Abstract
N-methyl-D-aspartate receptors (NMDARs) are mediators of synaptic plasticity and learning and are implicated in the pathophysiology of neuropsychiatric disease and age-related cognitive dysfunction. NMDARs are heteromers, but the relative contribution of specific subunits to NMDAR-mediated learning is not fully understood. We characterized pre-conditioning systemic treatment of the NR2B subunit-selective antagonist Ro 25-6981 for effects on multi-trial, one-trial and low-shock Pavlovian fear conditioning in C57BL/6J mice. Ro 25-6981 was also profiled for effects on novel open field exploration, elevated plus-maze anxiety-like behavior, startle reactivity, prepulse inhibition of startle, and nociception. Three-month (adult) and 12-month old C57BL/6Tac mice were compared for Ro 25-6981 effects on multi-trial fear conditioning, and corticolimbic NR2B protein levels. Ro 25-6981 moderately impaired fear learning in the multi-trial and one-trial (but not low-shock) conditioning paradigms, but did not affect exploratory or anxiety-related behaviors or sensory functions. Memory impairing effects of Ro 25-6981 were absent in 12-month old mice, although NR2B protein levels were not significantly altered. Present data provide further evidence of the memory impairing effects of selective blockade of NR2B-containing NMDARs, and show loss of these effects with ageing. This work could ultimately have implications for elucidating the pathophysiology of learning dysfunction in neuropsychiatric disorders and ageing.
Keywords: aged, anxiety, glutamate, learning, memory, NR2A, prepulse inhibition, open field, elevated plus-maze, one-trial, Western blot, C57BL/6
Introduction
There is compelling evidence that glutamatergic neurotransmission at N-methyl-D-aspartate receptors (NMDARs) is a major molecular mechanism underlying multiple forms of learning and memory. Activation of NMDARs initiates a cascade of molecular events that underlie synaptic plasticity which are strongly implicated in learning and memory, and NMDAR blockade prevents the induction of some forms of long-term potentiation (LTP) and long-term depression (LTD) (Bliss and Collingridge, 1993; Malenka and Bear, 2004). Behaviorally, systemic or intracerebral administration of NMDAR antagonists impairs learning and memory performance on various tasks, including Pavlovian fear conditioning (reviewed in Bannerman et al., 2006; Morris et al., 1990; Nakazawa et al., 2004). Pavlovian fear conditioning is a behavioral paradigm in which rodents learn to associate an innocuous stimulus (e.g., auditory tone) with footshock. Fear learning is impaired by pre-training systemic or intra-amygdala administration of NMDAR antagonists (e.g., D,L-AP5, MK-801/dizocilpine) probably via disruption of plastic changes at thalamo-amygdala synapses (e.g., Fanselow and Kim, 1994; Gewirtz and Davis, 1997; Lee and Kim, 1998; Walker and Davis, 2000; Walker et al., 2005).
NMDARs are heteromers composed of an obligatory NR1 subunit and at least one or more NR2 (NR2A–NR2D) subunits (Laube et al., 1998; Rosenmund et al., 1998; Schorge and Colquhoun, 2003). An important but as yet unresolved issue is the relative contribution of NMDAR subtypes to NMDAR-mediation of learning and memory. NR2A and NR2B subunits are both highly expressed in forebrain regions implicated in fear conditioning, including the amygdala, but contribute distinct physiological and molecular properties to NMDARs (Cull-Candy et al., 2001; Liu et al., 2004; Loftis and Janowsky, 2003; Perez-Otano and Ehlers, 2005; Radley et al., 2007). Sensory experience and discrimination learning increases the ratio of NR2A/NR2B and the threshold for LTP-induction (Carmignoto and Vicini, 1992; Kirkwood et al., 1996; Quinlan et al., 2004). A similar profile is seen during ontogeny where NR2B expression decreases in favor of NR2A during late postnatal development causing shortening of excitatory postsynaptic potentials (EPSCs) and increasing the threshold for LTP-induction (Hestrin, 1992; Liu et al., 2004; Lopez de Armentia and Sah, 2003). Collectively, these findings support a working model in which the NR2B facilitates NMDAR-mediated synaptic plasticity and new learning, while NR2A may support memory stabilization by preventing excessive plasticity (Quinlan et al., 2004; Tang et al., 1999).
