Abstract
NF-κB activation in bronchial epithelial cells is important for the development of allergic airway inflammation, and may control the expression of critical mediators of allergic inflammation such as thymic stromal lymphopoietin (TSLP) and the chemokine CCL20. Members of the caspase recruitment domain (CARD) family of proteins are differentially expressed in tissue and help mediate NF-κB activity in response to numerous stimuli. Here we demonstrate that CARMA3 (CARD10) is specifically expressed in human airway epithelial cells, and that expression of CARMA3 in these cells leads to activation of NF-κB. CARMA3 has recently been shown to mediate NF-κB activation in embryonic fibroblasts after stimulation with lysophosphatidic acid (LPA), a bioactive lipid-mediator that is elevated in the lungs of individuals with asthma. Consistent with this, we demonstrate that stimulation of airway epithelial cells with LPA leads to increased expression of TSLP and CCL20. We then show that inhibition of CARMA3 activity in airway epithelial cells reduces LPA-mediated NF-κB activity and the production of TSLP and CCL20. In conclusion, these data demonstrate that LPA stimulates TSLP and CCL20 expression in bronchial epithelial cells via CARMA3-mediated NF-κB activation.
Keywords: bronchial epithelial cells, asthma, lysophosphatidic acid, thymic stromal lymphopoietin, CARMA3
CLINICAL RELEVANCE
We demonstrate that CARMA3 is highly expressed in airway epithelial cells and that it mediates thymic stromal lymphopoietin and CCL20 expression in response to lysophosphatidic acid stimulation. These data illuminate a novel inflammatory pathway that could be important in asthma pathogenesis.
Asthma is a syndrome broadly defined by inflammation of the airways associated with airway hyperresponsiveness and mucus hypersecretion (1). In most cases, the airway inflammation characteristic of asthma results from an allergic-type reaction to an inhaled substance from the environment (so-called allergic asthma). In response to antigen exposure, the airways develop a predominantly eosinophilic inflammation with prominent edema and mucus production. One of the earliest steps in the establishment of allergic sensitization is the generation of an antigen-specific T cell response, which results from engagement of T cells by antigen-presenting dendritic cells (2). A network of dendritic cells is located under the airway epithelium, where they can survey the airway for invading pathogens and inhaled antigens (3, 4). When properly stimulated, these dendritic cells will mature and present antigen with other secondary activating signals to T cells (5). It is thought that adjuvant signals from airway epithelium, generated in response to inhaled stimuli, influence the migration and maturation state of dendritic cells and T cells, and helps determine whether a particular allergen will trigger a Th2-type inflammatory response (3, 6–9). In particular, the production of thymic stromal lymphopoietin (TSLP) and the chemokine CCL20 by epithelial cells is critical for maturation of airway dendritic cells and for the homing of myeloid dendritic cells and T cells to the airways (10–19). Consistent with this, TSLP and CCL20 are expressed in the airways of individuals with asthma and are known to contribute to the development of allergic airway inflammation in murine models of asthma (10–19). These data suggest that epithelial cell production of TSLP and CCL20 is likely a critical mechanism for the establishment of allergic airway inflammation, and that understanding the mechanisms that regulate their production in epithelial cells may provide novel insight into the nature of the interaction between innate and adaptive immunity in asthma.
The transcription factor NF-κB regulates TSLP and CCL20 expression (20–23), and therefore is an ideal therapeutic target for inhibiting the production of these important cytokines. Much attention has recently focused on molecular scaffolds that organize and facilitate NF-κB activation downstream of receptor signaling. Many of these proteins contain caspase recruitment domain (CARD) sequences that facilitate protein–protein interactions. CARD-containing proteins are now recognized as key components of the pathways that link innate and adaptive immunity (24), especially in processes that use NF-κB signaling (25).
To investigate the role of CARD proteins in NF-κB signaling in airway epithelial cells, we performed a functional screen of CARD proteins in airway epithelial cells and A549 cells (an alveolar epithelial cell line). Intersection of expression data and NF-κB–induced luciferase activity in these cells suggests that the CARD protein CARMA3 (CARD10) may have a specific role for NF-κB activation in airway epithelial cells. CARMA3 is known to activate NF-κB through its interactions with Bcl10 and NEMO/IKKγ (26, 27), and has recently been shown to mediate G protein–coupled receptor NF-κB activation (28, 29). Specifically, deletion of CARMA3 in embryonic fibroblasts eliminated both lysophosphatidic acid (LPA)- and endothelin-1–stimulated NF-κB activation and IL-8 production (28, 30, 31). LPA is a bioactive lysophospholipid that is a component of normal plasma, binds to receptors expressed on leukocytes and structural lung cells (including airway epithelial cells), and is reported to affect cell growth and stimulate cytokine production (32–34). In addition, LPA is detectable in the lungs of humans at baseline and its expression increases during allergic inflammation, suggesting a possible role in the pathophysiology of asthma (35). On the basis of these findings, we investigated the role of CARMA3 in LPA-mediated signaling in airway epithelial cells.
MATERIALS AND METHODS
Reagents
LPA and sphingosine-1-phosphate (S1P) were purchased from Avanti Polar Lipids (Alabaster, AL) and prepared according to the manufacturer's instructions. The antibody to CARMA3 was purchased from Abcam (Cambridge, MA).
Expression Constructs
Full-length mouse CARMA3 (CARMA3 WT) was obtained from Open Biosystems (Huntsville, AL) and subcloned into the pCMV-Myc vector from Clontech (Mountain View, CA). The dominant-negative construct (CARMA3 L51R Myc) was generated by site-directed mutagenesis PCR. Sequences of all cloned cDNAs were confirmed by DNA sequencing by the MGH Sequencing Core. CARD gene identification information is provided in Table E1 in the online supplement. The NF-κB–dependent firefly luciferase reporter was described previously (36, 37). The pRL-TK Renilla construct was obtained from Promega (Madison, WI). DNA lentiviral constructs containing shRNA against human CARMA3 (TRCN107246-49) were obtained from the RNAi consortium (TRC, http://www.broad.mit.edu/genome_bio/trc/) supply at the Broad Institute (Cambridge, MA) (38). The shRNA target sequence of TRCN107247 is 5′-AAACCCAGGGCTGCCTTGGAAAAG-3′ and that for TRCN107248 is 5′-AAACCCAGGGCTGCCTTGGAAAAG-3′. Both are cloned into the pLKO.1puro vector. Empty pLKO.1puro was used as a control.
