Abstract
Cocaine blocks uptake of the monoamines dopamine, serotonin and norepinephrine, and monoamine uptake inhibitors constitute one class of drugs under consideration as candidate “agonist” medications for the treatment of cocaine abuse and dependence. The pharmacological selectivity of monoamine uptake inhibitors to block uptake of dopamine, serotonin and norepinephrine is one factor that may influence the efficacy and/or safety of these compounds as drug abuse treatment medications. To address this issue, the present study compared the effects of 7-day treatment with a non-selective monoamine uptake inhibitor (RTI-112) and a dopamine-selective uptake inhibitor (RTI-113) on cocaine- and food-maintained responding in rhesus monkeys. Monkeys (N=3) were trained to respond for cocaine injections (0.01 mg/kg/inj) and food pellets under a second-order schedule [FR2(VR16:S)] during alternating daily components of cocaine and food availability. Both RTI-112 (0.0032–0.01 mg/kg/hr) and RTI-113 (0.01–0.056 mg/kg/hr) produced dose-dependent, sustained and nearly complete elimination of cocaine self-administration. However, for both drugs, the potency to reduce cocaine self-administration was similar to the potency to reduce food-maintained responding. These findings do not support the hypothesis that pharmacological selectivity to block dopamine uptake is associated with behavioral selectivity to decrease cocaine- vs. food-maintained responding in rhesus monkeys.
Keywords: cocaine, self-administration, RTI-112, RTI-113, monoamine uptake inhibitor, rhesus monkey
1. Introduction
Indirect dopamine agonists constitute one class of compounds under investigation as candidate “agonist” medications for the treatment of cocaine dependence (Grabowski et al., 2004; Howell and Wilcox, 2001; Mello and Negus, 1996; Rothman and Glowa, 1995). In the preclinical evaluation of candidate pharmacotherapies, it has been argued that the most promising medications for treatment of cocaine dependence might be those that produce sustained and selective decreases in cocaine self-administration during chronic treatment (Haney and Spealman, 2008; Mello and Negus, 1996). Based on these criteria, there is some evidence to suggest that more selective dopamine indirect agonists may be preferred to non-selective compounds. For example, in studies evaluating effects of monoamine releasers administered for at least 7 consecutive days, the most selective reductions in cocaine- vs. food-maintained responding in rhesus monkeys were produced by treatment with compounds that selectively promoted release of dopamine and norepinephrine and had lower potency to release serotonin (Negus and Mello, 2003b; Negus et al., 2007). Similarly, among monoamine uptake inhibitors, sustained and selective decreases in cocaine- vs. food-maintained responding were achieved with the relatively dopamine-selective uptake inhibitor GBR12909 (Glowa et al., 1996; Glowa et al., 1995b). In contrast, treatment with the less-selective monoamine uptake inhibitors mazindol or indatraline produced non-selective decreases in both cocaine- and food-maintained responding (Kleven and Woolverton, 1993; Negus et al., 1999).
The purpose of the present study was to evaluate further the hypothesis that pharmacological selectivity to block dopamine uptake is associated with behavioral selectivity to decrease cocaine self-administration in comparison to food-maintained responding. Studies were conducted with the structurally related phenyltropanes RTI-113 [3β-(4-chlorophenyl)tropane-2β-carboxylic acid phenyl ester hydrochloride] and RTI-112 [3β-(3-methyl-4-chlorophenyl)tropane-2β-carboxylic acid methyl ester hydrochloride]. Both compounds block dopamine uptake with nanomolar potency; however, RTI-113 blocks dopamine uptake selectively, whereas RTI-112 non-selectively blocks uptake of dopamine, serotonin and norepinephrine (Kuhar et al., 1999), see Table 1). Both RTI-113 and RTI-112 have been shown previously to decrease cocaine self-administration for up to 3 consecutive days of treatment (Ginsburg, 2002; Howell et al., 2000; Lindsey et al., 2004; Wilcox et al., 2002). Moreover, in accordance with their in vitro selectivities for blocking monoamine uptake, RTI-113 occupied >70% of dopamine transporters at doses that decreased cocaine self-administration (Wilcox et al., 2002), whereas RTI-112 preferentially occupied serotonin transporters at doses that suppressed cocaine self-administration (Lindsey et al., 2004). The behavioral selectivities of RTI-113 and RTI-112 to decrease cocaine- vs. food-maintained responding during longer treatments have not been examined. Acute treatment with RTI-113 was reported to selectively decrease cocaine- vs. food-maintained responding in rats (Dworkin et al., 1998). However, in squirrel monkeys, 3-day treatment with either RTI-113 or RTI-112 produced non-selective decreases in responding maintained by cocaine or by a schedule of stimulus termination (Ginsburg, 2002; Ginsburg et al., 2005; Howell et al., 2000).
Table 1.