The respective roles of the NR2A and NR2B subunits at the behavioral level have yet to be fully elucidated, in part due to a paucity of subtype selective drugs, especially for NR2A (Bartlett et al., 2007; Kash and Winder, 2007; Neyton and Paoletti, 2006). Gene knockout of NR2A has been shown to produce deficits in hippocampal LTP and impair spatial working memory, instrumental discrimination learning and fear conditioning under certain conditions (Bannerman et al., 2008; Brigman et al., 2008; Kiyama et al., 1998; Sakimura et al., 1995; Sprengel et al., 1998). On the other hand, overexpressing NR2B in the mouse forebrain leads to superior fear conditioning and extinction (Tang et al., 1999). Furthermore, pre-training systemic or intra-amygdala administration of the NR2B-selective antagonists ifenprodil or CP101,606, or genetic disruption of tyrosine-phosphorylation of NR2B, impairs fear acquisition and extinction in rats (Bauer et al., 2002; Blair et al., 2005; Dalton et al., 2007; Nakazawa et al., 2006; Rodrigues et al., 2001; Sotres-Bayon et al., 2007; Walker and Davis, 2008). Recent data also show that pre-training siRNA knockdown or selective pharmacological blockade of NR2B with Ro 25-6981 in the anterior cingulate region of the prefrontal cortex impairs the acquisition of context (but not tone) fear memory in mice (Zhao et al., 2005). Finally, global knockdown of NR2B in mice (Takehara et al., 2004) or intra-hippocampal infusion of Ro 25-6981 in rats (Valenzuela-Harrington et al., 2007) impairs trace fear conditioning but not the delay form of tone (or context) fear conditioning in mice (Zhao et al., 2005). While these data support the role of NR2B to fear learning, they do not fully address the behavioral consequences of inactivating NR2B-containing NR2B receptors. For example, while the aforementioned work of Zhao and colleagues and Valenzuela-Harrington and co-workers demonstrates fear memory impairing effects of Ro 25-6981 injected directly into specific regions of cortex and hippocampus, the consequences of systemic treatment with the drug for this behavior is unclear. This issue is salient to the potential future clinical use of Ro 25-6981 or structurally similar compounds (Danysz and Parsons, 2002; Gogas, 2006).
The existing literature also does not adequately address the question of whether the functional contribution of NR2B is altered under certain ‘pathological’ conditions. In this context, glutamate and NMDAR dysfunction is associated in age-related cognitive decline that is seen on various assays for learning and memory in rodents (Magnusson, 1998; Rosenzweig and Barnes, 2003). Some studies have found that age-related learning deficits are concomitant with a loss of NMDARs, prominently NR2B (Magnusson, 2001; Ontl et al., 2004). Interestingly, there is also decreased NR2B expression in Alzheimer’s disease (Maragos et al., 1987; Sze et al., 2001; Wang et al., 2000). Providing preliminary evidence that these changes may be of functional importance, mice in which NR2B is transgenically overexpressed are protected against deterioration of age-related learning on tasks including cued Pavlovian fear conditioning (Cao et al., 2007). However, whether the fear memory related effects of Ro 25-6981 vary as a function of ageing has to our knowledge not been studied.
The main objective of the present study was to characterize pre-training systemic treatment of the selective NR2B antagonist Ro 25-6981 for effects on strong (multi-trial) and weaker (one-trial, low-shock) forms of Pavlovian fear conditioning in mice. There is growing evidence that NMDAR blockade exerts effects on rodent locomotor activity, anxiety-related behaviors and sensorimotor gating (Boyce-Rustay and Holmes, 2006; Cryan and Dev, 2007; Geyer et al., 2001). Therefore, we also evaluated systemic Ro 25-6981 for effects on exploratory locomotion (novel open field), anxiety-related (elevated plus-maze) and sensory (acoustic startle, sensorimotor gating, hotplate nociception) behaviors. A final objective was to test whether fear memory impairing effects of Ro 25-6981 were altered in ageing mice.
