Abstract
GABAA receptors have an age-adapted function in the brain. During early development, they mediate excitatory effects resulting in activation of calcium sensitive signaling processes that are important for the differentiation of the brain. In more mature stages of development and in adults, GABAA receptors transmit inhibitory signals. The maturation of GABAA signaling follows sex-specific patterns, which appear to also be important for the sexual differentiation of the brain.
The inhibitory effects of GABAA receptor activation have been widely exploited in the treatment of conditions where neuronal silencing is necessary. For instance, drugs that target GABAA receptors are the mainstay of treatment of seizures. Recent evidence suggests however that the physiology and function of GABAA receptors changes in the brain of a subject that has epilepsy or status epilepticus.
This review will summarize the physiology of and the developmental factors regulating the signaling and function of GABAA receptors; how these may change in the brain that has experienced prior seizures; what are the implications for the age and sex specific treatment of seizures and status epilepticus. Finally, the implications of these changes for the treatment of certain forms of medically refractory epilepsies and status epilepticus will be discussed.
Key Words: GABA, chloride, brain, development, seizure, hippocampus, expression, physiology.
INTRODUCTION
GABA (γ-aminobutyric acid) was discovered in the brain in 1950 [11, 265, 309] and has since been established as the primary inhibitory neurotransmitter in the brain [48, 155]. Paradoxically, GABA is derived from the prototypical excitatory neurotransmitter glutamate [265], declaring even from its early production steps its role as a shunt of excitatory inputs, in a network where the balance between excitation and inhibition is very sensitive. GABA can bind to metabotropic GABAB receptors [133, 142, 143, 157, 221, 324] or to iono-tropic GABAA or GABAC receptors. Activation of postsynaptic GABAB receptors increases membrane conductance to K+ leading to prolonged neuronal hyperpolarization. Presynaptic GABAB receptor activation reduces Ca++ conductance and neurotransmitter release. Ionotropic GABAA or GABAC receptors are in turn permeable to chloride and bicarbonate ions [69]. Classically, activation of GABAA or GABAC receptors allows the influx of Cl-, following its electrochemical gradient, resulting in neuronal hyperpolarization. However, early during development, ionotropic GABA receptors mediate depolarizing currents, which activate calcium sensitive signaling processes that are vital for neuronal differentiation and brain development. The importance of GABA-mediated inhibition in normal brain function and diseases stemming from imbalance of excitation and inhibition is well accepted, thanks to our increasing knowledge of brain physiology, pharmacological advances and the progress of genetics. This has rendered the GABA pathway a popular target of pharmacological interventions when excessive brain excitation needs to be averted. However, the changing role of GABA during development and under certain pathological conditions has triggered a line of research re-evaluating the acute and long term effects of GABAergic drugs in the naïve developing brain or the brain that has experienced insults such as seizures. The current review will discuss the current state of knowledge about the dual actions of GABA, specifically as they pertain to GABAA receptor signaling, in the context of normal brain development or of a brain that has experienced seizures.
GABAA RECEPTORS IN NORMAL BRAIN FUNCTION AND DEVELOPMENT
GABAA Receptors: Structure and Pharmacology
GABAA receptors are pentameric channels composed of different combinations of subunits, with distinct pharmacological, localizing or kinetic properties [17]. In mammalians, 16 GABAA receptor subunits are known (α1-α6, β1-β3, γ1-γ3, δ, ε, θ, and π) which form bicuculline-sensitive, ligand-gated ion channel complexes [7, 24, 94-96, 100, 108, 110, 113, 132, 150, 168, 174, 175, 186, 194, 208, 233, 269, 276, 277, 282, 289, 329, 330, 332, 335, 337-339]. Alternate splicing offers additional diversity [18, 55, 152, 160, 248, 325]. The inclusion of a ρ subunit (ρ1 – ρ3) distinguishes the bicuculline-insensitive GABAC receptor family [27, 36, 50, 67, 91, 176, 228, 229, 322, 344]. Two more subunits (β4 and γ4) have been identified in chicken [101, 161]. The obligatory components of a functional GABAA receptor complex are the α and β subunits, typically 2 from each type. Channels formed only as a combination of α and β subunits can be functional [64, 250], but most frequently, a γ or δ subunit is also included. αxβyγ complexes are usually synaptically located GABAa receptors, mediating phasic inhibition [206, 208], although similar extrasynaptic complexes have also been reported [46, 68, 225, 226]. They are activated upon the spontaneous or triggered vesicular release of GABA and are therefore responsible for phasic GABAA receptor inhibitory postsynaptic currents (IPSCs). Alternatively, a δ, θ, π, or ε subunit may be included in the receptor complex. The presence of a δ subunit typically directs the GABAA receptor complex to extrasynaptic locations, where GABAA receptors are tonically activated by ambient GABA [226]. Ambient GABA may rise in cases of excessive synaptic GABA release and spillover, as occurs in seizures, or through pharmacological blockade of GABA re-uptake mechanisms. Less frequently extrasynaptic receptors are composed of αβ or α5β 3γ2 or a1β2γ2 or α3β3γ2, if highly expressed [21, 131, 211, 226, 296].
The combination of different subunits determines the pharmacological characteristics, kinetics, and subcellular localization of the GABAA receptors (reviewed in [208]). For example, zolpidem is an α1-selective agonist [207, 271]; 4,5,6,7-tetrahydroisoxazolo-[5,4-c]pyridin-3-ol (THIP or gaboxadol) has highest affinity for δ-subunit containing receptors [2, 298]. Their modulatory domains include binding sites for benzodiazepines (BZ site), GABA, barbiturates, nonbarbiturate anesthetics and ethanol, neurosteroids, picrotoxin, penicillin, and zinc. Among these, the BZ site is best characterized. Typical agonists at the BZ site are diazepam and lorazepam, whereas typical antagonist is flumazenil. Each receptor complex may have up to 2 BZ sites, each at the interface of an α and γ subunit, and up to 2 GABA sites, between an α and β subunit. Depending upon the subunit composition, BZ sites may have different ligand affinities, highest at type I sites (preferably α1-containing), intermediate at type IIM (preferably α2- or α3- containing) and low at type IIL (α5-containing) [197]. Among the γ subunits, γ2 is preferred for high BZ affinity [208]. Benzodiazepines have high affinity for most α and γ subunit containing receptors with exception of α4 and α6, and very low affinity for δ-containing receptors [207, 208]). Affinity to barbiturates affinity is determined by the β-subunit [99] and the α-subunit present [307], whereas ε-containing receptors are insensitive to barbiturates and other anaesthetic agents [54]. Neurosteroids typically act upon a δ-containing receptor complex, although α1β1γ2 or α3β1γ2 complexes may be responsive to their effects [21, 208]. GABAA receptor agonists can act as GABA-modulatory drugs, altering the effects of GABA binding, such as benzodiazepines, or GABAmimetic, which directly activate the receptors in the absence of GABA, such as muscimol, barbiturates or neurosteroids at high doses [185, 187].
Apart from GABA, several naturally occurring GABAA receptor-acting compounds have been identified. The benzodiazepine-like compounds diazepam and N-desmethyldiaze-pam have been detected in rat brain and adrenals [326], bovine cerebral cortex and milk [195], human milk [243] and have been localized into synaptic vesicles with immunocytochemical assays [195]. It is yet unclear whether these can be synthesized in these organisms in vivo or whether they are ingested from food products, such as wheat (diazepam [328]) or potato (lormetazepam, desmethyldiazepam, delorazepam, lorazepam, delormetazepam [272, 327]). In vivo biosynthetic pathways for N-desmethyldiazepam have been described in the fungus Penicillium verrucosum [31, 32]. In rat brain, active benzodiazepines can be generated in vivo from tryptophan [196] or during in vitro incubation [245]. Acinetobacter lwoffii, a bacterium of the intestinal or skin flora can produce inactive precursors of BZ-like molecules [341]. Pathological accumulation of their active benzodiazepine derivatives has been described in cases of hepatic failure and may contribute to hepatic encephalopathy.
Developmental Changes in GABAA Receptor Structure and Pharmacology
Most studies describing developmental changes in GABAAergic signaling have been done in rats. To better understand how might these reflect changes in humans, it is generally thought that brain development in a postnatal day 8-10 (PN8-10) rat is almost equivalent to a newborn human baby. The infantile stage in rats spans from PN7-21 and is followed by the juvenile stage. Puberty onset in rats occurs at approximately P32-37, whereas adulthood is reached at 2 months [230, 342, 343]. GABA is present in the embryonic neural system from the very early days [105, 162]. In the embryonic rat neocortex, GABA is detected diffusely as early as embryonic day 10 (E10) but after E14 its presence is limited to the subplate, cortical plate, marginal and intermediate zones [105]. In parallel, GABAA receptors are expressed, even before the establishment of GABAergic synapses, to permit the autocrine and paracrine actions of GABA on brain development [164, 183, 278]. Regional differences in subunit expression have been reported in rats, with α4, β1, γ1 detected in the premigratory neuroblasts of the ventricular zone [164, 183] and α2, α3, β3, γ2 at the cortical or subcortical plate [164, 183, 190]. The spatiotemporal developmental patterns of GABA / GABAA receptor expression are thought to be important in the orchestration of the normal GABA-related regulation of proliferation and migration or neural and glial progenitors [105]. The high levels of GABA in the early stages of development promote the proliferation of ventricular zone progenitors [105], whereas the subsequent decline and restriction of GABAAergic influence within the outer neocortical layers inhibits proliferation [8, 105, 177], enhances migration [20], and may therefore permit further neuronal differentiation. GABAAergic signaling is also important for neuronal survival at this stage [128]. In further support of the importance of GABAAergic signaling for brain development, in utero exposure to GABAA receptor inhibitors decreases the number of parvalbumin-immuno-reactive GABAergic neurons in the striatum, by impairing the survival or differentiation of these neurons [182]. Moreover, focal application of GABAAergic agonists in the cortex of newborn rats may induce abnormal migration and heterotopias [107].
Age-related, species, and region-specific changes, gradual or transient, continue through postnatal development, adulthood and ageing for GABAA receptor subunits like α1, α2, α3, α4, α5, γ1, γ2 [138, 171, 214, 255, 260, 340]. Fritschy et al. have proposed that during the early postnatal life, a gradual parallel decrease in α2 / α3 and increase in α1 expression occurs in rat brain [74, 120] (Fig. 1). Similar developmental switch from α2 / α3 to α1 subunit predominance has been observed in mouse superior colliculus [111] and visual cortex [37, 109]. Functionally, the postnatal increase in α1 has been linked to increased sensitivity to neurosteroids [214], zolpidem [111] and benzodiazepines [140], and acquisition of mature type postsynaptic IPSCs with shorter duration [29]. The latter may be important for a brain that learns to respond appropriately to novel patterns of neuronal activation. Using α1 knockout mice, Bosman et al. have elegantly shown that lack of α1 subunits leads to preservation of juvenile, long duration IPSCs and impairs spatiotemporal excitation patterns to local high frequency stimulation in the visual cortex [28, 29]. In the dentate granule cells of the rat hippocampus, the developmental switch from α5 to α1, α4, and γ2 subunits correlates with decreasing sensitivity to zinc and increase in the affinity for benzodiazepines [34, 140]. Sensitivity to zinc is important in the functional regulation of GABAAergic transmission, particularly in immature neurons. Large amounts of zinc can be stored in synaptic vesicles of nerve terminals, as in the hippocampal mossy fibers of the immature hippocampus. Stimulation-dependent zinc release in this system may therefore be useful to keep under control the excessive depolarizing effects of GABA, in a subunit-specific pattern [16, 53, 166, 285, 331]. This may be less important in adult neurons, which lose their sensitivity to zinc, as GABAA receptor mediated inhibition is more efficient.
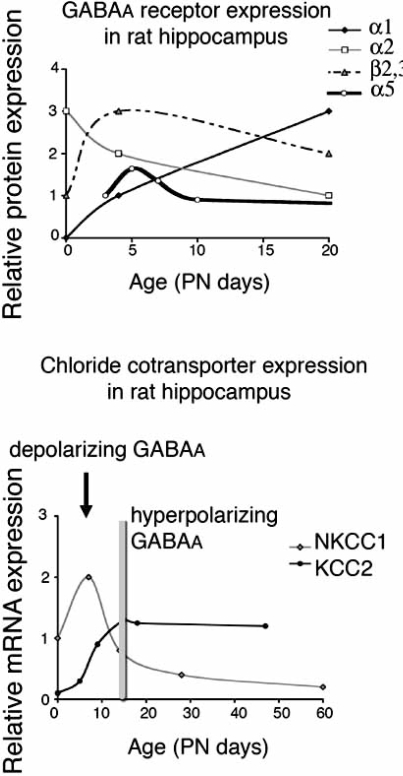
Fig. (1).
Developmental changes in selected GABAA receptor subunits, chloride cotransporters (KCC2 and NKCC1) and GABAA receptor physiology in rat hippocampus.Upper panel: A developmental decrease in α2 and α5 in parallel with an increase in a1 has been described in rat hippocampus. Age dependent changes in other subunits, such as β2,3 has also been reported. Results are from studies [34, 74, 89, 120, 140, 253, 204]. The scale is arbitrary and intends to depict relative changes in expression of a given subunit across ages, and not the relative abundance of one subunit vs another. Lower panel: A switch from an NKCC1-dominant to a KCC2-dominant state occurs in infantile rat hippocampus and has been implicated in the functional switch of GABAA receptor signaling from depolarizing to hyperpolarizing. Results are compiled from studies in male rats or rats of undetermined sex [247, 261]. The vertical bar indicates the age when hyperpolarizing GABAA receptor signaling occurs.
There is though regional specificity of the evolution of these changes [56]. Sex differences in GABAA receptor subunit expression further increase the diversity. These include increased expression of α1 subunit in the female substantia nigra of infantile and juvenile rats [255] and increased γ1 expression in the male rat juvenile medial preoptic area [219]. At the cellular level, GABAA receptor trafficking also evolves. Early in development and before synaptic integration occurs, receptor complexes can be diffusely expressed at the cell membrane and can be tonically activated in the presence of GABA [61, 172, 177, 236, 311]. As the establishment and differentiation of GABAergic synapses begins, they initially occupy both extrasynaptic and synaptic sites; finally targeting and clustering at synaptic sites and dendritic processes increases with maturation and spontaneous IPSCs can be detected [1, 236, 253].
The temporal, regional, sex, and species specific variability in the expression of these subunits in the brain emphasizes that generalization across brain regions, species, genders, and ages is not possible, but one needs to specifically study each structure, age, and condition independently. To further complicate these studies, handling, caloric restriction, and even swim stress regulate GABAA receptor subunit expression, at times with a lasting effect, suggesting that epigenetic influences may be as important in shaping the GABAA receptor related differentiation and communication patterns [122,170, 202, 238].
Developmental Aspects of GABAA Receptor Signaling
GABAA receptors, almost universally, depolarize very immature neurons [22, 23, 130, 177, 181, 198, 215, 235, 256, 275, 305]. The GABAAergic depolarizations can activate voltage sensitive calcium channels, increase intracellular calcium and therefore activate calcium sensitive signaling cascades [23, 235, 256] (Fig. 2). These are important for normal brain development, as they can control DNA synthesis, proliferation, migration, synaptic growth and integration and neuronal differentiation (Fig. 2). For most of the studied neuronal types, there is a time in their maturation process, usually by the end of the first postnatal month in rats, when GABAAergic signaling switches to hyperpolarizing [6, 15, 42, 79, 82, 97, 124, 127, 147, 149, 158, 167, 258, 261, 279, 295, 348, 349]. The ionic mechanisms implicated in this switch are related to the homeostatic regulation of chloride and bicarbonate ions, the main ions flowing through the chan-nel under normal conditions [4, 204, 220, 264, 303, 305].
Fig. (2) .
The developmental switch in chloride cotransporter (CCC) expression drives the functional switch of GABAA receptors from depolarizing to hyperpolarizing.The developmental increase in KCC2 and, in certain tissues, the decrease in NKCC1 triggers the switch from depolarizing to hyperpolarizing GABAAergic signaling [247, 261]. GABA-mediated depolarizations activate L-type voltage sensitive calcium channels(L-VSCC) and release the Mg++ block of NMDA receptors, increasing intracellular Ca++. This can activate calciumregulated signaling pathways, which are important in neuronal development, migration, proliferation, synaptogenesis and differentiation. The GABA-mediated activation of calcium signaling does not occur in neurons with hyperpolarizing GABAA receptor responses.
As shown in Fig. (3), intracellular accumulation of chloride ions is favored when high levels of expression of sodium chloride cotransporters (NCCs), sodium potassium chloride cotransporters (NKCCs) or sodium-independent anion exchangers (i.e. AE3) occurs. In contrast, decrease in intracellular chloride occurs when potassium chloride cotransporters (KCCs), sodium dependent anion exchangers (NDAE) or chloride channel 2 (Clc2) are overexpressed. The ionic permeability of these proteins is graphically depicted in Fig. (2) and described in Table 1.
Fig. (3) Schematic depiction of selected proteins involved in the regulation of Cl- homeostasis.
Upper panel: Cl- accumulation is effected by the presence of the Na+/Cl- Cotransporter NCC and Na+/K+/Cl- Cotransporter NKCCs, with main representative being NKCC1. Their function is dependent upon the supply of Na+ by the Na+/K+ ATPase. In contrast, Anion Exchangers, like AE3, favor Cl- accumulation in a sodium independent manner. Lower panel: Low intracellular Cl- concentration occurs as a result of K+/Cl- Cotransporters, such as KCC2, which export Cl- and K+. As a result, their function is also dependent upon K+ supply by Na+/K+ ATPases. The Sodium Dependent Anion Exchangers NDAE and Chloride Channel 2 (Clc2) also decrease intracellular Cl-[69, 268].
Table 1.
| Protein | Ion Permeability | Features | Inhibitors | Disease Linkage | References | |
|---|---|---|---|---|---|---|
| Cation chloride cotransporters | ||||||
| Potassium Chloride Cotransporters (KCCs) | [57, 77, 86, 134, 259] | |||||
| KCC1 (SLC12A4) | Efflux of K+, Cl- | Ubiquitous | Inhibited by: Furosemide > bumetanide, DIOA, ATP, Hypertonic media, Disulfonic acid stilbene High extracellular K+, WNK, SPAK Activated by: Hypotonic media;N-ethylmaleimide;PDGF (KCC2);PKA (KCC2) |
N/A | [86, 88, 119, 134, 227, 242, 299, 347, 350] | |
| KCC2 (SLC12A5) | Neuronal specific | N/A | [77, 86, 134, 234, 239, 263, 290, 346] | |||
| KCC3 (SLC12A6) | Widespread expression: heart, kidney, neurons, epithelia, red blood cells, muscle, placenta | ACCPN ; variants with bipolar disease | [86, 115, 121, 200, 201, 213, 252] | |||
| KCC4 (SLC12A7) | Widespread expression; weak in brain | N/A | [86, 169, 213, 312] | |||
| Sodium potassium chloride cotransporters (NKCCs) | ||||||
| NKCC1 (SLC12A2; BSC2) | Influx of Na+, K+, 2Cl- | Ubiquitous | Inhibited by: Bumetanide > Furosemide PP-1 Activated by: ATP, Hypertonic media, Calyculin A, Low Cl WNK, SPAK (NKCC1) |
N/A | [49, 59, 77, 134, 210, 240, 246, 247] | |
| NKCC2 (SLC12A1; BSC1) | Kidney | Bartter’s syndrome type I | [76, 126, 188, 259, 284] | |||
| Sodium chloride cotransporters (NCCs) | ||||||
| Sodium chloride co-transporter (SLC12A3; NCC) | Influx of Na+, Cl- | Kidney | Inhibited by: Thiazides Activated by: WNK |
Gitelman’s syndrome | [259] | |
| Chloride channels | ||||||
| Cl- channel 2 (Clcn2 or Clc2) | Efflux of Cl- | Brain (neurons), heart, pancreas, lung, liver, fibroblasts, epithelial | Inhibited by: PP-1 Activated by: Hyperpolarization, cell swelling, acidic pH, hypo-osmotic shock, arachidonic acid, omeprazole, p34(cdc2)/cyclin B; PKA |
Idiopathic generalized epilepsy | [25, 47, 52, 72, 75, 102, 222, 286, 306] | |
| Selected HCO3- transporters | ||||||
| Na+-dependent anion exchanger NDAE (SLC4A8; NDCBE) | Influx Na+, HCO3-; Efflux H+, Cl- |
Brain, testis, kidney, ovary | Inhibited by: DIDS Activated by: ATP requirement (squid) |
N/A | [92, 267,] | |
| Na+-independent anion exchanger AE3 (SLC4A3) | Influx Cl-; Efflux HCO3- | Brain, retina, heart, smooth muscle, epithelia | Activated by: Increased intracellular pH |
Idiopathic generalized epilepsy | [267, 273] | |
Abbreviations: ACCPN: Agenesis of Corpus Callosum with Peripheral Neuropathy; DIDS: 4,4’-diisothiocyano-2,2’-stilbene disulphonic acid; PP-1: protein phosphatase 1; PKA: protein kinase A; PDGF: platelet derived growth factor; WNK: With No lysine (K); SPAK: Serine Proline Alanine lysine (K) rich; OSR: oxidative stress responsive kinase; DIOA: dihydronindenyloxy alkanoic acid.
