cigarette smoke) or in vitro (ROS) induces MUC5B up-regulation. This ROS-induced MUC5B expression requires CD44 as well as EGFR and MAPK activation. In addition, we also provide evidence that hyaluronan fragments are sufficient to induce CD44/EGFR interaction and downstream signaling that results in MUC5B up-regulation, suggesting that hyaluronan depolymerization during inflammatory responses could be directly involved in the induction of mucus hypersecretion.
Keywords: MUC5B, hyaluronan fragments, CD44, airway epithelium
CLINICAL RELEVANCE
This study shows that oxidative stress induces MUC5B up-regulation in airway epithelium. In addition, it provides a mechanistic link between oxidative stress–induced hyaluronan depolymerization and increased MUC5B, characteristic of asthma and chronic obstructive pulmonary disease.
Mucus overproduction occurs in a variety of acute reactions to cigarette smoke (1) and microorganisms (2, 3), as well as in chronic airway inflammatory diseases such as chronic bronchitis (4–6), asthma (7–9), bronchiectasis (10), and cystic fibrosis (11). The major macromolecular components of mucus are large and heavily glycosylated mucin proteins that are encoded by various MUC genes (12–14), that maintain airways homeostasis by protecting the epithelial surface from environmental insults. MUC5B and MUC5AC are the major mucins present in airways secretions from patients with asthma (15–17), cystic fibrosis (18, 19), and chronic obstructive pulmonary disease (COPD) (1). In normal airways, MUC5AC and MUC5B are typically described as produced by goblet cells at the surface epithelium (20) and by mucous cells in submucosal glands (21), respectively. However, MUC5B mucin is also present at low levels in airway epithelium from normal lung donors (22) and up-regulated in patients with airways diseases and inflammation (1, 23–27). Nevertheless, it is not clear whether MUC5B and MUC5AC are produced by the same or by different cell populations in airway epithelium, since co-localization studies have not been done. Compared with MUC5AC expression regulation, the pathways involved in the regulation of MUC5B expression have been less explored. Recent studies have shown that MUC5B mRNA expression is up-regulated by a pyrimidine nucleotide triphosphate (UTP) and interleukin (IL)-17/IL-6 paracrine/autocrine loop through extracellular signal–regulated kinase (ERK)1/2, also known as 44/42MAPK (mitogen-activated protein kinase) (28, 29), or by phorbol 12-myristate 13-acetate (PMA) through a 44/42MAPK-independent pathway (30). We have previously shown that reactive oxygen species (ROS) increase MUC5AC production in normal human bronchial epithelial (NHBE) cells grown at the air–liquid interface (ALI) by activating epidermal growth factor receptor (EGFR) and 44/42MAPK pathway. Under these conditions, ROS, generated by X/XO, increase hyaluronan concentration in apical washes and decrease its average molecular size. These results are consistent with hyaluronan breakdown and release from the epithelial cell surface (31–33). Because MUC5B is present in airway epithelium, particularly in diseases associated with oxidative stress (1, 24, 26), we tested the ability of ROS to induce MUC5B mRNA and protein expression. In addition, hyaluronan fragments activate 44/42MAPK through the hyaluronan receptor CD44 (34). Interestingly, CD44 has been described as a co-receptor for EGFR, and their heterodimerization increases hyaluronan-mediated 42/44MAPK phosphorylation (35, 36). Therefore, we studied the role of hyaluronan and CD44 on ROS-induced MUC5B expression in airway epithelium.
In summary, the present work was focused on two aims: (1) to determine MUC5B protein expression in tissue sections obtained from normal and smoker lung donors, as well as in primary cultures of NHBE cells exposed to ROS; and (2) to evaluate the role that hyaluronan fragments and CD44 play on receptor activation and downstream signaling associated with oxidative stress–induced MUC5B expression in airway epithelium using primary cultures of NHBE cells.
MATERIALS AND METHODS
Materials
All materials were purchased from Sigma Chemical Co. (St. Louis, MO) unless otherwise specified.
Tracheobronchial Tissues
Tracheobronchial tissues from four nonsmoker and six smoker lung donors (22–59 yr old, and < 3 d of intubation) were provided by the University of Miami Life Alliance Organ Recovery Agency with approval from the local Institutional Review Board. Tissues were fixed in 4% paraformaldehyde and prepared for embedding in paraffin in a Microwave Tissue Processor (Microwave Materials Technologies, Inc., Knoxville, TN) according to the manufacturer's protocol. Embedding and sectioning were performed by the Histology Laboratory at the University of Miami Hospital and Clinics, Sylvester Comprehensive Cancer Center. A smoker donor was defined following two criteria. (1) According to the Centers for Disease Control (CDC): a person who had smoked at least 100 cigarettes or more and was currently a smoker either every day or some days (37). (2) Histologic criteria: lungs with histologic hallmarks of chronic bronchitis (enlargement of tracheobronchial submucosal glands, mucous cells metaplasia, and hyperplasia) (38). Smoker lung donors were compared with control group of lifelong nonsmokers.
MUC5B Immunofluorescence on Tissue Sections
Sections were deparaffinized with xylene, rehydrated by using graded ethanols, and subjected to heat-induced antigen retrieval with 10 mM of sodium citrate buffer (pH 6.0). Additional antigen retrieval by incubation with 0.25% pepsin (wt/vol) in 0.1 N HCl, 14 min, 40°C (39) was performed for MUC5B/cytokeratin co-labeling. After blocking for 1 hour with gelatin 1% (wt/vol) in PBS, tissues were incubated overnight with rabbit polyclonal anti-MUC5B antibody against amino acids 1201–1500 mapping near the C-terminus of MUC5B of human origin (1:100; Santa Cruz Biotechnology, Santa Cruz, CA) or nonimmune rabbit IgG (Santa Cruz). Then, sections were washed with PBS and Alexa 555–labeled anti-rabbit IgG (1 μg/ml; Invitrogen, Carlsbad, CA) was added. Co-labeling with MUC5AC (goblet cell marker), cytokeratins 5/14 (basal cell marker), or Foxj1 (ciliated cell marker) was achieved by the addition of mouse anti-MUC5AC (10 μg/ml; Chemicon, Billerica, MA), mouse anti cytokeratins 5/14 (1:25; Dako, Carpinteria, CA), or goat anti Foxj1 antibodies (1:100; R&D Systems, Minneapolis, MN), respectively. Nonimmune mouse or goat IgGs (Santa Cruz) were used as a negative controls. After washing, Alexa 647–conjugated anti-mouse or anti-goat IgGs (1 μg/ml) were added, and sections were incubated for 1 hour at room temperature. Nuclei were visualized with 4′,6-diamidine-2-phenylindole (DAPI) and slides were mounted with Gel/Mount (Biomeda, Foster City, CA). Fluorescent images were obtained using a fluorescence microscope Axiovert 200M and optically sectioned with the slider module Apotome (Carl Zeiss Meditec, Inc., Thornwood, NY).
Primary Cultures of NHBE Cells Grown at the ALI
Primary cultures of NHBE cells were obtained from normal lung donors, through the University of Miami Life Alliance Organ Recovery Agency with approval from the local Institutional Review Board, and cultures were established as previously reported (31, 40). Briefly, passage 0 (P0) cells were expanded in submerged conditions and then P1 cells were re-differentiated in 24-mm Transwell-clear culture inserts (Corning Costar Corporation, Cambridge, MA) coated with human placental collagen, at 37°C in humidified air supplemented with 5% (vol/vol) CO2 as described previously (31, 40). The apical surface was exposed to air as soon as the cells reached confluence. Cultures were used for experiments after reaching full differentiation (∼ 3 wk on air), assessed by visual confirmation of beating cilia and mucus. Under these conditions, ALI cultures had 89 ± 4% of ciliated cells (n = 10 lung donors), measured by labeling ciliated cells with anti-acetylated tubulin monoclonal antibodies and counted with Metamorph software (Universal Imaging Corporation, Molecular Devices, Sunnyvale, CA) as described previously (31).
