Abstract
Neurons are excitable cells that require large amounts of energy to support their survival and functions and are therefore prone to excitotoxicity, which involves energy depletion. By examining bioenergetic changes induced by glutamate, we found that the cellular nicotinamide adenine dinucleotide (NAD+) level is a critical determinant of neuronal survival. The bioenergetic effects of mitochondrial uncoupling and caloric restriction were also examined in cultured neurons and rodent brain. 2, 4-dinitrophenol (DNP) is a chemical mitochondrial uncoupler that stimulates glucose uptake and oxygen consumption on cultured neurons, which accelerates oxidation of NAD(P)H to NAD+ in mitochondria. The NAD+-dependent histone deacetylase sirtulin 1 (SIRT1) and glucose transporter 1 (GLUT1) mRNA are upregulated mouse brain under caloric restriction. To examine whether NAD+ mediates neuroprotective effects, nicotinamide, a precursor of NAD+ and inhibitor of SIRT1 and poly (ADP-ribose) polymerase 1 (PARP1) (two NAD+-dependent enzymes), was employed. Nicotinamide attenuated excitotoxic death and preserved cellular NAD+ levels to support SIRT1 and PARP 1 activities. Our findings suggest that mild mitochondrial uncoupling and caloric restriction exert hormetic effects by stimulating bioenergetics in neurons thereby increasing tolerance of neurons to metabolic stress.
Keywords: excitotoxicity, bioenergetics, mitochondrial uncoupling, caloric restriction, NAD+/NADH, SIRT1, GLUT1, nicotinamide
Excitotoxicity and Neuronal Energy Deficits
Compromised brain energy metabolism plays a critical role in the pathogenesis of neurological diseases, such as stroke and Alzheimer's disease. Neurons are excitable cells that require large amounts of energy to support their multiple ion-motive enzymes to maintain ion homeostasis, electrochemical membrane potential, and signaling functions.1 The energy demand of neurons in the brain is increased when brain neurons are stimulated by glutamate, an excitatory neurotransmitter critical for cognition, motor function, and other behaviors.2 Abnormally high concentrations of synaptic glutamate or prolonged stimulation of glutamate receptors, particularly N-methyl-d-aspartate (NMDA) receptors in pathological conditions, could result in perturbed ion homeostasis, energy depletion, and the degeneration and death of neurons in a process called excitotoxicity.3,4 Excitotoxicity is implicated in the degeneration of neurons that occurs in a range of neurological disorders, including epilepsy, stroke, and Alzheimer's disease.3,5,6
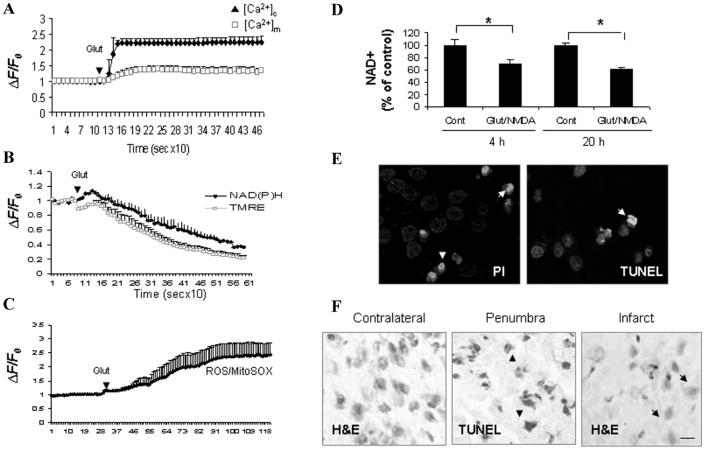
Cellular energy stores are depleted during excitotoxicity with decreases in levels of ATP and nicotinamide adenine dinucleotide (NAD+). NAD+ is an important energy substrate and cofactor involved in multiple metabolic reactions,7 including glycolysis, DNA repair processes, and the function of several NAD+-dependent enzymes, such as the histondeacetylase SIRT1 and poly (ADP-ribose) polymerase 1 (PARP1). In cultured rat hippocampal or cortical neurons, transient or chronic exposure to glutamate/NMDA induces an immediate influx of Ca2+ and Na+ and increased cytosolic and mitochondrial calcium accumulation (Fig. 1A). Cellular NAD(P)H levels rise transiently and then decline with increased oxygen consumption (data not show) and reactive oxygen species (ROS) production (Fig. 1B, C). Although a decrease in the ATP level could be detected within minutes following excitotoxic insults in neuronal cultures,8 a significant decrease of total cellular NAD+ levels occurred within 4 h (Fig. 1D), just prior to significant neuronal death (not shown). Increased percentages of propidium iodide (PI) positive cells and TUNEL positive cells were observed (Fig. 1E), and decreased levels of full-length SIRT1 and PARP1 (see Fig. 4A, B) associated with increased PAR accumulation (data not shown) were detected. The degradation of full-length PARP1 and SIRT1 could be the result of caspases activated during excitotoxic apoptosis and NAD+ depletion from DNA damage and PARP1 activation, which could further reduce SIRT1 activity. Our studies suggest that neuronal death is closely related to the cellular NAD+ levels because the depletion of NAD+ indicates the failure of glycolysis to restore ATP levels, dysfunction of the DNA repair process involving PARP1 activation, and failure of NAD+-dependent signaling pathway.