Epithelial Cell Culture
Mouse tracheal epithelial cells were cultured using a published protocol (39). Briefly, tracheas were removed and digested overnight with pronase. The released cells were collected and then further selected by removing cells that adhered to a culture dish. The cells were then plated onto collagen-coated Transwells (Fisher Scientific, Pittsburgh, PA) and allowed to grow in media supplemented with epidermal growth factor and retinoic acid as described previously (39). After 5 to 7 days, an air–liquid interface was created and the cells were allowed to grow for an additional 7 to 10 days. Purity of the culture was determined by the ability to maintain an air–liquid interface, the presence of beating cilia, and expression of the airway epithelial cell transcription factor (TTF-1). Normal human bronchial epithelial (NHBE) cells and airway smooth muscle cells were obtained from Lonza (Basel, Switzerland) and grown in bronchial epithelial cell growth media (BEGM) or smooth muscle cell media (Lonza) according to the manufacturer's instructions. After growth in tissue flasks, the NHBE cells were plated onto collagen-coated Transwells (Fisher Scientific) and allowed to grow in BEGM. After 7 days, an air-liquid interface was created and the cells were allowed to grow for an additional 7 to 10 days, before being used in experiments. A549 cells, a human alveolar epithelial cell line, were obtained from ATCC (Manassas, VA) and were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum plus supplemental iron. All cells were grown in a humidified 5% CO2 incubator at 37°C.
Mouse Lung Cell Isolation
Mouse tracheal epithelial (MTE) cells, lung endothelial cells, and lung fibroblasts were isolated and cultured as previously described (39–41). Pulmonary macrophages and dendritic cells were isolated from single cell suspensions of mouse lungs by cell sorting using the marker CD11c and autofluorescence as previously reported (42).
Epithelial Cell Stimulation
For all cell types, normal culture media was replaced with low serum (1%) media for 24 hours. The cells were then stimulated with LPA, 10 ng/ml TNF-α, or 1 ng/ml IL-1β (both from R&D Systems, Minneapolis, MN) for the indicated times. Concentrations were based on previously reported work with these cells (20, 28).
Functional Assay: CARD Domain NF-κB Screen
A549 cells were transfected with 250 ng of indicated CARD plasmids, plus NF-κB luciferase reporter (5 ng) and Renilla (0.05 ng) plasmids. cDNA clones were obtained from Open Biosystems (Hunstville, AL) and Origene (Rockville, MD). After 24 hours, the cells were lysed and luciferase activities were determined with a dual-luciferase reporter assay system (Promega, Madison, WI) according to the manufacturer's instructions. NF-κB activity was assessed by normalization of firefly luciferase activity to Renilla luciferase activity.
Immunohistochemistry
Normal C57BL/6 mice were killed by CO2 inhalation. The lungs were flushed free of blood by slowly injecting 10 ml of PBS into the right ventricle before excision. The left lung was inflated with 10% buffered formalin to 25 cm H2O pressure and transferred into vials containing 10% buffered formalin. Multiple paraffin-embedded 5-μm sections of the entire mouse lung were prepared. Lung sections were dewaxed in xylene, hydrated, and incubated in 5% normal horse serum to pre-absorb nonspecific immunoglobulin binding sites. The section was flooded with a rabbit polyclonal primary antibody to CARMA3 (1:300; Abcam) and incubated in a humid chamber overnight, followed by a biotinylated rabbit anti-sheep secondary antibody (1:200 dilution; Jackson Immunoresearch, West Grove, PA) for 1 hour and then 1:1,000 streptavidin-labeled Alexa Flour 568 (Molecular Probes/Invitrogen, Carlsbad, CA) for 1 hour.
A549 Stimulation and Luciferase Assay
A quantity of 5 × 105 A549 cells was seeded into single wells of a 12-well plate; 24 hours later, the cells were transfected as follows: 500 ng cDNA, 10 ng NF-κB luciferase reporter, and 0.1 ng Renilla luciferase reporter using Lipofectamine 2000 (Invitrogen) according to the manufacturer's recommended protocol. Cells were stimulated as outlined. The cells were then lysed and luciferase activity was determined with a dual-luciferase reporter assay system (Promega) according to the manufacturer's instructions. NF-κB activity was assessed by normalization of firefly luciferase activity to Renilla luciferase activity.
Transfection of NHBE Cells with shRNA Containing DNA Constructs
A quantity of 5 × 105 NHBE cells was seeded into single wells of a 6-well plate and cultured for 48 hours. The cells were then transfected using Primefect reagent (Lonza) and 1 μg of DNA construct containing a puromycin resistance gene and an shRNA specific to CARMA3 or a control vector according to the manufacturer's protocol. After 48 hours, the medium was changed and puromycin was added (2 mg/ml). After 24 to 48 hours, the cells were stimulated as described above and then harvested for analysis.
Quantitative PCR
RNA from various resting, unstimulated, and stimulated cell lines was isolated (RNeasy, Qiagen, Valencia, CA), and cDNA was prepared using the iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA). Gene expression was quantified on an iQ5 RT PCR Detection System (Bio-Rad) using SYBR green (iQ SYBR Green Supermix; Bio-Rad) according to the manufacturer's suggested protocol. All values are shown normalized to GAPDH values. Samples were assayed in duplicate. Primer sequences used were selected using the Massachusetts General Hospital (MGH) PrimerBank (pga.mgh.harvard.edu/primerbank/). All PCR products were visualized on a 2% agarose gel, and the bands were confirmed to be the correct sizes.
Protein Quantification
Supernatants from cell culture assays were collected and then used undiluted in commercial ELISA kits for IL-8, CCL20, and TSLP (R&D Systems) according to the manufacturer's protocol.