Protocols
Exogenous EGF was removed from culture media 48 hours before the studies. After treatment, apical supernatants were collected, and cells were lysed with 50 mM HEPES (pH 7.5), 150 mM NaCl, 20 mM MgCl2, 1.0% (vol/vol) Nonidet P-40, 0.2 mM Na3VO4, 0.2 mM phenylmethylsulfonyl fluoride, 10 μg/ml leupeptin, and 5 μg/ml aprotinin for 30 minutes at 4°C. Note that CD44/EGFR heterodimers are not dissociated under these conditions (36). To remove insoluble material, cell lysates were centrifuged at 12,000 × g for 5 minutes at 4°C. and supernatants and pellets were frozen for later analysis at −20°C. Protein concentration was measured with BCA Protein Assay Kit (Thermo Scientific, Rockford, IL), following the manufacturer's instructions.
In experiments designed to test the effects of ROS on EGFR/44/42MAPK signaling or MUC5B gene and protein expression, NHBE cells (n = 3 different lung donors, in duplicate wells for each experimental condition) were apically exposed to PBS or 0.6 mM xanthine plus 0.05 units of xanthine oxidase (X/XO) for 30 minutes (Day 0), in the presence or absence of catalase (150 U/ml) and then returned to air. In experiments aimed at determining whether ROS effects were mediated by EGFR and/or CD44, replicate cultures were pretreated with functionally blocking mouse anti-EGFR (4 μg/ml; Calbiochem, San Diego, CA) or/and rat anti-CD44 antibodies (5 μg/ml; Pierce, Rockford, IL) for 15 minutes before X/XO exposure. Control cultures were treated only with blocking antibodies to determine if basal EGFR/MAPK activation levels were modified. Samples were collected at Day 0, 30 minutes after initial treatment (EGFR/44/42MAPK activation), Day 1 (MUC5B mRNA expression), and Day 3 (MUC5B protein expression). In experiments designed to determine whether hyaluronan fragments were involved in EGFR/44/42MAPK signaling and MUC5B mRNA and protein expression, NHBE cultures were exposed to PBS (control) or 10 μg/ml of a hyaluronan of 14 mer oligosaccharide (HA14, 2.67 kD; generated from Hylumed Medical hyaluronan, Genzyme, Cambridge, MA as described in Ref. 41) in the presence or the absence of blocking antibodies against CD44, EGFR, or both, using the concentrations described above. Samples were collected 30 minutes after initial ROS exposure (Day 0) for the assessment of EGFR/44/42MAPK activation, at Day 1 for MUC5B mRNA expression, and at Day 3 for MUC5B protein expression. Control cultures were pretreated with isotype control antibodies from rat or mouse before PBS, X/XO, or HA14 exposure. MUC5B gene expression was assessed after 24 hours by qPCR. Effect of CD44 and EGFR blocking antibodies was evaluated as described before.
MTT Assay
To study whether ROS exposure was not associated with cytotoxicity, cell viability was assessed by reduction of the tetrazolium salt 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) to an insoluble formazan dye by mitochondrial enzymes associated with metabolic activity, indicating living cells (42). Briefly, NHBE cells were treated with PBS or X/XO as before, followed by MTT (2 mg/ ml) added to each well for 4 hours at 37°C. MTT was removed, 200 μl of DMSO was added to each well, and absorbance at 570 nm was read in a plate reader. Each treatment with the corresponding control was performed in triplicate. Viability was set as 100% in control cells (PBS).
MUC5B Immunofluorescence on NHBE Cells
To test whether ROS induce MUC5B protein expression on NHBE cells cultures grown at the ALI, and whether this mucin is synthesized by goblet cells, MUC5AC, a marker for goblet cells, was used for co-localization studies with MUC5B by immunofluorescence. Replicate cell culture inserts treated as described in Protocols were fixed and permeabilized with cold acetone-methanol (1:1) for 1 minute. Then, sections were deglycosylated following the MUC5B antibody manufacturer's protocol (Invitrogen). After blocking for 1 hour with gelatin 1% (wt/vol) in PBS, tissues were incubated overnight with 10 μg/ml of anti-MUC5B antibody (Invitrogen) or nonimmune mouse IgG (Santa Cruz) as a negative control. Sections were then washed with PBS, and Alexa 488–labeled anti-mouse IgG (1 μg/ ml; Invitrogen) was added. Co-localization with MUC5AC was achieved by the addition of rabbit anti-MUC5AC (1:100; Santa Cruz) for 2 hours at room temperature. Nonimmune rabbit IgG (Santa Cruz) was used as a negative control. After washing, sections were incubated for 1 hour with Alexa 555–conjugated anti-rabbit IgG (1 μg/ml). Transwell inserts were excised and mounted on slides with Gel/Mount (Biomeda). Images were captured with a fluorescence microscope Axiovert 200M and optically sectioned with the slider module Apotome (Carl Zeiss Meditec).
Phosphorylated and Total EGFR Immunoblotting
Cells lysates aliquots (n = 3 different lung donors) containing 1 mg of protein were used for relative estimation of total and phosphorylated EGFR (pEGFR) by Western blots. EGFR was immunoprecipitated using an agarose conjugated mouse anti-EGFR antibody (4 μg/ml; Santa Cruz). Pellets from immunoprecipitation were electrophoresed on 4 to 15% Tris-HCl Ready Gels (Bio-Rad, Hercules, CA) and then transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Billerica, MA). Membranes were blocked with 1% (wt/vol) gelatin in TBS containing 0.05% (vol/vol) Tween-20 (1 h) followed by anti-phosphotyrosine mAb (PY99, 2 μg/ml; Santa Cruz) or anti-EGFR mAb (1 μg/ml; Cell Signaling Technology, Beverly, MA). Secondary antibody was an alkaline phosphatase (AP)-conjugated goat anti-mouse IgG (0.5 μg/ml; KPL, Gaithersburg, MA). For visualization, Lumi-Phos (Pierce) was used as substrate. A digitized image of each blot was obtained using a ChemiDoc XRS imaging system (Bio-Rad), and quantification of the pEGFR to EGFR ratio was assessed using Quantity One software (Bio-Rad). Blot images correspond to one lung donor that is representative of three lung donors examined. Bar graphs represent the mean ± SE obtained from three different lung donors examined.
Phosphorylated and Total 44/42 MAPK Immunoblotting
Cells lysates aliquots containing 15 μg of protein (n = 3 different lung donors) were run on 4 to 15% Tris-HCl Ready Gels (Bio-Rad) and then transferred to PVDF membranes (Millipore). Visualization of 44/42MAPK and phosphorylated 44/42MAPK (pMAPK) was achieved using the PhosphoPlus p44/p42 MAP kinase (Thr202/Thr204) antibodies kit according to the manufacturer's instructions (Cell Signaling Technology). Developed blots were photographed, and pMAPK/MAPK ratios were quantified as described above for pEGFR/EGFR.
CD44/EGFR Co-Immunoprecipitation
Cells were lysated as described in protocols with a nonionic detergent (Nonidet P-40) that preserves association of CD44/growth factor receptor in heterodimers (36). Cell lysates containing 1 mg of protein were incubated 2 hours at 4°C with agarose-conjugated anti-EGFR antibodies raised against a peptide mapping at the C-terminus of human EGFR (5 μg/ml; Santa Cruz). These antibodies were different from these used for EGFR neutralization that were raised against its extracellular domain. Immunoprecipitates were washed with cold buffer, resuspended in Laemmli buffer, and examined for CD44 by Western blot. Immunodetection was performed using rat anti-CD44 (2 μg/ml; Pierce) followed by horseradish peroxidase–conjugated anti-rat antibodies (10,000 dilution; KPL). Blots were developed with an enhanced chemiluminescence kit (SuperSignal West Pico Maximum Sensitivity; Pierce) according to the manufacturer's instructions. A digitized image of each blot was obtained and a relative quantification of proteins was performed as described above.
