Abstract
Hippocampal sharp waves (SPWs) are among the earliest neural population patterns observed in infant mammals. Similarly, startles are among the earliest behavioral events observed. Here we provide evidence indicating that these two events are linked mechanistically soon after birth in freely moving and head-fixed 1-4-day-old rats. EMG electrodes and intrahippocampal silicon depth electrodes were used to detect the presence of startles and SPWs, respectively. In intact pups, the majority of sharp waves were preceded by startles (average latency: 161 ms). When the hippocampal formation was surgically separated from the brainstem, however, sharp waves and startles still occurred, but now independently. In addition, unrelated to startles or SPWs, gamma oscillations were detected in several subjects, as were neocortical “spindles” that propagated passively into the hippocampus. The co-occurrence of sharp waves and startles provides the opportunity for Hebbian changes in synaptic efficacy and, thus, is poised to contribute to the assembly of neural circuits early in development.
Hippocampal sharp waves (SPWs) and their putative in vitro counterpart, giant depolarizing potentials (GDPs), are synchronous, high-amplitude neural population events and are the earliest such patterns yet detected in neonates (Leinekugel et al., 2002). In contrast, startles—sudden, spontaneous, and simultaneous contractions of skeletal muscles throughout the body—are among the first behavioral events observed in human fetuses (de Vries et al., 1982; de Vries et al., 1985) and neonatal rats (Gramsbergen et al., 1970). In the present report, we provide evidence of a mechanistic link between these two prominent manifestations of infant neural activity.
When external input to the hippocampus is depressed, SPWs are initiated within the CA3 recurrent network. SPWs rapidly invade other hippocampal subfields and result in increased excitability throughout the hippocampal formation (Buzsáki, 1986; Buzsáki, 1996). SPWs are incompatible with rhythmic activity within the hippocampus (i.e., theta, gamma) that depend on excitatory hippocampal inputs (Bland, 1986; Bland and Colom, 1993; Buzsaki, 2002) and, thus, are more likely following periods of decreased neocortical input (Battaglia et al., 2004). In adults, SPWs occur intermittently during slow-wave sleep, waking immobility, and various consummatory behaviors (Buzsáki, 1986; Suzuki and Smith, 1987; Vanderwolf, 1969).
As high-amplitude events produced by the synchronized activation of a large population of neurons, SPWs are well-poised to play a role in Hebbian neuroplasticity (Hebb, 1961). Indeed, the pairing of cellular activation with naturally occurring SPWs and GDPs has been shown to be sufficient to induce long-term potentiation and enhance synaptic efficacy (Kasyanov et al., 2004; King et al., 1999). Thus, it has been argued that SPWs play a role in altering synaptic efficacy during memory consolidation (Buzsáki, 1998; King et al., 1999), as well as during forgetting (Colgin et al., 2004). The prominence of SPWs in infancy undergirds the notion that they (and GDPs) play a role in early development (Hanse et al., 1997; Leinekugel, 2003; Leinekugel et al., 2002).
Startles, occurring at a rate of approximately 1-2 per min in infant rats, are easily distinguished from other infantile behaviors, such as the more frequent myoclonic twitching that characterizes active sleep (de Vries et al., 1982; Gramsbergen et al., 1970; Roodenburg et al., 1991; Seelke et al., 2005). The neural basis of startles remains unknown, although the simultaneous activation of multiple muscle groups that characterizes a startle suggests a supraspinal source of initiation (O'Donovan, 1999).
Prior observations of freely moving infant rats indicated that SPWs and startles typically co-occur (Karlsson and Blumberg, 2003), suggesting a causal link between the two events. Because single-ended electrodes were used in that study, it was not possible to detect a phase reversal in the SPW signal so as to confirm that the events being recorded were indeed SPWs (e.g., as opposed to events generated in the neocortex and transmitted passively to the hippocampus). It was also not possible in that previous study to establish the precise temporal relationship between the SPWs and the behaviorally scored startles. Subsequently, we have determined that startles reliably exhibit an EMG “signature” comprising simultaneous activation of multiple muscle groups, usually during sleep (Viana Di Prisco and Blumberg, 2005). Building on this observation, we show here that SPWs, now verified using intrahippocampal silicon depth electrodes, are reliably preceded by startles. Moreover, when the neural connectivity between the hippocampus and brainstem is severed, SPWs and startles are effectively de-coupled. We suggest that the co-occurrence of SPWs and startles during early infancy represents a novel mechanism conducive to Hebbian plasticity in the immature nervous system.
MATERIALS AND METHODS
All experiments were performed under National Institutes of Health guidelines for the care of animals in research and were approved by the Institutional Animal Care and Use Committee of the University of Iowa.