The developmental change in chloride gradient across the open channel has long been implicated as a determining factor for the depolarizing and hyperpolarizing effects of GABA [4, 204, 220, 303, 305]. Very immature neurons have high intracellular chloride concentrations ([Cl-]i) that shift the equilibrium potential for Cl-(ECl-) to values less negative than the resting membrane potential (Vm). As a result, opening of a GABAA receptor channel leads to efflux of Cl -, which depolarizes the neuron in an attempt to reach ECl-. Mature neurons have low [Cl-]i, ECl- values more negative than Vm, and hyperpolarizing responses to GABAA receptor activation. The molecular characterization of chloride cotransport mechanisms (Table 1, Fig. 3) offered a first insight into the developmental regulation of GABAAergic signaling. Cation chloride cotransporters (CCCs) mediate the electroneutral transport of Cl-along with either K+ (potassium chloride cotransporters, KCCs) or K+ and Na+ (sodium potassium chloride cotransporters, NKCCs) or Na+ only (sodium chloride cotransporters, NCCs) [60, 268]. Under normal conditions, KCCs extrude K+ / Cl-, decreasing intracellular Cl-, whereas NKCCs and NCCs import cations and Cl- into the cell, increasing intracellular Cl-. During the developmental period when GABAA receptor signaling switches from depolarizing to hyperpolarizing in the hippocampus, the expression of key representatives of these families changes: NKCC1 decreases [247] whereas KCC2 increases [179, 261], with net result the decrease in intracellular Cl-. Furthermore, they are sufficient to trigger the switch as shown with in vitro or in vivo antisense inhibition [124, 261, 302,351], overexpression of KCC2 or NKCC1 [6, 39, 165] or pharmacological inhibitors of CCCs [333]. Similar age- and maturity-related changes in the expression of these cotransporters have been described in many neuronal structures [43, 79, 169, 179, 203, 281, 295, 316, 319]. Other factors that may contribute to the increased functionality of KCC2-mediated Cl-export in mature neurons is its more efficient localization at the plasma membrane and oligomerization [14, 26]. For NKCC1, a shift from a neuronal pattern to a glial-dominant pattern of expression has been described in the developing murine nervous system [123].
In normal humans, it is obviously difficult to identify the timing of the GABAA receptor switch and related changes in CCCs. Using human brain tissue from patients deceased from non-neurological disorders, similar developmental increase in KCC2 over NKCC1 was identified in the cortex postnatally, suggesting that a similar gradient of GABAAergic responses may occur [65]. Comorbid conditions and medical treatments, which are known to influence CCC expression and GABAA receptor signaling, may, to an extent, have influenced the expression of these proteins. However, the resemblance of these patterns with the biology of the system in the experimental studies strongly supports the hypothesis that depolarizing GABAAergic responses may indeed occur at least in prematurely born neonates.
Another level of complexity stems form recent findings that the maturation of GABAAergic signaling and its regulators may occur earlier in females than in males. In the substantia nigra pars reticulata (SNR), the expression of KCC2 mRNA is always higher in female than in male GABAergic SNR neurons (infantile and juvenile period) [79]. This explains the earlier appearance of hyperpolarizing GABAAergic responses in females than in males [79, 158]. Similarly, earlier appearance of hyperpolarizing GABAAergic signaling was seen in dopaminergic neurons of the female rat substantia nigra pars compacta (SNC) [82]. As a result, during the sensitive developmental windows of divergent GABAAergic signaling, physiological or pathological activation of these receptors may have distinct translational consequences in males and females. For instance, in male infantile (PN15) SN neurons, GABAAergic depolarizations increase intracellular calcium, the expression of the phosphorylated form of the transcriptional factor CREB (cAMP responsive element binding protein), as well as the expression of calcium regulated mRNAs, such as KCC2 [79, 80, 82]. These do not happen in female PN15 SN neurons, in which GABAAergic activation downregulates KCC2 mRNA [79]. Furthermore, GABAA receptor signaling also interferes with estradiol signaling. Estradiol downregulates KCC2 mRNA only in neurons which are depolarized by GABAA receptors [80] but not in neurons which are either hyperpolarized by them or in which GABAA receptors have been blocked [80, 218] (Fig. 4). These direct and indirect actions support that, in normal development, GABAA receptors can act as broadcasters of sexually differentiating signals in the brain promoting its sexual differentiation [81].
Fig. (4) .
Differential regulation of KCC2 in neurons with depolarizing or hyperpolarizing GABAAergic signaling.GABAA receptor activation and BDNF increase KCC2 in immature neurons with depolarizing GABAA ergic responses, but decrease it in neurons with hyperpolarizing GABAA ergic signaling. Estradiol (E) downregulates KCC2 only in neurons with depolarizing GABA. Testosterone and its androgenic products (T) increase KCC2 in both conditions [3, 35, 81, 262, 263, 317].
In addition, the intracellular concentrations of Cl-and HCO3- are regulated by anion exchangers (AE). The sodium independent electroneutral AEs exchange HCO3- for extracellular Cl-, lowering intracellular pH and increasing Cl- [112, 300, 336]. Sodium Dependent Anion (Cl- / HCO3-) Exchangers (NDAE), also called sodium-dependent Cl-/HCO3- exchangers (NDCBE or NCBE) function in the opposite direction increasing intracellular pH and lowering intracellular Cl-[87, 92, 151, 287, 288, 315, 321]. The expression of NCBE precedes KCC2 in the embryonic mouse brain and, unlike KCC2, NCBE is expressed in the peripheral nervous system and epithelial non-neuronal tissues [125].
Finally, the hyperpolarization-activated chloride channel Clc2 has been implicated in maintaining low intracellular Cl- [41, 102,283, 292]. Clc2 mediated Cl- efflux is also enhanced by extracellular acidosis. Low expression of functional Clc2 has been reported in the rat neonatal hippocampus and has been correlated with the depolarizing actions of GABAA receptors at this age [205]. Clc2 has also been proposed to be a key factor in maintaining low intracellular Cl- in adult dopaminergic neurons of the rat SNC [93].
GABAA receptors are also permeable to HCO3-. As the equilibrium potential for HCO3-is approximately 50mV less negative than the resting potential, HCO3-flux is usually outward [294]. This renders the reversal potential of GABAAergic inhibitory postsynaptic currents (EGABA) less negative than the ECl-, although its contribution is much smaller compared to Cl- [5, 135, 136]. Upregulation of a cytosolic carbonic anhydrase (CAVII), which catalyzes the production of HCO3-from CO2, occurs in hippocampal pyramidal neurons around PN12, promoting the depolarizing GABAAergic responses following high frequency repetitive stimulation [264].
An important distinction should be made though between the ability of GABAA receptor activation to depolarize a neuron as opposed to excite a network into epileptiform discharges or seizure activity. GABA-mediated depolarizations can often reach the threshold for activation of voltage gated calcium channels, such as the L-type channels, or for release of Mg++ block of NMDA receptors (Fig. 2). As a result these processes can increase intracellular calcium and activate calcium-sensitive signaling, with its known impact on brain development and differentiation. However, if the level of neuronal activation begins to exceed EGABA, which is very close to ECl-, the open GABAA receptors start to shunt excitation, by reversing Cl- flux, in an effort to maintain the neuronal potential close to EGABA [293]. Undoubtedly, in conditions when EGABA has shifted to significantly more positive values, even shunting inhibition can fail and this may explain reports of ictogenic properties of GABA [145, 209].
Regulation of Chloride Cotransporters and GABAA Receptor Signaling Switch
The functional importance of the switch of GABAAergic signaling generated a lot of interest around regulatory factors underlying this process. Karadsheh and Delpire sequenced portions of the 5’ region upstream to KCC2 gene and identified a 21bp element with 80% similarity to the neuronal-restrictive silencing factor binding consensus sequence (NRSE) that may function as gene silencer [141]. Although this finding is in good alignment with the neuronal specificity of KCC2, further studies showed that KCC2 lacking this NRSE sequence remains neuronal specific; moreover, in the absence of this NRSE, KCC2 shows similar developmental increase as the normal gene [310].
A number of studies have also investigated the regulation of KCC2 and GABAAergic switch by GABA signaling, showing that depolarizing GABAAergic signaling is a positive drive for the developmental upregulation of KCC2 and switch of GABAA receptors, mediating its effects through activation of voltage-sensitive calcium channels and activation of calcium signaling. Nevertheless it is not necessary, since in its absence the increase in KCC2 and GABAAergic switch still occurs, albeit at a later timepoint. These have been shown in vitro using E18 dissociated rat hippocampal cultures [85] and rat E14 ventral midbrain neurons [308]. Further support has been provided with the effects of in vivo administration of GABAAergic agonists and antagonists on KCC2 and GABAA receptor switch in rat SNR [79] and turtle retina [167]. The sexually dimorphic features of the PN15 rat SNR have provided us with a convenient in vivo system to study KCC2 regulation in normal neurons with similar chronological age, which have either depolarizing (male) or hyperpolarizing (female) GABAAergic signaling. The GABAAergic agonist muscimol increases KCC2 mRNA in male neurons, via activation of voltage sensitive calcium channels and calcium signaling [79, 80]; in contrast, muscimol decreases KCC2 mRNA in female SNR neurons with hyperpolarizing GABAAergic responses [79]. These indicate that the maturational state of a neuron, as it relates to the mode of GABAAergic signaling, is critical in defining its reaction to stimuli that tend to disturb its GABA-related developmental pathway. On a separate note, Ludwig et al. did not observe any changes in KCC2 immunoreactivity in cultured PN0-1 hippocampal mouse neurons chronically treated with either picrotoxin and the sodium channel inhibitor tetrodotoxin (TTX) or combinations of TTX with glutamate receptor inhibitors, proposing that these are not necessary for the developmental increase in KCC2, in hippocampus [180]. Some of the differences in these results may be due to a combination of factors, such as the different maturational stages of the studied cells (embryonic rat vs postnatal mouse hippocampal), or the different combinations and doses of inhibitors.
Another approach to dissect whether neuronal activation promotes the maturation of the GABAAergic system has been through sensory deprivation or lesioning of the natural afferent stimulatory pathways to sensory nuclei. Unilateral or bilateral cochlear ablations prior to the onset of hearing, maintained KCC2 expression at low levels and prevented the developmental decrease in intracellular chloride – at least within the time frame of the study -, within the lateral superior olivary nucleus of the developing rat (~PN15) [280]. In turtle retina, dark rearing inhibited the developmental increase in KCC2 and prolonged the period of excitatory GABAAergic responses [279].
Brain derived neurotrophic factor (BDNF) is a neurotrophic factor that has been implicated both in normal neuronal activity patterns, as well as in the mediation of long term effects of excessive and pathological patterns of neuronal excitability [3]. BDNF expression is high in the first 2-3 postnatal weeks and subsequently declines to adult levels (limbic system, rat ventral mesencephalon of voles) [173, 224]. BDNF exerts opposite effects on KCC2 expression, depending on the developmental stage of the target neuron. In developing neurons, BDNF increases KCC2 expression [3, 35], whereas in mature neurons BDNF decreases KCC2 and causes a positive shift of EGABA [262, 263, 317]. Rivera et al. identified trkB as the receptor involved in BDNF-mediated downregulation of KCC2 [263]. The PLCγ (phospholipase Cγ) signaling downregulates whereas Shc signaling upregulates KCC2 in their system [263]. The developmental and cell type specific expression of these signaling pathways may be therefore important in the developmental regulation of KCC2 by BDNF.
CCCs are also functionally regulated by post-translational modifications. Tyrosine phosphorylation of KCC2 by insulin-like growth factor (IGF-1) and tyrosine kinases increase its activity [144]. Members of the serine-threonine kinase WNK (With No lysine (K)), SPAK (Serine Proline Alanine lysine (K) rich), and oxidative stress responsive (OSR) kinase families have drawn much focus in related research, showing that they are important, cooperatively or independently, in the activation of NKCC1, NKCC2, NCCs and de-activation of KCCs [77, 86, 134] [259] [57]. These interactions are important for the volume-regulation of CCC activity. Activation of protein kinase A pathway (PKA) pathway, through its effects on protein phosphatases, has been implicated in the activation of KCC2 following high frequency stimulation of PN2-3 rat neurons at the deep cerebellar nuclei [234]. Platelet-derived growth factor (PDGF) activates KCC2 via the PI 3-K / PP-1 pathway (phosphoinositide 3-kinase / protein phosphatase-1) [346]. Although certain systems may be more sensitive to modulators of the activity of similar kinases [148], it is not yet known how they contribute to the developmental changes in CCC activity and Cl- regulation.
Hormonal regulation of CCC function is also important during development, given the ongoing neuroendocrine changes occurring at this period, which are important for brain development. We have studied the regulation of KCC2 by sex hormones in PN15 SNR, using in vivo injections. Testosterone and its androgenic derivative dihydrotestosterone both increased KCC2 mRNA expression acutely and this effect was sustained after repetitive doses. This androgenic effect was observed both in male and female SNR, suggesting that it can occur regardless of the direction of GABAAergic signaling [80]. Interestingly, 17β-estradiol was effective in decreasing KCC2 mRNA only in SNR neurons with depolarizing GABAAergic signaling, suggesting an interaction of the two pathways [80]. In accordance with these findings, estrogens failed to regulate KCC2 expression in the pyramidal region of the hippocampus of adult ovariectomized females, which likely have mature GABAAergic responses [218].
Implications for Normal Development and Physiology
In embryonic and immature neurons, GABA has neurotrophic properties: it regulates the proliferation, migration and differentiation of neurons, dendritogenesis and synaptogenesis, increases the number of neurotubules, rough endoplasmic reticulum, Golgi apparatus, synaptic vesicles [20, 23, 39, 105, 291]. As the functional recruitment and requirements of each neuronal structure during development changes with different tempos, it is not surprising that the maturation of the GABAAergic signaling pathway occurs at different timepoints for each cell type. The sensitive regulation of GABAAergic signaling by neuronal activity, patterns of sensory input, epigenetic factors, hormonal influences, interaction with other signaling pathways ensures that brain development will occur in a patterned but also time-, context-, sex-, and experience-driven fashion. This asynchronous maturation may at times be important for structured communication between different cell types [320] or generation of specific activity patterns [279]. On the other hand, it also renders it very vulnerable to dysfunction in case of pathological influences, as will be described in the subsequent sections.
GABAA RECEPTORS IN SEIZURES AND EPILEPSY
GABAA ergic drugs are the mainstay of treatments to suppress seizures [118, 199, 249, 266, 323]. They are primary or secondary targets of many of the available anticonvulsants [199]. These include drugs enhancing GABAA receptor action through a direct interaction with the receptor (benzodiazepines, barbiturates, propofol, stiripentol, topiramate, carbamazepine, phenytoin, felbamate) or indirectly by increasing the available GABA (tiagabine, vigabatrine, gabapentin, valproate) [51, 90, 156, 199, 251]. Furthermore, anticonvulsants can reduce the depolarizing effects of GABAA receptors by inhibiting carbonic anhydrase (topiramate, zonisamide, acetazolamide) [58, 63, 192, 223, 257].
GABAA receptors may influence the susceptibility to seizures. A variety of epileptic or seizure syndromes have been linked to genetic mutations of GABAA receptors, which compromise their function (Table 1). Seizures are most prevalent during the neonatal and infantile period, a time when the brain has not fully matured [103, 104, 212]. Although it is difficult to extrapolate experimental data to humans, this is the time when expression, efficacy, subcellular localization of GABAA receptors, and functional maturation of GABA-driven subcortical seizure-controlling networks have not been fully optimized [314]. For example, GABAAergic activation of the anterior SNR exerts proconvulsant effects in PN15 rats but anticonvulsant effects in PN30 male rats [313]. Moreover, shunting inhibition, due to the depolarizing EGABA is expected to be less efficient. This was nicely demonstrated both in vitro and in vivo by Dzhala et al. who showed that bumetanide, an NKCC1 inhibitor, can suppress ictal activity in very young rats [65]. Its efficacy dropped though in older ages, probably due to the decreased expression of NKCC1. In older preparations or subjects, anticonvulsant efficacy has been demonstrated for compounds potentially inhibiting other age-appropriate mechanisms mediating GABAA-depolarizations. These include thiazides and carbonic anhydrase inhibitors (acetazolamide) [114, 189, 274]. Furosemide, a loop diuretic preferentially inhibiting KCC2 over NKCC1, has been shown to have anticonvulsant activity, but its effect has been linked to a decrease in neuronal synchronization and cell volume regulation [116].
By far the most common type in patients with intractable epilepsy is temporal lobe epilepsy (TLE) [12, 30, 66, 78]. In most cases, TLE has not been linked to genetic factors. TLE patients commonly have a history of an initial precipitating event (IPI), including prolonged neonatal seizures [193]. As a result, intense research is undergoing to reveal how changes in GABAAergic signaling interfere with the acquired mechanisms of ictogenesis, epileptogenesis, and medical refractoriness. In human specimens from resected temporal lobes of patients with TLE and hippocampal sclerosis, the associated neuronal loss results in decreased cell counts of GABAA receptor immunoreactive cells in the vulnerable regions (CA1, CA3, hilus) [178]. The surviving neurons and interneurons show changes in morphology, expression and subcellular distribution of GABAA receptor subunits that partially correspond to patterns seen in younger age groups, based in the experimental studies. Specifically, these changes include increase in α2, α1, β2, β3, γ2 subunit expression in the somata and apical dendrites but reduction in basal dendrites, decreased α1 expression in sectors CA1, CA2, and CA3, decrease of α1 and increase of α2 in CA2 [178]. Pharmacologically, these studies may be interpreted as suggesting that the epileptic state may be associated with less sensitivity to GABAAergic drugs, specifically to benzodiazepines, at least in certain hippocampal neurons. Using flumazenil (benzodiazepine antagonist) PET study, Chugani et al. studied a cohort of patients with epilepsy (2-17 years old) and found an age-related decrease in flumazenil volume of distribution; this change occurred earlier in the subcortical regions [40]. From the experimental models, it is obvious that some of these changes may occur after prolonged seizures and may, at least in certain cases, be long lasting or permanent (Fig. 5). Interestingly, the effects of prolonged seizures on GABAA receptor expression and function are different in younger rats (Fig. 5), an observation that may partially explain the different outcomes of SE in very young vs older subjects. Despite its higher susceptibility to seizures, the immature brain is relatively more resilient to acute injury, epileptogenesis and long term cognitive dysfunction than the mature brain [78, 212].
Fig. (5)Schematic depiction of the timeline of changes in GABAA receptor subunit mRNA expression in the hippocampus, in rodent models of temporal lobe seizures and epilepsy.
The effects of SE change according to age of induction, model, and species. In most cases, the results stem from the lithium-pilocarpine or pilocarpine SE model, except for the results marked with an asterisk, which were described after kainic acid SE. Adulthood starts at PN60. The time scale used for the effects of SE in adult rats is approximate and is meant to reflect changes during the latent phase of epileptogene-sis, prior to the onset of spontaneous seizures, and during the epileptic phase, ie after the occurrence of 2 spontaneous seizures. The diagrams are based on a review of the pertinent literature [33, 73, 163, 254, 244, 345].