IC50 values (nM) for blockade of monoamine reuptake by RTI-112 and RTI-113 in rat tissue. Data from (Kuhar et al., 1999)
| Drug | DA | NE | 5HT | 5HT/DA | NE/DA |
|---|---|---|---|---|---|
| RTI-112 | 1.1 | 0.8 | 1.4 | 1.3 | 0.7 |
| RTI-113 | 3.0 | 31 | 229 | 76.3 | 10.3 |
The present study directly compared the effects of 7-day treatment with RTI-113 and RTI-112 in an assay of cocaine- vs. food-maintained responding in rhesus monkeys. This assay has been used previously to examine the time course and selectivity of effects produced by treatment with other indirect dopaminergic agonists (Negus et al., 1999; Negus and Mello, 2003b; Negus et al., 2007) and with non-dopaminergic compounds (Mello et al., 1990; Mello et al., 1989; Mello and Negus, 1998; Mello et al., 2006; Negus and Mello, 2002; Pereira-Do Carmo et al., 2006). We hypothesized that RTI-113 would produce a sustained and selective decrease in cocaine self-administration at doses that did not reduce food-maintained responding, whereas RTI-112 would display similar potencies for reducing both cocaine- and food-maintained responding.
2. Methods
2.1. Subjects
Studies were conducted in 3 adult male rhesus monkeys (Macaca mulatta) that weighed 6–12 kg. All monkeys had an experimental history involving the evaluation of dopaminergic and/or opioid compounds in assays of drug self-administration. Monkeys were maintained on a diet of multiple vitamins, fresh fruit and Lab Diet Jumbo Monkey biscuits (PMI Feeds, Inc., St. Louis, MO). In addition, monkeys could receive 1 gm banana flavored pellets (Precision Primate Pellets Formula L/I Banana Flavor, P. J. Noyes Co., Lancaster, NH) during daily operant sessions as described below. Water was continuously available. A 12 hr light-dark cycle was in effect (lights on from 7 a.m. to 7 p.m.).
Animal maintenance and research were conducted in accordance with the guidelines provided by the NIH Committee on Laboratory Animal Resources. The facility was licensed by the United States Department of Agriculture, and protocols were approved by the Institutional Animal Care and Use Committee. The health of the monkeys was periodically monitored by consulting veterinarians. Monkeys had visual, auditory and olfactory contact with other monkeys throughout the study. Operant procedures and foraging toys provide an opportunity for environmental manipulation and enrichment.
2.2. Apparatus
Each monkey was housed individually in a well-ventilated stainless steel chamber (64 × 64 × 79 cm). The home cages of all monkeys were modified to include an operant panel (28 × 28 cm) mounted on the front wall. Three round translucent response keys (5.1 cm in diameter) were arranged 3.5 cm apart in a horizontal row 9 cm from the top of the operant panel. Each key could be transilluminated by red or green stimulus lights (Superbright LED’s, Fairchild Semiconductor, San Jose, CA). In addition, three circular translucent panels (1.9 cm in diameter) were located in a vertical column below the center response key and could be transilluminated by red or green stimulus lights. The operant panel also supported an externally-mounted pellet dispenser (Gerbrands, Model G5210, Arlington, MA) that delivered 1 gm food pellets to a food receptacle mounted on the cage beneath the operant response panel. Operation of the operant panel and pellet dispenser and data collection were accomplished with custom-written software operating on microprocessors and software purchased from Med Associates Inc. (Georgia, VT) and located in a separate room.
A double-lumen catheter was surgically implanted into each monkey under aseptic conditions as described previously (Negus and Mello, 2003b). The intravenous catheter was protected by a tether system consisting of a custom-fitted nylon vest connected to a flexible stainless steel cable and fluid swivel (Lomir Biomedical, Malone, NY). Two syringe pumps (Model B5P-lE, Braintree Scientific, Braintree, MA; or Model 980210, Harvard Apparatus, South Natick, MA) were mounted above each cage for delivery of saline or drug solutions through the two lumen of the intravenous catheters. One syringe pump (the self-administration pump) was used to deliver self-administered cocaine injections through one lumen of the double-lumen catheter. The second syringe pump (the treatment pump) was used for non-contingent delivery of saline or test drugs through the second lumen of the double-lumen catheter. The treatment pump delivered injections every 20 min from 10:30 a.m. each day until 9:30 a.m. the next morning for a total of 3 injections/hr and 69 injections/day. No treatment injections were delivered between 9:30 a.m. and 10:30 a.m. During this period, monkeys received their morning ration of food, and their health status was evaluated by the technical staff. Catheter patency was periodically evaluated by i.v. administration of ketamine (5 mg/kg) or the short-acting barbiturate methohexital (3 mg/kg) through the catheter lumen. The catheter was considered to be patent if i.v. administration of ketamine or methohexital produced a loss of muscle tone within 10 sec. Operation of the operant panel, pellet dispenser and drug pumps and data collection were accomplished with custom-written software operating on microprocessors and software purchased from Med Associates Inc. (Georgia, VT) and located in a separate room.