Additional experiments were made to determine whether EGFR-neutralizing antibodies, although directed to its extracellular domain, interfere with CD44 pull-down by EGFR. In this case, immunoprecipitation was also done using EGFR antibodies followed by protein A/G agarose beads, which bind both EGFR antibodies (the neutralizing antibodies, and those used for immunoprecipitation). We did not find differences between both procedures (results not shown).
MUC5B mRNA Expression: RNA Isolation and Quantitative Real-Time PCR
RNA was extracted using Trizol (Invitrogen). cDNA from these samples was obtained using SuperScript First Strand Synthesis System for RT/PCR kit (Invitrogen). MUC5B mRNA expression was assessed using a pre-made TaqMan Gene Expression Assay (Applied Biosystems, Foster City, CA) with a TaqMan MGB probe, FAM labeled (Hs00861588_m1). The quantitative PCR (qPCR) was performed using Pre-developed TaqMan Universal PCR Master Mix (Applied Biosystems) following the manufacturer's instructions. Thermal cycling was performed using ICycler IQ apparatus (Bio-Rad). Samples were normalized using the housekeeping gene GAPDH (Hs99999905_m1). The comparative CT method (ΔΔCT) was used for relative quantization (43).
MUC5B Enzyme-Linked Immunosorbent Assay
MUC5B enzyme-linked immunosorbent assay (ELISA) assay was performed on cell lysates incubated with bicarbonate-carbonate buffer, pH 9.2, at 37°C in a MaxiSorp 96-well plate (Nunc, Rochester, NY) until dry. Then, plates were washed with TBS and blocked with 1% (wt/vol) gelatin for 1 hour at room temperature. Plates were again washed three times with TBS and then incubated overnight with a monoclonal anti-MUC5B antibody (2 μg/ml; Invitrogen). Wells were washed with TBS, and 100 μl of AP-conjugated goat anti-mouse IgG (0.2 μg/ml; KPL) was dispensed into each well. After one additional hour, plates were washed with TBS. Color was developed with p-nitrophenyl phosphate and stopped with 3 N NaOH. Absorbance was read at 410 nm. Results were expressed as % changes at A410 above the PBS control. This was necessary since purified MUC5B is not available to calibrate the assay.
Statistical Analysis
Data were expressed as mean ± SEM. Differences between multiple groups were compared using a one-way ANOVA followed by the Tuckey Kramer honestly significant difference test. Levene test was used to analyze the homogeneity of variances. Significance was accepted at P < 0.05.
RESULTS
MUC5B Protein Expression Is Increased in Airway Epithelium from Smokers
Since chronic bronchitis due to cigarette smoke is associated with mucus hypersecretion, we evaluated MUC5B protein expression on tracheobronchial tissues obtained from normal and smoker lung donors by immunofluorescence. Tissue sections shown in Figure 1 were obtained from two individual lung donors and are representative of the variability of all samples tested (n = 10). Figure 1A shows that in nonsmokers MUC5B is limited to submucosal glands. In contrast, in smokers (Figure 1B) MUC5B expression was also present in surface epithelium. Figure 1C shows nonimmune controls.
Figure 1.
MUC5B protein expression is increased in individual smoker donors. Sections of human trachea from (A) nonsmoker and (B) smoker donors were incubated with polyclonal antibody against human MUC5B or nonimmune rabbit IgG (C ), followed by anti-rabbit IgG coupled to AlexaFluor 555, and scanned using fluorescent microscopy (magnification: ×10). Nuclei were labeled with DAPI.
MUC5B Expression in Smokers' Airways
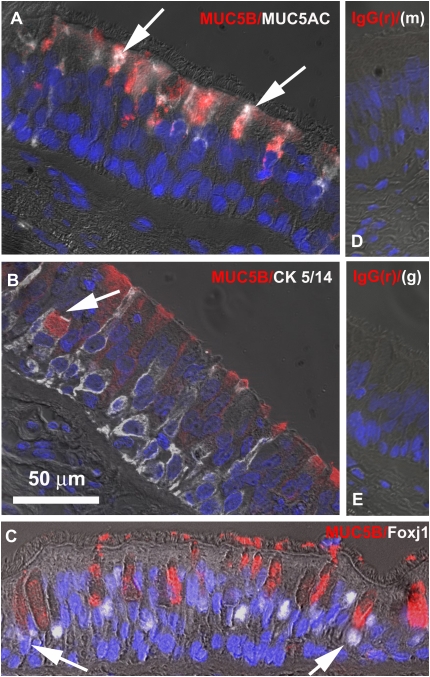
To further study MUC5B protein localization in surface airway epithelium from smokers, we assessed MUC5B double immunolabeling with MUC5AC (goblet cell marker), cytokeratin 5/14 (basal cell marker), or Foxj1 (ciliated cell marker) as described in Materials and Methods. White arrows in Figure 2A point toward MUC5B (red labeling) co-localization with MUC5AC (white labeling), indicating that MUC5B is produced by goblet cells. In addition, MUC5B/cytokeratin 5/14 immunofluorescence image suggests that this mucin is also expressed in basal cells (white arrow in Figure 2B). Foxj1 co-labeling results were not completely conclusive since few ciliated cells seem to be labeled with MUC5B (white arrows in Figure 2C), even though this could be due to overlapping of ciliated and goblet cells in these sections.
Figure 2.
Nonglandular MUC5B is mostly expressed by airway surface goblet cells in smoker donors. Sections of three smoker's human tracheas were double-labeled with MUC5B and (A) MUC5AC, (B) cytokeratin (CK) 5/14, or (C ) Foxj1 and visualized using fluorescent microscopy (magnification: ×63). Nonimmune controls using nonimmune IgGs from rabbit (r), mouse (m), and goat (g) are shown in D and E.
MUC5B Protein Expression Is Induced by ROS
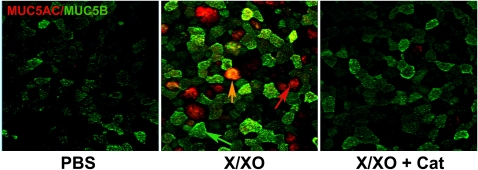
Since MUC5B is up-regulated in smokers and ROS are main components of cigarette smoke, we assessed whether oxidative stress was able to induce MUC5B expression in our cultures. NHBE cells were exposed to ROS generated by X/XO, and mucin expression was assessed by immunofluorescence. The concentration of X/XO used to generate ROS did not induce cytotoxicity in our cultures (see Figure E1 in the online supplement). As observed in smoker tissue sections, ROS induced MUC5B expression that co-localized with MUC5AC (Figure 3). Since oxidative stress (31, 44) also induced goblet cell metaplasia, these results give us confidence that this in vitro model can be used to explore the mechanisms of ROS-induced MUC5B expression.
Figure 3.
Reactive oxygen species (ROS) induce MUC5B protein. Normal human bronchial epithelial (NHBE) cells exposed to PBS or X/XO in the presence or the absence of catalase (Cat) were labeled using antibodies against MUC5B (green) and MUC5AC (red) and analyzed using fluorescence microscopy (magnification: ×20). Red arrow indicates a cell immunolabeled only with MUC5AC; green arrow shows a cell only positive for MUC5B; and orange arrow denotes a cell where MUC5AC and MUC5B are co-localized.