Beyond its role in suppressing seizures, several groups have proposed that GABAAergic signaling, under certain conditions, contributes to the appearance of interictal epileptic discharges by increasing neuronal synchronization [13, 153, 154]. An important study that re-focused the interest upon the role of depolarizing GABA in human mesial TLE was published in 2002 [44]. The authors showed that interictal-like activity detected in vitro from the subiculum of patients who underwent resective surgery for mesial TLE was blocked by either GABAA or glutamate receptor antagonists. This was associated with a positive shift in EGABA of the pyramidal subicular neurons. Palma et al. independently concluded that cell membranes from human epileptic tissue, when injected into Xenopus oocytes, elicited depolarizing GABAAergic currents, which was linked to upregulation of NKCC1 and downregulation of KCC2 [237]. Bertelli et al. have recently reported decreased levels of a Clc2 isoform in epileptic temporal lobes [25]. Furthermore, in patients with cortical dysplasias and intractable epilepsy, high expression of NKCC1 and abnormal subcellular distribution of KCC2 has been shown by 2 groups [9, 216]. Similar observations have been obtained in experimental models of seizures. Hippocampal kindling of adult mice decreased KCC2 expression in the hippocampus, through activation of the BDNF / trkB pathway [262]. Amygdalar kindling of adult rats increased NKCC1 in the piriform cortex [231] and dentate gyrus [232]; decreased KCC1 and Clc2 in the dentate gyrus and Clc2 in the CA1 pyramidal region of the hippocampus, but had no effect on KCC2 [232]. In their in vitro model of mirror epileptogenic focus, Khalilov et al. describe that seizure propagation to a drug-naïve hippocampus aberrantly switches GABAAergic signaling back to its immature depolarizing mode and this is important for the generation of ictal patterns [145, 146]. It should be noted that all these studies pertain to neurons, which at the time of seizures had mature-type, hyperpolarizing GABAAergic responses. Given the divergent patterns of regulation of KCC2 in neurons with depolarizing vs hyperpolarizing GABAAergic signaling (Fig. 2), can these observations be extended to neonatal and pediatric epilepsies, when the brain is still immature?
Isaeva et al. induced repetitive but brief flurothyl-induced seizures (not SE) in neonatal rats [129]. In their model, they failed to see any significant effect of neonatal seizures on the timing of GABAAergic switch in the CA3 region of the hippocampus, albeit the amplitude of IPSCs was reduced. More severe and prolonged seizures induced as 3 neonatal episodes of kainic acid-induced SE, increase KCC2 mRNA expression in the CA3 pyramidal region of the male rat hippocampus [83]. A possible explanation is that activation of BDNF and GABAAergic signaling during neonatal SE may actually upregulate KCC2 (Fig. 2). Both these studies support that the seizure-induced re-appearance of depolarizing GABAAergic responses observed in mature neurons is unlikely to occur in the neonatal brain with immature GABAAergic signaling. If indeed the epileptic state is linked with aberrant maintenance of depolarizing GABAAergic signaling, these findings may explain why neonatal rats are relatively resistant to the development of epilepsy following neonatal SE. Further studies are underway to fully characterize the effects of neonatal seizures on chloride homeostasis and GABAAergic switch and determine how these may contribute to the different outcome of neonatal seizures on brain development [78, 117].
Finally, a number of conditions that increase risk of subsequent epilepsy induce aberrant switch of hyperpolarizing to depolarizing GABAAergic signaling in mature neurons. These include hypoxia [84], axonal injury [217]. Interestingly, hypoxia in immature neurons decreases intracellular Cl- [334].
CONCLUSIONS
There is undoubtedly wide region, sex, age, species, experience-driven diversity in GABAAergic signaling. These differences may seem subtle, often identified only with sensitive pharmacological, electrophysiological or immunological tools, suggesting that its complex components serve as fail-safe mechanisms to preserve an important homeostatic mechanism. Their temporal evolution serves sex-, cell type-, and age-appropriate functions: neurotrophic and morphogenetic early in development; activity-driven plasticity at the time when environmental cues make their maximal imprint on the structural and functional organization of the brain; and finally inhibitory and neuromodulatory when the mature brain needs to homeostatically preserve its learned patterns of activity. A key feature of the immature type function of GABAA receptors is the depolarizing signaling, attributed to the inability of young neurons to maintain low intracellular chloride. This is critical for age- and sex-appropriate brain development and differentiation. Of equal importance is to keep in mind that the regulation of GABAAergic switch is different in neurons with depolarizing vs hyperpolarizing GABAAergic signaling. In mature neurons, recurrent and prolonged seizures may trigger a pathological reemergence of immature features of GABAA receptors, which compromises the efficacy of GABA-mediated inhibition. In immature neurons with depolarizing GABAAergic signaling, the physiological and pathological regulation of this system is completely different, possibly contributing to the different outcomes of early life seizures. Moreover, since disturbing the timing of GABAAerging switch can potentially have long lasting effects on brain development and differentiation, it becomes increasingly more urgent to design sex- and age-specific pharmacological interventions adapted for the maturational stage of the targeted brain region, so as to limit side effects. Of particular relevance is the further characterization of the long-term effects on naïve fetuses exposed in utero to maternal use of drugs acting on this system.
Table 2.
Epileptic and Seizure Syndromes Associated with Abnormalities in GABAAergic Signaling
| Gene Defect | Epileptic Syndrome | Proposed Dysfunction | Reference |
|---|---|---|---|
| GABAA receptor subunits | |||
| α 1 (GABRA1) | Autosomal dominant juvenile myoclonic epilepsy (ADJME) | Low amplitude GABA currents; reduced surface expression; increased GABA EC50 | [45, 184] |
| β3 (GABRB3) | Childhood absence epilepsy | [71] | |
| Deletion in 15q11-q13 (includes β 3,α 5, γ 3) | Angelman syndrome | [159, 301] | |
| γ 2 (GABRG2) | Autosomal dominant epilepsy with febrile seizures plus (ADEFS+) | Impaired Cl- influx or potentiation by endozepine | [19] |
| Febrile seizures + childhood absence epilepsy | Impaired sensitivity to benzodiazepines; accumulation of desensitized receptors; endoplasmic reticulum retention; temperature-sensitive trafficking defect | [70, 137, 139, 184, 191, 270, 318] | |
| ADEFS+ including a patient with severe myoclonic epilepsy of infancy (SMEI) | endoplasmic reticulum retention | [98] | |
| Febrile seizures | Increased fast phase desensitization, reduced sensitivity to diazepam | [10, 38] | |
| δ (GABRD | ADEFS+ | Low amplitude GABA currents | [62] |
| ADEFS+, idiopathic generalized epilepsies (IGE), febrile seizures, but also controls | Low amplitude GABA currents | [62] | |
| ADJME | Low amplitude GABA currents | [62] | |
| Proteins involved in Cl- regulation | |||
| AE3 (SLC4A3) | IGE | Abnormal Cl- homeostasis ? | [273] |
| Clc2 (Clcn2) | IGE | Lower transmembrane Cl- gradient, altered voltage-dependent gating | [52, 102, 297] |
ACKNOWLEDGEMENT
I would like to acknowledge the financial support by NIH NINDS (grants NS45243 and NS20253) and the Rett Syndrome Research Foundation, the excellent technical assistance by Mrs Qianyun Li, and the invaluable support by Dr Solomon L. Moshé. I have no conflicts of interest.
REFERENCES
- 1.Adelson PD, Black PM, Madsen JR, Kramer U, Rockoff MA, Riviello JJ, Helmers SL, Mikati M, Holmes GL. Use of subdural grids and strip electrodes to identify a seizure focus in children. Pediatr. Neurosurg. 1995;22(4):174–80. doi: 10.1159/000120898. [DOI] [PubMed] [Google Scholar]
- 2.Adkins CE, Pillai GV, Kerby J, Bonnert TP, Haldon C, McKernan RM, Gonzalez JE, Oades K, Whiting PJ, Simpson PB. Alpha4beta3delta GABA(A) receptors characterized by fluorescence resonance energy transfer-derived measurements of membrane potential. J. Biol. Chem. 2001;276(42):38934–9. doi: 10.1074/jbc.M104318200. [DOI] [PubMed] [Google Scholar]
- 3.Aguado F, Carmona MA, Pozas E, Aguilo A, Martinez-Guijarro FJ, Alcantara S, Borrell V, Yuste R, Ibanez CF, Soriano E. BDNF regulates spontaneous correlated activity at early developmental stages by increasing synaptogenesis and expression of the K+/Cl- co-transporter KCC2. Development. 2003;130(7):1267–80. doi: 10.1242/dev.00351. [DOI] [PubMed] [Google Scholar]
- 4.Aickin CC, Deisz RA, Lux HD. Mechanisms of chloride transport in crayfish stretch receptor neurones and guinea pig vas deferens: implications for inhibition mediated by GABA. Neurosci. Lett. 1984;47(3):239–44. doi: 10.1016/0304-3940(84)90520-2. [DOI] [PubMed] [Google Scholar]
- 5.Akaike N, Inomata N, Yakushiji T. Differential effects of extra- and intracellular anions on GABA-activated currents in bullfrog sensory neurons. J. Neurophysiol. 1989;62(6):1388–99. doi: 10.1152/jn.1989.62.6.1388. [DOI] [PubMed] [Google Scholar]
- 6.Akerman CJ, Cline HT. Depolarizing GABAergic conductances regulate the balance of excitation to inhibition in the developing retinotectal circuit in vivo. J. Neurosci. 2006;26(19):5117–30. doi: 10.1523/JNEUROSCI.0319-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Angelotti TP, Tan F, Chahine KG, Macdonald RL. Molecular and electrophysiological characterization of a allelic variant of the rat alpha 6 GABAA receptor subunit. Brain Res. Mol. Brain Res. 1992;16(1-2):173–8. doi: 10.1016/0169-328x(92)90209-t. [DOI] [PubMed] [Google Scholar]
- 8.Antonopoulos J, Pappas IS, Parnavelas JG. Activation of the GABAA receptor inhibits the proliferative effects of bFGF in cortical progenitor cells. Eur. J. Neurosci. 1997;9(2):291–8. doi: 10.1111/j.1460-9568.1997.tb01399.x. [DOI] [PubMed] [Google Scholar]
- 9.Aronica E, Boer K, Redeker S, Spliet WG, van Rijen PC, Troost D, Gorter JA. Differential expression patterns of chloride transporters, Na(+)-K(+)-2Cl(-)-cotransporter and K(+)-Cl(-)-cotransporter, in epilepsy-associated malformations of cortical development. Neuroscience. 2007;145(1):185–96. doi: 10.1016/j.neuroscience.2006.11.041. [DOI] [PubMed] [Google Scholar]
- 10.Audenaert D, Schwartz E, Claeys KG, Claes L, Deprez L, Suls A, Van Dyck T, Lagae L, Van Broeckhoven C, Macdonald RL, De Jonghe P. A novel GABRG2 mutation associated with febrile seizures. Neurology. 2006;67(4):687–90. doi: 10.1212/01.wnl.0000230145.73496.a2. [DOI] [PubMed] [Google Scholar]
- 11.Awapara J. Free gamma-aminobutyric acid in brain. J. Biol. Chem. 1950;187:35–39. [PubMed] [Google Scholar]
- 12.Babb TL, Brown WJ. Pathological findings in epilepsy. In: Engel JJ, editor. Surgical Treatment of the Epilepsies. NY: Raven Press; 1987. pp. 511–540. [Google Scholar]
- 13.Babb TL, Pretorius JK, Kupfer WR, Crandall PH. Glutamate decarboxylase-immunoreactive neurons are preserved in human epileptic hippocampus. J. Neurosci. 1989;9(7):2562–74. doi: 10.1523/JNEUROSCI.09-07-02562.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balakrishnan V, Becker M, Lohrke S, Nothwang HG, Guresir E, Friauf E. Expression and function of chloride transporters during development of inhibitory neurotransmission in the auditory brainstem. J. Neurosci. 2003;23(10):4134–45. doi: 10.1523/JNEUROSCI.23-10-04134.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banke TG, McBain CJ. GABAergic input onto CA3 hippocampal interneurons remains shunting throughout development. J. Neurosci. 2006;26(45):11720–5. doi: 10.1523/JNEUROSCI.2887-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barberis A, Cherubini E, Mozrzymas JW. Zinc inhibits miniature GABAergic currents by allosteric modulation of GABAA receptor gating. J. Neurosci. 2000;20(23):8618–27. doi: 10.1523/JNEUROSCI.20-23-08618.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barnard EA, Skolnick P, Olsen RW, Mohler H, Sieghart W, Biggio G, Braestrup C, Bateson AN, Langer SZ International Union of Pharmacology.XV. Subtypes of gamma-aminobutyric acidA receptors classification on the basis of subunit structure and receptor function. Pharmacol. Rev. 1998;50(2):291–313. [PubMed] [Google Scholar]
- 18.Bateson AN, Lasham A, Darlison MG. gamma-Aminobutyric acidA receptor heterogeneity is increased by alternative splicing of a novel beta-subunit gene transcript. J. Neurochem. 1991;56(4):1437–40. doi: 10.1111/j.1471-4159.1991.tb11443.x. [DOI] [PubMed] [Google Scholar]
- 19.Baulac S, Huberfeld G, Gourfinkel-An I, Mitropoulou G, Beranger A, Prud'homme JF, Baulac M, Brice A, Bruzzone R, LeGuern E. First genetic evidence of GABA(A) receptor dysfunction in epilepsy a mutation in the gamma2-subunit gene. Nat. Genet. 2001;28(1):46–8. doi: 10.1038/ng0501-46. [DOI] [PubMed] [Google Scholar]
- 20.Behar TN, Li YX, Tran HT, Ma W, Dunlap V, Scott C, Barker JL. GABA stimulates chemotaxis and chemokinesis of embryonic cortical neurons via calcium-dependent mechanisms. J. Neurosci. 1996;16(5):1808–18. doi: 10.1523/JNEUROSCI.16-05-01808.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABA(A) receptor. Nat. Rev. Neurosci. 2005;6(7):565–75. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- 22.Ben-Ari Y, Cherubini E, Corradetti R, Gaiarsa JL. Giant synaptic potentials in immature rat CA3 hippocampal neurones. J. Physiol. 1989;416:303–25. doi: 10.1113/jphysiol.1989.sp017762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ben-Ari Y. Excitatory actions of gaba during development: the nature of the nurture. Nat. Rev. Neurosci. 2002;3(9):728–39. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- 24.Benke D, Mertens S, Trzeciak A, Gillessen D, Mohler H. Identification and immunohistochemical mapping of GABAA receptor subtypes containing the delta-subunit in rat brain. FEBS Lett. 1991;283(1):145–9. doi: 10.1016/0014-5793(91)80573-l. [DOI] [PubMed] [Google Scholar]
- 25.Bertelli M, Cecchin S, Lapucci C, de Gemmis P, Danieli D, d'Amore ES, Buttolo L, Giunta F, Mortini P, Pandolfo M. Quantification of chloride channel 2 (CLCN2) gene isoforms in normal versus lesion- and epilepsy-associated brain tissue. Biochim. Biophys. Acta. 2007;1772(1):15–20. doi: 10.1016/j.bbadis.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 26.Blaesse P, Guillemin I, Schindler J, Schweizer M, Delpire E, Khiroug L, Friauf E, Nothwang HG. Oligomerization of KCC2 correlates with development of inhibitory neurotransmission. J. Neurosci. 2006;26(41):10407–19. doi: 10.1523/JNEUROSCI.3257-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bormann J. The 'ABC' of GABA receptors. Trends Pharmacol Sci. 2000;21(1):16–9. doi: 10.1016/s0165-6147(99)01413-3. [DOI] [PubMed] [Google Scholar]
- 28.Bosman L, Lodder JC, van Ooyen A, Brussaard AB. Role of synaptic inhibition in spatiotemporal patterning of cortical activity. Prog. Brain Res. 2005;147:201–4. doi: 10.1016/S0079-6123(04)47015-0. [DOI] [PubMed] [Google Scholar]
- 29.Bosman LW, Heinen K, Spijker S, Brussaard AB. Mice lacking the major adult GABAA receptor subtype have normal number of synapses, but retain juvenile IPSC kinetics until adulthood. J. Neurophysiol. 2005;94(1):338–46. doi: 10.1152/jn.00084.2005. [DOI] [PubMed] [Google Scholar]
- 30.Bourgeois BF. Temporal lobe epilepsy in infants and children. Brain Dev. 1998;20(3):135–41. doi: 10.1016/s0387-7604(98)00010-2. [DOI] [PubMed] [Google Scholar]
- 31.Bringmann G. A first biosynthetic proposal for the in vivo formation of naturally occurring diazepam-like 1,4-benzodiazepines. J. Neural Transm. Gen. Sect. 1992;88(1):77–82. doi: 10.1007/BF01245038. [DOI] [PubMed] [Google Scholar]
- 32.Bringmann G, Mader T. In vivo formation of diazepam-like 1,4-benzodiazepines by Penicillium verrucosum var.verrucosum after administration of 2-aminobenzophenones and glycine. J. Neural Transm. Gen. Sect. 1995;101(1-3):169–81. doi: 10.1007/BF01271554. [DOI] [PubMed] [Google Scholar]
- 33.Brooks-Kayal AR, Shumate MD, Jin H, Rikhter TY, Coulter DA. Selective changes in single cell GABA(A) receptor subunit expression and function in temporal lobe epilepsy. Nat. Med. 1998;4(10):1166–72. doi: 10.1038/2661. [DOI] [PubMed] [Google Scholar]
- 34.Brooks-Kayal AR, Shumate MD, Jin H, Rikhter TY, Kelly ME, Coulter DA. Gamma-Aminobutyric acid(A) receptor subunit expression predicts functional changes in hippocampal dentate granule cells during postnatal development. J. Neurochem. 2001;77(5):1266–78. doi: 10.1046/j.1471-4159.2001.00329.x. [DOI] [PubMed] [Google Scholar]