Subjects
Nineteen Sprague-Dawley male and female rats from 19 litters were used. All subjects were tested at postnatal days [P]1-4. Body weights were ??? g (you guys have the folder with this info: but, is it necessary?). The pups were raised in litters which were culled to 8 pups within 3 days after birth (day of birth = Day 0). Mothers and their litters were housed and raised in standard laboratory cages (48 cm long × 20 cm wide × 26 cm high) in the animal colony at the University of Iowa. Food and water were available to the animals ad libitum. All animals were maintained on a 12 hour light and dark schedule with lights on at 7:00 am. All experiments were conducted during the lights-on phase.
Surgery
Surgeries were performed under isoflurane anesthesia. For recordings in freely moving animals, 7 P4 rats were implanted with bipolar EMG electrodes, inserted into the right nuchal and the left vastus lateralis (hind leg) muscles. Of these 7 subjects, 2 were also implanted with intrahippocampal 16-site silicon electrodes for chronic recording (100 μm vertical separation between recording sites; Neuronexus Technologies, Ann Arbor, MI). For implantation of the silicon electrode, the infant's skull was bleached, dried, and then coated with Vetbond (3M, St. Paul, MN) to add strength. Finally, a burr hole drilled over the dorsal hippocampus and the silicone electrode was inserted into the CA1-dentate gyrus axis and affixed to the skull.
For recordings in head-fixed animals, 12 P1-4 rats were used. In these subjects, EMG electrodes were implanted and skulls were prepared as described above. Next, a custom built, T-shaped, stainless steel head-plant, designed to attach to the earbar and nosebar holders of a stereotaxic apparatus, was attached to the skull over the pretreated area using cyanoacrylate adhesive gel (Karlsson and Blumberg, 2005; Karlsson et al., 2005). Hippocampal activity was recorded using a 16-site silicon electrode for acute recordings (Neuronexus Technologies, Ann Arbor, MI). Six of these head-fixed pups were also decerebrated at the precollicular level. The decerebration was accomplished by inserting a blunted 25 g needle through the skull on the midline just caudal to lambda and with a slight (i.e., approximately 10°) forward angle; the needle was then gently dragged along the base of the skull, using a side-to-side motion (Karlsson et al., 2004). All pups recovered from surgery for 2-4 h before recording.
Procedure and data acquisition
Recordings in freely moving pups
After recovery from surgery, the pups were moved to an electrically shielded, humidified, double-walled glass recording chamber (height = 17.0 cm; i.d. = 12.5 cm) which was maintained at thermoneutrality (i.e., 35.5°C). The EMG electrodes were connected to differential amplifiers (A-M Systems, Carlsborg, WA) and their signals were amplified (×10 k) and filtered (300-5000 Hz). The silicon electrode was connected to a unity gain headstage and digital amplifier (Tucker-Davis Technologies, Alachua, Florida). Data were sampled in 10- or 30-min recording sessions, with 2– 6 sessions for each pup. The signals were filtered (5 kHz low-pass) and amplified (×10 k) before being sampled continuously (at 12.5 kHz) and stored for off-line analysis using Spike2 software (Cambridge Electronic Design, Ltd., Cambridge, UK). A microcamera placed above the chamber lid allowed for monitoring and recording of behavior. The EMG signals were stored with the video data onto digital videotape using a data recorder (Model DV8; WinTron Technologies, Rebersberg, PA).
Recordings in head-fixed pups
After recovery from surgery, the pups were secured in a stereotaxic apparatus and brain and body temperature were maintained at approximately 37°C (Karlsson and Blumberg, 2004). The EMG and hippocampal electrode signals were sampled, recorded, and stored as described above. Previous experiments (Karlsson and Blumberg, 2005; Karlsson et al., 2005) have shown that, within 2-3 hrs after surgery, pups exhibit frequent transitions between sleep and wakefulness, as judged by oscillations in muscle tone as well as myoclonic twitches against a background of nuchal atonia. When a pup began exhibiting sleep-wake cyclicity, the silicon electrode was lowered into the CA1-dentate gyrus axis of the hippocampus while neurophysiological activity was monitored using an oscilloscope and audio analyzer (FHC, Bowdoinham, Maine, United States). The electrode remained at the recording site for 5–10 min before data collection began. At the end of the experiment, the recording sites were marked by passing a 50 μA anodal current for 3-5 s through one electrode at the deepest and shallowest recording sites on the silicon electrode (1.6 mm total separation).
Data analysis
Recordings in freely moving pups
Using recordings from pups implanted with EMGs only, startles, defined behaviorally as abrupt, high-amplitude, synchronous movements of at least 3 limbs, were scored from the digital video. The EMG signature of startles was defined as EMG spikes, occurring in both nuchal and vastus lateralis leads, no more than 70 ms apart, with an amplitude of at least 30 μV, a duration of at least 100 ms, and in the absence of other EMG activity for at least 500 ms. SPWs were identified based on the presence of a characteristic local field potential including a pronounced phase reversal across the CA1 field and a negative peak signal-to-noise ratio of at least 20:1 (measured in the stratum radiatum; filtered 1-35 Hz).