Materials and methods
Subjects
With the exception of the ageing experiment, subjects were 2-4-month old male C57BL/6J mice obtained from The Jackson Laboratory (Bar Harbor, ME). Because it was not possible to obtain 12-month old mice from The Jackson Laboratory, C57BL/6 mice (C57BL/6Tac) were obtained from Taconic Farms (Germantown, NY). To provide appropriate controls for the age-comparison study, younger mice used in this experiment were 3-month old counterparts obtained from Taconic Farms in the same shipment as the 12-month old mice. Mice were pair-housed in a temperature and humidity controlled vivarium under a 12 h light/dark cycle (lights on 0600 h). All experimental procedures were approved by the National Institute on Alcohol Abuse and Alcoholism Animal Care and Use Committee and strictly followed the NIH guidelines ‘Using Animals in Intramural Research.’
Pavlovian fear conditioning
The memory impairing effects of Ro 25-6981 on Pavlovian fear conditioning were tested using 3 different paradigms (in separate groups of mice). One cohort was tested on a standard multi-trial delay conditioning paradigm (Kim and Fanselow, 1992; Yang et al., 2008) (for schematic, see Figure 1A). Previous studies have shown that pharmacologic and genetic inactivation of NMDARs or other glutamate receptors can preferentially impair fear learning under one-trial and low-shock forms of conditioning (Cravens et al., 2006; Feyder et al., 2008; Nakazawa et al., 2003; Walker and Davis, 2000). Therefore, additional experiments were conducted to test the memory impairing effects of Ro 25-6981 using a one-trial delay paradigm (for schematic, see Figure 1C) and multi-trial low-shock paradigm (for schematic, see Figure 1E).
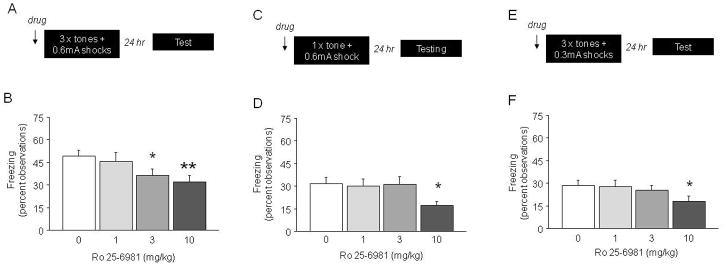
Figure 1.
Fear memory impairing effects of Ro 25-6981 on multi-trial, one-trial and low-lock shock fear conditioning. (A) Schematic of the multi-trial high shock protocol. (B) Pre-training treatment with 10 mg/kg Ro 25-6981 reduced fear recall relative to vehicle (0) (n=16–18/dose). (C) Schematic of the one-trial high-shock conditioning protocol. (D) Pre-training treatment with 10 mg/kg Ro 25-6981 reduced fear recall relative to vehicle (0) (n=13–14/dose). (E) Schematic of multi-trial low-shock protocol. (F) Pre-training treatment with 10 mg/kg Ro 25-6981 reduced fear recall relative to vehicle (0) (n=15–16/dose). **p<.01, *p<.05 vs. vehicle (0). Data in Figures 1–4 are Means ±SEM.
Memory impairing effects of Ro 25-6981 (multi-trial fear conditioning)
The procedure was similar to that previously employed in our laboratory (Hefner and Holmes, 2006). Conditioning took place in a 35 × 25 × 22 cm chamber with transparent walls and a metal rod floor. To provide a distinctive olfactory environment, the chamber was cleaned between subjects with a 79.5% water/19.5% ethanol/1% vanilla extract solution. After a 180 sec acclimation period, the mouse received 3 pairings (60–90 sec interval) between a tone (30 sec, 80 dB white noise) and footshock (2 sec, 0.6 mA scrambled footshock), in which the shock was presented during the last 2 sec of the tone. The presentation of stimuli was controlled by the Med Associates Video Freeze system (Med Associates Inc., St. Albans, VT). Twenty-four hr after conditioning, tone-recall was tested in a novel context, in a different room from training. The novel context was a square chamber or cylinder with black/white-checkered walls and a solid-Plexiglas, opaque floor, cleaned between subjects with a 1% acetic acid/99% water solution. After a 180 sec acclimation period, the tone was presented for 180 sec. Freezing in all experiments was defined as absence of any visible movement except that required for respiration, and scored at 5 sec intervals by an observer blind to experimental group. The number of observations scored as freezing were converted to a percentage ([number of freezing observations/total number of observations] × 100) for analysis.
Memory impairing effects of Ro 25-6981 (one-trial fear conditioning)
Mice were conditioned and tested as for multi-trial high-shock fear conditioning with the exception that there was only 1 tone-shock pairing as opposed to 3 pairings.