- 35.Carmona MA, Pozas E, Martinez A, Espinosa-Parrilla JF, Soriano E, Aguado F. Age-dependent spontaneous hyperexcitability and impairment of GABAergic function in the hippocampus of mice lacking trkB. Cereb. Cortex. 2006;16(1):47–63. doi: 10.1093/cercor/bhi083. [DOI] [PubMed] [Google Scholar]
- 36.Chebib M, Johnston GA. GABA-Activated ligand gated ion channels: medicinal chemistry and molecular biology. J. Med. Chem. 2000;43(8):1427–47. doi: 10.1021/jm9904349. [DOI] [PubMed] [Google Scholar]
- 37.Chen L, Yang C, Mower GD. Developmental changes in the expression of GABA(A) receptor subunits (alpha(1), alpha(2), alpha(3)) in the cat visual cortex and the effects of dark rearing. Brain Res. Mol. Brain Res. 2001;88(1-2):135–43. doi: 10.1016/s0169-328x(01)00042-0. [DOI] [PubMed] [Google Scholar]
- 38.Chou IC, Peng CT, Huang CC, Tsai JJ, Tsai FJ, Tsai CH. Association analysis of gamma 2 subunit of gamma- aminobutyric acid type A receptor polymorphisms with febrile seizures. Pediatr. Res. 2003;54(1):26–9. doi: 10.1203/01.PDR.0000069696.96041.34. [DOI] [PubMed] [Google Scholar]
- 39.Chudotvorova I, Ivanov A, Rama S, Hubner CA, Pellegrino C, Ben-Ari Y, Medina I. Early expression of KCC2 in rat hippocampal cultures augments expression of functional GABA synapses. J. Physiol. 2005;566(Pt 3):671–9. doi: 10.1113/jphysiol.2005.089821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chugani DC, Muzik O, Juhasz C, Janisse JJ, Ager J, Chugani HT. Postnatal maturation of human GABAA receptors measured with positron emission tomography. Ann. Neurol. 2001;49(5):618–26. [PubMed] [Google Scholar]
- 41.Cid LP, Montrose-Rafizadeh C, Smith DI, Guggino WB, Cutting GR. Cloning of a putative human voltage-gated chloride channel (CIC-2) cDNA widely expressed in human tissues. Hum. Mol. Genet. 1995;4(3):407–13. doi: 10.1093/hmg/4.3.407. [DOI] [PubMed] [Google Scholar]
- 42.Clarkson J, Herbison AE. Development of GABA and glutamate signaling at the GnRH neuron in relation to puberty. Mol. Cell Endocrinol. 2006;254-255:32–8. doi: 10.1016/j.mce.2006.04.036. [DOI] [PubMed] [Google Scholar]
- 43.Clayton GH, Owens GC, Wolff JS, Smith RL. Ontogeny of cation-Cl- cotransporter expression in rat neocortex. Brain Res. Dev. Brain Res. 1998;109(2):281–92. doi: 10.1016/s0165-3806(98)00078-9. [DOI] [PubMed] [Google Scholar]
- 44.Cohen I, Navarro V, Clemenceau S, Baulac M, Miles R. On the origin of interictal activity in human temporal lobe epilepsy in vitro. Science. 2002;298(5597):1418–21. doi: 10.1126/science.1076510. [DOI] [PubMed] [Google Scholar]
- 45.Cossette P, Liu L, Brisebois K, Dong H, Lortie A, Vanasse M, Saint-Hilaire JM, Carmant L, Verner A, Lu WY, Wang YT, Rouleau GA. Mutation of GABRA1 in an autosomal dominant form of juvenile myoclonic epilepsy. Nat. Genet. 2002;31(2):184–9. doi: 10.1038/ng885. [DOI] [PubMed] [Google Scholar]
- 46.Craig AM, Blackstone CD, Huganir RL, Banker G. Selective clustering of glutamate and gamma-aminobutyric acid receptors opposite terminals releasing the corresponding neurotransmitters. Proc. Natl. Acad. Sci. USA. 1994;91(26):12373–7. doi: 10.1073/pnas.91.26.12373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cuppoletti J, Tewari KP, Sherry AM, Kupert EY, Malinowska DH. ClC-2 Cl- channels in human lung epithelia: activation by arachidonic acid, amidation, and acid-activated omeprazole. Am. J. Physiol. Cell Physiol. 2001;281(1):C46–54. doi: 10.1152/ajpcell.2001.281.1.C46. [DOI] [PubMed] [Google Scholar]
- 48.Curtis DR, Duggan AW, Felix D, Johnston GA. GABA, bicuculline and central inhibition. Nature. 1970;226(5252):1222–4. doi: 10.1038/2261222a0. [DOI] [PubMed] [Google Scholar]
- 49.Cutler CP, Cramb G. Two isoforms of the Na+/K+/2Cl- cotransporter are expressed in the European eel (Anguilla anguilla) Biochim. Biophys. Acta. 2002;1566(1-2):92–103. doi: 10.1016/s0005-2736(02)00596-5. [DOI] [PubMed] [Google Scholar]
- 50.Cutting GR, Lu L, O'Hara BF, Kasch LM, Montrose-Rafizadeh C, Donovan DM, Shimada S, Antonarakis SE, Guggino WB, Uhl GR, Kazazian HH Jr. Cloning of the gamma-aminobutyric acid (GABA) rho 1 cDNA: a GABA receptor subunit highly expressed in the retina. Proc. Natl. Acad. Sci. USA. 1991;88(7):2673–7. doi: 10.1073/pnas.88.7.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Czuczwar SJ, Patsalos PN. The new generation of GABA enhancers.Potential in the treatment of epilepsy. CNS Drugs. 2001;15(5):339–50. doi: 10.2165/00023210-200115050-00001. [DOI] [PubMed] [Google Scholar]
- 52.D'Agostino D, Bertelli M, Gallo S, Cecchin S, Albiero E, Garofalo PG, Gambardella A, St Hilaire JM, Kwiecinski H, Andermann E, Pandolfo M. Mutations and polymorphisms of the CLCN2 gene in idiopathic epilepsy. Neurology. 2004;63(8):1500–2. doi: 10.1212/01.wnl.0000142093.94998.1a. [DOI] [PubMed] [Google Scholar]
- 53.Davies MF, Maguire PA, Loew GH. Zinc selectively inhibits flux through benzodiazepine-insensitive gamma-aminobutyric acid chloride channels in cortical and cerebellar microsacs. Mol. Pharmacol. 1993;44(4):876–81. [PubMed] [Google Scholar]
- 54.Davies PA, Hanna MC, Hales TG, Kirkness EF. Insensitivity to anaesthetic agents conferred by a class of GABA(A) receptor subunit. Nature. 1997;385(6619):820–3. doi: 10.1038/385820a0. [DOI] [PubMed] [Google Scholar]
- 55.Davies PA, McCartney MR, Wang W, Hales TG, Kirkness EF. Alternative transcripts of the GABA(A) receptor epsilon subunit in human and rat. Neuropharmacology. 2002;43(4):467–75. doi: 10.1016/s0028-3908(02)00153-3. [DOI] [PubMed] [Google Scholar]
- 56.Davis AM, Penschuck S, Fritschy JM, McCarthy MM. Developmental switch in the expression of GABA(A) receptor subunits alpha(1) and alpha(2) in the hypothalamus and limbic system of the rat. Brain Res. Dev. Brain Res. 2000;119(1):127–38. doi: 10.1016/s0165-3806(99)00150-9. [DOI] [PubMed] [Google Scholar]
- 57.de Los Heros P, Kahle KT, Rinehart J, Bobadilla NA, Vazquez N, San Cristobal P, Mount DB, Lifton RP, Hebert SC, Gamba G. WNK3 bypasses the tonicity requirement for K-Cl cotransporter activation via a phosphatase-dependent pathway. Proc. Natl. Acad. Sci. USA. 2006;103(6):1976–81. doi: 10.1073/pnas.0510947103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.De Simone G, Di Fiore A, Menchise V, Pedone C, Antel J, Casini A, Scozzafava A, Wurl M, Supuran CT. Carbonic anhydrase inhibitors.Zonisamide is an effective inhibitor of the cytosolic isozyme II and mitochondrial isozyme V: solution and X-ray crystallographic studies. Bioorg. Med. Chem. Lett. 2005;15(9):2315–20. doi: 10.1016/j.bmcl.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 59.Delpire E, Rauchman MI, Beier DR, Hebert SC, Gullans SR. Molecular cloning and chromosome localization of a putative basolateral Na(+)-K(+)-2Cl- cotransporter from mouse inner medullary collecting duct (mIMCD-3) cells. J. Biol. Chem. 1994;269(41):25677–83. [PubMed] [Google Scholar]
- 60.Delpire E. Cation-Chloride Cotransporters in Neuronal Communication. News Physiol. Sci. 2000;15:309–312. doi: 10.1152/physiologyonline.2000.15.6.309. [DOI] [PubMed] [Google Scholar]
- 61.Demarque M, Represa A, Becq H, Khalilov I, Ben-Ari Y, Aniksztejn L. Paracrine intercellular communication by a Ca2+- and SNARE-independent release of GABA and glutamate prior to synapse formation. Neuron. 2002;36(6):1051–61. doi: 10.1016/s0896-6273(02)01053-x. [DOI] [PubMed] [Google Scholar]
- 62.Dibbens LM, Feng HJ, Richards MC, Harkin LA, Hodgson BL, Scott D, Jenkins M, Petrou S, Sutherland GR, Scheffer IE, Berkovic SF, Macdonald RL, Mulley JC. GABRD encoding a protein for extra- or peri-synaptic GABAA receptors is a susceptibility locus for generalized epilepsies. Hum. Mol. Genet. 2004;13(13):1315–9. doi: 10.1093/hmg/ddh146. [DOI] [PubMed] [Google Scholar]
- 63.Dodgson SJ, Shank RP, Maryanoff BE. Topiramate as an inhibitor of carbonic anhydrase isoenzymes. Epilepsia. 2000;41 (Suppl 1):S35–9. doi: 10.1111/j.1528-1157.2000.tb06047.x. [DOI] [PubMed] [Google Scholar]
- 64.Drewe JA, Chen JS, Reyes AA, Lan NC. Stable high expression of human gamma-aminobutyric acidA receptors composed of alpha and beta subunits. Life Sci. 1995;57(12):1175–82. doi: 10.1016/0024-3205(95)02063-o. [DOI] [PubMed] [Google Scholar]
- 65.Dzhala VI, Talos DM, Sdrulla DA, Brumback AC, Mathews GC, Benke TA, Delpire E, Jensen FE, Staley KJ. NKCC1 transporter facilitates seizures in the developing brain. Nat. Med. 2005;11(11):1205–13. doi: 10.1038/nm1301. [DOI] [PubMed] [Google Scholar]
- 66.Engel J. Jr. Introduction to temporal lobe epilepsy. Epilepsy Res. 1996;26(1):141–50. doi: 10.1016/s0920-1211(96)00043-5. [DOI] [PubMed] [Google Scholar]
- 67.Enz R. GABA(C) receptors a molecular view. Biol. Chem. 2001;382(8):1111–22. doi: 10.1515/BC.2001.141. [DOI] [PubMed] [Google Scholar]
- 68.Essrich C, Lorez M, Benson JA, Fritschy JM, Luscher B. Postsynaptic clustering of major GABAA receptor subtypes requires the gamma 2 subunit and gephyrin. Nat. Neurosci. 1998;1(7):563–71. doi: 10.1038/2798. [DOI] [PubMed] [Google Scholar]
- 69.Farrant M, Kaila K, Tepper J, Abercrombie E, Bolam J. The cellular molecular and ionic basis of GABAA receptor signalling GABA and the basal ganglia. Elsevier. 2007;160:59–87. doi: 10.1016/S0079-6123(06)60005-8. [DOI] [PubMed] [Google Scholar]
- 70.Fedi M, Berkovic SF, Marini C, Mulligan R, Tochon-Danguy H, Reutens DC. A GABAA receptor mutation causing generalized epilepsy reduces benzodiazepine receptor binding. Neuroimage. 2006;32(3):995–1000. doi: 10.1016/j.neuroimage.2006.05.059. [DOI] [PubMed] [Google Scholar]
- 71.Feucht M, Fuchs K, Pichlbauer E, Hornik K, Scharfetter J, Goessler R, Fureder T, Cvetkovic N, Sieghart W, Kasper S, Aschauer H. Possible association between childhood absence epilepsy and the gene encoding GABRB3. Biol. Psychiatry. 1999;46(7):997–1002. doi: 10.1016/s0006-3223(99)00039-6. [DOI] [PubMed] [Google Scholar]
- 72.Flis K, Hinzpeter A, Edelman A, Kurlandzka A. The functioning of mammalian ClC-2 chloride channel in Saccharomyces cerevisiae cells requires an increased level of Kha1p. Biochem. J. 2005;390((Pt 3)):655–64. doi: 10.1042/BJ20050480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Friedman LK, Pellegrini-Giampietro DE, Sperber EF, Bennett MV, Moshé SL, Zukin RS. Kainate-induced status epilepticus alters glutamate and GABAA receptor gene expression in adult rat hippocampus: an in situ hybridization study. J. Neurosci. 1994;14((5 Pt 1)):2697–707. doi: 10.1523/JNEUROSCI.14-05-02697.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fritschy JM, Paysan J, Enna A, Mohler H. Switch in the expression of rat GABAA-receptor subtypes during postnatal development: an immunohistochemical study. J. Neurosci. 1994;14(9):5302–24. doi: 10.1523/JNEUROSCI.14-09-05302.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Furukawa T, Ogura T, Zheng YJ, Tsuchiya H, Nakaya H, Katayama Y, Inagaki N. Phosphorylation and functional regulation of ClC-2 chloride channels expressed in Xenopus oocytes by M cyclin-dependent protein kinase. J. Physiol. 2002;540(Pt 3):883–93. doi: 10.1113/jphysiol.2001.016188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gagnon E, Forbush B, Flemmer AW, Gimenez I, Caron L, Isenring P. Functional and molecular characterization of the shark renal Na-K-Cl cotransporter: novel aspects. Am. J.Physiol. Renal Physiol. 2002;283(5):F1046–55. doi: 10.1152/ajprenal.00107.2002. [DOI] [PubMed] [Google Scholar]
- 77.Gagnon KB, England R, Delpire E. Volume sensitivity of cation-Cl- cotransporters is modulated by the interaction of two kinases Ste20-related proline-alanine-rich kinase and WNK4. Am. J. Physiol. Cell Physiol. 2006;290(1):C134–42. doi: 10.1152/ajpcell.00037.2005. [DOI] [PubMed] [Google Scholar]
- 78.Galanopoulou AS, Vidaurre J, Moshé SL. Under what circumstances can seizures produce hippocampal injury: evidence for age-specific effects. Dev. Neurosci. 2002;24(5):355–63. doi: 10.1159/000069047. [DOI] [PubMed] [Google Scholar]
- 79.Galanopoulou AS, Kyrozis A, Claudio OI, Stanton PK, Moshé SL. Sex-specific KCC2 expression and GABAA receptor function in rat substantia nigra. Exp. Neurol. 2003;183(2):628–637. doi: 10.1016/s0014-4886(03)00213-9. [DOI] [PubMed] [Google Scholar]
- 80.Galanopoulou AS, Moshé SL. Role of sex hormones in the sexually dimorphic expression of KCC2 in rat substantia nigra. Exp. Neurol. 2003;184(2):1003–9. doi: 10.1016/S0014-4886(03)00387-X. [DOI] [PubMed] [Google Scholar]
- 81.Galanopoulou AS. GABA receptors as broadcasters of sexually differentiating signals in the brain. Epilepsia. 2005;46 (Suppl 5):107–12. doi: 10.1111/j.1528-1167.2005.01007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Galanopoulou AS. Sex- and cell-type-specific patterns of GABAA receptor and estradiol-mediated signaling in the immature rat substantia nigra. Eur. J. Neurosci. 2006;23(9):2423–30. doi: 10.1111/j.1460-9568.2006.04778.x. [DOI] [PubMed] [Google Scholar]
- 83.Galanopoulou AS. Developmental factors in the regulation of chloride homeostasis and GABA(A) receptor function by seizures. Epilepsia. 2007;48(Suppl 5):14–8. doi: 10.1111/j.1528-1167.2007.01284.x. [DOI] [PubMed] [Google Scholar]
- 84.Galeffi F, Sah R, Pond BB, George A, Schwartz-Bloom RD. Changes in intracellular chloride after oxygen-glucose deprivation of the adult hippocampal slice effect of diazepam. J. Neurosci. 2004;24(18):4478–88. doi: 10.1523/JNEUROSCI.0755-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ganguly K, Schinder AF, Wong ST, Poo M. GABA itself promotes the developmental switch of neuronal GABAergic responses from excitation to inhibition. Cell. 2001;105(4):521–32. doi: 10.1016/s0092-8674(01)00341-5. [DOI] [PubMed] [Google Scholar]
- 86.Garzon-Muvdi T, Pacheco-Alvarez D, Gagnon KB, Vazquez N, Ponce-Coria J, Moreno E, Delpire E, Gamba G. WNK4 kinase is a negative regulator of K+-Cl- cotransporters. Am. J. Physiol. Renal Physiol. 2006;292(4):F1197–207. doi: 10.1152/ajprenal.00335.2006. [DOI] [PubMed] [Google Scholar]
- 87.Giffard RG, Lee YS, Ouyang YB, Murphy SL, Monyer H. Two variants of the rat brain sodium-driven chloride bicarbonate exchanger (NCBE) developmental expression and addition of a PDZ motif. Eur. J. Neurosci. 2003;18(11):2935–45. doi: 10.1046/j.1460-9568.2003.03053.x. [DOI] [PubMed] [Google Scholar]
- 88.Gillen CM, Brill S, Payne JA, Forbush B 3rd. Molecular cloning and functional expression of the K-Cl cotransporter from rabbit, rat, and human.A new member of the cation-chloride cotransporter family. J. Biol. Chem. 1996;271(27):16237–44. doi: 10.1074/jbc.271.27.16237. [DOI] [PubMed] [Google Scholar]
- 89.Golshani P, Truong H, Jones EG. Developmental expression of GABA(A) receptor subunit and GAD genes in mouse somatosensory barrel cortex. J. Comp. Neurol. 1997;383(2):199–219. doi: 10.1002/(sici)1096-9861(19970630)383:2<199::aid-cne7>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 90.Granger P, Biton B, Faure C, Vige X, Depoortere H, Graham D, Langer SZ, Scatton B, Avenet P. Modulation of the gamma-aminobutyric acid type A receptor by the antiepileptic drugs carbamazepine and phenytoin. Mol. Pharmacol. 1995;47(6):1189–96. [PubMed] [Google Scholar]
- 91.Greka A, Koolen JA, Lipton SA, Zhang D. Cloning and characterization of mouse GABA(C) receptor subunits. Neuroreport. 1998;9(2):229–32. doi: 10.1097/00001756-199801260-00010. [DOI] [PubMed] [Google Scholar]
- 92.Grichtchenko II, Choi I, Zhong X, Bray-Ward P, Russell JM, Boron WF. Cloning, characterization, and chromosomal mapping of a human electroneutral Na(+)-driven Cl-HCO3 exchanger. J.Biol. Chem. 2001;276(11):8358–63. doi: 10.1074/jbc.C000716200. [DOI] [PubMed] [Google Scholar]
- 93.Gulacsi A, Lee CR, Sik A, Viitanen T, Kaila K, Tepper JM, Freund TF. Cell type-specific differences in chloride-regulatory mechanisms and GABA(A) receptor-mediated inhibition in rat substantia nigra. J. Neurosci. 2003;23(23):8237–46. doi: 10.1523/JNEUROSCI.23-23-08237.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hadingham KL, Wingrove P, Le Bourdelles B, Palmer KJ, Ragan CI, Whiting PJ. Cloning of cDNA sequences encoding human alpha 2 and alpha 3 gamma-aminobutyric acidA receptor subunits and characterization of the benzodiazepine pharmacology of recombinant alpha 1-, alpha 2-, alpha 3-, and alpha 5-containing human gamma-aminobutyric acidA receptors. Mol. Pharmacol. 1993;43(6):970–5. [PubMed] [Google Scholar]
- 95.Hadingham KL, Wingrove PB, Wafford KA, Bain C, Kemp JA, Palmer KJ, Wilson AW, Wilcox AS, Sikela JM, Ragan CI. Role of the beta subunit in determining the pharmacology of human gamma-aminobutyric acid type A receptors. Mol. Pharmacol. 1993;44(6):1211–8. [PubMed] [Google Scholar]
- 96.Hadingham KL, Garrett EM, Wafford KA, Bain C, Heavens RP, Sirinathsinghji DJ, Whiting PJ. Cloning of cDNAs encoding the human gamma-aminobutyric acid type A receptor alpha 6 subunit and characterization of the pharmacology of alpha 6-containing receptors. Mol. Pharmacol. 1996;49(2):253–9. [PubMed] [Google Scholar]