Recordings in head-fixed pups
For each record, the occurrence of SPWs and startles was quantified as described above and the hippocampal recordings were inspected for the occurrence of other activity such as rhythms in the theta or gamma range. The rates of SPWs and startles in intact and decerebrate pups were compared using unpaired t tests. When both startles and SPWs occurred, the latency between startle and SPW was determined from the startle-associated peak amplitude in the nuchal EMG and the SPW peak. Co-occurrence was defined as a latency of less than 300 ms. The number of startles co-occurring with SPWs was compared using unpaired t tests within groups and paired t tests between groups. Finally, the the chi-square statistic was used to assess the likelihood of co-occurrence between startles and SPWs in both intact and decerebrate groups
All means are presented with their standard errors.
Histology
After the recording the pups were overdosed with sodium pentobarbital (approximately 100 mg/kg intraperitoneally) and perfused transcardially with physiological saline followed by 3% formalin. Brains were postfixed in the skull for 1–2 d in a formalin-sucrose solution and then removed from the skull and fixed for at least 2 more days in a fresh solution. After fixation, the brains were sliced in 50-μm coronal sections with a sliding microtome (model SM 2000 R, Leica, Bensheim, Germany). The locations of the deepest and shallowest marking lesions were determined by examining serial sections, from which the locations of recording sites were determined.
RESULTS
Sharp waves are preceded by startles
In the two freely moving pups, frame-by-frame video analysis of behavior in relation to hippocampal field potentials showed that approximately 80% of SPWs are synchronized with startles. Due to the inherent technical challenges of recording hippocampal activity in freely moving neonates, we used the head-fixed method in subsequent experiments (Karlsson et al., 2005).
A representative recording of SPWs and associated EMG activity in a head-fixed intact P2 pup is shown in Figure 1. In intact pups, SPWs occurred with a frequency of 1.4 ± 0.3 per min (range: 0.6–2.0 per min), which is within the range reported previously in infant and adult rats (Buzsáki, 1986; Leinekugel et al., 2002). We, and others (Gramsbergen et al., 1970), have found similar startle frequencies for intact, freely moving infant rats.
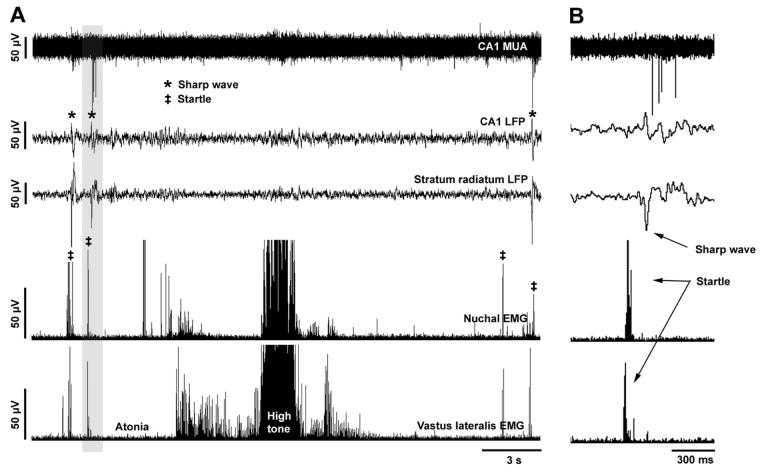
Figure 1.
(A) Representative recording of SPWs preceded by startles in a head-fixed P2 rat. Traces from top: CA1 multiunit activity (MUA; filter: 500-5000 Hz); local field potentials (LFP) recorded from CA1 and stratum radiatum (filter: >100 Hz); EMG activity in the nuchal and vastus lateralis muscles. Individual instances of SPWs and startles are indicated. (B) Shaded area from (A) expanded to reveal temporal structure of the startle-SPW complex in detail.
A temporal association between SPWs and startles became apparent when we examined EMG records around the time when SPWs occurred. Specifically, 78 of 92 (84.8%) recorded SPWs (5-19 SPWs observed in each pup during 10-min recording sessions) were immediately preceded by abrupt, phasic EMG activation. Of these EMG events, 76% comprised activation of both muscles which, as indicated above, provides strong positive evidence of the occurrence of a startle (the remaining 24% comprised activation of only one of the recorded muscles, which does not necessarily preclude its being produced by a startle). When startles preceded SPWs, the latency from peak EMG activation to the peak of a SPW was 161.0 ± 54.8 ms (range: 104.2-224.1 ms). The remaining 14 of 92 SPWS (15.2%) occurred either without any preceding phasic EMG activation or during periods of sustained high nuchal muscle tone (indicative of wakefulness).