Memory impairing effects of Ro 25-6981 (multi-trial low-shock fear conditioning)
Mice were conditioned and tested using the same procedure as described for multi-trial high-shock conditioning with the exception that footshock intensity was 0.3 mA as opposed to 0.6 mA.
Novel open field
Mice were tested on a novel open field apparatus as previously described (Wiedholz et al., 2008). The mouse was placed in the perimeter of a white Plexiglas 40 × 40 × 35 cm square arena (50 lux) under 65 dB white noise to minimize external disturbances (Sound Screen, Marpac Corporation, Rocky Point, NC), and allowed to explore for 60 min. Total distance traveled in the whole arena and time spent in the center (20 × 20 cm) was measured by the Ethovision videotracking system (Noldus Information Technology Inc., Leesburg, VA).
Elevated plus-maze
One week after novel open field testing, mice were tested on the elevated plus-maze test for anxiety-like behavior with assignment of drug doses randomized. Testing was conducted as previously described (Handley and Mithani, 1984; Holmes et al., 2000). The apparatus consisting of 2 open arms (30 × 5 cm; 90 lux) and 2 closed arms (30 × 5 × 15 cm; 20 lux) extending from a 5 × 5 cm central area and elevated 20 cm from the ground (San Diego Instruments, San Diego, CA). The walls were made from black ABS plastic and the floor from white ABS plastic. A 0.5 cm raised lip around the perimeter of the open arms prevented mice from falling off the maze. Testing was conducted under 65 dB white noise to minimize external disturbances (Sound Screen, Marpac Corporation, Rocky Point, NC). The mouse was placed in the center facing an open arm and allowed to explore the apparatus for 5 min. Time spent in the open arms, and entries into the open and closed arms was measured by the Ethovision videotracking system (Noldus Information Technology Inc., Leesburg, VA).
Acoustic startle and prepulse inhibition of startle
Acoustic startle and prepulse inhibition of the startle response was measured as previously described (Millstein et al., 2006). Mice were placed in a clear Plexiglas cylinder in 1 of 4 SR-Lab System startle chambers (San Diego Instruments, San Diego, CA) for a 5 min acclimation period. A 65 dB broadband background noise was delivered during acclimation and throughout testing. During the test session, mice were presented with startle trials (40 msec, 120 dB broadband sound pulse) and prepulse+startle trials (20 msec noise prepulse sound followed, 100 msec later, by a 40 msec 120 dB broadband sound pulse). The prepulse+startle trials were preceded and followed by 5 pulse alone trials, which were not included in the analyses. Test trials consisted of 10 trials of 3 different intensities (3, 6, and 12 dB above background). Each trial type was presented 10 times with a variable interval of 12–30 sec between each presentation. Basal activity in the startle chambers was measured during no-stimulus trials. Startle amplitude was measured every 1 msec, over a 65 msec period beginning at the onset of the startle stimulus. The maximum startle amplitude over the sampling period was taken as the dependent variable. Whole-body startle responses were measured via vibrations transduced into analog signals by a piezoelectric unit attached to the platform on which the cylinders rested. Prepulse inhibition of startle was calculated as 100- [(startle response for prepulse+startle trials/startle response for startle-alone trials) ×100].
Hot plate nociception
The hot plate test apparatus was a flat plate (Columbus Instruments, Columbus, OH) heated to 55°C on which the mouse was placed (Boyce-Rustay and Holmes, 2006). The latency to show a hind paw shake or lick was timed by an observer, with a maximum response latency of 30 sec.
Memory impairing effects of Ro 25-6981 in 3-month and 12-month old mice
Three-month and 12-month old mice were treated with Ro 25-6981 and tested using the multi-trial high-shock fear conditioning procedure described above (for schematic of the procedure, see Figure 2A). Twelve months was chosen as an age we hypothesized to be characterized by loss of NR2B function without the marked decrement in NMDAR function and learning that would occur in older mice (Magnusson et al., 2007). At completion of the tone-recall test, the vehicle-treated mice in this group were sacrificed by rapid cervical dislocation and decapitation. Brains were removed, flash-frozen in ice-cold isopentane and stored at −80°C for Western blot analysis of NMDAR levels as described below.