2.3. Training Procedure
Procedures for the evaluation of cocaine- and food-maintained responding were identical to those used in our previous studies of monoamine releasers (Negus and Mello, 2003b; Negus et al., 2007). Alternating daily sessions of food and cocaine availability were associated with different colored stimulus lights projected on the center response key of the operant response panel. Red stimulus lights signaled food availability, and green stimulus lights signaled the availability of cocaine injections. Cocaine injections were delivered in a volume of 0.1 ml in 1 sec, and dose was adjusted by varying the concentration of the cocaine solution. Under the terminal schedule, the completion of a variable ratio of 16 responses on the center response key resulted in the illumination for 1 sec of an appropriately colored stimulus light (red for food, green for drug) underneath the center key (VR16:S). In addition, completion of this VR response requirement a fixed ratio of two times (FR2) resulted in delivery of the available reinforcer and the initiation of a 10 sec time-out period, during which the stimulus light illuminating the center response key was turned off and responding had no scheduled consequences. This terminal second-order schedule is designated as FR2(VR16:S). The two side keys were not transilluminated during sessions of food and cocaine availability, and responding on these keys had no scheduled consequences. Four food sessions and four drug sessions were conducted during each experimental day. Food sessions began at 11 a.m., 3 p.m. 7 p.m. and 6 a.m. the next morning, and drug sessions began at 12 noon, 4 p.m., 8 p.m. and 7 a.m. the next morning. At all other times, responding had no scheduled consequences. Each food and drug session lasted one hour or until 25 food pellets or 20 injections had been delivered, whichever occurred first. Thus, monkeys could earn a maximum of 100 food pellets per day and 80 injections per day. Studies were conducted seven days a week.
During initial training, responding was maintained by delivery of 1gm food pellets during food sessions and by 0.032 mg/kg/inj cocaine injections during drug sessions. Training continued until monkeys met the following criteria for stable food and cocaine self-administration under the terminal schedule: (1) three consecutive days during which the number of drug injections/day differed by no more than 20% from the mean number of drug injections/day during those three days and there was no upward or downward trend; and (2) during the same three consecutive days, the mean number of both drug injections per day and food pellets per day was greater than 50.
2.4. Testing Procedures
Once cocaine- and food-maintained responding stabilized, testing began. Each dose of each drug was tested for a period of 7 consecutive days. During the 7-day test period, the unit dose of cocaine was changed to 0.01 mg/kg/inj, and saline or a dose of a test drug was administered by the treatment pump through one lumen of the double lumen catheter as described above (one injection every 20 min from 10:30 am each day until 9:30 a.m. the next day unless). A unit dose of 0.01 mg/kg/injection cocaine was used for these studies, because previous studies have demonstrated that this is the lowest dose to reliably maintain high rates of cocaine self-administration in all monkeys in this procedure, and because behavior maintained by this unit dose of cocaine is often more sensitive to the effects of treatment compounds than behavior maintained by higher unit doses of cocaine (Mello and Negus, 1998; Negus and Mello, 2002; Negus et al., 1996; Negus et al., 1995; Stevenson et al., 2004). Consequently, studies with this unit dose of cocaine are most likely to reveal selective reductions in cocaine- vs. food-maintained responding. The infusion rate for test drugs was identical to that used in our previous studies with monoamine releasers and opioids, and a similar infusion rate was employed in the present study to permit direct comparison with those previous studies (Negus et al., 1999; Negus and Mello, 2003b, 2002; Negus et al., 2007; Negus et al., 1997; Pereira-Do Carmo et al., 2006). The dose ranges for each test drug were as follows: RTI-112 (0.0032–0.01 mg/kg/hr) and RTI-113 (0.01–0.056 mg/kg/hr). These dose ranges were empirically determined to cover a range from doses that produced little or no effect to doses that decreased rates of cocaine self-administration to less than 20% of control. At the conclusion of each test period, the maintenance dose of cocaine (0.032 mg/kg/inj) and saline control treatment were reinstated for a period of at least four days and until the number of reinforcers per day maintained by cocaine and food returned to baseline levels. This interval between successive treatments was designed to reduce the possibility of carry-over effects from one treatment condition to the next. RTI-112 was tested before RTI-113, and doses of each drug were tested in a mixed order across monkeys.
2.5. Data Analysis
The primary dependent variables were the total injections per day and total pellets per day delivered during the last three days of each 7-day test period. For statistical analysis, values for cocaine- and food-maintained responding during drug treatments were expressed as a percentage of control values for cocaine- and food-maintained responding obtained during saline treatment. Test drug effects were then analyzed by two-factor ANOVA, with test drug dose as one factor and reinforcer type (cocaine or food) as the other factor. The time course of effects produced by saline and the highest doses of RTI-112 and RTI-113 were evaluated using a separate two-factor ANOVA, with time of treatment and reinforcer type as the two factors. A significant analysis of variance was followed by individual means comparison using the Bonferroni post hoc test (Prism 4.0c for Macintosh, GraphPad Software Inc, San Diego, CA). The criterion for significance was set at p<0.05.
In addition, ED50 values were calculated as the dose of RTI-112 or RTI-113 to reduce cocaine- or food-maintained responding to 50% of control levels. ED50 values were determined for each drug in each monkey by interpolation when only two data points were available (one below and one above 50% control) or by linear regression when at least three data points were available on the linear portion of the dose-effect curve. Individual ED50s were then averaged to yield means and 95% confidence limits, and ED50s were considered to be significantly different if confidence limits did not overlap. In one monkey, RTI-112 failed to reduce food-maintained responding below 50% of control across the dose range tested, and as a result an ED50 could not be determined. A higher dose was not tested to avoid potential toxicity that might be associated with high-dose RTI-112 treatment. For the purposes of statistical analysis, a minimum possible ED50 value was estimated for this monkey by assuming that a 0.25 log unit increase in RTI-112 dose would eliminate food-maintained responding.