Data Analysis
Data are expressed as mean ± SEM. Differences between means were tested for statistical significance using unpaired t tests as appropriate to the experiment. For comparisons of multiple time-points or dose–responses a two-way ANOVA was used. From such comparisons, differences yielding P < 0.05 were judged to be significant.
RESULTS
An Integrative Genomics Approach Can Identify CARD Proteins Involved in NF-κB Signaling in Lung Epithelial Cells
We hypothesized that CARD proteins are critical mediators of NF-κB activation in airway epithelium (43, 44). We found expression of a number of CARD proteins in normal human bronchial epithelial (NHBE) cells (Figure 1A), including PYCARD (CARD5 or ASC), DDX58 (RIG-I), IFIH1 (MDA-5), Bcl10, and CARMA3. To determine the function of these CARD proteins in epithelial cells, we performed a gain-of-function cellular screen. Expression constructs for individual CARD proteins along with a NF-κB reporter plasmid were introduced into A549 cells, and 24 hours later reporter activities were measured. Expression of these CARD proteins in this cell line led to enhanced NF-κB–dependent luciferase activity (Figure 1B). Among the activators, CARMA3 enhanced reporter activity more than 80-fold and was expressed specifically in NHBE cells when compared with other cell types.
Figure 1.
Caspase recruitment domain (CARD) protein expression and activity in airway epithelium. (A) Expression of a subset of CARD-containing genes in human primary bronchial epithelial cells, human airway smooth muscle cells, Jurkat, and 293 cells. Cells were transfected with constructs expressing the indicated genes. QPCR was preformed using RNA isolated from the cells. GAPDH was used to normalize the values of CARD genes tested. (B) NF-κB activation mediated by expression of a series of CARD genes in A549 cells, as measured by luciferase activation. All experiments were performed at least two times.
CARMA3 Is Expressed in Mouse Airway Epithelial Cells
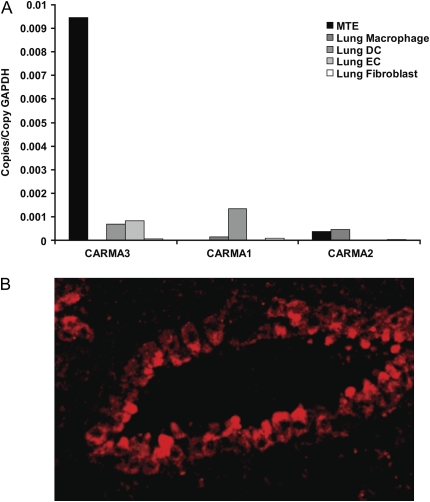
Our data are consistent with prior data demonstrating high expression of CARMA3 in human lung (27). In addition, our data suggest that relative to other CARD proteins, CARMA3 is specifically expressed in airway epithelium. To investigate the specificity of CARMA3 in several different lung cells, we used a panel of cell types isolated from murine lungs, including mouse tracheal epithelial cells (MTE), endothelial cells (EC), fibroblasts, macrophages, and dendritic cells (DC). We isolated RNA from these cells and measured CARMA gene expression. CARMA3 was most highly expressed in the MTE cells, with 9-fold greater expression in these cells compared with the other cell-types (Figure 2A). In addition, CARMA1 and CARMA2 were not expressed in the MTE cells. We next performed immunofluorescent staining with an antibody to CARMA3 on sections of normal mouse lung. There was prominent staining for CARMA3 in the airway epithelium on these sections but not in other cell-types in the lung (Figure 2B).
Figure 2.
CARMA3 is expressed in murine airway epithelial cells. (A) CARMA1, CARMA2, and CARMA3 RNA levels measured by QPCR and normalized to GAPDH in cell-types isolated and cultured from the lungs of mice. MTE, mouse tracheal epithelial cells; DC, dendritic cell; EC, endothelial cell. (B) Immunohistochemistry of normal mouse lung with stained with an antibody to CARMA3. There is impressive staining of bronchial epithelium.
LPA Stimulation of Bronchial Epithelial Cells Up-Regulates TSLP and CCL20 Expression
Our data suggest that airway epithelial cells may use CARMA3 for NF-κB activation in response to specific stimuli. Since TSLP and CCL20 expression in epithelial cells is controlled by NF-κB activation (20, 23, 28), we hypothesized that LPA, a mediator known to signal via CARMA3 (28, 29), will stimulate epithelial cells to express these mediators. We cultured NHBE cells on an air–liquid interface (ALI) and then stimulated them with 10 μM LPA for 4, 6, 18, 24, and 48 hours. We used 10 μM LPA for our initial experiments, as this was the concentration shown to be effective in prior studies (31, 32), and recent data from humans with asthma has demonstrated that airway concentrations are around 1 to 10 μM after allergen challenge (35). RNA expression of TSLP was increased in the NHBE cells after 4 hours of stimulation and peaked at 6 hours of stimulation to a level 49-fold greater than in unstimulated cells (Figure 3A). CCL20 RNA levels peaked with 4 hours of stimulation to a level 20-fold greater than in unstimulated cells. The levels then remained relatively elevated for 24 hours (4- to 6-fold greater than in unstimulated cells) (Figure 3B). After this, we stimulated the NHBE cells with increasing concentrations of LPA or the sphingolipid S1P for 6 hours and then measured the RNA levels of TSLP and CCL20. There was a nearly 6-fold increase in TSLP expression with treatment with 1 μM LPA and 40-fold increase in TSLP expression with 10 μM LPA treatment (Figure 3C). This increase was not seen with S1P stimulation (Figure 3C). Similarly, the expression of CCL20 and IL-8 (as shown by others [32, 45]) was up-regulated by 10 μM LPA stimulation by 10-fold and 4-fold, respectively, but not by S1P treatment (Figure 3D and data not shown). The protein level of TSLP in the supernatant 6 hours after 10 μM LPA stimulation was also increased 2.5-fold over unstimulated cells (Figure 3E) and was comparable to levels induced by TNF-α, which has been previously reported to stimulate TSLP production in epithelial cells (12, 20). We also measured the RNA expression levels of the five known LPA receptors (LPA1–5) in NHBE cells, which demonstrated expression of LPA1, LPA2, and LPA3, consistent with data from others (data not shown) (45).