Figure 1.
Bioenergetic failure in glutamate/ N-methyl-d-aspartate (NMDA)-mediated excitotoxicity. (A) Primary rat cortical neurons (7–10 days) were loaded with the fluorescence cytosolic calcium ([Ca2+]c) indicator dye Fluo4 and the mitochondrial calcium ([Ca2+]m) indicator dye Rhod-2. (B & C) Mitochondrial membrane potential (Δψm) measured using delta Psi m-sensitive dye tetramethylrhodamine, ethyl ester (TMRE), NAD(P)H autofluorescence and reactive oxygen species (ROS) measured using MitoSOX were also monitored. Time-lapse confocal images were acquired following addition of glutamate (100 μmol/L). Fluorescence intensity data from multiple cells were processed to baseline intensity (ΔF/F0). (D) Total cellular NAD+ levels decreased within 4 h and continued to be depressed through 20 h after exposure to excitotoxic insults (100 μmol/L glutamate combined with 80 μmol/L NMDA). *P < 0.05, compared to the corresponding control value. (E) Significant decrease of cell survival was evident around 6 h following excitotoxic insults with increased percentages of propidium iodide (PI; red) positive and TUNEL positive (green) cells. Cells were counterstained with Hoechst (blue). (F) TUNEL positive cells (brown cells) detected in penumbra region and eosinphilic “red neuron” in infarct core at 12 h following focal cerebral ischemia (MCAO) in rodent brain. Scale bar, 25 μmol/L.
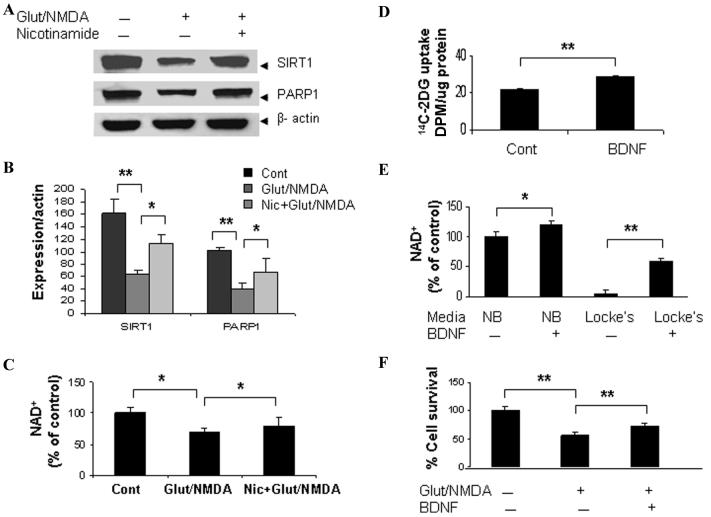
Figure 4.