- 97.Han SK, Abraham IM, Herbison AE. Effect of GABA on GnRH neurons switches from depolarization to hyperpolarization at puberty in the female mouse. Endocrinology. 2002;143(4):1459–66. doi: 10.1210/endo.143.4.8724. [DOI] [PubMed] [Google Scholar]
- 98.Harkin LA, Bowser DN, Dibbens LM, Singh R, Phillips F, Wallace RH, Richards MC, Williams DA, Mulley JC, Berkovic SF, Scheffer IE, Petrou S. Truncation of the GABA(A)-receptor gamma2 subunit in a family with generalized epilepsy with febrile seizures plus. Am. J. Hum. Genet. 2002;70(2):530–6. doi: 10.1086/338710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Harris BD, Wong G, Moody EJ, Skolnick P. Different subunit requirements for volatile and nonvolatile anesthetics at gamma-aminobutyric acid type A receptors. Mol. Pharmacol. 1995;47(2):363–7. [PubMed] [Google Scholar]
- 100.Harvey RJ, Vreugdenhil E, Barnard EA, Darlison MG. Cloning of genomic and cDNA sequences encoding an invertebrate gamma-aminobutyric acidA receptor subunit. Biochem. Soc. Trans. 1990;18(3):438–9. doi: 10.1042/bst0180438. [DOI] [PubMed] [Google Scholar]
- 101.Harvey RJ, Kim HC, Darlison MG. Molecular cloning reveals the existence of a fourth gamma subunit of the vertebrate brain GABAA receptor. FEBS Lett. 1993;331(3):211–6. doi: 10.1016/0014-5793(93)80339-v. [DOI] [PubMed] [Google Scholar]
- 102.Haug K, Warnstedt M, Alekov AK, Sander T, Ramirez A, Poser B, Maljevic S, Hebeisen S, Kubisch C, Rebstock J, Horvath S, Hallmann K, Dullinger JS, Rau B, Haverkamp F, Beyenburg S, Schulz H, Janz D, Giese B, Muller-Newen G, Propping P, Elger CE, Fahlke C, Lerche H, Heils A. Mutations in CLCN2 encoding a voltage-gated chloride channel are associated with idiopathic generalized epilepsies. Nat. Genet. 2003;33(4):527–32. doi: 10.1038/ng1121. [DOI] [PubMed] [Google Scholar]
- 103.Hauser WA. Status epilepticus: epidemiologic considerations. Neurology. 1990;40(5 Suppl 2):9–13. [PubMed] [Google Scholar]
- 104.Hauser WA, Annegers JF, Kurland LT. Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota 1935-1984. Epilepsia. 34(3):453–68. doi: 10.1111/j.1528-1157.1993.tb02586.x. [DOI] [PubMed] [Google Scholar]
- 105.Haydar TF, Wang F, Schwartz ML, Rakic P. Differential modulation of proliferation in the neocortical ventricular and subventricular zones. J. Neurosci. 2000;20(15):5764–74. doi: 10.1523/JNEUROSCI.20-15-05764.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hebert SC, Mount DB, Gamba G. Molecular physiology of cation-coupled Cl- cotransport: the SLC12 family. Pflugers Arch. 2004;447(5):580–93. doi: 10.1007/s00424-003-1066-3. [DOI] [PubMed] [Google Scholar]
- 107.Heck N, Kilb W, Reiprich P, Kubota H, Furukawa T, Fukuda A, Luhmann HJ. GABA-A receptors regulate neocortical neuronal migration in vitro and in vivo. Cereb. Cortex. 2007;17(1):138–48. doi: 10.1093/cercor/bhj135. [DOI] [PubMed] [Google Scholar]
- 108.Hedblom E, Kirkness EF. A novel class of GABAA receptor subunit in tissues of the reproductive system. J. Biol. Chem. 1997;272(24):15346–50. doi: 10.1074/jbc.272.24.15346. [DOI] [PubMed] [Google Scholar]
- 109.Heinen K, Bosman LW, Spijker S, van Pelt J, Smit AB, Voorn P, Baker RE, Brussaard AB. GABAA receptor maturation in relation to eye opening in the rat visual cortex. Neuroscience. 2004;124(1):161–71. doi: 10.1016/j.neuroscience.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 110.Henderson JE, Soderlund DM, Knipple DC. Characterization of a putative gamma-aminobutyric acid (GABA) receptor beta subunit gene from Drosophila melanogaster. Biochem. Biophys. Res. Commun. 1993;193(2):474–82. doi: 10.1006/bbrc.1993.1648. [DOI] [PubMed] [Google Scholar]
- 111.Henneberger C, Juttner R, Schmidt SA, Walter J, Meier JC, Rothe T, Grantyn R. GluR- and TrkB-mediated maturation of GABA receptor function during the period of eye opening. Eur. J. Neurosci. 2005;21(2):431–40. doi: 10.1111/j.1460-9568.2005.03869.x. [DOI] [PubMed] [Google Scholar]
- 112.Hentschke M, Wiemann M, Hentschke S, Kurth I, Hermans-Borgmeyer I, Seidenbecher T, Jentsch TJ, Gal A, Hubner CA. Mice with a targeted disruption of the Cl-/HCO3- exchanger AE3 display a reduced seizure threshold. Mol. Cell Biol. 2006;26(1):182–91. doi: 10.1128/MCB.26.1.182-191.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Herb A, Wisden W, Luddens H, Puia G, Vicini S, Seeburg PH. The third gamma subunit of the gamma-aminobutyric acid type A receptor family. Proc. Natl. Acad. Sci. USA. 1992;89(4):1433–7. doi: 10.1073/pnas.89.4.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hesdorffer DC, Stables JP, Hauser WA, Annegers JF, Cascino G. Are certain diuretics also anticonvulsants? Ann. Neurol. 2001;50(4):458–62. doi: 10.1002/ana.1136. [DOI] [PubMed] [Google Scholar]
- 115.Hiki K, D'Andrea RJ, Furze J, Crawford J, Woollatt E, Sutherland GR, Vadas MA, Gamble JR. Cloning, characterization, and chromosomal location of a novel human K+-Cl- cotransporter. J. Biol. Chem. 1999;274(15):10661–7. doi: 10.1074/jbc.274.15.10661. [DOI] [PubMed] [Google Scholar]
- 116.Hochman DW, Baraban SC, Owens JW, Schwartzkroin PA. Dissociation of synchronization and excitability in furosemide blockade of epileptiform activity. Science. 1995;270(5233):99–102. doi: 10.1126/science.270.5233.99. [DOI] [PubMed] [Google Scholar]
- 117.Holmes GL, Gairsa JL, Chevassus-Au-Louis N, Ben-Ari Y. Consequences of neonatal seizures in the rat: morphological and behavioral effects. Ann. Neurol. 1998;44(6):845–57. doi: 10.1002/ana.410440602. [DOI] [PubMed] [Google Scholar]
- 118.Holmes GL, Riviello JJ Jr. Midazolam and pentobarbital for refractory status epilepticus. Pediatr. Neurol. 1999;20(4):259–64. doi: 10.1016/s0887-8994(98)00155-6. [DOI] [PubMed] [Google Scholar]
- 119.Holtzman EJ, Kumar S, Faaland CA, Warner F, Logue PJ, Erickson SJ, Ricken G, Waldman J, Kumar S, Dunham PB. Cloning, characterization, and gene organization of K-Cl cotransporter from pig and human kidney and C.elegans. Am. J. Physiol. 1998;275(4 Pt 2):F550–64. doi: 10.1152/ajprenal.1998.275.4.F550. [DOI] [PubMed] [Google Scholar]
- 120.Hornung JP, Fritschy JM. Developmental profile of GABAA-receptors in the marmoset monkey expression of distinct subtypes in pre- and postnatal brain. J. Comp. Neurol. 1996;367(3):413–30. doi: 10.1002/(SICI)1096-9861(19960408)367:3<413::AID-CNE7>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 121.Howard HC, Mount DB, Rochefort D, Byun N, Dupre N, Lu J, Fan X, Song L, Riviere JB, Prevost C, Horst J, Simonati A, Lemcke B, Welch R, England R, Zhan FQ, Mercado A, Siesser WB, George AL Jr, McDonald MP, Bouchard JP, Mathieu J, Delpire E, Rouleau GA. The K-Cl cotransporter KCC3 is mutant in a severe peripheral neuropathy associated with agenesis of the corpus callosum. Nat. Genet. 2002;32(3):384–92. doi: 10.1038/ng1002. [DOI] [PubMed] [Google Scholar]
- 122.Hsu FC, Zhang GJ, Raol YS, Valentino RJ, Coulter DA, Brooks-Kayal AR. Repeated neonatal handling with maternal separation permanently alters hippocampal GABAA receptors and behavioral stress responses. Proc. Natl. Acad. Sci. USA. 2003;100(21):12213–8. doi: 10.1073/pnas.2131679100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hubner CA, Lorke DE, Hermans-Borgmeyer I. Expression of the Na-K-2Cl-cotransporter NKCC1 during mouse development. Mech. Dev. 2001;102(1-2):267–9. doi: 10.1016/s0925-4773(01)00309-4. [DOI] [PubMed] [Google Scholar]
- 124.Hubner CA, Stein V, Hermans-Borgmeyer I, Meyer T, Ballanyi K, Jentsch TJ. Disruption of KCC2 reveals an essential role of K-Cl cotransport already in early synaptic inhibition. Neuron. 2001;30(2):515–24. doi: 10.1016/s0896-6273(01)00297-5. [DOI] [PubMed] [Google Scholar]
- 125.Hubner CA, Hentschke M, Jacobs S, Hermans-Borgmeyer I. Expression of the sodium-driven chloride bicarbonate exchanger NCBE during prenatal mouse development. Gene Expr. Patterns. 2004;5(2):219–23. doi: 10.1016/j.modgep.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 126.Igarashi P, Vanden Heuvel GB, Payne JA, Forbush B 3rd. Cloning, embryonic expression, and alternative splicing of a murine kidney-specific Na-K-Cl cotransporter. Am. J. Physiol. 1995;269(3 Pt 2):F405–18. doi: 10.1152/ajprenal.1995.269.3.F405. [DOI] [PubMed] [Google Scholar]
- 127.Ikeda M, Toyoda H, Yamada J, Okabe A, Sato K, Hotta Y, Fukuda A. Differential development of cation-chloride cotransporters and Cl- homeostasis contributes to differential GABAergic actions between developing rat visual cortex and dorsal lateral geniculate nucleus. Brain Res. 2003;984(1-2):149–59. doi: 10.1016/s0006-8993(03)03126-3. [DOI] [PubMed] [Google Scholar]
- 128.Ikeda Y, Nishiyama N, Saito H, Katsuki H. GABAA receptor stimulation promotes survival of embryonic rat striatal neurons in culture. Brain Res. Dev.Brain Res. 1997;98(2):253–8. doi: 10.1016/s0165-3806(96)00183-6. [DOI] [PubMed] [Google Scholar]
- 129.Isaeva E, Isaev D, Khazipov R, Holmes GL. Selective impairment of GABAergic synaptic transmission in the flurothyl model of neonatal seizures. Eur. J. Neurosci. 2006;23(6):1559–66. doi: 10.1111/j.1460-9568.2006.04693.x. [DOI] [PubMed] [Google Scholar]
- 130.Janigro D, Schwartzkroin PA. Effects of GABA and baclofen on pyramidal cells in the developing rabbit hippocampus an 'in vitro' study. Brain Res. 1988;469(1-2):171–84. doi: 10.1016/0165-3806(88)90180-0. [DOI] [PubMed] [Google Scholar]
- 131.Jia F, Pignataro L, Schofield CM, Yue M, Harrison NL, Goldstein PA. An extrasynaptic GABAA receptor mediates tonic inhibition in thalamic VB neurons. J. Neurophysiol. 2005;94(6):4491–501. doi: 10.1152/jn.00421.2005. [DOI] [PubMed] [Google Scholar]
- 132.Jin P, Zhang J, Rowe-Teeter C, Yang J, Stuve LL, Fu GK. Cloning and characterization of a GABAA receptor gamma2 subunit variant. J. Biol. Chem. 2004;279(2):1408–14. doi: 10.1074/jbc.M308656200. [DOI] [PubMed] [Google Scholar]
- 133.Jones KA, Borowsky B, Tamm JA, Craig DA, Durkin MM, Dai M, Yao WJ, Johnson M, Gunwaldsen C, Huang LY, Tang C, Shen Q, Salon JA, Morse K, Laz T, Smith KE, Nagarathnam D, Noble SA, Branchek TA, Gerald C. GABA(B) receptors function as a heteromeric assembly of the subunits GABA(B)R1 and GABA(B)R2. Nature. 1998;396(6712):674–9. doi: 10.1038/25348. [DOI] [PubMed] [Google Scholar]
- 134.Kahle KT, Rinehart J, de Los Heros P, Louvi A, Meade P, Vazquez N, Hebert SC, Gamba G, Gimenez I, Lifton RP. WNK3 modulates transport of Cl- in and out of cells: implications for control of cell volume and neuronal excitability. Proc. Natl. Acad. Sci. USA. 2005;102(46):16783–8. doi: 10.1073/pnas.0508307102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kaila K, Voipio J. Postsynaptic fall in intracellular pH induced by GABA-activated bicarbonate conductance. Nature. 1987;330(6144):163–5. doi: 10.1038/330163a0. [DOI] [PubMed] [Google Scholar]
- 136.Kaila K, Voipio J, Paalasmaa P, Pasternack M, Deisz RA. The role of bicarbonate in GABAA receptor-mediated IPSPs of rat neocortical neurones. J. Physiol. 1993;464:273–89. doi: 10.1113/jphysiol.1993.sp019634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kananura C, Haug K, Sander T, Runge U, Gu W, Hallmann K, Rebstock J, Heils A, Steinlein OK. A splice-site mutation in GABRG2 associated with childhood absence epilepsy and febrile convulsions. Arch. Neurol. 2002;59(7):1137–41. doi: 10.1001/archneur.59.7.1137. [DOI] [PubMed] [Google Scholar]
- 138.Kanaumi T, Takashima S, Iwasaki H, Mitsudome A, Hirose S. Developmental changes in the expression of GABAA receptor alpha 1 and gamma 2 subunits in human temporal lobe, hippocampus and basal ganglia: an implication for consideration on age-related epilepsy. Epilepsy Res. 2006;71(1):47–53. doi: 10.1016/j.eplepsyres.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 139.Kang JQ, Shen W, Macdonald RL. Why does fever trigger febrile seizures? GABAA receptor gamma2 subunit mutations associated with idiopathic generalized epilepsies have temperature-dependent trafficking deficiencies. J. Neurosci. 2006;26(9):2590–7. doi: 10.1523/JNEUROSCI.4243-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kapur J, Macdonald RL. Postnatal development of hippocampal dentate granule cell gamma-aminobutyric acidA receptor pharmacological properties. Mol. Pharmacol. 1999;55(3):444–52. [PubMed] [Google Scholar]
- 141.Karadsheh MF, Delpire E. Neuronal restrictive silencing element is found in the KCC2 gene: molecular basis for KCC2-specific expression in neurons. J. Neurophysiol. 2001;85(2):995–7. doi: 10.1152/jn.2001.85.2.995. [DOI] [PubMed] [Google Scholar]
- 142.Kaupmann K, Huggel K, Heid J, Flor PJ, Bischoff S, Mickel SJ, McMaster G, Angst C, Bittiger H, Froestl W, Bettler B. Expression cloning of GABA(B) receptors uncovers similarity to metabotropic glutamate receptors. Nature. 1997;386(6622):239–46. doi: 10.1038/386239a0. [DOI] [PubMed] [Google Scholar]
- 143.Kaupmann K, Malitschek B, Schuler V, Heid J, Froestl W, Beck P, Mosbacher J, Bischoff S, Kulik A, Shigemoto R, Karschin A, Bettler B. GABA(B)-receptor subtypes assemble into functional heteromeric complexes. Nature. 1998;396(6712):683–7. doi: 10.1038/25360. [DOI] [PubMed] [Google Scholar]
- 144.Kelsch W, Hormuzdi S, Straube E, Lewen A, Monyer H, Misgeld U. Insulin-like growth factor 1 and a cytosolic tyrosine kinase activate chloride outward transport during maturation of hippocampal neurons. J. Neurosci. 2001;21(21):8339–47. doi: 10.1523/JNEUROSCI.21-21-08339.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Khalilov I, Holmes GL, Ben-Ari Y. In vitro formation of a secondary epileptogenic mirror focus by interhippocampal propagation of seizures. Nat. Neurosci. 2003;6(10):1079–85. doi: 10.1038/nn1125. [DOI] [PubMed] [Google Scholar]
- 146.Khalilov I, Le Van Quyen M, Gozlan H, Ben-Ari Y. Epileptogenic actions of GABA and fast oscillations in the developing hippocampus. Neuron. 2005;48(5):787–96. doi: 10.1016/j.neuron.2005.09.026. [DOI] [PubMed] [Google Scholar]
- 147.Khazipov R, Khalilov I, Tyzio R, Morozova E, Ben-Ari Y, Holmes GL. Developmental changes in GABAergic actions and seizure susceptibility in the rat hippocampus. Eur. J. Neurosci. 2004;19(3):590–600. doi: 10.1111/j.0953-816x.2003.03152.x. [DOI] [PubMed] [Google Scholar]
- 148.Khirug S, Huttu K, Ludwig A, Smirnov S, Voipio J, Rivera C, Kaila K, Khiroug L. Distinct properties of functional KCC2 expression in immature mouse hippocampal neurons in culture and in acute slices. Eur. J. Neurosci. 2005;21(4):899–904. doi: 10.1111/j.1460-9568.2005.03886.x. [DOI] [PubMed] [Google Scholar]
- 149.Kirischuk S, Juttner R, Grantyn R. Time-matched pre- and postsynaptic changes of GABAergic synaptic transmission in the developing mouse superior colliculus. J. Physiol. 2005;563(Pt 3):795–807. doi: 10.1113/jphysiol.2004.081216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kirkness EF, Kusiak JW, Fleming JT, Menninger J, Gocayne JD, Ward DC, Venter JC. Isolation, characterization, and localization of human genomic DNA encoding the beta 1 subunit of the GABAA receptor (GABRB1) Genomics. 1991;10(4):985–95. doi: 10.1016/0888-7543(91)90189-l. [DOI] [PubMed] [Google Scholar]
- 151.Ko YP, Lang HJ, Loh SH, Chu KC, Wu ML. Cl--dependent and Cl--independent Na+/ HCO3- acid extrusion in cultured rat cerebellar astrocytes. Chin. J. Physiol. 1999;42(4):237–48. [PubMed] [Google Scholar]
- 152.Kofuji P, Wang JB, Moss SJ, Huganir RL, Burt DR. Generation of two forms of the gamma-aminobutyric acidA receptor gamma 2-subunit in mice by alternative splicing. J. Neurochem. 1991;56(2):713–5. doi: 10.1111/j.1471-4159.1991.tb08209.x. [DOI] [PubMed] [Google Scholar]
- 153.Kohling R, Lucke A, Straub H, Speckmann EJ, Tuxhorn I, Wolf P, Pannek H, Oppel F. Spontaneous sharp waves in human neocortical slices excised from epileptic patients. Brain. 1998;121(Pt 6):1073–87. doi: 10.1093/brain/121.6.1073. [DOI] [PubMed] [Google Scholar]
- 154.Kostopoulos G, Antoniadis G. Active role of cortical inhibition in the development of generalized epilepsy with spike-and-wave discharges evidence from electrophysiological, microiontophoretic and simulation studies. Epilepsy Res. . 1992;8(Suppl):125–33. doi: 10.1016/b978-0-444-89710-7.50021-5. [DOI] [PubMed] [Google Scholar]
- 155.Krnjevic K, Phillis JW. Iontophoretic studies of neurones in the mammalian cerebral cortex. J. Physiol. 1963;165:274–304. doi: 10.1113/jphysiol.1963.sp007057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kume A, Greenfield LJ Jr, Macdonald RL, Albin RL. Felbamate inhibits [3H]t-butylbicycloorthobenzoate (TBOB) binding and enhances Cl- current at the gamma-aminobutyric AcidA (GABAA) receptor. J. Pharmacol. Exp. Ther. 1996;277(3):1784–92. [PubMed] [Google Scholar]
- 157.Kuner R, Kohr G, Grunewald S, Eisenhardt G, Bach A, Kornau HC. Role of heteromer formation in GABAB receptor function. Science. 1999;283(5398):74–7. doi: 10.1126/science.283.5398.74. [DOI] [PubMed] [Google Scholar]
- 158.Kyrozis A, Chudomel O, Moshé SL, Galanopoulou AS. Sex-dependent maturation of GABAA receptor-mediated synaptic events in rat substantia nigra reticulata. Neurosci. Lett. 2006;398(1-2):1–5. doi: 10.1016/j.neulet.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 159.Lalande M, Minassian BA, DeLorey TM, Olsen RW. Parental imprinting and Angelman syndrome. Adv. Neurol. 1999;79:421–9. [PubMed] [Google Scholar]