Figure 3.
Thymic stromal lymphopoietin (TSLP) and CCL20 expression in normal human epithelial cells in response to LPA stimulation. (A and B) Normal human bronchial epithelial (NHBE) cells were grown on an air–liquid interface and stimulated with 10 μM lysophosphatidic acid (LPA) for the indicated time period. RNA was isolated from the cells and the levels of (A) TSLP and (B) CCL20 were determined by QPCR. Values are the mean of three to six samples ± SEM. *P < 0.05 versus unstimulated cells by unpaired t test. This experiment was repeated two times. (C and D) NHBE cells grown on an air–liquid interface were stimulated with increasing concentrations of LPA or sphongosine-1-phosphate (S1P) for 6 hours. RNA was isolated from the cells and the levels of (C) TSLP and (D) CCL20 were determined by QPCR. Values are the mean of three to six samples ± SEM. *P < 0.05 versus S1P stimulated cells by ANOVA. This experiment was repeated two times. (E) NHBE cells were grown on an air–liquid interface and stimulated for 6 hours with media alone (serum-free), 10 ng/ml TNF-α, 1 ng/ml IL-1β, or 10 μM LPA. TSLP expression was then analyzed by commercial enzyme-linked immunosorbent assay (ELISA). Values are the mean of four samples ± SEM. *P < 0.05 versus unstimulated cells by unpaired t test.
CARMA3 Mediates LPA-Stimulated TSLP and CCL20 Production by Lung Epithelial Cells
To determine if CARMA3 mediates LPA-stimulated TSLP production, we used the human alveolar epithelial cell line A549. These cells expressed CARMA3, but at levels 3-fold lower than the MTE or NHBE cells (Figure 4A), and did not have robust production of TSLP in response to LPA (data not shown). We transfected A549 cells with Myc-tagged wild-type (WT) murine CARMA3, a Myc-tagged dominant-negative mutant form of CARMA3 (L51R), or empty vector. As with CARMA1, the CARD motif of CARMA3 binds to Bcl10 directly and is required to induce NF-κB activation. A point mutation in the CARD sequence of CARMA1 (L39R) disrupted binding to Bcl10 and acted as a dominant-negative form of the protein (46). We mutated the corresponding conserved L51 to R in CARMA3 to mimic the CARMA1 dominant-negative mutant. Consistent with this, expression of the CARMA3 L51R construct led to minimal NF-κB–induced luciferase activity, while overexpression of CARMA3 WT protein led to a 25-fold increase in NF-κB activity (Figure 4B). When the transfected A549 cells were stimulated with LPA, there was a 15-fold increase in NF-κB–induced luciferase activity in cells trasnfected with a myc-control vector, while the cells transfected with WT CARMA3 had a greater than 70-fold in increase in NF-κB–induced luciferase activity. LPA stimulation of the cells transfected with the dominant-negative form of CARMA3, however, did not lead to an increase in NF-κB activation compared with the A549 cells transfected with the control vector (Figure 4B). We also measured TSLP expression and found that LPA increased TSLP RNA levels greater than 2-fold in cells transfected with WT CARMA3, but not in the cells transfected with the mutant dominant-negative form of CARMA3 (Figure 4C). TNF-α–mediated expression of TSLP was not affected (data not shown).
Figure 4.
CARMA3 mediates LPA-induced NF-κB activity and TSLP expression. (A) MTE, NHBE, and A549 cells were grown in culture for 1 to 2 weeks and then harvested for RNA isolation. CARMA3 RNA levels were measured by QPCR and normalized to GAPDH in the indicated cell types. (B) A549 cells were transfected with WT CARMA3, the L51R dominant-negative mutant form of CARMA3, or a control vector plus NF-κB luciferase reporter and Renilla plasmids. NF-κB activity in these cells was assessed by normalization of firefly luciferase activity to Renilla luciferase activity at baseline and after incubation for 6 hours with 10 μM LPA. This experiment was repeated two times. (C) TSLP RNA levels measured by QPCR and normalized to GAPDH in transfected A549 cells at baseline and after incubation for 6 hours with 10 μM LPA. Values are the mean of three samples ± SEM. *P < 0.05 by unpaired t test. This experiment was repeated two times.
As a final proof of concept, we sought to determine if shRNA-mediated knockdown of CARMA3 would inhibit LPA-mediated TSLP and CCL20 production in NHBE cells. We obtained four expression constructs containing shRNA sequences specific for human (and also murine) CARMA3. We then co-transfected A549 cells with Myc-tagged WT CARMA3 and the shRNA constructs, and then measured CARMA3 RNA and protein expression. We used an empty vector (pLKO.1puro) as a control (shRNA control). Two of the constructs, TRCN107247 and TRCN107248 (labeled shRNA47 and 48), led to 70 to 80% knockdown of CARMA3 at the RNA level and a dramatic decrease in CARMA3 protein production (Figure 5A). A similar amount of CARMA3 knockdown was seen in transfected NHBE cells (Figure E1). Based on this analysis, we used the shRNA48 construct to transfect NHBE cells for subsequent experiments. After transfection and puromycin selection, there was a 2.5-fold decrease in CARMA3 RNA levels in the NHBE cells transfected with shRNA48 compared with cells transfected with the control vector (Figure 5B). When the transfected cells were stimulated with 10 μM LPA for 6 hours, there was a greater than 900-fold increase in TSLP RNA levels and a greater than 15-fold increase in CCL20 RNA levels in the cells transfected with control vector, while the cells transfected with the shRNA48 construct had an attenuated response to LPA with 6-fold less TSLP RNA and 3-fold less CCL20 RNA compared with the cells transfected with the control vector (Figure 5C). Analysis of TSLP and CCL20 protein levels in the supernatents from these cells demonstrated 4-fold less TSLP and nearly 2-fold less CCL20 in the supernatants from LPA-stimulated cells transfected with the shRNA48 construct compared with the cells transfected with the control vector (Figure 5D).
Figure 5.