ROS-Induced EGFR and 44/42MAPK Activation in Human Airway Epithelial Cells Is Mediated by CD44
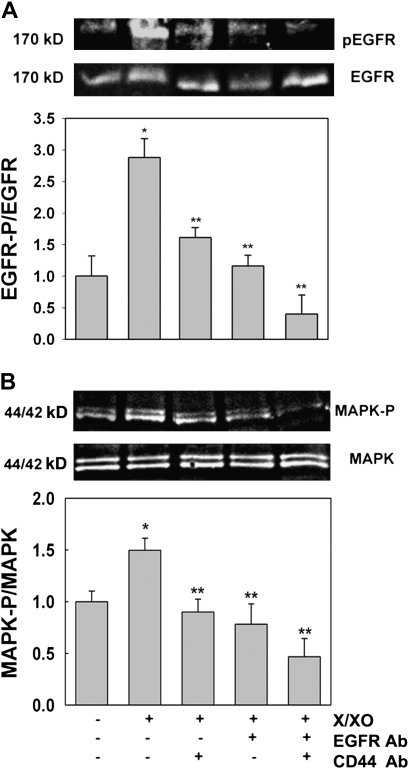
We have previously shown in NHBE cells that ROS induced hyaluronan depolymerization and EGFR/44/42MAPK activation that results in MUC5AC up-regulation (31). To further study whether the hyaluronan receptor CD44 was involved in this mechanism, NHBE cells were treated apically with PBS or X/XO for 30 minutes in the presence or the absence of CD44- and/or EGFR-blocking antibodies and assessed for EGFR and 44/42MAPK activation (measured as EGFR and 44/42MAPK phosphorylation, respectively) as described in Materials and Methods. Treatment with PBS containing only EGFR- and/or CD44-blocking antibodies did not modify the basal levels of EGFR and 44/42MAPK activation in our cultures (results not shown). Figure 4A shows that X/XO induced EGFR activation (2.9- ± 0.3-fold change versus PBS 1.0 ± 0.3, P < 0.05). This effect was reduced by blocking CD44 or EGFR (1.6 ± 0.2 and 1.2 ± 0.2, respectively; all P < 0.05 versus X/XO). Moreover, when both antibodies were used simultaneously, X/XO effect was completely blocked (0.4 ± 0.3, P < 0.05 versus X/XO). Figure 4B shows that these findings correlated with 44/42MAPK downstream signaling. We found that 44/42MAPK phosphorylation increased 1.5 ± 0.1 versus PBS 1.0 ± 0.1 (P < 0.05). This effect was blocked by inhibiting CD44 (0.9 ± 0.1, P < 0.05 versus X/XO), EGFR (0.8 ± 0.2, P < 0.05 versus X/XO), or both (0.5 ± 0.2, P < 0.05 versus X/XO). These results indicate that both CD44 and EGFR are involved in ROS-induced 44/42MAPK activation.
Figure 4.
ROS-induced epidermal growth factor receptor (EGFR) and 44/42MAPK (mitogen-activated protein kinase) activation is mediated by CD44 in NHBE cultures. Cells were exposed for 30 minutes to apical PBS (control), X/XO or X/XO plus catalase (Cat), or functionally blocking anti-CD44 or/and anti-EGFR antibodies (CD44 Ab and EGFR Ab respectively). Cell lysates were analyzed by Western blot. (A) Top gel, pEGFR; bottom gel, total EGFR; graph, pEGFR/EGFR ratios. (B) Top gel, p44/42MAPK; bottom gel, total 44/42MAPK, graph, pMAPK/MAPK ratios. Blot images correspond to one lung donor that is representative of three lung donors. Bar graphs represent the mean ± SE obtained from three different lung donors examined. *P < 0.05 compared with PBS (nontreated control); **P < 0.05 compared with X/XO.
X/XO Induce CD44/EGFR Co-Immunoprecipitation
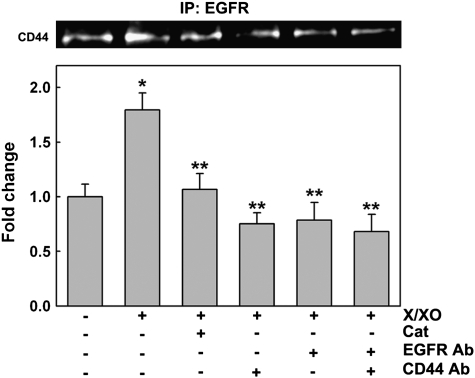
To address the question of whether oxidative stress induces a direct interaction between CD44 and EGFR, NHBE cells were exposed to PBS (control) or X/XO, in the presence or the absence of catalase or blocking antibodies against CD44 and/or EGFR as described above. As has been described previously (36), cells were lysed under special conditions in which CD44/EGFR heterodimers were not dissociated (see Materials and Methods). Cell lysates were immunoprecipitated with an agarose-conjugated anti-EGFR antibody, followed by immunoblotting with anti-CD44 antibody. Figure 5 shows that X/XO treatment increased CD44/EGFR co-immunoprecipitation (1.8- ± 0.2-fold change) compared with the basal level of CD44/EGFR association present on PBS (1.0 ± 0.1, P < 0.05). This effect was inhibited by catalase (0.9 ± 0.1, P < 0.05 versus X/XO) and by blocking CD44, EGFR, or both receptors together (0.8 ± 0.1, 0.8 ± 0.2, and 0.7 ± 0.2, respectively; all P < 0.05 versus X/XO), confirming that oxidative stress induces CD44/EGFR interaction in NHBE cells.
Figure 5.
ROS induce CD44/EGFR interaction on NHBE cells. NHBE cells treated 30 minutes with PBS or X/XO in the presence or absence of catalase (Cat), blocking antibodies against CD44 (CD44 Ab), and/or EGFR (EGFR Ab) were lysed and immunoprecipitated (IP) with an agarose-conjugated anti-EGFR antibody, followed by immunoblotting using anti-CD44 antibody. Results were expressed as fold change versus PBS. Blot images correspond to one lung donor that is representative of three lung donors examined. Mean ± SE was obtained from three different lung donors. *P < 0.01 compared with PBS (nontreated control), ** P < 0.05 compared with XXO.
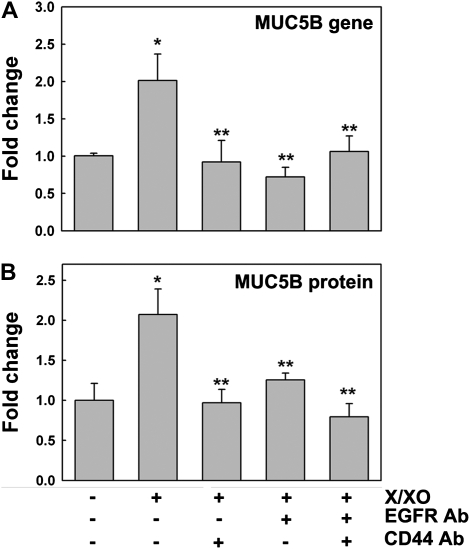
Oxidative Stress Induces MUC5B mRNA and Protein Expression that Is Mediated by CD44/EGFR
To confirm that X/XO-induced MUC5B mRNA and protein expression is mediated by CD44/EGFR, qPCR and ELISA were used as described in Materials and Methods. Figure 6B shows that ROS induced MUC5B mRNA expression (2.0- ± 0.4-fold, P < 0.05) compared with PBS control (1.0 ± 0.1). The increase in MUC5B expression was inhibited by blocking CD44, EGFR, or both receptors (0.9 ± 0.3, 0.7 ± 0.1, 1.1 ± 0.2, respectively; all P < 0.05 versus X/XO). These blocking antibodies did not modify MUC5B mRNA basal levels. In addition, rat or mouse isotype control IgGs did not modify MUC5B gene expression in PBS- or X/XO-treated cells (Figure E2). Similar results were obtained for MUC5B protein expression (Figure 6B), since X/XO induced MUC5B (2.1- ± 0.3-fold change versus 1.0 ± 0.2 on PBS; P < 0.05), an effect also inhibited by blocking CD44, EGFR, or both receptors (0.9 ± 0.2, 1.2 ± 0.1, 0.8 ± 0.2, respectively; all P < 0.05 versus X/XO). These results provide evidence that ROS-induced increases in MUC5B mRNA and protein expression are mediated by CD44/EGFR in NHBE cells, since blocking either receptor is sufficient to inhibit MUC5B up-regulation.