2.6. Drugs
Cocaine HCl was obtained from the National Institute on Drug Abuse (Bethesda, MD). RTI-112 [3β-(3-methyl-4-chlorophenyl)tropane-2β-carboxylic acid methyl ester hydrochloride] and RTI-113 [3β-(4-chlorophenyl)tropane-2β-carboxylic acid phenyl ester hydrochloride] were synthesized at Research Triangle Institute (kindly provided by Dr. F.I. Carroll). All drugs were dissolved in sterile saline, and solutions were filter-sterilized using a 0.22 micron Millipore filter. Doses were calculated using the forms of the drugs given above.
3. Results
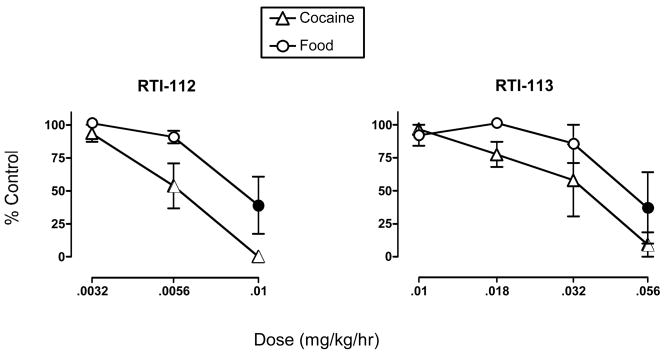
Figure 1 shows mean data from the last 3 days of 7-day treatments with RTI-112 and RTI-113 on responding maintained by 0.01 mg/kg/inj cocaine and food. For each drug, data were analyzed by two-factor ANOVA to evaluate effects of dose and reinforcer type, and results of statistical analysis are shown in the figure legend. Control levels of cocaine- and food-maintained responding during saline treatment were 80±0 cocaine injections/day (out of a maximum of 80 injections/day) and 98.6±1.4 food pellets/day (out of maximum of 100 pellets/day). Both monoamine reuptake inhibitors produced a dose-dependent, statistically significant and nearly complete elimination of cocaine self-administration. ED50 values are shown in Table 2. RTI-112 was approximately 5-fold more potent than RTI-113.
Figure 1. Effects of treatment with RTI-112 and RTI-113 on responding maintained by 0.01 mg/kg/inj cocaine and food.
Abscissae: Dose of test drug in mg/kg/hr, log scale. Ordinates: Percent control levels of cocaine- and food-maintained responding. All points show mean±SEM in 3 monkeys from the last 3 days of each 7-day treatment. The absence of error bars indicates that error bars were contained within the point. Filled points indicate a significant difference from control levels of cocaine- or food-maintained responding (p<0.05). There was not a significant effect of reinforcer type at any dose of either drug. ANOVA results for RTI-112. Main effect of dose: F(3,12)=27.17, p<0.0001. Main effect of reinforcer type: F(1,4)=8.09, p=0.047. Dose × reinforcer type interaction: F(3,12)=1.91, p=0.18. ANOVA results for RTI-113: Main effect of dose: F(4,16)=10.11, p=0.0003. Main effect of reinforcer type: F(1,4)=2.89, p=0.16. Dose × reinforcer type interaction: F(4,16)=0.66, p=0.63.
Table 2.
ED50 values (95%CL) in mg/kg/hr for RTI-112 and RTI-113 to decrease responding maintained by 0.01 mg/kg/inj cocaine and food in rhesus monkeys (N=3)
| Drug | Cocaine-Maintained Responding | Food-Maintained Responding |
|---|---|---|
| RTI-112 | 0.0058 (0.0047–0.0071) | 0.0088 (0.0070–0.011)* |
| RTI-113 | 0.029 (0.023–0.036) | 0.055 (0.028–0.11) |
Includes estimated ED50 in one of three monkeys. See “Methods” for details.
Both drugs also produced dose-dependent and statistically significant decreases in food-maintained responding, and potencies to decrease cocaine- and food-maintained responding were similar. For RTI-112, a dose of 0.0056 mg/kg/hr significantly reduced cocaine self-administration without altering food-maintained responding. However, a higher dose of 0.01 mg/kg/hr reduced both cocaine- and food-maintained responding, and effects of RTI-112 on cocaine- and food-maintained responding were not significantly different at any dose of RTI-112. In addition, ED50 values for RTI-112 to decrease cocaine- and food-maintained responding had overlapping 95% confidence limits. For RTI-113, the highest dose of 0.056 mg/kg/hr was required to significantly decrease both cocaine- and food-maintained responding, and ED50 values for RTI-113 to decrease cocaine- and food-maintained responding had overlapping 95% confidence limits.
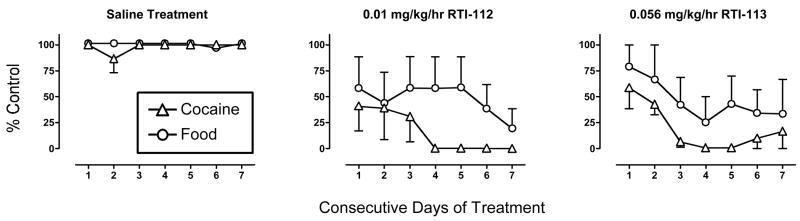
Figure 2 shows the full 7-day time course of effects produced by treatment with saline or the highest doses of RTI-112 (0.01 mg/kg/hr) or RTI-113 (0.056 mg/kg/hr). For each treatment, data were analyzed by a two-factor ANOVA to evaluate effects of time and reinforcer type, and statistical results are shown in the figure legend. During saline treatment, monkeys usually responded for the maximum numbers of cocaine injections and food pellets every day. Both RTI-112 and RTI-113 reduced cocaine self-administration, and peak effects of both drugs were achieved after 3–4 days of treatment. Food-maintained responding also tended to decline over time, and food-maintained responding was usually decreased less that cocaine self-administration. However, there was not a significant main effect of reinforcer type or a significant interaction between reinforcer type and time for either RTI-112 or RTI-113. Thus, effects of RTI-112 and RTI-113 on cocaine- vs. food-maintained responding could not be statistically differentiated at any time during treatment with either drug.