Knockdown of CARMA3 attenuates LPA induced TSLP and CCL20 expression in NHBE cells. (A) A549 cells were transfected with lentiviral vectors expressing shRNA sequences specific to CARMA3 or a control vector. CARMA3 RNA knockdown was assessed by measuring the level of CARMA3 expression by QCPR. Levels were normalized to A549 cells transfected with a control vector and expressed as relative expression compared with control levels. Values are the mean of three samples ± SEM. Western blot of cell lysates from A549 cells transfected with WT CARMA3 and constructs containing sequences for shRNA against CARMA3. Blots were probed with an antibody to Myc. (B) CARMA3 RNA levels measured by QPCR and normalized to GAPDH in NHBE cells after transfection with a construct (shRNA48) containing an shRNA specific to CARMA3 or a control vector. Values are the mean of three samples ± SEM. This experiment was repeated two times. (C) TSLP and CCL20 RNA levels measured by QPCR and normalized to GAPDH in transfected NHBE cells at baseline and after incubation for 6 hours with 10 μM LPA. There are at least six samples in each group pooled from two experiments. *P < 0.05 by unpaired t test. (D) TSLP and CCL20 protein levels in culture supernatants measured by ELISA in transfected NHBE cells at baseline and after incubation for 6 hours with 10 μM LPA. There are at least three samples in each group pooled from two experiments. *P < 0.05 by unpaired t test.
DISCUSSION
Activation of NF-κB is induced by a large and medically significant collection of signals that herald the onset of infection or injury and mobilize the resources of the immune system to meet the challenge. In addition, activation of NF-κB is seen in the context of immune system dysregulation in disorders such as allergic asthma, and thus is a therapeutic target for inflammatory diseases. Consistent with this, NF-κB activation in airway epithelium occurs quickly after allergen exposure in animal models and humans, and mediates expression of inflammatory cytokines (47–51), many of which play an important role in the pathogenesis of allergic airway inflammation (13, 21, 52–59). Furthermore, inhibition of NF-κB in airway epithelial cells effectively attenuates allergic inflammation in a murine model of asthma (60). Since complete inhibition of NF-κB would lead to profound immunosuppression (61), we have investigated whether disruption of accessory proteins in the NF-κB pathway may allow more cell- and pathway-specific inhibition of inflammation.
Many of the scaffold proteins that mediate NF-κB activation contain one or more CARD sequences that facilitate protein-protein interactions (25). A large family of CARD-containing proteins have now been identified that have very different functional roles and cell-specific expression profiles. For example, we have recently shown that CARMA1 helps mediate allergic airway inflammation via its role in T cell activation (62). In the current study, we investigated the expression of a panel of CARD proteins in airway epithelial cells. Our data demonstrate that CARMA3 is preferentially expressed in lung epithelium compared with other lung cell-types, and induces high-level NF-κB activation when expressed. Additional CARD proteins expressed in the bronchial epithelium include CARD domain containing helicase proteins DDX58 (RIG-I), melanoma-differentiation-associated gene 5 (MDA5/IFIH1/Helacard), and CARD5 (ASC). DDX58 and IFIH1 have an important role in the response to viral infections such as influenza and rhinovirus and may be particularly important in epithelial cell recognition of these viruses and the asthma response. CARD5 is important for formation of the inflammasome, which ultimately leads to the initiation of innate immunity. Although many of these proteins are likely important for allergic immune response in asthma, we chose to explore the role of CARMA3 in bronchial epithelial cells, based on its specificity to these cells and the high-level NF-κB activation induced by its expression.
The NF-κB–dependent cytokines, TSLP and CCL20, are produced by airway epithelial cells and are important for initiating allergic inflammation (14). Among its many functions, TSLP stimulates dendritic cell maturation, which in turn leads to effective antigen presentation to reactive T cells, thus initiating the adaptive immune response to an inhaled antigen. CCL20 is a chemokine that mediates chemotaxis via its receptor CCR6 (15, 23). CCR6 is expressed on memory T cells and dendritic cells, so it may help recruit these cells into the airway, further facilitating dendritic cell–T cell interactions (63, 64). Experiments in murine models of asthma have confirmed an important role for the CCL20-CCR6 axis in the pathogenesis of allergic airway inflammation (19). Therefore, understanding the mechanisms that lead to NF-κB–mediated TSLP and CCL20 production in epithelial cells after exposure to antigen may provide a novel means to prevent airway inflammation.
As mentioned previously, CARMA3 has been shown to mediate LPA-stimulated NF-κB activation in murine embryonic fibroblasts (28, 30, 31), and recent data suggest that LPA may have an important role in mediating airway epithelial cell function, including the release of inflammatory mediators (32–34). In addition, there are accumulating data suggesting a role for LPA in asthma (33–35). Based on this, we hypothesized that LPA stimulation of bronchial epithelial cells would activate NF-κB via CARMA3. Furthermore, since TSLP and CCL20 production from airway epithelial cells is controlled via NF-κB, we were interested in finding out if LPA would up-regulate TSLP and CCL20 production in airway epithelial cells (20–23). Our data clearly demonstrate that LPA, at concentrations seen in asthmatic airways, can stimulate TSLP and CCL20 production from airway epithelium. This was specific to LPA as stimulation with a related phopholipid, S1P, did not up-regulate these cytokines. This suggests that LPA, possibly released in response to allergen exposure, can stimulate epithelial cells via CARMA3-induced NF-κB activation to express TSLP and CCL20. These experiments reveal a novel biological pathway that may be important for the development of allergic inflammation in the lung.
Supplementary Material
This work was supported by an American Thoracic Society Unrestricted Research Award to B.D.M., and by National Institutes of Health grants AI062773 to R.J.X. and HL088297 to B.D.M. and R.J.X.