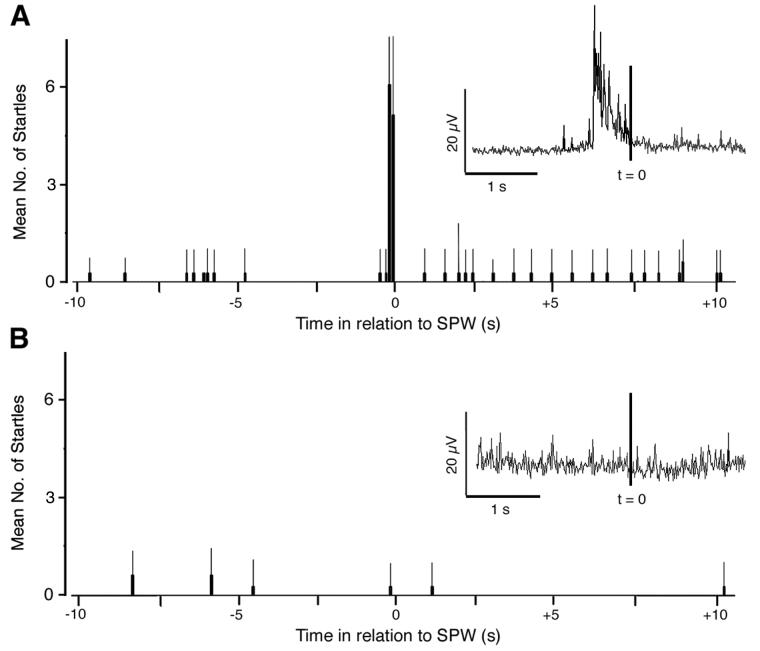
Figure 2A is a peristimulus histogram that depicts the temporal relation between startles and SPWs across all intact P1-4 subjects. A distinct cluster of startles is found immediately preceding the SPWs. Within a 300-ms time window, the mean number of startles was significantly higher during the period before than after the SPWs (t = 5.861, df = 3, P < 0.01). The inset depicts the averaged nuchal EMG trace in relation to SPWs (indicated by the vertical line).
Figure 2.
(A) Peristimulus histogram of mean (+ s.e.) number of startles, determined from EMG records and triggered by SPWs, in intact and head-fixed P1-4 rats (n = 4). The inset shows the averaged nuchal EMG amplitude in relation to the peak of a SPW (t = 0; vertical line). N = 92 SPWs. (B) Same as in (A) accept pups were decerebrated before testing (n = 6). N = 83 SPWs. The inset figures are produced by averaging nuchal EMG after downsampling to 250 Hz.
Decerebration decouples sharp waves and startles
To determine if the strong relationship between startles and SPWs requires neural connectivity between the hippocampal formation and the brainstem (a plausible source of spontaneous activity leading to a behavioral startle (Corner et al., 2002)), we recorded from head-fixed pups (n=6) that had previously received precollicular decerebrations. Though SPWs were not eradicated by decerebration (range: 5–33 SPWs per pup recorded from the 6 subjects during 30-min recording sessions), they were reduced in frequency. Specifically, SPWs occurred with a frequency of 0.5 ± 2.4 per min (range: 0.1-1.2 per min), which is significantly lower than in the intact pups (t = 3.62, df = 10, P < 0.005).
When SPWs did occur in decerebrates, however, and in marked contrast to intact pups, only 3 of 83 (3.6%) SPWs were preceded by startles within a window of 224 ms (i.e., the longest latency observed in the intact pups). Moreover, 3.7% of SPWs in the decerebrates were now followed by startles. In addition, 50.6% of SPWs occurred in the absence of any associated phasic muscle activity, and 42.2% occurred during prolonged periods of high nuchal muscle tone. The odds ratio of observing a SPW after a startle was 0.84 in intact pups and 0.04 in decerebrated pups (χ2 = 131.3; df = 1; P < 0.001). The sharp contrast between intact and decerebrated pups is also clearly visible in Figure 2B, where the SPW-triggered peristimulus histogram reveals no co-occurrence of SPWs and startles; as shown in the inset, the averaged nuchal EMG trace immediately preceding the SPW is flat. In addition, within a 300-ms time window, the mean number of startles was not significantly different between the periods before and after a SPW (t = 1.00, df = 5, P > 0.36). Finally, whereas there was no difference in the occurrence of startles in the 300-ms time window after a SPW between intact and decerebrated pups (t = 1.26, df = 8, P > 0.242), there was a significant difference between these groups for the 300-ms time window preceding SPWs (t = 6.97, df = 8, P < 0.0001).