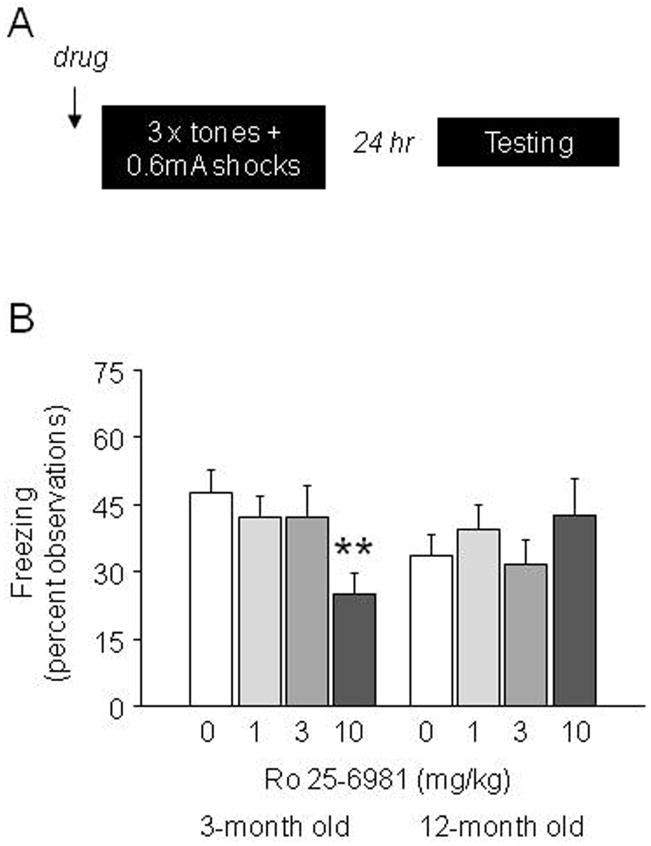
Figure 2.
Fear memory impairing effects of Ro 25-6981 on multi-trial high-shock fear conditioning as a function of ageing (A) Schematic of the multi-trial high-shock conditioning protocol. (B) Pre-training treatment with 10 mg/kg Ro 25-6981 significantly reduced fear recall relative to vehicle (0) in 3-month but not 12-month old mice (n=8–10/dose/age). **p<.01 vs. 0 (vehicle).
Corticolimbic NR2B protein levels in 3-month and 12-month old mice
Micropunches (Zivic Laboratories Inc., Pittsburgh, PA) were taken from medial prefrontal cortex (2.0 mm diameter punch), dorsal hippocampus (2.0 mm diameter punch) and basolateral amygdala (1.0 mm diameter punch) and were dissected on ice. Tissue was homogenized by sonication in protease and phosphatase inhibitors (Sigma protease inhibitor cocktail and phosphatase inhibitor cocktails 1 and 2, 10 μM NaF, 1% Triton-X 100, 25 mM Tris, pH 6.8) by sonication and protein concentration determined by the BCA method. Samples were diluted with 4X sample buffer (Laemmli, 1970). β-mercaptoethanol was added to a final concentration of 5% (v/v) and samples were boiled for 10 min. Twenty-five (amygdala) or 30 (hippocampus and prefrontal cortex) μg of protein was subjected to discontinuous pH 7.5% SDS-polyacrylamide gel electrophoresis (pH 8.3) with a 4% stacking gel (pH 6.8) (Laemmli, 1970) using a triple-wide electrophoresis apparatus (CBS Scientific, La Jolla, CA). Proteins were transferred to a PVDF membrane overnight at 50 mA as described for nitrocellulose membranes (Towbin et al., 1979). The blot was washed in TTBS (150 mM NaCl, 25 mM Tris, 0.05% Tween-20, pH 7.3) and blocked for 1 hr in 5% non-fat powdered milk. The NR2B antibody (Chemicon/Millipore/Upstate, catalogue # AB1557P) was used at 1:1000. The blot was incubated overnight in primary antibody, washed 3 times in TTBS, and then incubated in HRP-conjugated goat anti-rabbit (Pierce, Rockford, IL) for 1 hr. Following 3 washes, immunoreactivity was detected using SuperSignal West Dura chemiluminescence detection reagent and collected using a Kodak Image Station 4000.