Figure 2. Time course of effects produced by treatment with saline, RTI-112 (0.01 mg/kg/hr) and RTI-113 (0.056 mg/kg/hr) on responding maintained by 0.01 mg/kg/inj cocaine and food.
Abscissae: Consecutives days of treatment. All treatments were implemented for a period of 7 days. Ordinates: Percent control levels of cocaine- and food-maintained responding. All points show mean ± SEM in three monkeys. The absence of error bars indicates that error bars were contained within the point. There was not a significant difference in effects of RTI-112 or RTI-113 on cocaine- vs. food-maintained responding at any time during treatment. ANOVA results for saline treatment. Main effect of time: F(6,24)=0.89, p=0.39. Main effect of reinforcer type: F(1,4)=1.75, p=0.26. Time × reinforcer type interaction: F(6,24)=1.10, p=0.39. ANOVA results for 0.01 mg/kg/hr RTI-112 treatment. Main effect of time: F(6,24)=1.94, p=0.11. Main effect of reinforcer type: F(1,4)=1.46, p=0.29. Time × reinforcer type interaction: F(6,24)=1.00, p=0.45. ANOVA results for 0.056 mg/kg/hr RTI-113 treatment. Main effect of time: F(6,24)=5.08, p=0.0017. Main effect of reinforcer type: F(1,4)=1.22, p=0.33. Time × reinforcer type interaction: F(6,24)=0.24, p=0.96.
Overt behavioral effects of RTI-112 and RTI-113 were not formally monitored; however, the highest doses of RTI-112 (0.01 mg/kg/hr) and RTI-113 (0.056 mg/kg/hr) increased activity expressed either as increased whole-body locomotion (e.g. circling in cage) or increased manual or oral movements (e.g. grooming or manipulation of cage features, repeated mouth/tongue movements).
4. Discussion
The main finding of this study was that 7-day treatment with either RTI-112 or RTI-113 produced dose-dependent, sustained and relatively non-selective decreases in cocaine- vs. food-maintained responding in rhesus monkeys. These results do not support the hypothesis that pharmacological selectivity to block dopamine uptake is associated with behavioral selectivity to reduce cocaine- vs. food-maintained responding.
These findings confirm and extend previous studies that evaluated the effects of RTI-112, RTI-113 and other monoamine uptake inhibitors on cocaine self-administration. Regarding the time course of monoamine uptake inhibitor effects, both RTI-112 and RTI-113 have been reported previously to decrease cocaine self-administration for up to three days (Ginsburg, 2002; Howell et al., 2000; Lindsey et al., 2004), and the present study demonstrates that these effects can be sustained for up to seven days of treatment. Robust and sustained decreases in cocaine self-administration for ≥7 days have also been achieved with other selective and non-selective dopamine uptake inhibitors including GBR12909 (Glowa et al., 1995b), mazindol (Kleven and Woolverton, 1993) and indatraline (Negus et al., 1999). The sustained effects of dopamine uptake inhibitors contrast with the more transient decreases on cocaine self-administration produced by some other classes of compounds, such as dopamine receptor antagonists (Kleven and Woolverton, 1990; Negus et al., 1996).
The present findings also extend previous studies that evaluated the selectivity of monoamine uptake inhibitor effects on cocaine self-administration. Specifically, both RTI-112 and RTI-113 produced non-selective decreases in cocaine- vs. food-maintained responding. Although given doses of each drug tended to decrease cocaine self-administration more than food-maintained responding, there was not a significant difference in the effects of any dose of RTI-112 or RTI-113 on cocaine- vs. food-maintained responding, and there was not a significant difference in the ED50 for either drug to decrease cocaine- vs. food-maintained responding. These results agree with previous studies that found similar potencies for RTI-113 and RTI-112 to decrease responding maintained by cocaine delivery or by termination of a light associated with a noxious stimulus (Ginsburg, 2002; Ginsburg et al., 2005; Howell et al., 2000). Multi-day treatments with the less selective monoamine uptake inhibitors mazindol and indatraline also produced non-selective decreases in responding maintained by cocaine and food delivery in rhesus monkeys (Kleven and Woolverton, 1993; Negus et al., 1999). Taken together, these data suggest that dopamine uptake inhibitors with a broad range of pharmacological selectivities produces at best only marginally selective decreases in responding maintained by cocaine in comparison to responding maintained by non-drug reinforcers.