Figure 6.
ROS-mediated MUC5B gene and protein expression is dependent on CD44/EGFR interaction. NHBE cultures exposed to PBS or X/XO in the presence or absence of blocking antibodies against CD44 (CD44 Ab) and/or EGFR (EGFR Ab) were analyzed for (A) MUC5B gene (by qPCR) and (B) protein expression (by ELISA). Mean ± SE obtained from three different lung donors. *P < 0.01 compared with PBS (nontreated control), **P < 0.05 compared with XXO.
Hyaluronan Oligomers Induce EGFR and 44/42MAPK Activation
To investigate whether hyaluronan fragments (i.e., through their interaction with the hyaluronan receptor CD44) induce EGFR and 44/42MAPK signaling, NHBE cells (n = 3 lung donors) were exposed for 30 minutes to PBS (control) or 10 μg/ml of HA14 in the presence or the absence of CD44 and/or EGFR-neutralizing antibodies. EGFR and 44/42MAPK activation were analyzed by Western blot as described in Materials and Methods. EGFR phosphorylation increased significantly after HA14 treatment (3.1- ± 0.7-fold change versus 1.0 ± 0.2 on PBS; P < 0.05, Figures 7A and 7B). HA14-induced EGFR activation was prevented by blocking antibodies against CD44, EGFR, or both (1.0 ± 0.1, 0.6 ± 0.1, and 0.3 ± 0.1, respectively; all P < 0.05 versus HA14). These findings support the notion that the interaction of hyaluronan oligosaccharides with CD44 is sufficient to induce EGFR activation in NHBE cells. The same pattern was observed for 44/42MAPK (Figures 7C and 7D), where HA14 induced 44/42MAPK activation (4.3- ± 0.4-fold change versus 1.0 ± 0.2 on PBS; P < 0.05). This effect was inhibited significantly by blocking CD44 (1.7 ± 0.1; P < 0.05 versus HA14), EGFR (1.4 ± 0.2; P < 0.05 versus HA14), or both receptors (1.6 ± 0.2; P < 0.05 versus HA14). These results suggest that HA14/CD44 interaction promotes an EGFR-dependent 44/42MAPK phosphorylation in NHBE cells.
Figure 7.
Hyaluronan fragments induce EGFR and 44/42MAPK activation, and CD44/EGFR interaction in NHBE cultures. NHBE cells were exposed for 30 minutes to apical PBS (control) or HA14 in the presence or the absence of blocking antibodies against CD44 and/or EGFR (CD44 Ab and EGFR Ab, respectively). Cell lysates were analyzed by Western blot. (A) Top, pEGFR; bottom, total EGFR. (B) pEGFR/EGFR ratios. (C ) Top, p44/42MAPK; bottom, total 44/42MAPK. (D) pMAPK/ MAPK ratios. Cell lysates were also immunoprecipitated (IP) with an agarose conjugated anti-EGFR antibody, followed by immunoblotting using anti-CD44 antibody (E ). F shows the fold change versus PBS of CD44 pulled down by anti-EGFR antibody. Blot images correspond to one lung donor that is representative of three lung donors. Bar graphs represent the mean ± SE obtained from three different lung donors. *P < 0.05 compared with PBS (nontreated control); **P < 0.05 compared with HA14.
Hyaluronan Oligomers Induce CD44/EGFR Co-Immunoprecipitation
To determine whether HA14 induces CD44/EGFR direct interaction, as observed after ROS exposure, NHBE cells were exposed to PBS or HA14 (10 μg/ml), in the presence or the absence of blocking antibodies against CD44 and/or EGFR as showed above. Proteins were immunoprecipitated with agarose-conjugated anti-EGFR antibody and CD44 was assessed by Western blot. Figure 7F shows that HA14 increased CD44 co-immunoprecipitation with EGFR (3.9- ± 0.6-fold change versus 1.0 ± 0.1 on PBS; P < 0.05), an effect that was partially inhibited by blocking CD44 or EGFR individually, or both receptors together (2.0 ± 0.3, 1.4 ± 0.2, 1.6 ± 0.2, respectively; all P < 0.05 versus HA14). These results indicate that hyaluronan fragments induce CD44/EGFR interaction.
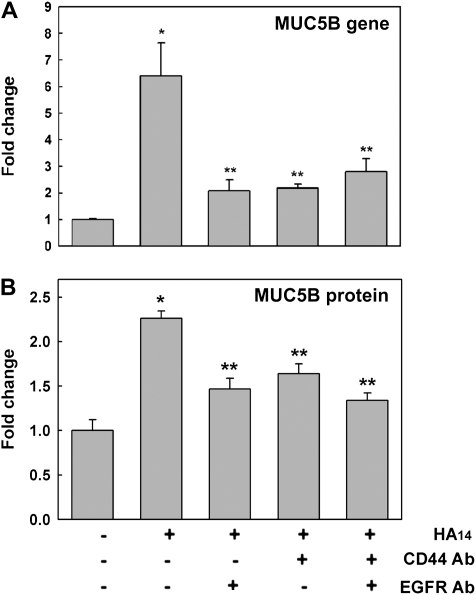
MUC5B mRNA and Protein Expression Induced by HA14 Is Mediated by CD44/EGFR
To investigate whether HA14-induced CD44/EGFR association and EGFR signaling are sufficient to induce MUC5B expression, mRNA was measured by qPCR at Day 1 and MUC5B protein was assessed by ELISA at Day 3 after X/XO removal as described above. Figure 8A shows that 1 day after exposure, HA14 increased MUC5B mRNA expression (6.4- ± 1.3-fold change; P < 0.05 compared with PBS control, 1.0 ± 0.1). This effect was inhibited by blocking EGFR, CD44, or both receptors (2.1 ± 0.4, 2.1 ± 0.2, 2.7 ± 0.5, respectively; all P < 0.05 versus HA14), but not by isotype control IgGs (Figure E2). Although there was a significant decrease of HA14-induced MUC5B mRNA expression by blocking CD44 and/or EGFR, MUC5B did not return to the baseline levels. These results suggest that other pathways may be involved in regulating HA14-induced MUC5B expression as well. A similar effect was observed in MUC5B protein levels (Figure 8B), where HA14 induced MUC5B (2.3- ± 0.1-fold change versus 1.0 ± 0.2 on PBS, P < 0.05), effect partially inhibited by blocking CD44, EGFR, or both (1.5 ± 0.1, 1.6 ± 0.1, 1.3 ± 0.1, respectively; all P < 0.05 versus HA14). Our observations strongly suggest that HA14-induced CD44/EGFR interaction is involved in the control of MUC5B mRNA and protein expression.
Figure 8.
MUC5B gene and protein expression induced by HA14 is mediated by CD44/EGFR. NHBE cultures exposed to PBS or HA14 in the presence or absence of blocking antibodies against CD44 (CD44 Ab) and/or EGFR (EGFR Ab) were analyzed for (A) MUC5B gene (by qPCR) and (B) protein expression (by ELISA). Mean ± SE obtained from four different lung donors. *P < 0.01 compared with PBS (nontreated control), **P < 0.05 compared with HA14.
DISCUSSION
In the present work, we explored MUC5B expression in airway epithelium under oxidative stress by localizing MUC5B in tissues obtained from smokers, and we used differentiated primary cultures of NHBE cells to explore the mechanisms of ROS-induced MUC5B induction. The data presented here support the hypothesis that MUC5B mRNA and protein expression induced by oxidative stress in NHBE cells is due, at least in part, to a cascade of events that include ROS-induced hyaluronan depolymerization, CD44/EGFR interaction, and 44/42MAPK activation.