Drugs
Ro 25-6981 (R-(R*,S*)-α-(4-hydroxyphenyl)-β-methyl-4-(phenylmethyl)-1-piperidine propranolol hydrochloride) (Tocris Cookson, Ellisville, MO) was prepared in a 0.9% saline vehicle and injected intraperitoneally in a volume of 10 mL/kg body weight. The doses and treatment-to-test interval for Ro 25-6981 were chosen on the basis of a previous study showing that i.p. injection of 10 mg/kg Ro 25-6981 30 min prior to conditioning impaired trace eyeblink conditioning in rats (Valenzuela-Harrington et al., 2007) and subcutaneous injection of 5 mg/kg Ro 25-6981 30 min prior to testing impaired spatial reversal learning in C57BL/6J mice (Duffy et al., 2008).
Statistical analysis
The effects of Ro 25-6981 treatment, age and brain region were analyzed by use of analysis of variance (ANOVA) and Fisher’s LSD post hoc tests, using StatView (SAS Institute, Inc., Cary, NC). Mice that had tone recall freezing scores standard 2 deviations different from the grand mean were identified as statistical outliers and removed. Statistical significance was set at p<.05.
Results
Memory impairing effects of Ro 25-6981 (multi-trial fear conditioning)
During conditioning, baseline freezing did not differ between treatment groups (0 mg/kg=1.1 ±1.1%, 1 mg/kg=0.0 ±0.0, 3 mg/kg=0.0 ±0. 0, 10 mg/kg=0.2 ±0.2). There was no significant effect of Ro 25-6981 on freezing to the final tone (0 mg/kg=54.5 ±7.4%, 1 mg/kg=50.6 ±6.9, 3 mg/kg=50.0 ±4.1, 10 mg/kg=42.5 ±6.6). During testing, baseline freezing did not differ between treatment groups (data not shown), while there was a significant effect of Ro 25-6981 treatment on freezing to tone (F3,63=3.13, p=0.03). Post hoc analysis showed that mice treated with 3 or 10 mg/kg Ro 25-6981 showed significantly less freezing than vehicle-treated controls (Figure 1B). These results demonstrate that pre-conditioning Ro 25-6981 treatment impaired fear recall in a multi-trial paradigm.
Memory impairing effects of Ro 25-6981 (one-trial fear conditioning)
During conditioning, Ro 25-6981 treatment had no effect on baseline freezing (0 mg/kg=0.0 ±0.0%, 1 mg/kg=0.2 ±0.2, 3 mg/kg=0.0 ±0.0, 10 mg/kg=0.40 ±0.40). As expected, freezing to the single (unconditioned at the time of presentation) tone in this paradigm was minimal and not different between doses (0 mg/kg=2.9 ±2.9%, 1 mg/kg=1.5 ±1.5, 3 mg/kg=1.4 ±1.4, 10 mg/kg=4.3 ±3.1). During testing, baseline freezing did not differ between treatment groups (data not shown). Ro 25-6981 had a significant effect on freezing to tone (F3,51=2.83, p=.05). Post hoc analysis showed that 10 mg/kg Ro 25-6981 significantly reduced freezing relative to vehicle (Figure 1D). These data demonstrate that pre-conditioning Ro 25-6981 treatment also impaired fear recall in a one-trial paradigm.
Memory impairing effects of Ro 25-6981 (multi-trial low-shock fear conditioning)
During conditioning, Ro 25-6981 treatment had no affect on baseline freezing (0 mg/kg=0.0 ±0.0%, 1 mg/kg=0.4 ±0.4, 3 mg/kg=0.9 ±0.2, 10 mg/kg=0.4 ±0.2). There was a significant effect of Ro 25-6981 on freezing to the final tone (F3,58=3.94, p<.01), due to higher freezing in mice treated with 3 mg/kg relative to vehicle (p<.05) (vehicle=26.7 ±6.9%, 1 mg/kg=36.3 ±6.6, 3 mg/kg=45.3 ±5.3, 10 mg/kg=17.5 ±5.1). During testing, baseline freezing did not differ between treatment groups (data not shown). There was no significant main effect of Ro 25-6981 on freezing during tone recall when all doses were included in the analysis (F3,58=1.76, p=.16). However, planned post hoc comparisons indicated significantly lesser freezing at the 10 mg/kg dose relative to vehicle (Figure 1F). These results indicate that pre-conditioning Ro 25-6981 treatment impaired fear recall in a low-shock paradigm.