Preventing NAD+ depletion preserved SIRT1 expression and attenuated excitotoxic neuronal death. (A & B) Immunoblot showed that SIRT1 and poly (ADP-ribose) polymerase 1 (PARP1) (full-length) levels were reduced at 6 h following excitotoxic insults (100 μmol/L glutamate with 80 μmol/L NMDA) in cortical neurons. (C) Cellular total NAD+ content was significantly decreased. Adding nicotinamide (2 mM), the precursor of NAD+ and an inhibitor of PARP1 and SIRT1, to the cultures preserved cellular NAD+ levels and SIRT1, PARP1 levels in cortical cells exposed to excitotoxic insults. (D) BDNF (100 nM) enhanced [14C]-2-deoxyglucose uptake in cortical cells. (E) Cellular NAD+ levels were significantly higher in BDNF-treated (24 h) cortical neurons in both neurobasal media (NB, higher glucose concentration) and Locke's media (lower glucose concentration) compared to vehicle-treated neuronal cultures. (F) BDNF-enhanced neuronal survival in neurons exposed to glutamate/NMDA. Cell survival was detected by MTT (3-[4, 5-dimethylthhylthiazol-2-yl]-2, 5-diphenyl-tetrazolium bromide) assay; *P < 0.05; **P < 0.01.
In a focal permanent cerebral ischemia model (MCAO) in rodents, we detected prominent TUNEL positive cells at 6 h post stroke and a further increase in TUNEL positive cells up to 24 and 48 h poststroke in the ipsilateral cortex (Fig. 1F).9,10 A decrease of ATP levels occurred within 1 h after cerebral ischemia.11 The time gap between initiation of ischemia and significant neuronal death suggests a therapeutic time window to rescue neurons, which might be accomplished by restoring cellular NAD+ levels to maintain ATP production, prevent NAD+ depletion from DNA damage-induced PARP1 activation, and support NAD+-dependent signaling pathways, such as SIRT1 and several mitochondrial sirtuins.12
Bioenergetic Effects of Mitochondrial Uncoupling and Caloric Restriction
Glucose is the main source of energy for neurons to generate ATP during mitochondrial oxidative phosphorylation, a process which also generates harmful free radicals. Alternatively, neurons can generate ATP by glycolysis, a pathway that may be particularly important as an energy compensatory mechanism when mitochondrial function is compromised.13
Mitochondrial uncoupling is increasingly recognized as a mechanism by which cells modulate their energy metabolism and redox state in response to environmental demands.14,15 The chemical mitochondrial uncoupler 2, 4-dinitrophenol (DNP) has been used as an anti-obesity therapy because of its energy dissipating effects, which mimic the actions of caloric restriction. An unanswered question is how mild mitochondrial uncoupling and caloric restriction are able to maintain sufficient energy to support the function of the various enzymes critical for neuronal survival, particularly in pathological conditions such as excitotoxicity.
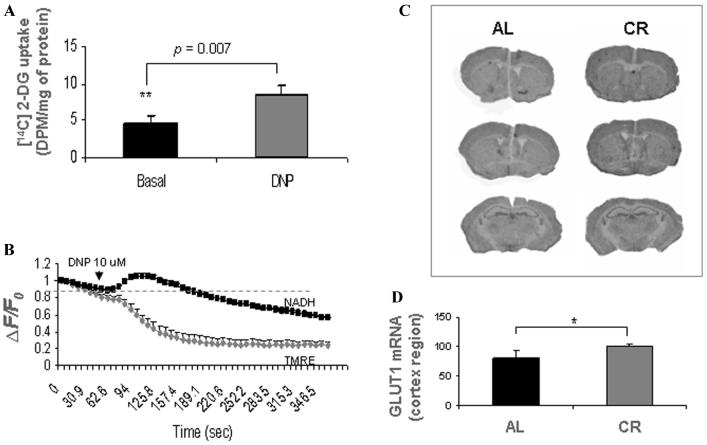
The possible bioenergetic effects of mild mitochondrial uncoupling and caloric restriction were studied in neuronal cultures and rodents. Using DNP at a noncytotoxic concentration, we found that mitochondrial uncoupling could stimulate energy metabolism through a mechanism involving mild metabolic stress responses. [14C] 2-deoxyglucose (DG) uptake and oxygen consumption are greatly stimulated and oxidation of NADH to NAD+ in mitochondrial is accelerated in cortical neurons treated with DNP (Fig. 2A-B) as the mitochondrial electron transport chain is one of the primary sites of NADH oxidation during respiration.16,17 Through stimulating energy metabolism and modulating cellular NAD+ levels and/or NAD+: NADH ratio, mitochondrial uncoupling could regulate SIRT1 activity, a NAD+-dependent histone deacetylase and, hence, the sirtuin signaling pathway. Indeed, carbonyl cyanide p-(trifluomethoxy) phenyl hydrazone, another commonly used chemical mitochondrial uncoupler, has been reported to induce a compensatory cellular energy homeostasis through peroxisome proliferators-activated receptor (PPAR) gamma coactivator-1alpha (PGC-1α), a downstream component of the sirtuin pathway.18
Figure 2.