We have found that MUC5B mucin, characteristic product of mucous cells from submucosal glands with no obvious inflammation or infection (20), is expressed in airway epithelium from smoker donors. This is in agreement with previous studies that showed MUC5B as the major mucin in sputum and bronchiolar lumen of patients with COPD (1, 16). However, other authors reported significant decreases (45) or no change (46) in MUC5B expression in smokers. These discrepancies may reflect differences in the methodologies used, including the choice of MUC5B antibodies and immunofluorescence protocols (fixation, antigen retrieval, deglycosilation, etc.). In addition, our results showed that MUC5B was not only produced by goblet cells in airway epithelium but also by basal cells. A similar result was reported by Lin and coworkers (47) in inflamed middle ear, where MUC5B mRNA was expressed in the entire epithelium. After confirming MUC5B up-regulation in smokers, we looked at the molecular mechanisms involved in MUC5B mRNA and protein expression induced by ROS. We used NHBE cells grown at the ALI and exposed to ROS generated by X/XO. We found that exogenous ROS increased not only MUC5AC protein expression as described previously (31, 48, 49) but also MUC5B. These data agreed with our observations on tracheobronchial tissue sections obtained by smoker donors. This up-regulation on MUC5B expression by oxidative stress was also observed by Wu and colleagues on NHBE cells that were exposed to PMA (30), a compound that induces endogenous H2O2 production (49). In contrast to our results, where the ROS-induced MUC5B expression was mediated by 44/42MAPK, the PMA effect was 44/42MAPK independent (30). Wu and colleagues also showed that IL-17/IL-6 induced MUC5B expression through 44/42MAPK (29), while UTP signals through 44/42MAPK-dependent and -independent pathways (28). In addition, differences between stimuli used to generate ROS (PMA instead of X/XO) and/or different cultures conditions (e.g., EGF concentration) may also contribute to the differential signaling pathways that promote MUC5B expression in NHBE cells. These results highlight the notion that the mechanisms responsible for regulation of MUC5B expression are complex, involving a number of biochemical and cellular pathways/events. We have previously showed that ROS-dependent 44/42MAPK activation is mediated by EGFR activation on NHBE cells (31). EGFR signaling requires homo- or heterodimerization followed by activation of its intrinsic tyrosine kinase activity (50). In the present work we provide evidence that ROS-dependent EGFR and 44/42MAPK activation was mediated by the hyaluronan receptor CD44. This transmembrane glycoprotein cooperates with tyrosine kinase receptors by mediating receptor activation or by modulating kinase activity (reviewed in Ref. 51). Heterodimerization between CD44 and growth factor receptors such as EGFR or erbB2 was found to be important for their activation in other cells (35, 52). In this report we provide evidence that CD44/EGFR interaction is involved in EGFR/44/42MAPK activation by ROS. Although CD44/EGFR interaction has been described previously in cell lines from oral squamous cell carcinoma, cervical carcinomas, human gliomas, and glioblastoma (35, 36, 52, 53), this is, to our knowledge, the first time it has been described in primary cells cultures from airways. Interestingly, these authors showed that CD44 acts a co-receptor for EGFR inducing hyaluronan-mediated 44/42MAPK phosphorylation. These findings agree with our observations that ROS-induced MUC5B mRNA and protein expression in NHBE cells is prevented by blocking CD44 and/or EGFR. Altogether, these results support the notion that ROS up-regulate MUC5B expression through a 44/42MAPK-dependent signaling pathway mediated by CD44/EGFR interaction.
Previously, we have shown that ROS induced hyaluronan depolymerization in NHBE and SMG cell cultures (31, 33, 54). Hyaluronan depolymerization is a relevant process, since hyaluronan biological functions are size dependent (55, 56). Whereas high-molecular-weight (MW) hyaluronan has been found to be anti-angiogenic, anti-inflammatory, and immunosuppressive; hyaluronan oligosaccharides have been reported to be angiogenic and proinflammatory (57), participating on development and maintenance of inflammatory events in the lung interstitium (58). Here, to evaluate whether hyaluronan oligomers could mediate ROS-induced MUC5B expression, we exposed NHBE cells to HA14 (corresponding to seven disaccharide repeats with a mass of 2,673 Da). This size of hyaluronan was chosen because it is larger than the minimum length (decasaccharide) that has been found to compete for the binding of high-MW hyaluronan to CD44 (59, 60). In addition, on the basis of recent structural analyses of a CD44/hyaluronan complex, it can be assumed that such oligomers will bind to CD44 molecules with a 1:1 stoichiometry and completely fill the ligand-binding site of the receptor (60). This MW is also within the range that has been associated with proinflammatory signaling (58, 61). We found that HA14 oligosaccharides induce 44/42MAPK and EGFR activation, an effect that we demonstrated to be dependent on the interaction of CD44 with EGFR; similar results have been reported previously in chondrosarcoma cells cultures (34). In this regard, we provide evidence that HA14 induces CD44/EGFR interaction (i.e., by co-immunoprecipitation). In addition, HA14 also induced MUC5B expression at both mRNA and protein levels. Therefore, we conclude that ROS-induced MUC5B expression is mediated by hyaluronan fragments via a 44/42MAPK-dependent pathway that requires CD44/EGFR interaction.
In addition, it is known that besides ROS, hyaluronidases (Hyals) also induce hyaluronan depolymerization (61). We have found that Hyal 1, 2, and 3 are expressed in airway epithelium and that they are up-regulated by TNF-α on NHBE cells (62). Since TNF-α also induce MUC5B up-regulation (63), it is likely that this effect could be mediated by hyaluronidase-generated hyaluronan fragments as well.
In summary, we found that oxidative stress in vivo (cigarette smoke) or in vitro (ROS) induces MUC5B up-regulation in airway epithelium. In addition, our studies provide a direct mechanistic link between airway oxidative stress responses and MUC5B hyperproduction, characteristic of asthma and COPD. This effect is mediated, at least in part, by hyaluronan fragments, CD44/EGFR association, and 44/42MAPK, suggesting that hyaluronan depolymerization during inflammatory responses could be directly involved in the induction of mucus hyperproduction.
Supplementary Material
Acknowledgments
The authors thank Drs. Matthias Salathe, Gregory Conner, and Nevis Fregein for critical comments and suggestions, and Nathalie Schmid for technical support.