- 160.Lasham A, Bateson AN, Darlison MG. Alternative splicing increases GABAA receptor heterogeneity. Biochem. Soc. Trans. 1991;19(1):9S–0. doi: 10.1042/bst019009s. [DOI] [PubMed] [Google Scholar]
- 161.Lasham A, Vreugdenhil E, Bateson AN, Barnard EA, Darlison MG. Conserved organization of gamma-aminobutyric acidA receptor genes: cloning and analysis of the chicken beta 4-subunit gene. J. Neurochem. 1991;57(1):352–5. doi: 10.1111/j.1471-4159.1991.tb02135.x. [DOI] [PubMed] [Google Scholar]
- 162.Lauder JM, Han VK, Henderson P, Verdoorn T, Towle AC. Prenatal ontogeny of the GABAergic system in the rat brain: an immunocytochemical study. Neuroscience. 1986;19(2):465–93. doi: 10.1016/0306-4522(86)90275-7. [DOI] [PubMed] [Google Scholar]
- 163.Lauren HB, Lopez-Picon FR, Korpi ER, Holopainen IE. Kainic acid-induced status epilepticus alters GABA receptor subunit mRNA and protein expression in the developing rat hippocampus. J. Neurochem. 2005;94(5):1384–94. doi: 10.1111/j.1471-4159.2005.03274.x. [DOI] [PubMed] [Google Scholar]
- 164.Laurie DJ, Wisden W, Seeburg PH. The distribution of thirteen GABAA receptor subunit mRNAs in the rat brain.III. Embryonic and postnatal development. . J. Neurosci. 1992;12(11):4151–72. doi: 10.1523/JNEUROSCI.12-11-04151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lee H, Chen CX, Liu YJ, Aizenman E, Kandler K. KCC2 expression in immature rat cortical neurons is sufficient to switch the polarity of GABA responses. Eur. J. Neurosci. 2005;21(9):2593–9. doi: 10.1111/j.1460-9568.2005.04084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Legendre P, Westbrook GL. Noncompetitive inhibition of gamma-aminobutyric acidA channels by Zn. Mol. Pharmacol. 1991;39(3):267–74. [PubMed] [Google Scholar]
- 167.Leitch E, Coaker J, Young C, Mehta V, Sernagor E. GABA type-A activity controls its own developmental polarity switch in the maturing retina. J. Neurosci. 2005;25(19):4801–5. doi: 10.1523/JNEUROSCI.0172-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Levitan ES, Schofield PR, Burt DR, Rhee LM, Wisden W, Kohler M, Fujita N, Rodriguez HF, Stephenson A, Darlison MG, Barnard EA, Seeburg PH. Structural and functional basis for GABAA receptor heterogeneity. Nature. 1988;335(6185):76–9. doi: 10.1038/335076a0. [DOI] [PubMed] [Google Scholar]
- 169.Li H, Tornberg J, Kaila K, Airaksinen MS, Rivera C. Patterns of cation-chloride cotransporter expression during embryonic rodent CNS development. Eur. J. Neurosci. 2002;16(12):2358–70. doi: 10.1046/j.1460-9568.2002.02419.x. [DOI] [PubMed] [Google Scholar]
- 170.Liu J, Brannen KC, Grayson DR, Morrow AL, Devaud LL, Lauder JM. Prenatal exposure to the pesticide dieldrin or the GABA(A) receptor antagonist bicuculline differentially alters expression of GABA(A) receptor subunit mRNAs in fetal rat brainstem. Dev. Neurosci. 1998;20(1):83–92. doi: 10.1159/000017302. [DOI] [PubMed] [Google Scholar]
- 171.Liu Q, Wong-Riley MT. Developmental changes in the expression of GABAA receptor subunits alpha1, alpha2, and alpha3 in brain stem nuclei of rats. Brain Res. 2006;1098(1):129–38. doi: 10.1016/j.brainres.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 172.Liu X, Wang Q, Haydar TF, Bordey A. Nonsynaptic GABA signaling in postnatal subventricular zone controls proliferation of GFAP-expressing progenitors. Nat. Neurosci. 2005;8(9):1179–87. doi: 10.1038/nn1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Liu Y, Fowler CD, Wang Z. Ontogeny of brain-derived neurotrophic factor gene expression in the forebrain of prairie and montane voles. Brain Res. Dev. Brain Res. 2001;127(1):51–61. doi: 10.1016/s0165-3806(01)00111-0. [DOI] [PubMed] [Google Scholar]
- 174.Lolait SJ, O'Carroll AM, Kusano K, Mahan LC. Pharmacological characterization and region-specific expression in brain of the beta 2- and beta 3-subunits of the rat GABAA receptor. FEBS Lett. 1989;258(1):17–21. doi: 10.1016/0014-5793(89)81605-9. [DOI] [PubMed] [Google Scholar]
- 175.Lolait SJ, O'Carroll AM, Kusano K, Muller JM, Brownstein MJ, Mahan LC. Cloning and expression of a novel rat GABAA receptor. FEBS Lett. 1989;246(1-2):145–8. doi: 10.1016/0014-5793(89)80271-6. [DOI] [PubMed] [Google Scholar]
- 176.Lopez-Chavez A, Miledi R, Martinez-Torres A. Cloning and functional expression of the bovine GABA(C) rho2 subunit.Molecular evidence of a widespread distribution in the CNS. Neurosci. Res. 2005;53(4):421–7. doi: 10.1016/j.neures.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 177.LoTurco JJ, Owens DF, Heath MJ, Davis MB, Kriegstein AR. GABA and glutamate depolarize cortical progenitor cells and inhibit DNA synthesis. Neuron. 1995;15(6):1287–98. doi: 10.1016/0896-6273(95)90008-x. [DOI] [PubMed] [Google Scholar]
- 178.Loup F, Wieser HG, Yonekawa Y, Aguzzi A, Fritschy JM. Selective alterations in GABAA receptor subtypes in human temporal lobe epilepsy. J. Neurosci. 2000;20(14):5401–19. doi: 10.1523/JNEUROSCI.20-14-05401.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lu J, Karadsheh M, Delpire E. Developmental regulation of the neuronal-specific isoform of K-Cl cotransporter KCC2 in postnatal rat brains. J. Neurobiol. 1999;39(4):558–68. [PubMed] [Google Scholar]
- 180.Ludwig A, Li H, Saarma M, Kaila K, Rivera C. Developmental up-regulation of KCC2 in the absence of GABAergic and glutamatergic transmission. Eur. J. Neurosci. 2003;18(12):3199–206. doi: 10.1111/j.1460-9568.2003.03069.x. [DOI] [PubMed] [Google Scholar]
- 181.Luhmann HJ, Prince DA. Postnatal maturation of the GABAergic system in rat neocortex. J. Neurophysiol. 1991;65(2):247–63. doi: 10.1152/jn.1991.65.2.247. [DOI] [PubMed] [Google Scholar]
- 182.Luk KC, Sadikot AF. GABA promotes survival but not proliferation of parvalbumin-immunoreactive interneurons in rodent neostriatum an in vivo study with stereology. Neuroscience. 2001;104(1):93–103. doi: 10.1016/s0306-4522(01)00038-0. [DOI] [PubMed] [Google Scholar]
- 183.Ma W, Barker JL. Complementary expressions of transcripts encoding GAD67 and GABAA receptor alpha 4, beta 1, and gamma 1 subunits in the proliferative zone of the embryonic rat central nervous system. J. Neurosci. 1995;15(3 Pt 2):2547–60. doi: 10.1523/JNEUROSCI.15-03-02547.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Macdonald RL, Gallagher MJ, Feng HJ, Kang J. GABA(A) receptor epilepsy mutations. Biochem. Pharmacol. 2004;68(8):1497–506. doi: 10.1016/j.bcp.2004.07.029. [DOI] [PubMed] [Google Scholar]
- 185.Malherbe P, Draguhn A, Multhaup G, Beyreuther K, Mohler H. GABAA-receptor expressed from rat brain alpha- and beta-subunit cDNAs displays potentiation by benzodiazepine receptor ligands. Brain Res. Mol. Brain Res. 1990;8(3):199–208. doi: 10.1016/0169-328x(90)90017-8. [DOI] [PubMed] [Google Scholar]
- 186.Malherbe P, Sigel E, Baur R, Persohn E, Richards JG, Mohler H. Functional expression and sites of gene transcription of a novel alpha subunit of the GABAA receptor in rat brain. FEBS Lett. 1990;260(2):261–5. doi: 10.1016/0014-5793(90)80118-3. [DOI] [PubMed] [Google Scholar]
- 187.Malherbe P, Sigel E, Baur R, Persohn E, Richards JG, Mohler H. Functional characteristics and sites of gene expression of the alpha 1, beta 1, gamma 2-isoform of the rat GABAA receptor. J. Neurosci. 1990;10(7):2330–7. doi: 10.1523/JNEUROSCI.10-07-02330.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Marek S, Tekesin I, Hellmeyer L, Komhoff M, Seyberth HW, Maier RF, Schmidt S. [Differential diagnosis of a polyhydramnion in hyperprostaglandin E syndrome: a case report] Z. Geburtshilfe Neonatol. 2004;208(6):232–5. doi: 10.1055/s-2004-835870. [DOI] [PubMed] [Google Scholar]
- 189.Margineanu DG, Klitgaard H. Differential effects of cation-chloride co-transport-blocking diuretics in a rat hippocampal slice model of epilepsy. Epilepsy Res. 2006;69(2):93–9. doi: 10.1016/j.eplepsyres.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 190.Maric D, Maric I, Ma W, Lahojuji F, Somogyi R, Wen X, Sieghart W, Fritschy JM, Barker JL. Anatomical gradients in proliferation and differentiation of embryonic rat CNS accessed by buoyant density fractionation: alpha 3, beta 3 and gamma 2 GABAA receptor subunit co-expression by post-mitotic neocortical neurons correlates directly with cell buoyancy. Eur. J. Neurosci. 1997;9(3):507–22. doi: 10.1111/j.1460-9568.1997.tb01628.x. [DOI] [PubMed] [Google Scholar]
- 191.Marini C, Harkin LA, Wallace RH, Mulley JC, Scheffer IE, Berkovic SF. Childhood absence epilepsy and febrile seizures: a family with a GABA(A) receptor mutation. Brain. 2003;126(Pt 1):230–40. doi: 10.1093/brain/awg018. [DOI] [PubMed] [Google Scholar]
- 192.Masereel B, Rolin S, Abbate F, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors: anticonvulsant sulfonamides incorporating valproyl and other lipophilic moieties. J. Med. Chem. 2002;45(2):312–20. doi: 10.1021/jm0109199. [DOI] [PubMed] [Google Scholar]
- 193.Mathern GW, Pretorius JK, Babb TL. Influence of the type of initial precipitating injury and at what age it occurs on course and outcome in patients with temporal lobe seizures. J. Neurosurg. 1995;82(2):220–7. doi: 10.3171/jns.1995.82.2.0220. [DOI] [PubMed] [Google Scholar]
- 194.McKinley DD, Lennon DJ, Carter DB. Cloning, sequence analysis and expression of two forms of mRNA coding for the human beta 2 subunit of the GABAA receptor. Brain Res. Mol. Brain Res. 1995;28(1):175–9. doi: 10.1016/0169-328x(94)00228-7. [DOI] [PubMed] [Google Scholar]
- 195.Medina JH, Pena C, Piva M, Paladini AC, De Robertis E. Presence of benzodiazepine-like molecules in mammalian brain and milk. Biochem. Biophys. Res. Commun. 1988;152(2):534–9. doi: 10.1016/s0006-291x(88)80070-6. [DOI] [PubMed] [Google Scholar]
- 196.Medina JH, Levi de Stein M, Wolfman C, Wasowski C, De Blas A, Paladini AC. In vivo formation of benzodiazepine-like molecules in mammalian brain. Biochem. Biophys. Res. Commun. 1993;195(2):1111–8. doi: 10.1006/bbrc.1993.2159. [DOI] [PubMed] [Google Scholar]
- 197.Mehta AK, Ticku MK. An update on GABAA receptors. Brain Res. Brain Res. Rev. 1999;29(2-3):196–217. doi: 10.1016/s0165-0173(98)00052-6. [DOI] [PubMed] [Google Scholar]
- 198.Meier J, Akyeli J, Kirischuk S, Grantyn R. GABA(A) receptor activity and PKC control inhibitory synaptogenesis in CNS tissue slices. Mol. Cell Neurosci. 2003;23(4):600–13. doi: 10.1016/s1044-7431(03)00079-4. [DOI] [PubMed] [Google Scholar]
- 199.Meldrum BS, Rogawski MA. Molecular targets for antiepileptic drug development. Neurotherapeatics. 2007;4(1):18–61. doi: 10.1016/j.nurt.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Mercado A, Vazquez N, Song L, Cortes R, Enck AH, Welch R, Delpire E, Gamba G, Mount DB. NH2-terminal heterogeneity in the KCC3 K+-Cl- cotransporter. Am. J. Physiol. Renal Physiol. 2005;289(6):F1246–61. doi: 10.1152/ajprenal.00464.2004. [DOI] [PubMed] [Google Scholar]
- 201.Meyer J, Johannssen K, Freitag CM, Schraut K, Teuber I, Hahner A, Mainhardt C, Mossner R, Volz HP, Wienker TF, McKeane D, Stephan DA, Rouleau G, Reif A, Lesch KP. Rare variants of the gene encoding the potassium chloride co-transporter 3 are associated with bipolar disorder. Int. J. Neuropsychopharmacol. 2005;8(4):495–504. doi: 10.1017/S1461145705005821. [DOI] [PubMed] [Google Scholar]
- 202.Mhatre MC, Ticku MK. Caloric restriction retards the aging associated changes in gamma-aminobutyric acidA receptor gene expression in rat cerebellum. Brain Res. Mol. Brain Res. 1998;54(2):270–5. doi: 10.1016/s0169-328x(97)00342-2. [DOI] [PubMed] [Google Scholar]
- 203.Mikawa S, Wang C, Shu F, Wang T, Fukuda A, Sato K. Developmental changes in KCC1, KCC2 and NKCC1 mRNAs in the rat cerebellum. Brain Res. Dev. Brain Res. 2002;136(2):93–100. doi: 10.1016/s0165-3806(02)00345-0. [DOI] [PubMed] [Google Scholar]
- 204.Misgeld U, Deisz RA, Dodt HU, Lux HD. The role of chloride transport in postsynaptic inhibition of hippocampal neurons. Science. 1986;232(4756):1413–5. doi: 10.1126/science.2424084. [DOI] [PubMed] [Google Scholar]
- 205.Mladinic M, Becchetti A, Didelon F, Bradbury A, Cherubini E. Low expression of the ClC-2 chloride channel during postnatal development: a mechanism for the paradoxical depolarizing action of GABA and glycine in the hippocampus. Proc. Biol. Sci. 1999;266(1425):1207–13. doi: 10.1098/rspb.1999.0764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Mody I, Pearce RA. Diversity of inhibitory neurotransmission through GABA(A) receptors. Trends Neurosci. 2004;27(9):569–75. doi: 10.1016/j.tins.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 207.Mohler H, Benke D, Mertens S, Fritschy JM. GABAA-receptor subtypes differing in alpha-subunit composition display unique pharmacological properties. Adv. Biochem. Psychopharmacol. 1992;47:41–53. [PubMed] [Google Scholar]
- 208.Mohler H. GABA(A) receptor diversity and pharmacology. Cell Tissue Res. 2006;326(2):505–16. doi: 10.1007/s00441-006-0284-3. [DOI] [PubMed] [Google Scholar]
- 209.Montenegro MA, Guerreiro MM, Caldas JP, Moura-Ribeiro MV, Guerreiro CA. Epileptic manifestations induced by midazolam in the neonatal period. Arq. Neuropsiquiatr. 2001;59(2-A):242–3. doi: 10.1590/s0004-282x2001000200018. [DOI] [PubMed] [Google Scholar]
- 210.Moore-Hoon ML, Turner RJ. Molecular and topological characterization of the rat parotid Na+-K+-2Cl- cotransporter1. Biochim. Biophys. Acta. 1998;1373(1):261–9. doi: 10.1016/s0005-2736(98)00112-6. [DOI] [PubMed] [Google Scholar]
- 211.Mortensen M, Smart TG. Extrasynaptic alphabeta subunit GABAA receptors on rat hippocampal pyramidal neurons. J. Physiol. 2006;577(Pt 3):841–56. doi: 10.1113/jphysiol.2006.117952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Moshé SL. Epileptogenesis and the immature brain. Epilepsia. 1987;28 (Suppl1):S3–15. doi: 10.1111/j.1528-1157.1987.tb05753.x. [DOI] [PubMed] [Google Scholar]
- 213.Mount DB, Mercado A, Song L, Xu J, George AL Jr, Delpire E, Gamba G. Cloning and characterization of KCC3 and KCC4, new members of the cation-chloride cotransporter gene family. J. Biol. Chem. 1999;274(23):16355–62. doi: 10.1074/jbc.274.23.16355. [DOI] [PubMed] [Google Scholar]
- 214.Mtchedlishvili Z, Sun CS, Harrison MB, Kapur J. Increased neurosteroid sensitivity of hippocampal GABAA receptors during postnatal development. Neuroscience. 2003;118(3):655–66. doi: 10.1016/s0306-4522(03)00043-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Mueller AL, Chesnut RM, Schwartzkroin PA. Actions of GABA in developing rabbit hippocampus, an in vitro study. Neurosci. Lett. 1983;39(2):193–8. doi: 10.1016/0304-3940(83)90076-9. [DOI] [PubMed] [Google Scholar]
- 216.Munakata M, Watanabe M, Otsuki T, Nakama H, Arima K, Itoh M, Nabekura J, Iinuma K, Tsuchiya S. Altered distribution of KCC2 in cortical dysplasia in patients with intractable epilepsy. Epilepsia. 2007;48(4):837–44. doi: 10.1111/j.1528-1167.2006.00954.x. [DOI] [PubMed] [Google Scholar]
- 217.Nabekura J, Ueno T, Okabe A, Furuta A, Iwaki T, Shimizu-Okabe C, Fukuda A, Akaike N. Reduction of KCC2 expression and GABAA receptor-mediated excitation after in vivo axonal injury. J. Neurosci. 2002;22(11):4412–7. doi: 10.1523/JNEUROSCI.22-11-04412.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Nakamura NH, Rosell DR, Akama KT, McEwen BS. Estrogen and ovariectomy regulate mRNA and protein of glutamic acid decarboxylases and cation-chloride cotransporters in the adult rat hippocampus. Neuroendocrinology. 2004;80(5):308–23. doi: 10.1159/000083657. [DOI] [PubMed] [Google Scholar]
- 219.Nett ST, Jorge-Rivera JC, Myers M, Clark AS, Henderson LP. Properties and sex-specific differences of GABAA receptors in neurons expressing gamma1 subunit mRNA in the preoptic area of the rat. J. Neurophysiol. 1999;81(1):192–203. doi: 10.1152/jn.1999.81.1.192. [DOI] [PubMed] [Google Scholar]
- 220.Newberry NR, Nicoll RA. Comparison of the action of baclofen with gamma-aminobutyric acid on rat hippocampal pyramidal cells in vitro. J. Physiol. 1985;360:161–85. doi: 10.1113/jphysiol.1985.sp015610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Ng GY, Clark J, Coulombe N, Ethier N, Hebert TE, Sullivan R, Kargman S, Chateauneuf A, Tsukamoto N, McDonald T, Whiting P, Mezey E, Johnson MP, Liu Q, Kolakowski LF Jr, Evans JF, Bonner TI, O'Neill GP. Identification of a GABAB receptor subunit, gb2, required for functional GABAB receptor activity. J. Biol. Chem. 1999;274(12):7607–10. doi: 10.1074/jbc.274.12.7607. [DOI] [PubMed] [Google Scholar]
- 222.Niemeyer MI, Yusef YR, Cornejo I, Flores CA, Sepulveda FV, Cid LP. Functional evaluation of human ClC-2 chloride channel mutations associated with idiopathic generalized epilepsies. Physiol. Genomics. 2004;19(1):74–83. doi: 10.1152/physiolgenomics.00070.2004. [DOI] [PubMed] [Google Scholar]