This article has an online supplement, available from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2008-0129OC on August 28, 2008
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Busse WW, Lemanske RF Jr. Asthma. N Engl J Med 2001;344:350–362. [DOI] [PubMed] [Google Scholar]
- 2.Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell 2001;106:255–258. [DOI] [PubMed] [Google Scholar]
- 3.Huh JC, Strickland DH, Jahnsen FL, Turner DJ, Thomas JA, Napoli S, Tobagus I, Stumbles PA, Sly PD, Holt PG. Bidirectional interactions between antigen-bearing respiratory tract dendritic cells (DCs) and T cells precede the late phase reaction in experimental asthma: DC activation occurs in the airway mucosa but not in the lung parenchyma. J Exp Med 2003;198:19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holt PG, Schon-Hegrad MA, Oliver J, Holt BJ, McMenamin PG. A contiguous network of dendritic antigen-presenting cells within the respiratory epithelium. Int Arch Allergy Appl Immunol 1990;91:155–159. [DOI] [PubMed] [Google Scholar]
- 5.de Jong EC, Smits HH, Kapsenberg ML. Dendritic cell-mediated T cell polarization. Springer Semin Immunopathol 2005;26:289–307. [DOI] [PubMed] [Google Scholar]
- 6.Eisenbarth SC, Cassel S, Bottomly K. Understanding asthma pathogenesis: linking innate and adaptive immunity. Curr Opin Pediatr 2004;16:659–666. [DOI] [PubMed] [Google Scholar]
- 7.Eisenbarth SC, Piggott DA, Bottomly K. The master regulators of allergic inflammation: dendritic cells in Th2 sensitization. Curr Opin Immunol 2003;15:620–626. [DOI] [PubMed] [Google Scholar]
- 8.Herrick CA, Bottomly K. To respond or not to respond: T cells in allergic asthma. Nat Rev Immunol 2003;3:405–412. [DOI] [PubMed] [Google Scholar]
- 9.Piggott DA, Eisenbarth SC, Xu L, Constant SL, Huleatt JW, Herrick CA, Bottomly K. Myd88-dependent induction of allergic Th2 responses to intranasal antigen. J Clin Invest 2005;115:459–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ritz SA, Cundall MJ, Gajewska BU, Alvarez D, Gutierrez-Ramos JC, Coyle AJ, McKenzie AN, Stampfli MR, Jordana M. Granulocyte macrophage colony-stimulating factor-driven respiratory mucosal sensitization induces Th2 differentiation and function independently of interleukin-4. Am J Respir Cell Mol Biol 2002;27:428–435. [DOI] [PubMed] [Google Scholar]
- 11.Stampfli MR, Wiley RE, Neigh GS, Gajewska BU, Lei XF, Snider DP, Xing Z, Jordana M. GM-CSF transgene expression in the airway allows aerosolized ovalbumin to induce allergic sensitization in mice. J Clin Invest 1998;102:1704–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allakhverdi Z, Comeau MR, Jessup HK, Yoon BR, Brewer A, Chartier S, Paquette N, Ziegler SF, Sarfati M, Delespesse G. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. J Exp Med 2007;204:253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bleck B, Tse DB, Jaspers I, Curotto de Lafaille MA, Reibman J. Diesel exhaust particle-exposed human bronchial epithelial cells induce dendritic cell maturation. J Immunol 2006;176:7431–7437. [DOI] [PubMed] [Google Scholar]
- 14.Liu YJ. Thymic stromal lymphopoietin: master switch for allergic inflammation. J Exp Med 2006;203:269–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reibman J, Hsu Y, Chen LC, Bleck B, Gordon T. Airway epithelial cells release mip-3alpha/ccl20 in response to cytokines and ambient particulate matter. Am J Respir Cell Mol Biol 2003;28:648–654. [DOI] [PubMed] [Google Scholar]
- 16.Weckmann M, Collison A, Simpson JL, Kopp MV, Wark PA, Smyth MJ, Yagita H, Matthaei KI, Hansbro N, Whitehead B, et al. Critical link between trail and CCL20 for the activation of Th2 cells and the expression of allergic airway disease. Nat Med 2007;13:1308–1315. [DOI] [PubMed] [Google Scholar]
- 17.Farrell E, O'Connor TM, Duong M, Watson RM, Strinich T, Gauvreau GM, OA'Byrne PM. Circulating myeloid and plasmacytoid dendritic cells after allergen inhalation in asthmatic subjects. Allergy 2007;62:1139–1145. [DOI] [PubMed] [Google Scholar]
- 18.Thomas SY, Banerji A, Medoff BD, Lilly CM, Luster AD. Multiple chemokine receptors, including CCR6 and CXCR3, regulate antigen-induced T cell homing to the human asthmatic airway. J Immunol 2007;179:1901–1912. [DOI] [PubMed] [Google Scholar]
- 19.Lukacs NW, Prosser DM, Wiekowski M, Lira SA, Cook DN. Requirement for the chemokine receptor ccr6 in allergic pulmonary inflammation. J Exp Med 2001;194:551–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee HC, Ziegler SF. Inducible expression of the proallergic cytokine thymic stromal lymphopoietin in airway epithelial cells is controlled by NFkappaB. Proc Natl Acad Sci USA 2007;104:914–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Newton R, Holden NS, Catley MC, Oyelusi W, Leigh R, Proud D, Barnes PJ. Repression of inflammatory gene expression in human pulmonary epithelial cells by small-molecule I{kappa}b kinase inhibitors. J Pharmacol Exp Ther 2007;321:734–742. [DOI] [PubMed] [Google Scholar]
- 22.Battaglia F, Delfino S, Merello E, Puppo M, Piva R, Varesio L, Bosco MC. Hypoxia transcriptionally induces macrophage-inflammatory protein-3{alpha}/ccl-20 in primary human mononuclear phagocytes through nuclear factor (nf)-{kappa}b. J Leukoc Biol 2008;83:648–662. [DOI] [PubMed] [Google Scholar]