This work was supported by AHA Scientist Development Grant 635093N (S.M.C.-M.), Biomedical Research Grants 07KN-02-12324 (S.M.C.-M.) and 07KB-02–12292 (R.F.), National Institutes of Health HL073156 (R.F.), and the James and Esther King Team Science Program (R.F.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2008-0073OC on August 28, 2008
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Caramori G, Di Gregorio C, Carlstedt I, Casolari P, Guzzinati I, Adcock IM, Barnes PJ, Ciaccia A, Cavallesco G, Chung KF, et al. Mucin expression in peripheral airways of patients with chronic obstructive pulmonary disease. Histopathology 2004;45:477–484. [DOI] [PubMed] [Google Scholar]
- 2.Dohrman A, Miyata S, Gallup M, Li JD, Chapelin C, Coste A, Escudier E, Nadel J, Basbaum C. Mucin gene (MUC 2 and MUC 5AC) upregulation by Gram-positive and Gram-negative bacteria. Biochim Biophys Acta 1998;1406:251–259. [DOI] [PubMed] [Google Scholar]
- 3.Hashimoto K, Graham BS, Ho SB, Adler KB, Collins RD, Olson SJ, Zhou W, Suzutani T, Jones PW, Goleniewska K, et al. Respiratory syncytial virus in allergic lung inflammation increases Muc5ac and gob-5. Am J Respir Crit Care Med 2004;170:306–312. [DOI] [PubMed] [Google Scholar]
- 4.Reid LM. Pathology of chronic bronchitis. Lancet 1954;266:274–278. [PubMed] [Google Scholar]
- 5.Wanner A. The role of mucus in chronic obstructive pulmonary disease. Chest 1990; 97(2, Suppl)11S–15S. [DOI] [PubMed] [Google Scholar]
- 6.Markewitz BA, Owens MW, Payne DK. The pathogenesis of chronic obstructive pulmonary disease. Am J Med Sci 1999;318:74–78. [DOI] [PubMed] [Google Scholar]
- 7.Dunnill MS. The pathology of asthma, with special reference to changes in the bronchial mucosa. J Clin Pathol 1960;13:27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aikawa T, Shimura S, Sasaki H, Ebina M, Takishima T. Marked goblet cell hyperplasia with mucus accumulation in the airways of patients who died of severe acute asthma attack. Chest 1992;101:916–921. [DOI] [PubMed] [Google Scholar]
- 9.Ordonez CL, Khashayar R, Wong HH, Ferrando R, Wu R, Hyde DM, Hotchkiss JA, Zhang Y, Novikov A, Dolganov G, et al. Mild and moderate asthma is associated with airway goblet cell hyperplasia and abnormalities in mucin gene expression. Am J Respir Crit Care Med 2001;163:517–523. [DOI] [PubMed] [Google Scholar]
- 10.Fahy JV, Schuster A, Ueki I, Boushey HA, Nadel JA. Mucus hypersecretion in bronchiectasis: the role of neutrophil proteases. Am Rev Respir Dis 1992;146:1430–1433. [DOI] [PubMed] [Google Scholar]
- 11.Hauber HP, Tsicopoulos A, Wallaert B, Griffin S, McElvaney NG, Daigneault P, Mueller Z, Olivenstein R, Holroyd KJ, Levitt RC, et al. Expression of HCLCA1 in cystic fibrosis lungs is associated with mucus overproduction. Eur Respir J 2004;23:846–850. [DOI] [PubMed] [Google Scholar]
- 12.Adler KB, Li Y. Airway epithelium and mucus: intracellular signaling pathways for gene expression and secretion. Am J Respir Cell Mol Biol 2001;25:397–400. [DOI] [PubMed] [Google Scholar]
- 13.Rose MC, Voynow JA. Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol Rev 2006;86:245–278. [DOI] [PubMed] [Google Scholar]
- 14.Voynow JA, Gendler SJ, Rose MC. Regulation of mucin genes in chronic inflammatory airway diseases. Am J Respir Cell Mol Biol 2006;34:661–665. [DOI] [PubMed] [Google Scholar]
- 15.Thornton DJ, Howard M, Khan N, Sheehan JK. Identification of two glycoforms of the MUC5B mucin in human respiratory mucus: evidence for a cysteine-rich sequence repeated within the molecule. J Biol Chem 1997;272:9561–9566. [DOI] [PubMed] [Google Scholar]
- 16.Kirkham S, Sheehan JK, Knight D, Richardson PS, Thornton DJ. Heterogeneity of airways mucus: variations in the amounts and glycoforms of the major oligomeric mucins MUC5AC and MUC5B. Biochem J 2002;361:537–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rose MC, Nickola TJ, Voynow JA. Airway mucus obstruction: mucin glycoproteins, MUC gene regulation and goblet cell hyperplasia. Am J Respir Cell Mol Biol 2001;25:533–537. [DOI] [PubMed] [Google Scholar]
- 18.Davies JR, Svitacheva N, Lannefors L, Kornfalt R, Carlstedt I. Identification of MUC5B, MUC5AC and small amounts of MUC2 mucins in cystic fibrosis airway secretions. Biochem J 1999;344:321–330. [PMC free article] [PubMed] [Google Scholar]
- 19.Henke MO, John G, Germann M, Lindemann H, Rubin BK. MUC5AC and MUC5B mucins increase in cystic fibrosis airway secretions during pulmonary exacerbation. Am J Respir Crit Care Med 2007;175:816–821. [DOI] [PubMed] [Google Scholar]
- 20.Hovenberg HW, Davies JR, Carlstedt I. Different mucins are produced by the surface epithelium and the submucosa in human trachea: identification of MUC5AC as a major mucin from the goblet cells. Biochem J 1996;318:319–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharma P, Dudus L, Nielsen PA, Clausen H, Yankaskas JR, Hollingsworth MA, Engelhardt JF. MUC5B and MUC7 are differentially expressed in mucous and serous cells of submucosal glands in human bronchial airways. Am J Respir Cell Mol Biol 1998;19:30–37. [DOI] [PubMed] [Google Scholar]
- 22.Wickstrom C, Davies JR, Eriksen GV, Veerman EC, Carlstedt I. MUC5B is a major gel-forming, oligomeric mucin from human salivary gland, respiratory tract and endocervix: identification of glycoforms and C-terminal cleavage. Biochem J 1998;334:685–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Groneberg DA, Eynott PR, Oates T, Lim S, Wu R, Carlstedt I, Nicholson AG, Chung KF. Expression of MUC5AC and MUC5B mucins in normal and cystic fibrosis lung. Respir Med 2002;96:81–86. [DOI] [PubMed] [Google Scholar]
- 24.Groneberg DA, Eynott PR, Lim S, Oates T, Wu R, Carlstedt I, Roberts P, McCann B, Nicholson AG, Harrison BD, et al. Expression of respiratory mucins in fatal status asthmaticus and mild asthma. Histopathology 2002;40:367–373. [DOI] [PubMed] [Google Scholar]
- 25.Chen Y, Zhao YH, Di YP, Wu R. Characterization of human mucin 5B gene expression in airway epithelium and the genomic clone of the amino-terminal and 5′-flanking region. Am J Respir Cell Mol Biol 2001;25:542–553. [DOI] [PubMed] [Google Scholar]
- 26.Kim DH, Chu HS, Lee JY, Hwang SJ, Lee SH, Lee HM. Up-regulation of MUC5AC and MUC5B mucin genes in chronic rhinosinusitis. Arch Otolaryngol Head Neck Surg 2004;130:747–752. [DOI] [PubMed] [Google Scholar]
- 27.Kamio K, Matsushita I, Hijikata M, Kobashi Y, Tanaka G, Nakata K, Ishida T, Tokunaga K, Taguchi Y, Homma S, et al. Promoter analysis and aberrant expression of the MUC5B gene in diffuse panbronchiolitis. Am J Respir Crit Care Med 2005;171:949–957. [DOI] [PubMed] [Google Scholar]
- 28.Chen Y, Zhao YH, Wu R. Differential regulation of airway mucin gene expression and mucin secretion by extracellular nucleotide triphosphates. Am J Respir Cell Mol Biol 2001;25:409–417. [DOI] [PubMed] [Google Scholar]
- 29.Chen Y, Thai P, Zhao YH, Ho YS, DeSouza MM, Wu R. Stimulation of airway mucin gene expression by interleukin (IL)-17 through IL-6 paracrine/autocrine loop. J Biol Chem 2003;278:17036–17043. [DOI] [PubMed] [Google Scholar]
- 30.Yuan-Chen Wu D, Wu R, Reddy SP, Lee YC, Chang MM. Distinctive epidermal growth factor receptor/extracellular regulated kinase-independent and -dependent signaling pathways in the induction of airway mucin 5B and mucin 5AC expression by phorbol 12-myristate 13-acetate. Am J Pathol 2007;170:20–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Casalino-Matsuda SM, Monzon ME, Forteza RM. Epidermal growth factor receptor activation by epidermal growth factor mediates oxidant-induced goblet cell metaplasia in human airway epithelium. Am J Respir Cell Mol Biol 2006;34:581–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Forteza R, Conner GE, Salathe M. Hyaluronan in the airways. In: Garg HG, Hales CA, editors. Chemistry and biology of hyaluronan. Kidlington, UK: Elsevier Ltd.; 2004. p. 323–338.