- 223.Nishimori I, Vullo D, Innocenti A, Scozzafava A, Mastrolorenzo A, Supuran CT. Carbonic anhydrase inhibitors: inhibition of the transmembrane isozyme XIV with sulfonamides. Bioorg. Med. Chem. Lett. 2005;15(17):3828–33. doi: 10.1016/j.bmcl.2005.06.055. [DOI] [PubMed] [Google Scholar]
- 224.Numan S, Gall CM, Seroogy KB. Developmental expression of neurotrophins and their receptors in postnatal rat ventral midbrain. J. Mol. Neurosci. 2005;27(2):245–60. doi: 10.1385/JMN:27:2:245. [DOI] [PubMed] [Google Scholar]
- 225.Nusser Z, Roberts JD, Baude A, Richards JG, Sieghart W, Somogyi P. Immunocytochemical localization of the alpha 1 and beta 2/3 subunits of the GABAA receptor in relation to specific GABAergic synapses in the dentate gyrus. Eur. J. Neurosci. 1995;7(4):630–46. doi: 10.1111/j.1460-9568.1995.tb00667.x. [DOI] [PubMed] [Google Scholar]
- 226.Nusser Z, Sieghart W, Somogyi P. Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J. Neurosci. 1998;18(5):1693–703. doi: 10.1523/JNEUROSCI.18-05-01693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Ochiai H, Higa K, Fujise H. Molecular identification of K-CL cotransporter in dog erythroid progenitor cells. J. Biochem. (Tokyo) 2004;135(3):365–74. doi: 10.1093/jb/mvh044. [DOI] [PubMed] [Google Scholar]
- 228.Ogurusu T, Taira H, Shingai R. Identification of GABAA receptor subunits in rat retina: cloning of the rat GABAA receptor rho 2-subunit cDNA. J. Neurochem. 1995;65(3):964–8. doi: 10.1046/j.1471-4159.1995.65030964.x. [DOI] [PubMed] [Google Scholar]
- 229.Ogurusu T, Shingai R. Cloning of a putative gamma-aminobutyric acid (GABA) receptor subunit rho 3 cDNA. Biochim. Biophys. Acta. 1996;1305(1-2):15–8. doi: 10.1016/0167-4781(95)00205-7. [DOI] [PubMed] [Google Scholar]
- 230.Ojeda SR, Andrews WW, Advis JP, White SS. Recent advances in the endocrinology of puberty. Endocr. Rev. 1980;1(3):228–57. doi: 10.1210/edrv-1-3-228. [DOI] [PubMed] [Google Scholar]
- 231.Okabe A, Ohno K, Toyoda H, Yokokura M, Sato K, Fukuda A. Amygdala kindling induces upregulation of mRNA for NKCC1, a Na(+), K(+)- 2Cl(-) cotransporter, in the rat piriform cortex. Neurosci. Res. 2002;44(2):225–9. doi: 10.1016/s0168-0102(02)00093-7. [DOI] [PubMed] [Google Scholar]
- 232.Okabe A, Yokokura M, Toyoda H, Shimizu-Okabe C, Ohno K, Sato K, Fukuda A. Changes in chloride homeostasis-regulating gene expressions in the rat hippocampus following amygdala kindling. Brain Res. 2003;990(1-2):221–6. doi: 10.1016/s0006-8993(03)03528-5. [DOI] [PubMed] [Google Scholar]
- 233.Olsen RW, Bureau MH, Endo S, Smith G. The GABAA receptor family in the mammalian brain. Neurochem. Res. 1991;16(3):317–25. doi: 10.1007/BF00966095. [DOI] [PubMed] [Google Scholar]
- 234.Ouardouz M, Sastry BR. Activity-mediated shift in reversal potential of GABA-ergic synaptic currents in immature neurons. Brain Res. Dev. Brain Res. 2005;160(1):78–84. doi: 10.1016/j.devbrainres.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 235.Owens DF, Boyce LH, Davis MB, Kriegstein AR. Excitatory GABA responses in embryonic and neonatal cortical slices demonstrated by gramicidin perforated-patch recordings and calcium imaging. J. Neurosci. 1996;16(20):6414–23. doi: 10.1523/JNEUROSCI.16-20-06414.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Owens DF, Liu X, Kriegstein AR. Changing properties of GABA(A) receptor-mediated signaling during early neocortical development. J. Neurophysiol. 1999;82(2):570–83. doi: 10.1152/jn.1999.82.2.570. [DOI] [PubMed] [Google Scholar]
- 237.Palma E, Amici M, Sobrero F, Spinelli G, Di Angelantonio S, Ragozzino D, Mascia A, Scoppetta C, Esposito V, Miledi R, Eusebi F. Anomalous levels of Cl- transporters in the hippocampal subiculum from temporal lobe epilepsy patients make GABA excitatory. Proc. Natl. Acad. Sci. USA. 2006;103(22):8465–8. doi: 10.1073/pnas.0602979103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Park CH, Hitri A, Lukacs LG, Deutsch SI. Swim stress selectively alters the specific binding of a benzodiazepine antagonist in mice. Pharmacol. Biochem. Behav. 1993;45(2):299–304. doi: 10.1016/0091-3057(93)90242-l. [DOI] [PubMed] [Google Scholar]
- 239.Payne JA, Stevenson TJ, Donaldson LF. Molecular characterization of a putative K-Cl cotransporter in rat brain.A neuronal-specific isoform. J. Biol. Chem. 1996;271(27):16245–52. doi: 10.1074/jbc.271.27.16245. [DOI] [PubMed] [Google Scholar]
- 240.Payne JA, Ferrell C, Chung CY. Endogenous and exogenous Na-K-Cl cotransporter expression in a low K-resistant mutant MDCK cell line. Am. J. Physiol. Cell Physiol . 001.;280(6):C1607–15. doi: 10.1152/ajpcell.2001.280.6.C1607. [DOI] [PubMed] [Google Scholar]
- 241.Payne JA, Rivera C, Voipio J, Kaila K. Cation-chloride co-transporters in neuronal communication, development and trauma. Trends Neurosci. 2003;26(4):199–206. doi: 10.1016/S0166-2236(03)00068-7. [DOI] [PubMed] [Google Scholar]
- 242.Pellegrino CM, Rybicki AC, Musto S, Nagel RL, Schwartz RS. Molecular identification and expression of erythroid K:Cl cotransporter in human and mouse erythroleukemic cells. Blood Cells Mol. Dis. 1998;24(1):31–40. doi: 10.1006/bcmd.1998.0168. [DOI] [PubMed] [Google Scholar]
- 243.Pena C, Medina JH, Piva M, Diaz LE, Danilowicz C, Paladini AC. Naturally occurring benzodiazepines in human milk. Biochem. Biophys. Res. Commun. 1991;175(3):1042–50. doi: 10.1016/0006-291x(91)91670-8. [DOI] [PubMed] [Google Scholar]
- 244.Peng Z, Huang CS, Stell BM, Mody I, Houser CR. Altered expression of the delta subunit of the GABAA receptor in a mouse model of temporal lobe epilepsy. J. Neurosci. 2004;24(39):8629–39. doi: 10.1523/JNEUROSCI.2877-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Piva MA, Medina JH, de Blas AL, Pena C. Formation of benzodiazepine-like molecules in rat brain. Biochem. Biophys. Res. Commun. 1991;180(2):972–81. doi: 10.1016/s0006-291x(05)81161-1. [DOI] [PubMed] [Google Scholar]
- 246.Plotkin MD, Kaplan MR, Peterson LN, Gullans SR, Hebert SC, Delpire E. Expression of the Na(+)-K(+)-2Cl- cotransporter BSC2 in the nervous system. Am. J. Physiol. 1997;272(1 Pt 1):C173–83. doi: 10.1152/ajpcell.1997.272.1.C173. [DOI] [PubMed] [Google Scholar]
- 247.Plotkin MD, Snyder EY, Hebert SC, Delpire E. Expression of the Na-K-2Cl cotransporter is developmentally regulated in postnatal rat brains a possible mechanism underlying GABA's excitatory role in immature brain. J. Neurobiol. 1997;33(6):781–95. doi: 10.1002/(sici)1097-4695(19971120)33:6<781::aid-neu6>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 248.Poulsen CF, Christjansen KN, Hastrup S, Hartvig L. Identification and cloning of a gamma 3 subunit splice variant of the human GABA(A) receptor. Brain Res. Mol. Brain Res. 2000;78(1-2):201–3. doi: 10.1016/s0169-328x(00)00085-1. [DOI] [PubMed] [Google Scholar]
- 249.Prasad AN, Seshia SS. Status epilepticus in pediatric practice: neonate to adolescent. Adv. Neurol. 2006;97:229–43. [PubMed] [Google Scholar]
- 250.Pritchett DB, Sontheimer H, Gorman CM, Kettenmann H, Seeburg PH, Schofield PR. Transient expression shows ligand gating and allosteric potentiation of GABAA receptor subunits. Science. 1988;242(4883):1306–8. doi: 10.1126/science.2848320. [DOI] [PubMed] [Google Scholar]
- 251.Quilichini PP, Chiron C, Ben-Ari Y, Gozlan H. Stiripentol, a putative antiepileptic drug, enhances the duration of opening of GABA-A receptor channels. Epilepsia. 2006;47(4):704–16. doi: 10.1111/j.1528-1167.2006.00497.x. [DOI] [PubMed] [Google Scholar]
- 252.Race JE, Makhlouf FN, Logue PJ, Wilson FH, Dunham PB, Holtzman EJ. Molecular cloning and functional characterization of KCC3, a new K-Cl cotransporter. Am. J. Physiol. 1999;277(6 Pt 1):C1210–9. doi: 10.1152/ajpcell.1999.277.6.C1210. [DOI] [PubMed] [Google Scholar]
- 253.Ramos B, Lopez-Tellez JF, Vela J, Baglietto-Vargas D, del Rio JC, Ruano D, Gutierrez A, Vitorica J. Expression of alpha 5 GABAA receptor subunit in developing rat hippocampus. Brain Res. Dev. Brain Res. 2004;151(1-2):87–98. doi: 10.1016/j.devbrainres.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 254.Raol YH, Zhang G, Lund IV, Porter BE, Maronski MA, Brooks-Kayal AR. Increased GABA(A)-receptor alpha1-subunit expression in hippocampal dentate gyrus after early-life status epilepticus. Epilepsia. 2006;47(10):1665–73. doi: 10.1111/j.1528-1167.2006.00640.x. [DOI] [PubMed] [Google Scholar]
- 255.Ravizza T, Friedman LK, Moshé SL, Veliskova J. Sex differences in GABA(A)ergic system in rat substantia nigra pars reticulata. Int. J. Dev. Neurosci. 2003;21(5):245–54. doi: 10.1016/s0736-5748(03)00069-8. [DOI] [PubMed] [Google Scholar]
- 256.Reichling DB, Kyrozis A, Wang J, MacDermott AB. Mechanisms of GABA and glycine depolarization-induced calcium transients in rat dorsal horn neurons. J. Physiol. 1994;476(3):411–21. doi: 10.1113/jphysiol.1994.sp020142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Reiss WG, Oles KS. Acetazolamide in the treatment of seizures. Ann. Pharmacother. 1996;30(5):514–9. doi: 10.1177/106002809603000515. [DOI] [PubMed] [Google Scholar]
- 258.Ren J, Greer JJ. Modulation of respiratory rhythmogenesis by chloride-mediated conductances during the perinatal period. J. Neurosci. 2006;26(14):3721–30. doi: 10.1523/JNEUROSCI.0026-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Rinehart J, Kahle KT, de Los Heros P, Vazquez N, Meade P, Wilson FH, Hebert SC, Gimenez I, Gamba G, Lifton RP. WNK3 kinase is a positive regulator of NKCC2 and NCC, renal cation-Cl- cotransporters required for normal blood pressure homeostasis. Proc. Natl. Acad. Sci. USA. 2005;102(46):16777–82. doi: 10.1073/pnas.0508303102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Rissman RA, Nocera R, Fuller LM, Kordower JH, Armstrong DM. Age-related alterations in GABA(A) receptor subunits in the nonhuman primate hippocampus. Brain Res. 2006;1073-1074:120–30. doi: 10.1016/j.brainres.2005.12.036. [DOI] [PubMed] [Google Scholar]
- 261.Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, Pirvola U, Saarma M, Kaila K. The K+/Cl- co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999;397(6716):251–5. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- 262.Rivera C, Li H, Thomas-Crusells J, Lahtinen H, Viitanen T, Nanobashvili A, Kokaia Z, Airaksinen MS, Voipio J, Kaila K, Saarma M. BDNF-induced TrkB activation down-regulates the K+-Cl- cotransporter KCC2 and impairs neuronal Cl- extrusion. J. Cell Biol. 2002;159(5):747–52. doi: 10.1083/jcb.200209011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Rivera C, Voipio J, Thomas-Crusells J, Li H, Emri Z, Sipila S, Payne JA, Minichiello L, Saarma M, Kaila K. Mechanism of activity-dependent downregulation of the neuron-specific K-Cl cotransporter KCC2. J. Neurosci. 2004;24(19):4683–91. doi: 10.1523/JNEUROSCI.5265-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Rivera C, Voipio J, Kaila K. Two developmental switches in GABAergic signalling, the K+-Cl- cotransporter KCC2 and carbonic anhydrase CAVII. J. Physiol. 2005;562(Pt 1):27–36. doi: 10.1113/jphysiol.2004.077495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Roberts E, Frankel S. Gamma-Aminobutyric acid in brain, its formation from glutamic acid. J. Biol. Chem. 1950;187:55–63. [PubMed] [Google Scholar]
- 266.Rogawski MA. Principles of antiepileptic drug action . In: Levy RH, Mattson RH, Meldrum BS, Perucca E, editors. Antiepileptic drugs. Philadelphia, PA,: Williams & Wilkins; pp. 3–22. [Google Scholar]
- 267.Romero MF, Fulton CM, Boron WF. The SLC4 family of HCO 3 - transporters. Pflugers Arch. 2004;447(5):495–509. doi: 10.1007/s00424-003-1180-2. [DOI] [PubMed] [Google Scholar]
- 268.Russell JM. Sodium-potassium-chloride cotransport. Physiol. Rev. 2000;80(1):211–76. doi: 10.1152/physrev.2000.80.1.211. [DOI] [PubMed] [Google Scholar]
- 269.San Juan I, Alonso JM, Ovalle S, Negro A, Chinchetru MA, Calvo P. Immunodetection of the large form of the gamma 2 subunit of mammalian GABAA receptor. Brain Res. 1995;698(1-2):209–12. doi: 10.1016/0006-8993(95)00712-y. [DOI] [PubMed] [Google Scholar]
- 270.Sancar F, Czajkowski C. A GABAA receptor mutation linked to human epilepsy (gamma2R43Q) impairs cell surface expression of alphabetagamma receptors. J. Biol. Chem. 2004;279(45):47034–9. doi: 10.1074/jbc.M403388200. [DOI] [PubMed] [Google Scholar]
- 271.Sancar F, Ericksen SS, Kucken AM, Teissere JA, Czajkowski C. Structural determinants for high-affinity zolpidem binding to GABA-A receptors. Mol. Pharmacol. 2007;71(1):38–46. doi: 10.1124/mol.106.029595. [DOI] [PubMed] [Google Scholar]
- 272.Sand P, Kavvadias D, Feineis D, Riederer P, Schreier P, Kleinschnitz M, Czygan FC, Abou-Mandour A, Bringmann G, Beckmann H. Naturally occurring benzodiazepines: current status of research and clinical implications. Eur. Arch. Psychiatry Clin. Neurosci. 2000;250(4):194–202. doi: 10.1007/s004060070024. [DOI] [PubMed] [Google Scholar]
- 273.Sander T, Toliat MR, Heils A, Leschik G, Becker C, Ruschendorf F, Rohde K, Mundlos S, Nurnberg P. Association of the 867Asp variant of the human anion exchanger 3 gene with common subtypes of idiopathic generalized epilepsy. Epilepsy Res. 2002;51(3):249–55. doi: 10.1016/s0920-1211(02)00152-3. [DOI] [PubMed] [Google Scholar]
- 274.Sato J, Nioka M, Owada E, Ito K, Murata T. Effect of acetazolamide on the anticonvulsant potency of phenobarbital in mice. J. Pharmacobiodyn. 1981;4(12):952–60. doi: 10.1248/bpb1978.4.952. [DOI] [PubMed] [Google Scholar]
- 275.Schlesinger F, Meywirth J, Krampfl K, Grosskreutz J, Petri S, Mauth C, Just L, Bader A, Bufler J. Ligand-gated channels in early mesencephalic neuronal precursors: immunocytochemical and electrophysiological analysis. Eur. J. Neurosci. 2004;19(9):2371–6. doi: 10.1111/j.0953-816X.2004.03343.x. [DOI] [PubMed] [Google Scholar]
- 276.Schofield PR, Darlison MG, Fujita N, Burt DR, Stephenson FA, Rodriguez H, Rhee LM, Ramachandran J, Reale V, Glencorse TA, Seeburg PH, Barnard EA. Sequence and functional expression of the GABA A receptor shows a ligand-gated receptor super-family. Nature. 1987;328(6127):221–7. doi: 10.1038/328221a0. [DOI] [PubMed] [Google Scholar]
- 277.Schofield PR, Pritchett DB, Sontheimer H, Kettenmann H, Seeburg PH. Sequence and expression of human GABAA receptor alpha 1 and beta 1 subunits. FEBS Lett. 1989;244(2):361–4. doi: 10.1016/0014-5793(89)80563-0. [DOI] [PubMed] [Google Scholar]
- 278.Serafini R, Ma W, Maric D, Maric I, Lahjouji F, Sieghart W, Barker JL. Initially expressed early rat embryonic GABA(A) receptor Cl- ion channels exhibit heterogeneous channel properties. Eur. J. Neurosci. 1998;10(5):1771–83. doi: 10.1046/j.1460-9568.1998.00187.x. [DOI] [PubMed] [Google Scholar]
- 279.Sernagor E, Young C, Eglen SJ. Developmental modulation of retinal wave dynamics, shedding light on the GABA saga. J. Neurosci. 2003;23(20):7621–9. doi: 10.1523/JNEUROSCI.23-20-07621.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Shibata S, Kakazu Y, Okabe A, Fukuda A, Nabekura J. Experience-dependent changes in intracellular Cl- regulation in developing auditory neurons. Neurosci. Res. 2004;48(2):211–20. doi: 10.1016/j.neures.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 281.Shimizu-Okabe C, Yokokura M, Okabe A, Ikeda M, Sato K, Kilb W, Luhmann HJ, Fukuda A. Layer-specific expression of Cl- transporters and differential [Cl-]i in newborn rat cortex. Neuroreport. 2002;13(18):2433–7. doi: 10.1097/00001756-200212200-00012. [DOI] [PubMed] [Google Scholar]
- 282.Shivers BD, Killisch I, Sprengel R, Sontheimer H, Kohler M, Schofield PR, Seeburg PH. Two novel GABAA receptor subunits exist in distinct neuronal subpopulations. Neuron. 1989;3(3):327–37. doi: 10.1016/0896-6273(89)90257-2. [DOI] [PubMed] [Google Scholar]
- 283.Sik A, Smith RL, Freund TF. Distribution of chloride channel-2-immunoreactive neuronal and astrocytic processes in the hippocampus. Neuroscience. 2000;101(1):51–65. doi: 10.1016/s0306-4522(00)00360-2. [DOI] [PubMed] [Google Scholar]
- 284.Simon DB, Karet FE, Hamdan JM, DiPietro A, Sanjad SA, Lifton RP. Bartter's syndrome, hypokalaemic alkalosis with hypercalciuria, is caused by mutations in the Na-K-2Cl cotransporter NKCC2. Nat. Genet. 1996;13(2):183–8. doi: 10.1038/ng0696-183. [DOI] [PubMed] [Google Scholar]
- 285.Smart TG, Constanti A. Differential effect of zinc on the vertebrate GABAA-receptor complex. Br. J. Pharmacol. 1990;99(4):643–54. doi: 10.1111/j.1476-5381.1990.tb12984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Smith RL, Clayton GH, Wilcox CL, Escudero KW, Staley KJ. Differential expression of an inwardly rectifying chloride conductance in rat brain neurons: a potential mechanism for cell-specific modulation of postsynaptic inhibition. J. Neurosci. 1995;15(5 Pt 2):4057–67. doi: 10.1523/JNEUROSCI.15-05-04057.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Soleimani M, Burnham CE. Na+:HCO(3-) cotransporters (NBC): cloning and characterization. J. Membr. Biol. 2001;183(2):71–84. doi: 10.1007/s00232-001-0055-8. [DOI] [PubMed] [Google Scholar]
- 288.Soleimani M. Na+:HCO3- cotransporters (NBC), expression and regulation in the kidney. J. Nephrol. 2002;15 (Suppl 5):S32–40. [PubMed] [Google Scholar]