At first glance, the results of the present study appear to contrast with a study concluding that RTI-113 selectively decreased cocaine- vs. food-maintained responding in rats (Dworkin et al., 1998). However, that study reported effects of only one relatively low dose of RTI-113 dose on responding for food, and this dose produced a relatively small decrease in cocaine self-administration. Higher RTI-113 doses that produced greater reductions in cocaine self-administration were not examined for their effects on rates of food-maintained responding, and the relative potency of RTI-113 to decrease cocaine- vs. food-maintained responding in rats was not determined. Thus, it is possible that RTI-113 might have produced only a marginally selective reduction in cocaine self-administration in rats as well.
The present results provide a more significant contrast with the reported effects of the dopamine-selective uptake inhibitor GBR12909. Both repeated daily treatments with GBR12909 and single treatments with a long-acting decanoate salt of GBR12909 produced sustained and highly selective decreases in cocaine self-administration in comparison to food-maintained responding (Glowa et al., 1996; Glowa et al., 1995b). However, these authors also demonstrated that the behavioral selectivity of GBR12909 was sensitive to changes in both the cocaine unit dose and the schedules of cocaine and food availability (Glowa et al., 1995a; Stafford et al., 2000). More specifically, the behavioral selectivity of GBR12909 to reduce cocaine- vs. food-maintained responding was reduced when the unit dose of cocaine was increased or the fixed-ratio response requirement for cocaine was decreased. Thus, even GBR12909 appears to produce selective decreases in cocaine- vs. food-maintained responding under a limited range of conditions.
The sensitivity of GBR12909 effects to such variables as reinforcer magnitude and schedule requirement raise the possibility that behavioral selectivity of RTI-113 and RTI-112 in the present study might have been limited by the selection of unfavorable parameters for these variables. However, at least three findings argue against this possibility. First, the reinforcer types and magnitudes used in the present study were virtually identical to those used in studies demonstrating selective reductions in cocaine self-administration by GBR12909 (i.e. cocaine unit dose of 0.01 mg/kg/inj cocaine; 1 gm, banana-flavored food pellets) (Glowa et al., 1996; Glowa et al., 1995b). Second, comparison of the efficacy of these reinforcers using either choice or progressive ratio schedules suggests that a unit dose of 0.01 mg/kg/inj cocaine is a weaker reinforcer than a single 1gm food pellet in rhesus monkeys (Negus, 2003; Negus and Mello, 2003a). Thus, monkeys given a choice between 0.01 mg/kg/inj cocaine and 1gm food pellets will typically chose food, and 1 gm food pellets maintained higher break points under a progressive ratio schedule than 0.01 mg/kg/inj cocaine. In general, behavior maintained by smaller/weaker reinforcers is more sensitive to disruption than behavior maintained by larger/stronger reinforcers (Egli et al., 1992; Glowa et al., 1995a). Application of this principle to the present study would predict selective reductions in responding maintained by the weaker reinforcer--i.e. selective reductions in cocaine self-administration. Indeed, the modest selectivity page 14 in effects of RTI-113 and RTI-112 on cocaine- vs. food-maintained responding may have resulted from the lower efficacy of the cocaine reinforcer rather than from any selective effects of RTI-113 or RTI-112 on the mechanisms of cocaine reinforcement (see also (Glowa et al., 1995a) for an application of this same line of reasoning to results of studies with GBR12909).
Finally, the relative lack of behavioral selectivity observed with RTI-113 and RTI-112 contrasts with the finding that dopamine-selective monoamine releasers produced more selective reductions in cocaine self-administration under identical conditions (Negus and Mello, 2003b; Negus et al., 2007). Moreover, dopamine-selective releasers have produced selective reductions in cocaine- vs. food-maintained responding across a broad range of cocaine doses, under a variety of different schedules of reinforcement, and in both rhesus monkeys and rats (Barrett et al., 2005; Negus, 2003; Negus and Mello, 2003a, b; Negus et al., 2007). These findings suggest that dopamine-selective monoamine releasers may produce more selective reductions in cocaine-vs. food-maintained responding than dopamine-selective uptake inhibitors. However, the degree and generality of this potentially important difference between dopamine releasers and dopamine uptake inhibitors remains to be determined. For example, monoamine releasers often produce an initial, transient reduction in food-maintained responding, and behavioral selectivity may then emerge over time as food-maintained responding recovers while cocaine self-administration remains suppressed (Negus and Mello, 2003b; Negus et al., 2007). It is possible that behavioral selectivity of RTI-112 and RTI-113 may also have increased if treatment had continued beyond 7 days. Subtle differences in pharmacologic selectivity can also influence apparent behavioral selectivity. Thus, studies in our laboratory with a series of nine compounds found that monoamine releasers with approximately 30- to 40-fold selectivity for dopamine vs. serotonin release produced more selective reductions in cocaine self-administration than releasers with lower or higher selectivities (Negus and Mello, 2003b; Negus et al., 2007, unpublished observations). By extension, it remains possible that monoamine uptake inhibitors with pharmacological selectivities different from those of RTI-112 and RTI-113 (e.g. intermediate DAT selectivity) may produce more selective reductions in cocaine self-administration.