- 33.Manzanares D, Monzon ME, Savani RC, Salathe M. Apical oxidative hyaluronan degradation stimulates airway ciliary beating via RHAMM and RON. Am J Respir Cell Mol Biol 2007;37:160–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kobayashi H, Suzuki M, Kanayama N, Nishida T, Takigawa M, Terao T. CD44 stimulation by fragmented hyaluronic acid induces upregulation of urokinase-type plasminogen activator and its receptor and subsequently facilitates invasion of human chondrosarcoma cells. Int J Cancer 2002;102:379–389. [DOI] [PubMed] [Google Scholar]
- 35.Tsatas D, Kanagasundaram V, Kaye A, Novak U. EGF receptor modifies cellular responses to hyaluronan in glioblastoma cell lines. J Clin Neurosci 2002;9:282–288. [DOI] [PubMed] [Google Scholar]
- 36.Wakahara K, Kobayashi H, Yagyu T, Matsuzaki H, Kondo T, Kurita N, Sekino H, Inagaki K, Suzuki M, Kanayama N, et al. Bikunin down-regulates heterodimerization between CD44 and growth factor receptors and subsequently suppresses agonist-mediated signaling. J Cell Biochem 2005;94:995–1009. [DOI] [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention. Cigarette smoking among adults–United States, 1992, and changes in the definition of current cigarette smoking. MMWR Morb Mortal Wkly Rep 1994;43:342–346. [PubMed] [Google Scholar]
- 38.Jeffery PK. Histological features of the airways in asthma and COPD. Respiration 1992;59:13–16. [DOI] [PubMed] [Google Scholar]
- 39.Voynow JA, Fischer BM, Roberts BC, Proia AD. Basal-like cells constitute the proliferating cell population in cystic fibrosis airways. Am J Respir Crit Care Med 2005;172:1013–1018. [DOI] [PubMed] [Google Scholar]
- 40.Nlend MC, Bookman RJ, Conner GE, Salathe M. Regulator of G-protein signaling protein 2 modulates purinergic calcium and ciliary beat frequency responses in airway epithelia. Am J Respir Cell Mol Biol 2002;27:436–445. [DOI] [PubMed] [Google Scholar]
- 41.Mahoney DJ, Aplin RT, Calabro A, Hascall VC, Day AJ. Novel methods for the preparation and characterization of hyaluronan oligosaccharides of defined length. Glycobiology 2001;11:1025–1033. [DOI] [PubMed] [Google Scholar]
- 42.El-Bayoumy K, Das A, Narayanan B, Narayanan N, Fiala ES, Desai D, Rao CV, Amin S, Sinha R. Molecular targets of the chemopreventive agent 1,4-phenylenebis (methylene)-selenocyanate in human non-small cell lung cancer. Carcinogenesis 2006;27:1369–1376. [DOI] [PubMed] [Google Scholar]
- 43.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001;25:402–408. [DOI] [PubMed] [Google Scholar]
- 44.Takeyama K, Dabbagh K, Jeong Shim J, Dao-Pick T, Ueki IF, Nadel JA. Oxidative stress causes mucin synthesis via transactivation of epidermal growth factor receptor: role of neutrophils. J Immunol 2000;164:1546–1552. [DOI] [PubMed] [Google Scholar]
- 45.Innes AL, Woodruff PG, Ferrando RE, Donnelly S, Dolganov GM, Lazarus SC, Fahy JV. Epithelial mucin stores are increased in the large airways of smokers with airflow obstruction. Chest 2006;130:1102–1108. [DOI] [PubMed] [Google Scholar]
- 46.O'Donnell RA, Richter A, Ward J, Angco G, Mehta A, Rousseau K, Swallow DM, Holgate ST, Djukanovic R, Davies DE, et al. Expression of ErbB receptors and mucins in the airways of long term current smokers. Thorax 2004;59:1032–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin J, Tsuprun V, Kawano H, Paparella MM, Zhang Z, Anway R, Ho SB. Characterization of mucins in human middle ear and Eustachian tube. Am J Physiol Lung Cell Mol Physiol 2001;280:L1157–L1167. [DOI] [PubMed] [Google Scholar]
- 48.Fischer BM, Voynow JA. Neutrophil elastase induces MUC5AC gene expression in airway epithelium via a pathway involving reactive oxygen species. Am J Respir Cell Mol Biol 2002;26:447–452. [DOI] [PubMed] [Google Scholar]
- 49.Shao MX, Nadel JA. Dual oxidase 1-dependent MUC5AC mucin expression in cultured human airway epithelial cells. Proc Natl Acad Sci USA 2005;102:767–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Olayioye MA, Neve RM, Lane HA, Hynes NE. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J 2000;19:3159–3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ponta H, Sherman L, Herrlich PA. CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol 2003;4:33–45. [DOI] [PubMed] [Google Scholar]
- 52.Wobus M, Kuns R, Wolf C, Horn LC, Kohler U, Sheyn I, Werness BA, Sherman LS. CD44 mediates constitutive type I receptor signaling in cervical carcinoma cells. Gynecol Oncol 2001;83:227–234. [DOI] [PubMed] [Google Scholar]
- 53.Wang SJ, Bourguignon LY. Hyaluronan and the interaction between CD44 and epidermal growth factor receptor in oncogenic signaling and chemotherapy resistance in head and neck cancer. Arch Otolaryngol Head Neck Surg 2006;132:771–778. [DOI] [PubMed] [Google Scholar]
- 54.Casalino-Matsuda SM, Monzon ME, Conner GE, Salathe M, Forteza RM. Role of hyaluronan and reactive oxygen species in tissue kallikrein-mediated epidermal growth factor receptor activation in human airways. J Biol Chem 2004;279:21606–21616. [DOI] [PubMed] [Google Scholar]
- 55.Powell JD, Horton MR. Threat matrix: low-molecular-weight hyaluronan (HA) as a danger signal. Immunol Res 2005;31:207–218. [DOI] [PubMed] [Google Scholar]
- 56.Wuthrich RP. The proinflammatory role of hyaluronan-CD44 interactions in renal injury. Nephrol Dial Transplant 1999;14:2554–2556. [DOI] [PubMed] [Google Scholar]
- 57.Camenisch TD, McDonald JA. Hyaluronan: is bigger better? Am J Respir Cell Mol Biol 2000;23:431–433. [DOI] [PubMed] [Google Scholar]
- 58.McKee CM, Penno MB, Cowman M, Burdick MD, Strieter RM, Bao C, Noble PW. Hyaluronan (HA) fragments induce chemokine gene expression in alveolar macrophages: the role of HA size and CD44. J Clin Invest 1996;98:2403–2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tammi R, MacCallum D, Hascall VC, Pienimaki JP, Hyttinen M, Tammi M. Hyaluronan bound to CD44 on keratinocytes is displaced by hyaluronan decasaccharides and not hexasaccharides. J Biol Chem 1998;273:28878–28888. [DOI] [PubMed] [Google Scholar]
- 60.Banerji S, Wright AJ, Noble M, Mahoney DJ, Campbell ID, Day AJ, Jackson DG. Structures of the Cd44-hyaluronan complex provide insight into a fundamental carbohydrate-protein interaction. Nat Struct Mol Biol 2007;14:234–239. [DOI] [PubMed] [Google Scholar]
- 61.Stern R, Asari AA, Sugahara KN. Hyaluronan fragments: an information-rich system. Eur J Cell Biol 2006;85:699–715. [DOI] [PubMed] [Google Scholar]
- 62.Monzon ME, Manzanares D, Schmid N, Casalino-Matsuda SM, Forteza RM. Hyaluronidase expression and activity is regulated by pro-inflammatory cytokines in human airway epithelial cells. Am J Respir Cell Mol Biol 2008;39:289–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smirnova MG, Kiselev SL, Birchall JP, Pearson JP. Up-regulation of mucin secretion in HT29-MTX cells by the pro-inflammatory cytokines tumor necrosis factor-alpha and interleukin-6. Eur Cytokine Netw 2001;12:119–125. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.