Memory impairing effects of Ro 25-6981 on fear memory in 3-month and 12-month old mice
During conditioning, there was no effect of Ro 25-6981 or age on baseline freezing (3-month olds, 0 mg/kg=0.0 ±0.0%, 1 mg/kg=0.0 ±0.0, 3 mg/kg=0.0 ±0.0, 10 mg/kg=0.0 ±0.0, 12-month olds, 0 mg/kg=0.0 ±0.0%, 1 mg/kg=0.3 ±0.3, 3 mg/kg=0.0 ±0.0, 10 mg/kg=0.3 ±0.3). There was a significant Ro 25-6981 × age interaction for freezing to the final tone (F3,68=3.01, p=.04). Post hoc analysis showed that 10 mg/kg Ro 25-6981 significantly reduced freezing in 3-month, but not 12-month, old mice (p<.01) (3-month olds, 0 mg/kg=54.0 ±8.5%, 1 mg/kg=58.0 ±9.6, 3 mg/kg=57.5 ±7.0, 10 mg/kg=13.3 ±5.8, 12-month olds, 0 mg/kg=42.0 ±7.6, 1 mg/kg=52.0 ±8.5, 3 mg/kg=44.4 ±6.5, 10 mg/kg=42.0 ±8.7).
During testing, there was significant effect of age (F3,68=5.17, p=.03) but not Ro 25-6981 on baseline freezing. Post hoc analysis collapsed across dose showed that 12-month old mice generally froze more than 3-month old mice, although levels of freezing were actually very low in both groups (3-month old= 2.79 ±0.74%, 12-month old= 5.72 ±1.08%). There was a significant interaction between age and Ro 25-6981 treatment for freezing during tone recall (F3,68=3.01, p=.04). Post hoc tests showed that 10 mg/kg Ro 25-6981 reduced freezing relative to vehicle in 3-month, but not 12-month, old mice (Figure 2B). Freezing in vehicle-treated 3-month and 12-month old mice did not differ. These findings show that the memory impairing effects of preconditioning Ro 25-6981 treatment in a multi-trial paradigm were lost in 12-month old mice.
Corticolimbic NR2B protein levels in 3-month and 12-month old mice
There was no significant main effect of region or age and no region × age interaction for NR2B levels (Table 1). Thus, loss of sensitivity to the memory impairing effects of Ro 25-6981 in 12-month old mice was not associated with significant loss of NR2B protein levels in various regions mediating this behavior.
Table 1.
NR2B protein levels in amygdala, hippocampus and medial prefrontal cortex of 3-month old and 12-month old mice. Data are mean ±SEM net intensity of immunoblots. n=6–9/region/age group.
| 3-month old | 12-month old | |
|---|---|---|
| Amygdala | 10737 ±2548 | 18817 ±6519 |
| Hippocampus | 21130 ±3575 | 34634 ±8875 |
| Medial prefrontal cortex | 23942 ±8496 | 33015 ±8737 |
Novel open field
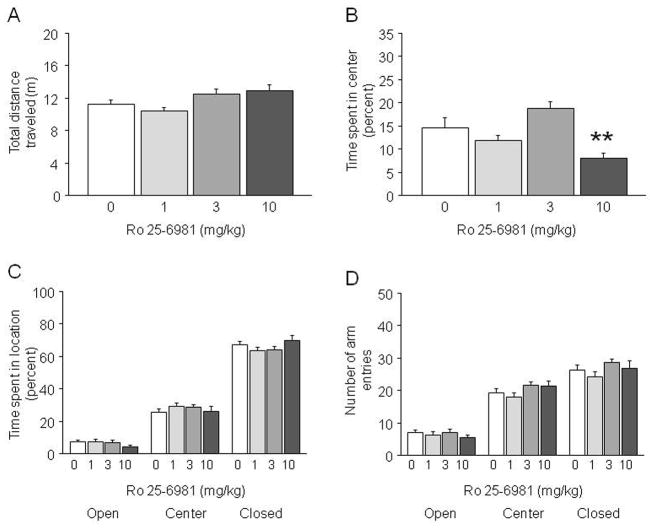
Ro 25-6981 treatment had a significant effect on total distance traveled (F3,36=3.41, p<.03). However, post hoc analysis found no significant effect of any dose of Ro 25-6981 relative to vehicle (Figure 3A). Drug treatment significantly affected percent center time (F3,36=9.51, p<.01). Post hoc tests indicated significantly lesser center time in mice treated with 10 mg/kg Ro 25-6981 as compared to vehicle controls (Figure 3B). These results demonstrate that Ro 25-6981 treatment affected a measure of anxiety-like behavior but not exploratory locomotion in a novel open field.