Gamma rhythms occur as early as p2
Surprisingly, in 4 of the 14 head-fixed pups (3 P2 intact and 1 P4 decerebrate) implanted with intrahippocampal electrodes, bursts of gamma-frequency rhythms (20-100 Hz) were detected. Previously, gamma rhythms have been recorded as early as P5 (Lahtinen et al., 2002). Nine of 21 (43%) instances of gamma occurred during periods of high muscle tone (indicative of wakefulness) and 12 of 21 (57%) instances occurred during periods of EMG quiescence (indicative of sleep).
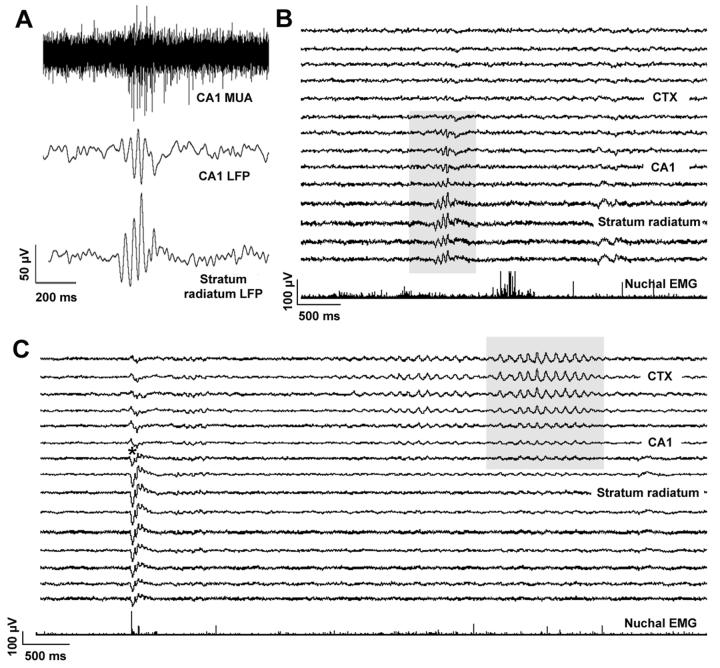
Gamma bursts occurred at rates of 0.20-0.47 per min, were 178 ± 15.4 ms in duration, and had a maximum peak-to-peak amplitude of 62.4 ± 35.2 μV and a frequency of 23.3 ± 4.1 Hz. Figure 3A depicts a representative gamma burst recorded in an intact P2 rat during sleep. In Figure 3B, the laminar profile of a gamma burst is depicted; a phase reversal occurs across CA1 and the highest amplitude is observed within stratum radiatum.
Figure 3.
(A) Representative gamma burst in a head-fixed P2 rat during sleep. Traces from top: CA1 multiunit activity (MUA; filter: 500-5000 Hz); local field potentials (LFP) recorded from CA1 and stratum radiatum (filter: >100 Hz). Note the phase reversal across the two hippocampal layers and the concurrent increase in multiunit activity. (B) Depth profile during a gamma burst (shaded region) for the same P2 subject. Note that the highest amplitude is recorded in the middle portion of the stratum radiatum. Each recording site has a vertical separation of 100 μm. (C) Depth profile of a startle-related SPW (left) and a neocortical “spindle” (right) from another P2 rat during sleep. For the SPW, note the phase reversal between CA1 and stratum radiatum (asterisk). For the spindle, note the absence of phase reversals and the diminishing amplitude as the wave travels from neocortex toward hippocampus.
Other well-known hippocampal population patterns, such as theta rhythm and dentate spikes, were not observed in these subjects. We did, however, find evidence of neocortical “spindles,” as defined by others in infant rats (Khazipov et al., 2004). Figure 3C depicts the depth and amplitude profile of a cortical spindle and, for comparison, a SPW. The SPW exhibits a phase reversal across CA1 and its amplitude is greatest within stratum radiatum. In contrast, the spindle does not exhibit a phase reversal and its amplitude is highest within neocortex. The diminishing amplitude of the spindle between neocortex and hippocampus likely reflects passive propagation of the waveform as a result of volume conduction (Khazipov et al., 2004).
DISCUSSION
Using silicon depth electrodes to verify the occurrence of SPWs and multiple EMG electrodes to detect the presence of startles, we have shown here that SPWs are reliably preceded by startles during the early postnatal period in rats. This relationship between SPWs and startles is temporary, as it is not seen in adults (Vanderwolf, 1969). Consistent with our current understanding of the neural origins of SPWs (Buzsáki, 1986), the fact that startles precede SPWs precludes the possibility that startles are produced by SPWs. Also, because precollicular decerebration decoupled SPWs from startles, it cannot be that startles are necessary to produce SPWs. We must look, then, for an alternative explanation for the temporal link between these two events.