Acknowledgments
This work was supported by grants R01-DA02519 and P01-DA14528 (NKM and SSN), K05-DA00101 (NKM), K02-DA00517 (LLH), K01-DA15092 (HLK), and R01-DA05477 (FIC) from NIDA, NIH. The authors would like to thank Peter A. Fivel and Cara Sylvester for outstanding technical support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barrett AC, Negus SS, Newman AH, Grundt P, Caine SB. Effects of standard dopaminergics and comparison of D3 and D2 receptor ligands on choice between concurrently available cocaine and food in rats. Presented at the 2005 meeting of the American College of Neuropsychopharmacology; 2005. [Google Scholar]
- Dworkin SI, Lambert P, Sizemore GM, Carroll FI, Kuhar MJ. RTI-113 administration reduces cocaine self-administration at high occupancy of dopamine transporter. Synapse. 1998;30:49–55. doi: 10.1002/(SICI)1098-2396(199809)30:1<49::AID-SYN6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Egli M, Schaal DW, Thompson T, Cleary J. Opioid-induced response-rate decrements in pigeons responding under variable-interval schedules: reinforcement mechanisms. Behav Pharmacol. 1992;3:581–591. [PubMed] [Google Scholar]
- Ginsburg BC. Graduate Program in Molecular and Systems Pharmacology. Atlanta, GA: Emory University; 2002. Behavioral and extracellelular dopamine effects of monoamine uptake inhibitors alone and in combination with cocaine in squirrel monkeys. [Google Scholar]
- Ginsburg BC, Kimmel HL, Carroll FI, Goodman MM, Howell LL. Interaction of cocaine and dopamine transporter inhibitors on behavior and neurochemistry in monkeys. Pharmacol Biochem Behav. 2005;80:481–491. doi: 10.1016/j.pbb.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Glowa JR, Fantegrossi WE, Lewis DB, Matecka D, Rice KC, Rothman RB. Sustained decrease in cocaine-maintained responding in rhesus monkeys with 1-[2-[bis(4-fluorophenyl)methoxy]ethyl]-4-(3-hydroxy-3-phenylpropyl) piperazinyl decanoate, a long-acting ester derivative of GBR 12909. J Med Chem. 1996;39:4689–4691. doi: 10.1021/jm960551t. [DOI] [PubMed] [Google Scholar]
- Glowa JR, Wojnicki FHE, Matecka D, Bacher JD. Effects of dopamine reuptake inhibitors on food- and cocaine-maintained responding: I. Dependence on unit dose of cocaine. Exp Clin Psychopharmacol. 1995a;3:1–13. [Google Scholar]
- Glowa JR, Wojnicki FHE, Matecka D, Rice KC, Rothman RB. Effects of dopamine reuptake inhibitors on food- and cocaine-maintained responding: II. Comparisons with other drugs and repeated administrations. Exp Clin Psychopharmacol. 1995b;3:1–8. [Google Scholar]
- Grabowski J, Shearer J, Merrill J, Negus SS. Agonist-like, replacement pharmacotherapy for stimulant abuse and dependence. Addict Behav. 2004;29:1439–1464. doi: 10.1016/j.addbeh.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Haney M, Spealman R. Controversies in translational research: drug self-administration. Psychopharmacology (Berl) 2008 doi: 10.1007/s00213-008-1079-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell LL, Czoty PW, Kuhar MJ, Carroll FI. Comparative behavioral pharmacology of cocaine and the selective dopamine uptake inhibitor RTI-113 in the squirrel monkey. J Pharmacol Exp Ther. 2000;292:521–529. [PubMed] [Google Scholar]
- Howell LL, Wilcox KM. The dopamine transporter and cocaine medication development: drug self-administration in nonhuman primates. J Pharmacol Exp Ther. 2001;298:1–6. [PubMed] [Google Scholar]
- Kleven MS, Woolverton WL. Effects of continuous infusions of SCH 23390 on cocaine- or food-maintained behavior in rhesus monkeys. Behav Pharmacol. 1990;1:365–373. doi: 10.1097/00008877-199000140-00010. [DOI] [PubMed] [Google Scholar]
- Kleven MS, Woolverton WL. Effects of three monoamine uptake inhibitors on behavior maintained by cocaine or food presentation in rhesus monkeys. Drug Alcohol Depend. 1993;31:149–158. doi: 10.1016/0376-8716(93)90067-z. [DOI] [PubMed] [Google Scholar]
- Kuhar MJ, McGirr KM, Hunter RG, Lambert PD, Garrett BE, Carroll FI. Studies of selected phenyltropanes at monoamine transporters. Drug Alcohol Depend. 1999;56:9–15. doi: 10.1016/s0376-8716(99)00005-8. [DOI] [PubMed] [Google Scholar]
- Lindsey KP, Wilcox KM, Votaw JR, Goodman MM, Plisson C, Carroll FI, Rice KC, Howell LL. Effects of dopamine transporter inhibitors on cocaine self-administration in rhesus monkeys: relationship to transporter occupancy determined by positron emission tomography neuroimaging. J Pharmacol Exp Ther. 2004;309:959–969. doi: 10.1124/jpet.103.060293. [DOI] [PubMed] [Google Scholar]