- 23.Kao CY, Huang F, Chen Y, Thai P, Wachi S, Kim C, Tam L, Wu R. Up-regulation of CC chemokine ligand 20 expression in human airway epithelium by IL-17 through a JAK-independent but MEK/NF-kappaB-dependent signaling pathway. J Immunol 2005;175:6676–6685. [DOI] [PubMed] [Google Scholar]
- 24.Palsson-McDermott EM, O'Neill LA. Building an immune system from nine domains. Biochem Soc Trans 2007;35:1437–1444. [DOI] [PubMed] [Google Scholar]
- 25.Bouchier-Hayes L, Martin SJ. Card games in apoptosis and immunity. EMBO Rep 2002;3:616–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stilo R, Liguoro D, Di Jeso B, Formisano S, Consiglio E, Leonardi A, Vito P. Physical and functional interaction of CARMA1 and CARMA3 with Ikappa kinase gamma-NFkappaB essential modulator. J Biol Chem 2004;279:34323–34331. [DOI] [PubMed] [Google Scholar]
- 27.Wang L, Guo Y, Huang WJ, Ke X, Poyet JL, Manji GA, Merriam S, Glucksmann MA, DiStefano PS, Alnemri ES, et al. CARD10 is a novel caspase recruitment domain/membrane-associated guanylate kinase family member that interacts with Bcl10 and activates NF-kappa b. J Biol Chem 2001;276:21405–21409. [DOI] [PubMed] [Google Scholar]
- 28.Grabiner BC, Blonska M, Lin PC, You Y, Wang D, Sun J, Darnay BG, Dong C, Lin X. CARMA3 deficiency abrogates G protein-coupled receptor-induced NF-{kappa}B activation. Genes Dev 2007;21:984–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mahanivong C, Chen HM, Yee SW, Pan ZK, Dong Z, Huang S. Protein kinase C alpha-CARMA3 signaling axis links Ras to NF-kappa B for lysophosphatidic acid-induced urokinase plasminogen activator expression in ovarian cancer cells. Oncogene 2008;27:1273–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang D, You Y, Lin PC, Xue L, Morris SW, Zeng H, Wen R, Lin X. Bcl10 plays a critical role in NF-kappab activation induced by G protein-coupled receptors. Proc Natl Acad Sci USA 2007;104:145–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klemm S, Zimmermann S, Peschel C, Mak TW, Ruland J. Bcl10 and malt1 control lysophosphatidic acid-induced NF-kappaB activation and cytokine production. Proc Natl Acad Sci USA 2007;104:134–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cummings R, Zhao Y, Jacoby D, Spannhake EW, Ohba M, Garcia JG, Watkins T, He D, Saatian B, Natarajan V. Protein kinase cdelta mediates lysophosphatidic acid-induced NF-kappaB activation and interleukin-8 secretion in human bronchial epithelial cells. J Biol Chem 2004;279:41085–41094. [DOI] [PubMed] [Google Scholar]
- 33.Rubenfeld J, Guo J, Sookrung N, Chen R, Chaicumpa W, Casolaro V, Zhao Y, Natarajan V, Georas S. Lysophosphatidic acid enhances interleukin-13 gene expression and promoter activity in T cells. Am J Physiol Lung Cell Mol Physiol 2006;290:L66–L74. [DOI] [PubMed] [Google Scholar]
- 34.Zhao Y, He D, Zhao J, Wang L, Leff AR, Spannhake EW, Georas S, Natarajan V. Lysophosphatidic acid induces interleukin-13 (IL-13) receptor alpha2 expression and inhibits IL-13 signaling in primary human bronchial epithelial cells. J Biol Chem 2007;282:10172–10179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Georas SN, Berdyshev E, Hubbard W, Gorshkova IA, Usatyuk PV, Saatian B, Myers AC, Williams MA, Xiao HQ, Liu M, et al. Lysophosphatidic acid is detectable in human bronchoalveolar lavage fluids at baseline and increased after segmental allergen challenge. Clin Exp Allergy 2007;37:311–322. [DOI] [PubMed] [Google Scholar]
- 36.Ishiguro K, Avruch J, Landry A, Qin S, Ando T, Goto H, Xavier R. Nore1b regulates tcr signaling via ras and CARMA1. Cell Signal 2006;18:1647–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ting AT, Pimentel-Muinos FX, Seed B. Rip mediates tumor necrosis factor receptor 1 activation of NF-kappab but not Fas/APO-1-initiated apoptosis. EMBO J 1996;15:6189–6196. [PMC free article] [PubMed] [Google Scholar]
- 38.Moffat J, Grueneberg DA, Yang X, Kim SY, Kloepfer AM, Hinkle G, Piqani B, Eisenhaure TM, Luo B, Grenier JK, et al. A lentiviral RNAI library for human and mouse genes applied to an arrayed viral high-content screen. Cell 2006;124:1283–1298. [DOI] [PubMed] [Google Scholar]
- 39.You Y, Richer EJ, Huang T, Brody SL. Growth and differentiation of mouse tracheal epithelial cells: selection of a proliferative population. Am J Physiol Lung Cell Mol Physiol 2002;283:L1315–L1321. [DOI] [PubMed] [Google Scholar]
- 40.Allport JR, Lim YC, Shipley JM, Senior RM, Shapiro SD, Matsuyoshi N, Vestweber D,Luscinskas FW. Neutrophils from MMP-9- or neutrophil elastase-deficient mice show no defect in transendothelial migration under flow in vitro. J Leukoc Biol 2002;71:821–828. [PubMed] [Google Scholar]
- 41.Tager AM, Kradin RL, LaCamera P, Bercury SD, Campanella GS, Leary CP, Polosukhin V, Zhao LH, Sakamoto H, Blackwell TS, et al. Inhibition of pulmonary fibrosis by the chemokine IP-10/CXCL10. Am J Respir Cell Mol Biol 2004;31:395–404. [DOI] [PubMed] [Google Scholar]
- 42.Vermaelen K, Pauwels R. Accurate and simple discrimination of mouse pulmonary dendritic cell and macrophage populations by flow cytometry: methodology and new insights. Cytometry A 2004;61:170–177. [DOI] [PubMed] [Google Scholar]
- 43.Hamilton LM, Davies DE, Wilson SJ, Kimber I, Dearman RJ, Holgate ST. The bronchial epithelium in asthma–much more than a passive barrier. Monaldi Arch Chest Dis 2001;56:48–54. [PubMed] [Google Scholar]