Bioenergetic effects of mild mitochondrial uncoupling and caloric restriction. (A) [14C]-2-deoxyglucose uptake was significantly increased in cortical neurons treated with the mitochondrial uncoupler 2, 4-DNP (20 μmol/L). Values are the mean and SD of measurements made in 4 cultures. (B) Cultured cortical neurons were loaded with TMRE and time-lapse confocal images of TMRE fluorescence (gray) and NAD(P)H autofluorescence (black) were acquired sequentially at 10-s intervals. DNP induced a transient increase of NAD(P)H autofluorescence followed by a prolonged decrease from enhanced NAD(P)H oxidation. (C, D)In-creased glucose transporter 1 (GLUT1) mRNA expression (in situ hybridization with 35S-labeled riboprobes) was detected in caloric restricted (CR) mouse brains (6-month-old, 3 months under alternate-day fasting) compared to noncaloric restricted control (AL, ad libitum); n = 5 animals in each group.
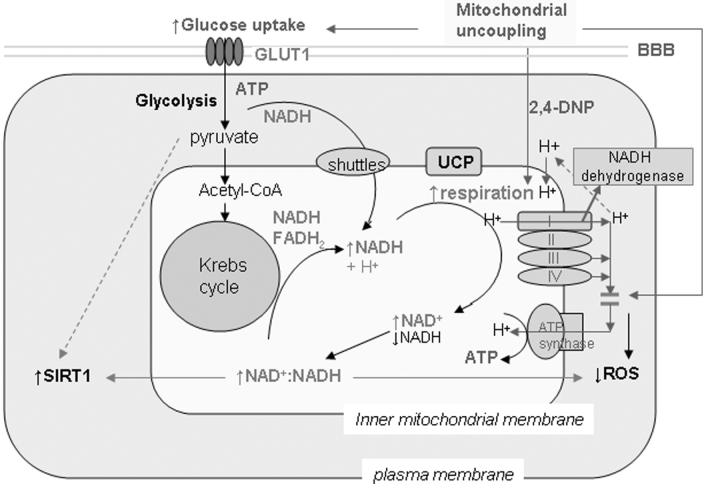
Compensatory changes in energy metabolism associated with mild metabolic stress have also been observed in the brain in response to caloric restriction. We detected significant increases of glucose transporter 1 (GLUT1) expression in the brains of caloric restricted mice (Fig. 2C, D). The NAD+-dependent deacetylase SIRT1, the sensor of energy and redox states, was increased in the brain and other tissues in response to caloric restriction.19 Our studies suggest that mild metabolic stress, such as mild mitochondrial uncoupling and caloric restriction, stimulates glucose utilization and modulates NAD+/NADH redox state through respiration. The possible bioenergetic effects of mild mitochondrial uncoupling are shown in Figure 3. The cellular metabolic stress response induced by mitochondrial uncoupling and caloric restriction may prevent energy deficits through a compensatory mechanism, such as stimulating glucose uptake, enhancing ATP production from glycolysis,13 modulating NAD+/NADH redox state to activate sirtuin pathway, or increasing mitochondrial biogenesis.20 The uncoupling activity and function of various mitochondrial uncoupling proteins expressed in brain cells needs further study. In this regard, we recently showed that UCP4, a brain mitochondrial protein, modifies neuronal energy metabolism and ROS production.13 In pheochromocytoma (PC12) cells overexpressing human UCP4, the total ATP level was maintained even though there was increased ATP production from glycolysis, suggesting a shift in energy metabolism.13
Figure 3.