Startles likely originate within the caudal brainstem and their initiation does not require intact connections with the forebrain, whereas SPWs are triggered within the hippocampus and their initiation does not require intact connections with any extrahippocampal structures (Buzsáki, 1986; Buzsáki, 1996). Thus, although the neural mechanisms that produce startles do not directly cause SPWs, startles and SPWs do appear mechanistically linked. Consequently, we suggest that spontaneous startle-generating activity within caudal brainstem neurons (Corner et al., 2002; O'Donovan, 1999) also produces transient network depression (Fedirchuk et al., 1999; Tabak et al., 2001) that ultimately releases hippocampal CA3 neurons to trigger SPWs (Buzsáki, 1986; Buzsáki, 1996). This notion is consistent with the transient depression of neocortical activity, perhaps reflecting decreased cortical input to the hippocampal formation, that precedes the occurrence of SPWs in adults (Battaglia et al., 2004).
Regardless of the exact mechanism that links these two events, however, the near-simultaneous occurrence of startles and SPWs opens the window to further examination of the mechanism whereby SPWs produce Hebbian-like changes in synaptic efficacy during development (Hanse et al., 1997; Kasyanov et al., 2004; King et al., 1999). In this regard, it is interesting that startles decline in frequency with age until they disappear at the end of the third postnatal week (de Vries et al., 1985; Gramsbergen et al., 1970) whereas SPWs persist throughout the lifetime (Buzsáki, 1986; Leinekugel et al., 2002). This observation suggests a transitory functional role for the startle-SPW association in early development.
To our surprise, we did record rhythms in the gamma range in rats as young as P2. Previously, gamma-waves have been reported in rats as young as P5 (Lahtinen et al., 2002). The gamma waves appeared to be of hippocampal origin: they exhibited a phase reversal across the CA1 layer, they diminished rapidly in amplitude with increasing distance from the hippocampus, and they were accompanied by increased unit activity within CA1. Thus, it is apparent that the neonatal hippocampus has the necessary structures to produce and sustain rhythmic activity, even at an age when it differs from the hippocampus of adults with regard to neurotransmitter function, receptor expression, and connectivity (Ben-Ari, 2002; Hanse et al., 1997; Leinekugel, 2003).
In a previous study in which we recorded hippocampal activity in freely moving pups using single-ended electrodes, bursts of theta activity were detected during periods of active sleep (Karlsson and Blumberg, 2003). To our surprise, we did not observe theta activity in the present study, either in head-fixed or freely moving subjects. We did, however, record occasional bursts of rhythmic activity with a frequency of approximately 8 Hz that, in contrast to the gamma activity, did not exhibit phase reversal across the CA1 layer, was not accompanied by increased unit activity, and whose amplitude diminished with distance from the cortical surface. This activity likely reflects passive volume conduction of “spindle” activity from neocortex (Khazipov et al., 2004). On one hand, it is possible that the theta activity reported in the previous study was actually neocortical activity transmitted passively to the hippocampus. On the other hand, the earlier-reported theta bursts were of lower mean frequency than spindles (8.2 ± 0.1 vs. 10.2 ± 1.2 Hz, respectively), and they were accompanied by synchronized CA1 multiunit activity. In addition, the spindles reported by Khazipov et al. (2004) were triggered by sensory feedback resulting from limb movements. The theta-range rhythms reported previously, however, occurred most frequently during behavioral quiescence interspersed with myoclonic twitching but did not seem to follow the high-amplitude movements that are most likely to generate sensory feedback. Additional recordings in freely moving subjects are needed before a final conclusion can be drawn.
Investigations of the development of behavior and neural activity in mammals have evolved in virtual isolation from each other under the rubric of developmental psychobiology and developmental neurophysiology, respectively. The relative independence of these two fields has resulted in part from the inherent technical difficulties of recording neurophysiological activity in unanesthetized infants, especially in the infants of small, altricial species. It is becoming apparent, however, that these technical difficulties can be overcome such that state-of-the-art recording techniques can be used in infants to examine the neural basis of behavior (Karlsson et al., 2005; Khazipov et al., 2004; Lahtinen et al., 2002; Leinekugel et al., 2002).
Whereas startles have long been identified as a developmentally primitive and easily detectable behavior in mammalian fetuses and neonates, neurophysiologists interested in hippocampal function have identified SPWs (and GDPs) as the earliest and most robust neural population pattern exhibited by this structure. Here, we demonstrate a surprisingly reliable temporal link between startles and SPWs in vivo, and we demonstrate that the two can be decoupled when the neural connections between the brainstem and the hippocampus are severed. We suggest that single neural events in the brainstem trigger startles and also contribute, via the spread of transient network depression, to the increased probability of a CA3-triggered SPWs. In turn, the pairing of neural activity that follows a startle—perhaps including sensory feedback arising from startle-related motor activity—with SPWs could result in enhanced efficacy at the affected synapses (Kasyanov et al., 2004; King et al., 1999). In other words, the present results may best be interpreted within the context of recent studies (Khazipov et al., 2004; Petersson et al., 2003) suggesting a role for sensory feedback from self-generated motor activity in the assembly of neural circuits—from spinal cord to forebrain—in the developing animal.