- 44.Takizawa H. Bronchial epithelial cells in allergic reactions. Curr Drug Targets 2005;4:305–311. [DOI] [PubMed] [Google Scholar]
- 45.Saatian B, Zhao Y, He D, Georas SN, Watkins T, Spannhake EW, Natarajan V. Transcriptional regulation of lysophosphatidic acid-induced interleukin-8 expression and secretion by p38 MAPK and JNK in human bronchial epithelial cells. Biochem J 2006;393:657–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gaide O, Favier B, Legler DF, Bonnet D, Brissoni B, Valitutti S, Bron C, Tschopp J, Thome M. CARMA1 is a critical lipid raft-associated regulator of TCR-induced NF-kappa B activation. Nat Immunol 2002;3:836–843. [DOI] [PubMed] [Google Scholar]
- 47.Poynter ME, Irvin CG, Janssen-Heininger YM. Rapid activation of nuclear factor-kappab in airway epithelium in a murine model of allergic airway inflammation. Am J Pathol 2002;160:1325–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hart LA, Krishnan VL, Adcock IM, Barnes PJ, Chung KF. Activation and localization of transcription factor, nuclear factor-kappaB, in asthma. Am J Respir Crit Care Med 1998;158:1585–1592. [DOI] [PubMed] [Google Scholar]
- 49.Stacey MA, Sun G, Vassalli G, Marini M, Bellini A, Mattoli S. The allergen Der p1 induces NF-kappaB activation through interference with IkappaB alpha function in asthmatic bronchial epithelial cells. Biochem Biophys Res Commun 1997;236:522–526. [DOI] [PubMed] [Google Scholar]
- 50.Vignola AM, Chiappara G, Siena L, Bruno A, Gagliardo R, Merendino AM, Polla BS, Arrigo AP, Bonsignore G, Bousquet J, et al. Proliferation and activation of bronchial epithelial cells in corticosteroid-dependent asthma. J Allergy Clin Immunol 2001;108:738–746. [DOI] [PubMed] [Google Scholar]
- 51.Zhao S, Qi Y, Liu X, Jiang Q, Liu S, Jiang Y, Jiang Z. Activation of NF-kappa B in bronchial epithelial cells from children with asthma. Chin Med J (Engl) 2001;114:909–911. [PubMed] [Google Scholar]
- 52.Howarth PH, Babu KS, Arshad HS, Lau L, Buckley M, McConnell W, Beckett P, Al Ali M, Chauhan A, Wilson SJ, et al. Tumour necrosis factor (TNFalpha) as a novel therapeutic target in symptomatic corticosteroid dependent asthma. Thorax 2005;60:1012–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jatakanon A, Uasuf C, Maziak W, Lim S, Chung KF, Barnes PJ. Neutrophilic inflammation in severe persistent asthma. Am J Respir Crit Care Med 1999;160:1532–1539. [DOI] [PubMed] [Google Scholar]
- 54.Kurashima K, Mukaida N, Fujimura M, Schroder JM, Matsuda T, Matsushima K. Increase of chemokine levels in sputum precedes exacerbation of acute asthma attacks. J Leukoc Biol 1996;59:313–316. [DOI] [PubMed] [Google Scholar]
- 55.Ying S, O'Connor B, Ratoff J, Meng Q, Mallett K, Cousins D, Robinson D, Zhang G, Zhao J, Lee TH, et al. Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. J Immunol 2005;174:8183–8190. [DOI] [PubMed] [Google Scholar]
- 56.Ying S, Meng Q, Zeibecoglou K, Robinson DS, Macfarlane A, Humbert M, Kay AB. Eosinophil chemotactic chemokines (eotaxin, eotaxin-2, RANTES, monocyte chemoattractant protein-3 (MCP-3), and MCP-4), and C–C chemokine receptor 3 expression in bronchial biopsies from atopic and nonatopic (intrinsic) asthmatics. J Immunol 1999;163:6321–6329. [PubMed] [Google Scholar]
- 57.Khan LN, Kon OM, Macfarlane AJ, Meng Q, Ying S, Barnes NC, Kay AB. Attenuation of the allergen-induced late asthmatic reaction by cyclosporin A is associated with inhibition of bronchial eosinophils, interleukin-5, granulocyte macrophage colony-stimulating factor, and eotaxin. Am J Respir Crit Care Med 2000;162:1377–1382. [DOI] [PubMed] [Google Scholar]
- 58.Lilly CM, Nakamura H, Belostotsky OI, Haley KJ, Garcia-Zepeda EA, Luster AD, Israel E. Eotaxin expression after segmental allergen challenge in subjects with atopic asthma. Am J Respir Crit Care Med 2001;163:1669–1675. [DOI] [PubMed] [Google Scholar]
- 59.Walker C, Bauer W, Braun RK, Menz G, Braun P, Schwarz F, Hansel TT, Villiger B. Activated T cells and cytokines in bronchoalveolar lavages from patients with various lung diseases associated with eosinophilia. Am J Respir Crit Care Med 1994;150:1038–1048. [DOI] [PubMed] [Google Scholar]
- 60.Poynter ME, Cloots R, van Woerkom T, Butnor KJ, Vacek P, Taatjes DJ, Irvin CG, Janssen-Heininger YM. NF-kappa B activation in airways modulates allergic inflammation but not hyperresponsiveness. J Immunol 2004;173:7003–7009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol 2002;2:725–734. [DOI] [PubMed] [Google Scholar]
- 62.Medoff BD, Seed B, Jackobek R, Zora J, Yang Y, Luster AD, Xavier R. CARMA1 is critical for the development of allergic airway inflammation in a murine model of asthma. J Immunol 2006;176:7272–7277. [DOI] [PubMed] [Google Scholar]
- 63.Demedts IK, Bracke KR, Van Pottelberge G, Testelmans D, Verleden GM, Vermassen FE, Joos GF, Brusselle GG. Accumulation of dendritic cells and increased CCL20 levels in the airways of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2007;175:998–1005. [DOI] [PubMed] [Google Scholar]
- 64.Le Borgne M, Etchart N, Goubier A, Lira SA, Sirard JC, van Rooijen N, Caux C, Ait-Yahia S, Vicari A, Kaiserlian D, et al. Dendritic cells rapidly recruited into epithelial tissues via CCR6/CCL20 are responsible for CD8+ t cell crosspriming in vivo. Immunity 2006;24:191–201. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.