- Mello NK, Lukas SE, Bree MP, Mendelson JH. Desipramine effects on cocaine self-administration by rhesus monkeys. Drug Alcohol Depend. 1990;26:103–116. doi: 10.1016/0376-8716(90)90117-w. [DOI] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH, Bree MP, Lukas SE. Buprenorphine suppresses cocaine self-administration by rhesus monkey. Science. 1989;245:859–862. doi: 10.1126/science.2772637. [DOI] [PubMed] [Google Scholar]
- Mello NK, Negus SS. Effects of kappa opioid agonists on cocaine- and food-maintained responding by rhesus monkeys. J Pharmacol Exp Ther. 1998;286:812–824. [PubMed] [Google Scholar]
- Mello NK, Negus SS. Preclinical evaluation of pharmacotherapies for treatment of cocaine and opioid abuse using drug self-administration procedures. Neuropsychopharmacology. 1996;14:375–424. doi: 10.1016/0893-133X(95)00274-H. [DOI] [PubMed] [Google Scholar]
- Mello NK, Negus SS, Rice KC, Mendelson JH. Effects of the CRF1 antagonist antalarmin on cocaine self-administration and discrimination in rhesus monkeys. Pharmacol Biochem Behav. 2006;85:744–751. doi: 10.1016/j.pbb.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Negus SS. Rapid assessment of choice between cocaine and food in rhesus monkeys: effects of environmental manipulations and treatment with d-amphetamine and flupenthixol. Neuropsychopharmacology. 2003;28:919–931. doi: 10.1038/sj.npp.1300096. [DOI] [PubMed] [Google Scholar]
- Negus SS, Brandt MR, Mello NK. Effects of the long-acting monoamine reuptake inhibitor indatraline on cocaine self-administration in rhesus monkeys. J Pharmacol Exp Ther. 1999;291:60–69. [PubMed] [Google Scholar]
- Negus SS, Mello NK. Effects of chronic d-amphetamine treatment on cocaine- and food-maintained responding under a progressive-ratio schedule in rhesus monkeys. Psychopharmacology (Berl) 2003a;167:324–332. doi: 10.1007/s00213-003-1409-y. [DOI] [PubMed] [Google Scholar]
- Negus SS, Mello NK. Effects of chronic d-amphetamine treatment on cocaine- and food-maintained responding under a second-order schedule in rhesus monkeys. Drug Alcohol Depend. 2003b;70:39–52. doi: 10.1016/s0376-8716(02)00339-3. [DOI] [PubMed] [Google Scholar]
- Negus SS, Mello NK. Effects of mu-opioid agonists on cocaine- and food-maintained responding and cocaine discrimination in rhesus monkeys: role of mu-agonist efficacy. J Pharmacol Exp Ther. 2002;300:1111–1121. doi: 10.1124/jpet.300.3.1111. [DOI] [PubMed] [Google Scholar]
- Negus SS, Mello NK, Blough BE, Baumann MH, Rothman RB. Monoamine Releasers with Varying Selectivity for Dopamine/Norepinephrine versus Serotonin Release as Candidate “Agonist” Medications for Cocaine Dependence: Studies in Assays of Cocaine Discrimination and Cocaine Self-Administration in Rhesus Monkeys. J Pharmacol Exp Ther. 2007;320:627–636. doi: 10.1124/jpet.106.107383. [DOI] [PubMed] [Google Scholar]
- Negus SS, Mello NK, Lamas X, Mendelson JH. Acute and chronic effects of flupenthixol on the discriminative stimulus and reinforcing effects of cocaine in rhesus monkeys. J Pharmacol Exp Ther. 1996;278:879–890. [PubMed] [Google Scholar]
- Negus SS, Mello NK, Lukas SE, Mendelson JH. Diurnal patterns of cocaine and heroin self-administration in rhesus monkeys responding under a schedule of multiple daily sessions. Behav Pharmacol. 1995;6:763–775. [PubMed] [Google Scholar]
- Negus SS, Mello NK, Portoghese PS, Lin CE. Effects of kappa opioids on cocaine self-administration by rhesus monkeys. J Pharmacol Exp Ther. 1997;282:44–55. [PubMed] [Google Scholar]
- Pereira-Do Carmo G, Mello NK, Rice KC, Folk JE, Negus SS. Effects of the selective delta opioid agonist SNC80 on cocaine- and food-maintained responding in rhesus monkeys. Eur J Pharmacol. 2006;547:92–100. doi: 10.1016/j.ejphar.2006.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman RB, Glowa JR. A review of the effects of dopaminergic agents on humans, animals, and drug-seeking behavior, and its implications for medication development: Focus on GBR 12909. Mol Neurobiol. 1995;10:1–19. doi: 10.1007/BF02740680. [DOI] [PubMed] [Google Scholar]
- Stafford D, Rice KC, Lewis DB, Glowa JR. Response requirements and unit dose modify the effects of GBR 12909 on cocaine-maintained behavior. Exp Clin Psychopharmacol. 2000;8:539–548. doi: 10.1037//1064-1297.8.4.539. [DOI] [PubMed] [Google Scholar]
- Stevenson GW, Wentland MP, Bidlack JM, Mello NK, Negus SS. Effects of the mixed-action kappa/mu opioid agonist 8-carboxamidocyclazocine on cocaine- and food-maintained responding in rhesus monkeys. Eur. J. Pharmacol. 2004;506:133–141. doi: 10.1016/j.ejphar.2004.10.051. [DOI] [PubMed] [Google Scholar]
- Wilcox KM, Lindsey KP, Votaw JR, Goodman MM, Martarello L, Carroll FI, Howell LL. Self-administration of cocaine and the cocaine analog RTI-113: relationship to dopamine transporter occupancy determined by PET neuroimaging in rhesus monkeys. Synapse. 2002;43:78–85. doi: 10.1002/syn.10018. [DOI] [PubMed] [Google Scholar]