- 289.Sommer B, Poustka A, Spurr NK, Seeburg PH. The murine GABAA receptor delta-subunit gene: structure and assignment to human chromosome 1. DNA Cel Biol. 1990;9(8):561–8. doi: 10.1089/dna.1990.9.561. [DOI] [PubMed] [Google Scholar]
- 290.Song L, Mercado A, Vazquez N, Xie Q, Desai R, George AL Jr, Gamba G, Mount DB. Molecular, functional, and genomic characterization of human KCC2, the neuronal K-Cl cotransporter. Brain Res. Mol. Brain Res. 2002;103(1-2):91–105. doi: 10.1016/s0169-328x(02)00190-0. [DOI] [PubMed] [Google Scholar]
- 291.Spoerri PE. Neurotrophic effects of GABA in cultures of embryonic chick brain and retina. Synapse. 1988;2(1):11–22. doi: 10.1002/syn.890020104. [DOI] [PubMed] [Google Scholar]
- 292.Staley K, Smith R, Schaack J, Wilcox C, Jentsch TJ. Alteration of GABAA receptor function following gene transfer of the CLC-2 chloride channel. Neuron. 1996;17(3):543–51. doi: 10.1016/s0896-6273(00)80186-5. [DOI] [PubMed] [Google Scholar]
- 293.Staley KJ, Mody I. Shunting of excitatory input to dentate gyrus granule cells by a depolarizing GABAA receptor-mediated postsynaptic conductance. J. Neurophysiol. 1992;68(1):197–212. doi: 10.1152/jn.1992.68.1.197. [DOI] [PubMed] [Google Scholar]
- 294.Staley KJ, Proctor WR. Modulation of mammalian dendritic GABA(A) receptor function by the kinetics of Cl- and HCO3- transport. J. Physiol. 1999;519 (Pt 3):693–712. doi: 10.1111/j.1469-7793.1999.0693n.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Stein V, Hermans-Borgmeyer I, Jentsch TJ, Hubner CA. Expression of the KCl cotransporter KCC2 parallels neuronal maturation and the emergence of low intracellular chloride. J. Comp. Neurol. 2004;468(1):57–64. doi: 10.1002/cne.10983. [DOI] [PubMed] [Google Scholar]
- 296.Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by delta subunit-containing GABAA receptors. Proc. Natl. Acad. Sci. USA. 2003;100(24):14439–44. doi: 10.1073/pnas.2435457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Stogmann E, Lichtner P, Baumgartner C, Schmied M, Hotzy C, Asmus F, Leutmezer F, Bonelli S, Assem-Hilger E, Vass K, Hatala K, Strom TM, Meitinger T, Zimprich F, Zimprich A. Mutations in the CLCN2 gene are a rare cause of idiopathic generalized epilepsy syndromes. Neurogenetics. 2006;7(4):265–8. doi: 10.1007/s10048-006-0057-x. [DOI] [PubMed] [Google Scholar]
- 298.Storustovu SI, Ebert B. Pharmacological characterization of agonists at delta-containing GABAA receptors: Functional selectivity for extrasynaptic receptors is dependent on the absence of gamma2. J. Pharmacol. Exp. Ther. 2006;316(3):1351–9. doi: 10.1124/jpet.105.092403. [DOI] [PubMed] [Google Scholar]
- 299.Su W, Shmukler BE, Chernova MN, Stuart-Tilley AK, de Franceschi L, Brugnara C, Alper SL. Mouse K-Cl cotransporter KCC1: cloning, mapping, pathological expression, and functional regulation. Am. J. Physiol. 1999;277(5 Pt 1):C899–912. doi: 10.1152/ajpcell.1999.277.5.C899. [DOI] [PubMed] [Google Scholar]
- 300.Su YR, Klanke CA, Houseal TW, Linn SC, Burk SE, Varvil TS, Otterud BE, Shull GE, Leppert MF, Menon AG. Molecular cloning and physical and genetic mapping of the human anion exchanger isoform 3 (SLC2C) gene to chromosome 2q36. Genomics. 1994;22(3):605–9. doi: 10.1006/geno.1994.1433. [DOI] [PubMed] [Google Scholar]
- 301.Sugimoto T, Araki A, Yasuhara A, Woo M, Nishida N, Sasaki T. Angelman syndrome in three siblings: genetic model of epilepsy associated with chromosomal DNA deletion of the GABAA receptor. Jpn. J. Psychiatry Neurol. 1994;48(2):271–3. doi: 10.1111/j.1440-1819.1994.tb03066.x. [DOI] [PubMed] [Google Scholar]
- 302.Sung KW, Kirby M, McDonald MP, Lovinger DM, Delpire E. Abnormal GABAA receptor-mediated currents in dorsal root ganglion neurons isolated from Na-K-2Cl cotransporter null mice. J. Neurosci. 2000;20(20):7531–8. doi: 10.1523/JNEUROSCI.20-20-07531.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Taraskevich PS, Douglas WW. Pharmacological and ionic features of gamma-aminobutyric acid receptors influencing electrical properties of melanotrophs isolated from the rat pars intermedia. Neuroscience. 1985;14(1):301–8. doi: 10.1016/0306-4522(85)90179-4. [DOI] [PubMed] [Google Scholar]
- 304.Temple JL, Wray S. Developmental changes in GABA receptor subunit composition within the gonadotrophin-releasing hormone-1 neuronal system. J. Neuroendocrinol. 2005;17(9):591–9. doi: 10.1111/j.1365-2826.2005.01348.x. [DOI] [PubMed] [Google Scholar]
- 305.Thalmann RH, Peck EJ, Ayala GF. Biphasic response of hippocampal pyramidal neurons to GABA. Neurosci. Lett. 1981;21(3):319–24. doi: 10.1016/0304-3940(81)90224-x. [DOI] [PubMed] [Google Scholar]
- 306.Thiemann A, Grunder S, Pusch M, Jentsch TJ. A chloride channel widely expressed in epithelial and non-epithelial cells. Nature. 1992;356(6364):57–60. doi: 10.1038/356057a0. [DOI] [PubMed] [Google Scholar]
- 307.Thompson SA, Whiting PJ, Wafford KA. Barbiturate interactions at the human GABAA receptor: dependence on receptor subunit combination. Br. J. Pharmacol. 1996;117(3):521–527. doi: 10.1111/j.1476-5381.1996.tb15221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Titz S, Hans M, Kelsch W, Lewen A, Swandulla D, Misgeld U. Hyperpolarizing inhibition develops without trophic support by GABA in cultured rat midbrain neurons. J. Physiol. 2003;550(Pt 3):719–30. doi: 10.1113/jphysiol.2003.041863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Udenfriend S. Identification of gamma-aminobutyric acid in brain by the isotope derivative method. J. Biol. Chem. 1950;187:65–69. [PubMed] [Google Scholar]
- 310.Uvarov P, Pruunsild P, Timmusk T, Airaksinen MS. Neuronal K+/Cl- co-transporter (KCC2) transgenes lacking neurone restrictive silencer element recapitulate CNS neurone-specific expression and developmental up-regulation of endogenous KCC2 gene. J. Neurochem. 2005;95(4):1144–55. doi: 10.1111/j.1471-4159.2005.03434.x. [DOI] [PubMed] [Google Scholar]
- 311.Valeyev AY, Cruciani RA, Lange GD, Smallwood VS, Barker JL. Cl- channels are randomly activated by continuous GABA secretion in cultured embryonic rat hippocampal neurons. Neurosci. Lett. 1993;155(2):199–203. doi: 10.1016/0304-3940(93)90707-r. [DOI] [PubMed] [Google Scholar]
- 312.Velazquez H, Silva T. Cloning and localization of KCC4 in rabbit kidney, expression in distal convoluted tubule. Am. J. Physiol. Renal Physiol. 2003;285(1):F49–58. doi: 10.1152/ajprenal.00389.2002. [DOI] [PubMed] [Google Scholar]
- 313.Veliskova J, Moshé SL. Sexual dimorphism and developmental regulation of substantia nigra function. Ann. Neurol. 2001;50(5):596–601. doi: 10.1002/ana.1248. [DOI] [PubMed] [Google Scholar]
- 314.Veliskova J, Claudio OI, Galanopoulou AS, Lado FA, Ravizza T, Velisek L, Moshé SL. Seizures in the developing brain. Epilepsia. 2004;45(Suppl 8):6–12. doi: 10.1111/j.0013-9580.2004.458002.x. [DOI] [PubMed] [Google Scholar]
- 315.Virkki LV, Choi I, Davis BA, Boron WF. Cloning of a Na+-driven Cl/HCO3 exchanger from squid giant fiber lobe. Am. J. Physiol. Cell Physiol. 2003;285(4):C771–80. doi: 10.1152/ajpcell.00439.2002. [DOI] [PubMed] [Google Scholar]
- 316.Vu TQ, Payne JA, Copenhagen DR. Localization and developmental expression patterns of the neuronal K-Cl cotransporter (KCC2) in the rat retina. J. Neurosci. 2000;20(4):1414–23. doi: 10.1523/JNEUROSCI.20-04-01414.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Wake H, Watanabe M, Moorhouse AJ, Kanematsu T, Horibe S, Matsukawa N, Asai K, Ojika K, Hirata M, Nabekura J. Early changes in KCC2 phosphorylation in response to neuronal stress result in functional downregulation. J. Neurosci. 2007;27(7):1642–50. doi: 10.1523/JNEUROSCI.3104-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Wallace RH, Marini C, Petrou S, Harkin LA, Bowser DN, Panchal RG, Williams DA, Sutherland GR, Mulley JC, Scheffer IE, Berkovic SF. Mutant GABA(A) receptor gamma2-subunit in childhood absence epilepsy and febrile seizures. Nat. Genet. 2001;28(1):49–52. doi: 10.1038/ng0501-49. [DOI] [PubMed] [Google Scholar]
- 319.Wang C, Shimizu-Okabe C, Watanabe K, Okabe A, Matsuzaki H, Ogawa T, Mori N, Fukuda A, Sato K. Developmental changes in KCC1, KCC2, and NKCC1 mRNA expressions in the rat brain. Brain Res. Dev. Brain Res. 2002;139(1):59–66. doi: 10.1016/s0165-3806(02)00536-9. [DOI] [PubMed] [Google Scholar]
- 320.Wang C, Ohno K, Furukawa T, Ueki T, Ikeda M, Fukuda A, Sato K. Differential expression of KCC2 accounts for the differential GABA responses between relay and intrinsic neurons in the early postnatal rat olfactory bulb. Eur. J. Neurosci. 2005;21(5):1449–55. doi: 10.1111/j.1460-9568.2005.03975.x. [DOI] [PubMed] [Google Scholar]
- 321.Wang CZ, Yano H, Nagashima K, Seino S. The Na+-driven Cl-/HCO3- exchanger. J. Biol. Chem. 2000;275(45):35486–90. doi: 10.1074/jbc.C000456200. [DOI] [PubMed] [Google Scholar]
- 322.Wang TL, Guggino WB, Cutting GR. A novel gamma-aminobutyric acid receptor subunit (rho 2) cloned from human retina forms bicuculline-insensitive homooligomeric receptors in Xenopus oocytes. J. Neurosci. 1994;14(11 Pt 1):6524–31. doi: 10.1523/JNEUROSCI.14-11-06524.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Wheless JW, Clarke DF, Carpenter D. Treatment of pediatric epilepsy: expert opinion, 2005. J. Child. Neurol quiz S59-60. 2005;20(Suppl 1):S1–56. doi: 10.1177/088307380502000101. [DOI] [PubMed] [Google Scholar]
- 324.White JH, Wise A, Main MJ, Green A, Fraser NJ, Disney GH, Barnes AA, Emson P, Foord SM, Marshall FH. Heterodimerization is required for the formation of a functional GABA(B) receptor. Nature. 1998;396(6712):679–82. doi: 10.1038/25354. [DOI] [PubMed] [Google Scholar]
- 325.Whiting P, McKernan RM, Iversen LL. Another mechanism for creating diversity in gamma-aminobutyrate type A receptors RNA splicing directs expression of two forms of gamma 2 phosphorylation site. Proc. Natl. Acad. Sci. USA. 1990;87(24):9966–70. doi: 10.1073/pnas.87.24.9966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Wildmann J, Mohler H, Vetter W, Ranalder U, Schmidt K, Maurer R. Diazepam and N-desmethyldiazepam are found in rat brain and adrenal and may be of plant origin. J. Neural Transm. 1987;70(3-4):383–98. doi: 10.1007/BF01253613. [DOI] [PubMed] [Google Scholar]
- 327.Wildmann J. Increase of natural benzodiazepines in wheat and potato during germination. Biochem. Biophys. Res. Commun. 1988;157(3):1436–43. doi: 10.1016/s0006-291x(88)81036-2. [DOI] [PubMed] [Google Scholar]
- 328.Wildmann J, Vetter W, Ranalder UB, Schmidt K, Maurer R, Mohler H. Occurrence of pharmacologically active benzodiazepines in trace amounts in wheat and potato. Biochem. Pharmacol. 1988;37(19):3549–59. doi: 10.1016/0006-2952(88)90384-x. [DOI] [PubMed] [Google Scholar]
- 329.Wingrove P, Hadingham K, Wafford K, Kemp JA, Ragan CI, Whiting P. Cloning and expression of a cDNA encoding the human GABA-A receptor alpha 5 subunit. Biochem. Soc. Trans. 1992;20(1):18S. doi: 10.1042/bst020018s. [DOI] [PubMed] [Google Scholar]
- 330.Wisden W, Herb A, Wieland H, Keinanen K, Luddens H, Seeburg PH. Cloning, pharmacological characteristics and expression pattern of the rat GABAA receptor alpha 4 subunit. FEBS Lett. 1991;289(2):227–30. doi: 10.1016/0014-5793(91)81076-k. [DOI] [PubMed] [Google Scholar]
- 331.Xie XM, Smart TG. A physiological role for endogenous zinc in rat hippocampal synaptic neurotransmission. Nature. 1991;349(6309):521–4. doi: 10.1038/349521a0. [DOI] [PubMed] [Google Scholar]
- 332.Xue H. Identification of major phylogenetic branches of inhibitory ligand-gated channel receptors. J. Mol. Evol. 1998;47(3):323–33. doi: 10.1007/pl00006390. [DOI] [PubMed] [Google Scholar]
- 333.Yamada J, Okabe A, Toyoda H, Kilb W, Luhmann HJ, Fukuda A. Cl- uptake promoting depolarizing GABA actions in immature rat neocortical neurones is mediated by NKCC1. J. Physiol. 2004;557(Pt 3):829–41. doi: 10.1113/jphysiol.2004.062471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Yamada Y, Fukuda A, Tanaka M, Shimano Y, Nishino H, Muramatsu K, Togari H, Wada Y. Optical imaging reveals cation--Cl(-) cotransporter-mediated transient rapid decrease in intracellular Cl(-) concentration induced by oxygen--glucose deprivation in rat neocortical slices. Neurosci. Res. 2001;39(3):269–80. doi: 10.1016/s0168-0102(00)00221-2. [DOI] [PubMed] [Google Scholar]
- 335.Yang W, Drewe JA, Lan NC. Cloning and characterization of the human GABAA receptor alpha 4 subunit: identification of a unique diazepam-insensitive binding site. Eur. J. Pharmacol. 1995;291(3):319–25. doi: 10.1016/0922-4106(95)90072-1. [DOI] [PubMed] [Google Scholar]
- 336.Yannoukakos D, Stuart-Tilley A, Fernandez HA, Fey P, Duyk G, Alper SL. Molecular cloning, expression, and chromosomal localization of two isoforms of the AE3 anion exchanger from human heart. Circ. Res. 1994;75(4):603–14. doi: 10.1161/01.res.75.4.603. [DOI] [PubMed] [Google Scholar]
- 337.Ymer S, Draguhn A, Kohler M, Schofield PR, Seeburg PH. Sequence and expression of a novel GABAA receptor alpha subunit. FEBS Lett. 1989;258(1):119–22. doi: 10.1016/0014-5793(89)81630-8. [DOI] [PubMed] [Google Scholar]
- 338.Ymer S, Schofield PR, Draguhn A, Werner P, Kohler M, Seeburg PH. GABAA receptor beta subunit heterogeneity functional expression of cloned cDNAs. EMBO J. 1989;8(6):1665–70. doi: 10.1002/j.1460-2075.1989.tb03557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Ymer S, Draguhn A, Wisden W, Werner P, Keinanen K, Schofield PR, Sprengel R, Pritchett DB, Seeburg PH. Structural and functional characterization of the gamma 1 subunit of GABAA/benzodiazepine receptors. EMBO J. 1990;9(10):3261–7. doi: 10.1002/j.1460-2075.1990.tb07525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Yu ZY, Wang W, Fritschy JM, Witte OW, Redecker C. Changes in neocortical and hippocampal GABAA receptor subunit distribution during brain maturation and aging. Brain Res. 2006;1099(1):73–81. doi: 10.1016/j.brainres.2006.04.118. [DOI] [PubMed] [Google Scholar]
- 341.Yurdaydin C, Walsh TJ, Engler HD, Ha JH, Li Y, Jones EA, Basile AS. Gut bacteria provide precursors of benzodiazepine receptor ligands in a rat model of hepatic encephalopathy. Brain Res. 1995;679(1):42–8. doi: 10.1016/0006-8993(95)00241-h. [DOI] [PubMed] [Google Scholar]
- 342.Zapatero-Caballero H, Sanchez-Franco F, Guerra-Perez N, Fernandez-Mendez C, Fernandez-Vazquez G. Gonadotropin-releasing hormone receptor gene expression during pubertal development of male rats. Biol. Reprod. 2003;68(5):1764–70. doi: 10.1095/biolreprod.102.008821. [DOI] [PubMed] [Google Scholar]
- 343.Zapatero-Caballero H, Sanchez-Franco F, Fernandez-Mendez C, Garcia-San Frutos M, Botella-Cubells LM, Fernandez-Vazquez G. Gonadotropin-releasing hormone receptor gene expression during pubertal development of female rats. Biol. Reprod. 2004;70(2):348–55. doi: 10.1095/biolreprod.103.020818. [DOI] [PubMed] [Google Scholar]
- 344.Zhang D, Pan ZH, Zhang X, Brideau AD, Lipton SA. Cloning of a gamma-aminobutyric acid type C receptor subunit in rat retina with a methionine residue critical for picrotoxinin channel block. Proc. Natl. Acad. Sci. USA. 1995;92(25):11756–60. doi: 10.1073/pnas.92.25.11756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Zhang G, Raol YH, Hsu FC, Coulter DA, Brooks-Kayal AR. Effects of status epilepticus on hippocampal GABAA receptors are age-dependent. Neuroscience. 2004;125(2):299–303. doi: 10.1016/j.neuroscience.2004.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Zhang J, Lauf PK, Adragna NC. PDGF activates K-Cl cotransport through phosphoinositide 3-kinase and protein phosphatase-1 in primary cultures of vascular smooth muscle cells. Life Sci. 2005;77(9):953–65. doi: 10.1016/j.lfs.2004.08.046. [DOI] [PubMed] [Google Scholar]
- 347.Zhang JJ, Misri S, Adragna NC, Gagnon KB, Fyffe RE, Lauf PK. Cloning and expression of sheep renal K-CI cotransporter-1. Cell Physiol. Biochem. 2005;16(1-3):87–98. doi: 10.1159/000087735. [DOI] [PubMed] [Google Scholar]
- 348.Zhang LL, Pathak HR, Coulter DA, Freed MA, Vardi N. Shift of intracellular chloride concentration in ganglion and amacrine cells of developing mouse retina. J. Neurophysiol. 2006;95(4):2404–16. doi: 10.1152/jn.00578.2005. [DOI] [PubMed] [Google Scholar]
- 349.Zheng JJ, Lee S, Zhou ZJ. A developmental switch in the excitability and function of the starburst network in the mammalian retina. Neuron. 2004;44(5):851–64. doi: 10.1016/j.neuron.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 350.Zhou GP, Wong C, Su R, Crable SC, Anderson KP, Gallagher PG. Human potassium chloride cotransporter 1 (SLC12A4) promoter is regulated by AP-2 and contains a functional downstream promoter element. Blood. 2004;103(11):4302–9. doi: 10.1182/blood-2003-01-0107. [DOI] [PubMed] [Google Scholar]
- 351.Zhu L, Lovinger D, Delpire E. Cortical neurons lacking KCC2 expression show impaired regulation of intracellular chloride. J. Neurophysiol. 2005;93(3):1557–68. doi: 10.1152/jn.00616.2004. [DOI] [PubMed] [Google Scholar]