Acknowledgments
Supported by grants from the National Institute of Mental Health (MH50701, MH66424) to M.S.B.
References
- Battaglia FP, Sutherland GR, McNaughton BL. Hippocampal sharp wave bursts coincide with neocortical “up-state” transitions. Learning and Memory. 2004;11(6):697–704. doi: 10.1101/lm.73504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y. Excitatory actions of gaba during development: the nature of the nurture. Nature Reviews Neuroscience. 2002;3(9):728–39. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Bland B. The physiology and pharmacology of hippocampal theta rhythms. Progress in Neurobiology. 1986;26:1–54. doi: 10.1016/0301-0082(86)90019-5. [DOI] [PubMed] [Google Scholar]
- Bland B, Colom LV. Extrinsic and intrinsic properties underlying oscillation and synchrony in limbic cortex. Progress in Neurobiology. 1993;41:157–208. doi: 10.1016/0301-0082(93)90007-f. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Theta oscillations in the hippocampus. Neuron. 2002;33(3):325–40. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- Buzsáki G. Hippocampal sharp waves: their origin and significance. Brain Research. 1986;(398):242–252. doi: 10.1016/0006-8993(86)91483-6. [DOI] [PubMed] [Google Scholar]
- Buzsáki G. The hippocampo-neocortical dialogue. Cerebral Cortex. 1996;6:81–92. doi: 10.1093/cercor/6.2.81. [DOI] [PubMed] [Google Scholar]
- Buzsáki G. Memory consolidation during sleep: a neurophysiological perspective. Journal of Sleep Research. 1998;7(Suppl 1):17–23. doi: 10.1046/j.1365-2869.7.s1.3.x. [DOI] [PubMed] [Google Scholar]
- Colgin LL, Kubota D, Jia Y, Rex CS, Lynch G. Long-term potentiation is impaired in rat hippocampal slices that produce spontaneous sharp waves. Journal of Physiology. 2004;558(Pt 3):953–61. doi: 10.1113/jphysiol.2004.068080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corner MA, van Pelt J, Wolters PS, Baker RE, Nuytinck RH. Physiological effects of sustained blockade of excitatory synaptic transmission on spontaneously active developing neuronal networks--an inquiry into the reciprocal linkage between intrinsic biorhythms and neuroplasticity in early ontogeny. Neuroscience & Biobehavioral Reviews. 2002;26(2):127–85. doi: 10.1016/s0149-7634(01)00062-8. [DOI] [PubMed] [Google Scholar]
- de Vries JI, Visser GH, Prechtl HF. The emergence of fetal behaviour. I. Qualitative aspects. Early Human Development. 1982;7(4):301–22. doi: 10.1016/0378-3782(82)90033-0. [DOI] [PubMed] [Google Scholar]
- de Vries JI, Visser GH, Prechtl HF. The emergence of fetal behaviour. II. Quantitative aspects. Early Human Development. 1985;12(2):99–120. doi: 10.1016/0378-3782(85)90174-4. [DOI] [PubMed] [Google Scholar]
- Fedirchuk B, Wenner P, Whelan PJ, Ho S, Tabak J, O'Donovan MJ. Spontaneous network activity transiently depresses synaptic transmission in the embryonic chick spinal cord. Journal of Neuroscience. 1999;19(6):2102–12. doi: 10.1523/JNEUROSCI.19-06-02102.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramsbergen A, Schwartze P, Prechtl HFR. The postnatal development of behavioral states in the rat. Developmental Psychobiology. 1970;3(4):267–280. doi: 10.1002/dev.420030407. [DOI] [PubMed] [Google Scholar]
- Hanse E, Durand GM, Garaschuk O, Konnerth A. Activity-dependent wiring of the developing hippocampal neuronal circuit. Seminars in Cell and Developmental Biology. 1997;8(1):35–42. doi: 10.1006/scdb.1996.0119. [DOI] [PubMed] [Google Scholar]
- Hebb DO. The organization of behavior: a neuropsychological theory. Science Editions New York: 1961. [Google Scholar]
- Karlsson K,Æ, Kreider JC, Blumberg MS. Hypothalamic contributions to sleep-wake cycle development. Neuroscience. 2004;123:575–582. doi: 10.1016/j.neuroscience.2003.09.025. [DOI] [PubMed] [Google Scholar]
- Karlsson KÆ, Blumberg MS. Hippocampal theta in the newborn rat is revealed under conditions that promote REM sleep. Journal of Neuroscience. 2003;23(4):1114–1118. doi: 10.1523/JNEUROSCI.23-04-01114.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson KÆ, Blumberg MS. Temperature-induced reciprocal activation of hippocampal field activity. Journal of Neurophysiology. 2004;91(1):583–588. doi: 10.1152/jn.00953.2003. [DOI] [PubMed] [Google Scholar]