Proposed model for the bioenergetic effects of mild mitochondrial uncoupling on neurons. Mitochondrial uncoupling induces mild metabolic stress from dissipation of the hydrogen ion gradient across the inner mitochondrial membrane. As a stress response, glucose uptake and NADH oxidation are stimulated with increased respiration, which increases NAD+ level and the NAD+:NADH ratio in mitochondria. The NAD+-dependent deacetylase sirtulin 1 (SIRT1) is activated. The increased NAD+:NADH ratio could also reduce mitochondrial ROS production. UCP, uncoupling protein; BBB, blood-brain barrier; ROS, reactive oxygen species.
Brain-derived neurotrophic factor (BDNF), a neurotrophin which modulates brain energy metabolism, is increased in brains of calorie-restricted rodents.21 BDNF is involved in the preconditioning and regulation of brain energy metabolism in response to mild metabolic stress. We found that BDNF stimulates [14C] 2-DG uptake, increases cellular NAD+ levels, and protects cultured cortical neurons against excitotoxic insults (Fig. 4D–F). The bioenergetic effects of BDNF further support the concept that maintaining cellular energy homeostasis, particularly NAD+ levels, is a principle mechanism for neuronal survival during excitotoxic and ischemic conditions.
Bioenergetic Intervention in Excitotoxicity
Caloric restriction and mild mitochondrial uncoupling have been reported to be neuroprotective in models of several neurological disorders.20,22-24 To examine whether their neuroprotective effects are related to cellular bioenergetics and the NAD+-dependent histone deacetylase SIRT1 pathway, we employed nicotinamide (vitamin B3), the precursor of NAD+ in scavenging pathway and also an inhibitor of SIRT1 and PARP1. When glutamate receptors are over stimulated, particularly under conditions of metabolic or oxidative stress, their ability to maintain the function of vital ion pumps and NAD+-dependent enzymes is compromised. We found that total cellular NAD+ level and levels of SIRT1 and PARP1 of cultured neurons were reduced as early as 4–6 h following chronic exposure to glutamate/NMDA (Fig. 4A, B). Adding nicotinamide to neuronal cultures preserved the total cellular NAD+ level. Cellular SIRT1 and PARP1 expression levels were also preserved and excitotoxic neuronal death was attenuated (Fig. 4A-C).
Our findings suggest a therapeutic potential for neuroprotective interventions that maintain cellular NAD+ levels in pathological conditions involving excitotoxicity. In our focal permanent cerebral ischemia mouse model (MCAO), administration of nicotinamide i.p. after ischemia (30 min−1 h) significantly reduced infarct size. Importantly, the time window for infarct development was extended, which could be critical for therapeutic intervention in stroke. The ability of nicotinamide to restore ATP levels following ischemia–reperfusion has been reported.25
Summary
Our studies suggest that maintenance of cellular bioenergetic homeostasis and NAD+ levels is of critical importance to support the NAD+-dependent enzymes, such as SIRT1 and PARP1, and for protection against excitotoxicity. Preventing cellular energy deficits by preserving cellular NAD+ levels could be a valuable neuroprotective mechanism applied as therapeutic interventions for neurodegenerative diseases.26 Mild mitochondrial uncoupling and caloric restriction exert hormetic effects by enhancing energy metabolism, such as stimulating glucose uptake, enhancing NADH oxidation to NAD+, and inducing an adaptive shift in energy metabolism13 to prevent energy depletion. Furthermore, activation of sirtuin-related signaling pathways and increased levels of neurotrophins, such as BDNF, would increase the tolerance of neurons to metabolic and oxidative stress during excitotoxic conditions (Fig. 5).