- Karlsson KÆ, Blumberg MS. Active medullary control of atonia in week-old rats. Neuroscience. 2005;130:275–283. doi: 10.1016/j.neuroscience.2004.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson KÆ, Gall AJ, Mohns EJ, Seelke AM, Blumberg MS. The neural substrates of infant sleep in rats. PLoS Biology. 2005;3(5):891–901. doi: 10.1371/journal.pbio.0030143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasyanov AM, Safiulina VF, Voronin LL, Cherubini E. GABA-mediated giant depolarizing potentials as coincidence detectors for enhancing synaptic efficacy in the developing hippocampus. Proceedings of the National Academy of Sciences USA. 2004;101(11):3967–72. doi: 10.1073/pnas.0305974101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khazipov R, Sirota R, Leinekugel X, Holmes GL, Buzsaki G. Early motor activity drives spindle-bursts in the developing somatosensory cortex. Nature. 2004;432:758–761. doi: 10.1038/nature03132. [DOI] [PubMed] [Google Scholar]
- King C, Henze DA, Leinekugel X, Buzsáki G. Hebbian modification of hippocampal population pattern in the rat. Journal of Physiology. 1999;521(1):159–167. doi: 10.1111/j.1469-7793.1999.00159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahtinen H, Palva JM, Sumanen S, Voipio J, Kaila K, Taira T. Postnatal development of rat hippocampal gamma rhythm in vivo. Journal of Neurophysiology. 2002;88:1469–1474. doi: 10.1152/jn.2002.88.3.1469. [DOI] [PubMed] [Google Scholar]
- Leinekugel X. Developmental patterns and plasticities: the hippocampal model. Journal of Physiology Paris. 2003;97(1):27–37. doi: 10.1016/j.jphysparis.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Leinekugel X, Khazipov R, Cannon R, Hirase H, Ben-Ari Y, Buzsaki G. Correlated bursts of activity in the neonatal hippocampus in vivo. Science. 2002;296(5575):2049–52. doi: 10.1126/science.1071111. [DOI] [PubMed] [Google Scholar]
- O'Donovan MJ. The origin of spontaneous activity in developing networks of the vertebrate nervous system. Current Opinion in Neurobiology. 1999;9:94–104. doi: 10.1016/s0959-4388(99)80012-9. [DOI] [PubMed] [Google Scholar]
- Petersson P, Waldenstrom A, Fahraeus C, Schouenborg J. Spontaneous muscle twitches during sleep guide spinal self-organization. Nature. 2003;424(6944):72–5. doi: 10.1038/nature01719. [DOI] [PubMed] [Google Scholar]
- Roodenburg PJ, Wladimiroff JW, van Es A, Prechtl HF. Classification and quantitative aspects of fetal movements during the second half of normal pregnancy. Early Human Development. 1991;25(1):19–35. doi: 10.1016/0378-3782(91)90203-f. [DOI] [PubMed] [Google Scholar]
- Seelke AM, Karlsson KA, Gall AJ, Blumberg MS. Extraocular muscle activity, rapid ey. movements and the development of active and quiet sleep. European Journal of Neuroscience. 2005;22(4):911–20. doi: 10.1111/j.1460-9568.2005.04322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki SS, Smith GK. Spontaneous EEG spikes in the normal hippocampus. I. Behavioral correlates, laminar pr.files and bilateral synchrony. Electroencephalography and Clinical Neurophysiology. 1987;67(4):348–59. doi: 10.1016/0013-4694(87)90123-4. [DOI] [PubMed] [Google Scholar]
- Tabak J, Rinzel J, O'Donovan MJ. The role of activity-dependent network depression in the expression and self-regulation of spontaneous activity in the developing spinal cord. Journal of Neuroscience. 2001;21(22):8966–78. doi: 10.1523/JNEUROSCI.21-22-08966.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderwolf CH. Hippocampal electrical activity and voluntary movement in the rat. Electroencephalography and Clinical Neurophysiology. 1969;26(4):407–18. doi: 10.1016/0013-4694(69)90092-3. [DOI] [PubMed] [Google Scholar]
- Viana Di Prisco G, Blumberg MS. Effects of serotonin depletion on sleep-related motor activity in infant rats. Society for Neuroscience; Washington, DC: 2005. [Google Scholar]