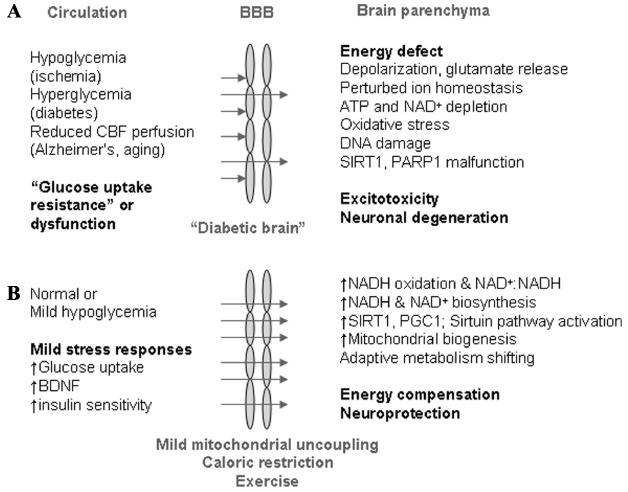
Figure 5.
Possible neuroprotective mechanisms of mild mitochondrial uncoupling and caloric restriction in neurodegenerative diseases. (A) Compromised bioenergetics in stroke (ischemia), Alzheimer's disease (reduced cerebral blood flow perfusion), or hyperglycemia in diabetic conditions results in an energy defect in neurons. (B) The interventions of mild mitochondrial uncoupling and caloric restriction cause a mild metabolic stress. Through a compensatory response, brain energy metabolism is stimulated. Glucose uptake is accelerated with increased expression of the brain glucose transporter 1 (GLUT1) and BDNF in the brain. NAD(P)H oxidation in mitochondria is enhanced with increased respiration, which modulates cellular NAD+ levels and the NAD+/NADH redox state, and activates SIRT1/sirtuin–PGC1α signaling pathway.
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Mattson MP, Liu D. Energetics and oxidative stress in synaptic plasticity and neurodegenerative disorders. Neuromol. Med. 2002;2:215–231. doi: 10.1385/NMM:2:2:215. [DOI] [PubMed] [Google Scholar]
- 2.Antzoulatos EG, Byrne JH. Learning insights transmitted by glutamate. Trends Neurosci. 2004;27:555–560. doi: 10.1016/j.tins.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 3.Mattson MP. Pathways towards and away from Alzheimer's disease. Nature. 2004;430:631–639. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aundine M, Tymianski M. Molecular mechanisms of calcium-dependent neurodegeneration in excitotoxicity. Cell Calcium. 2003;34:325–337. doi: 10.1016/s0143-4160(03)00141-6. [DOI] [PubMed] [Google Scholar]
- 5.Greene JG, Greenamyre JT. Bioenergetics and glutamate excitotoxicity. Prog. Neurobiol. 1996;48:613–634. doi: 10.1016/0301-0082(96)00006-8. [DOI] [PubMed] [Google Scholar]
- 6.Pieper AA, Blackshaw S, Clements EE, et al. Poly (ADP-ribosyl) ation basally activated by DNA strand breaks reflects glutamate-nitric oxide neuro-transmission. Proc. Natl. Acad. Sci. USA. 2000;97:1845–1850. doi: 10.1073/pnas.97.4.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brennan AM, Connor JA, Shuttleworth CW. NAD(P)H fluorescence transients after synaptic activity in brain slices: predominant role of mitochondrial function. J. Cereb. Blood Flow Metab. 2006;26:1389–1406. doi: 10.1038/sj.jcbfm.9600292. [DOI] [PubMed] [Google Scholar]
- 8.Ankarcrona M, Dypbukt JM, et al. Glutamate-induced neuronal death: a succession of necrosisi or apoptosis depending on mitochondrial function. Neuron. 1995;15:961–973. doi: 10.1016/0896-6273(95)90186-8. [DOI] [PubMed] [Google Scholar]
- 9.Liu D, Carolyn CL, Barone FC, et al. Astrocytic demise precedes delayed neuronal death in focal ischemia rat brain. Mol. Brain Res. 1999;68:29–41. doi: 10.1016/s0169-328x(99)00063-7. [DOI] [PubMed] [Google Scholar]
- 10.Liu D, Lu C, Wan R, et al. Activation of mitochondrial ATP-dependent potassium channels protects neurons against ischemia-induced death by a mechanism involving suppression of BAX translocation and cytochrome c release. J. Cereb. Blood Flow Metab. 2002;22:431–443. doi: 10.1097/00004647-200204000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Hata R, Maeda K, Hermann D, et al. Dynamics of regional brain metabolism and gene expression after middle cerebral artery occlusion. J. Cereb. Blood Flow Metab. 2000;20:306–315. doi: 10.1097/00004647-200002000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Guarente L, Picard F. Calorie restriction-the sir2 conection. Cell. 2005;120:473–482. doi: 10.1016/j.cell.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 13.Liu D, Chan SL, de Souza-Pinto NC, et al. Mitochondrial UCP4 mediates an adaptive shift in energy metabolism and increases the resistance of neurons to metabolic and oxidative stress. Neuromol. Med. 2006;8:389–414. doi: 10.1385/NMM:8:3:389. [DOI] [PubMed] [Google Scholar]
- 14.Andrews ZB, Diano S, Horvath TL. Mitochondrial uncoupling proteins in the CNS: in support of function and survival. Nat. Rev. Neurosci. 2005;6:829–840. doi: 10.1038/nrn1767. [DOI] [PubMed] [Google Scholar]
- 15.Wolkow CA, Iser WB. Uncoupling protein horologes may provide a link between mitochondria, metabolism and lifespan. Ageing Res. Rev. 2006;5:196–208. doi: 10.1016/j.arr.2006.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krebs HA, Gascoyne T. The redox state of the nicotinamide-adenine dinucleotides in rat liver homogenates. Biochem. J. 1968;108:1967–1968. doi: 10.1042/bj1080513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bakker BM, Overkamp KM, Maris AJA, et al. Stoichiometry and compartmentation of NADH metabolism in Saccharomyces cerevisiae. FEMS Microbiol. Rev. 2001;25:15–37. doi: 10.1111/j.1574-6976.2001.tb00570.x. [DOI] [PubMed] [Google Scholar]
- 18.Rohas LM, St-Pierre J, Uldry M, et al. A fundamental system of cellular energy homeostasis regulated by PGC-1α. Proc.Natl.Acad.Sci.USA. 2007;104:7933–7938. doi: 10.1073/pnas.0702683104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen HY, Miller C, Bitterman KJ, et al. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science. 2004;16:305–390. doi: 10.1126/science.1099196. [DOI] [PubMed] [Google Scholar]
- 20.Diano S, Matthews RT, Patrylo P, et al. Un-coupling protein 2 prevents neuronal death including that occurring during seizures: a mechanism for precondition. Endocrinology. 2003;144:5014–5021. doi: 10.1210/en.2003-0667. [DOI] [PubMed] [Google Scholar]
- 21.Lee J, Duan W, Mattson MP. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus. J. Neurochem. 2002;82:1367–1375. doi: 10.1046/j.1471-4159.2002.01085.x. [DOI] [PubMed] [Google Scholar]
- 22.Bruce-Keller AJ, Umberger G, McFall R, Mattson MP. Food restriction reduces brain damage and improves behavioral outcome following excitotoxic and metabolic insults. Ann. Neurol. 1999;45:8–15. [PubMed] [Google Scholar]
- 23.Mattiasson G, Shamloo M, Gido G, et al. Uncoupling protein-2 prevents neuronal death and diminishes brain dysfunction after stroke and brain trauma. Nat. Med. 2003;9:1062–1068. doi: 10.1038/nm903. [DOI] [PubMed] [Google Scholar]
- 24.McLeod CJ, Aziz A, Hoyt RF, Jr, et al. Uncoupling proteins 2 and 3 function in concert to augment tolerance to cardiac ischemia. J. Biol. Chem. 2005;280:33470–33476. doi: 10.1074/jbc.M505258200. [DOI] [PubMed] [Google Scholar]
- 25.Klaidman L, Morales M, Kem S, et al. Nicotinamide offers multiple protective mechanisms in stroke and as a precursor for NAD+, as a PARP inhibitor and by partial restoration of mitochondrial function. Pharmacology. 2003;69:150–157. doi: 10.1159/000072668. [DOI] [PubMed] [Google Scholar]
- 26.Mattson MP. Excitotoxic and excitoprotective mechanisms: abundant targets for the prevention and treatment of neurodegenerative disorders. Neuromol. Med. 2003;3:65–94. doi: 10.1385/NMM:3:2:65. [DOI] [PubMed] [Google Scholar]