Figure 3.
Effects of Ro 25-6981 on exploratory locomotion and anxiety-like behavior. In the novel open field, Ro 25-6981 did not alter total distance traveled (A), while the highest dose decreased percent center time (B) (n=10/dose). In the elevated plus-maze, Ro 25-6981 did not alter time spent in the open arms, center square or closed arms (C), or open, closed or total arm entries (D) (n=11/dose). **p<.01 vs. vehicle (0).
Elevated plus-maze
Ro 25-6981 treatment did not significantly affect time spent in the open arm, center square or closed arms (Figure 3C), or the number of open, closed or total arm entries (Figure 3D). Thus, Ro 25-6981 treatment did not alter anxiety-like behavior in this assay.
Acoustic startle and prepulse inhibition of startle
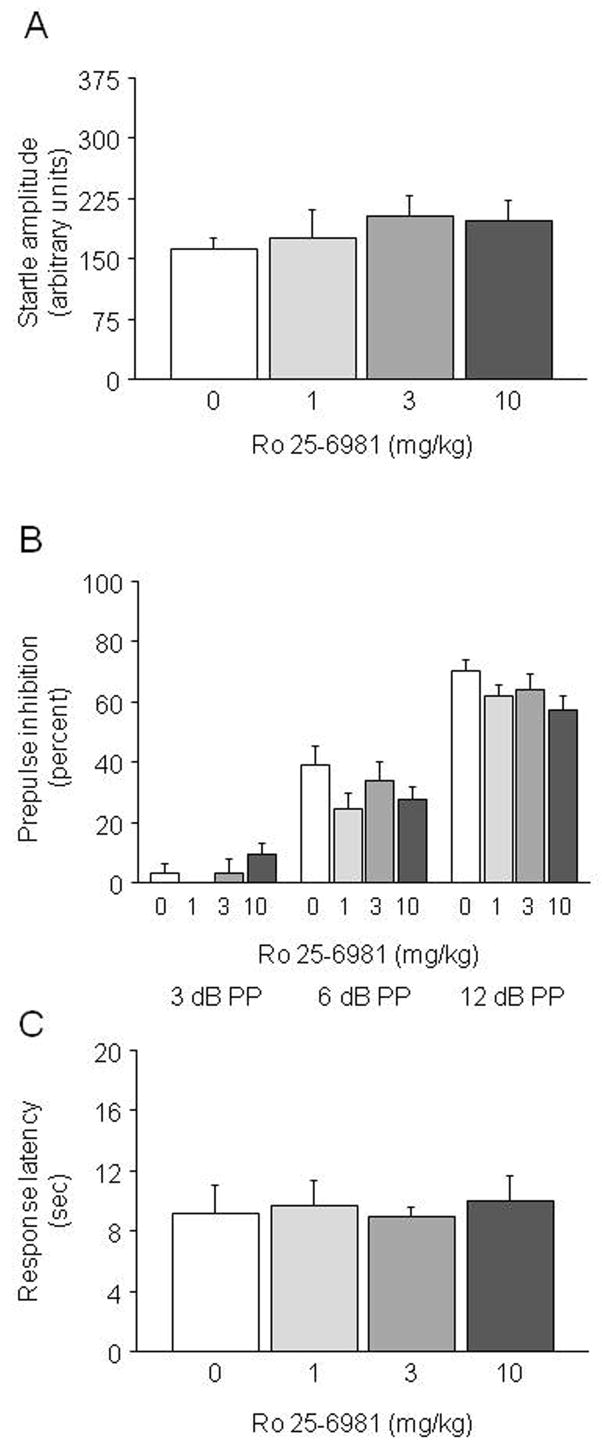
Ro 25-6981 treatment had no effect on acoustic startle amplitude (Figure 4A) or baseline movement (data not shown). There was a significant effect of prepulse intensity (F2,60=265.82, p<.01) but no effect of Ro 25-6981 treatment and no drug × prepulse intensity interaction for percent prepulse inhibition (Figure 4B). These data show that Ro 25-6981 treatment did not affect startle or sensorimotor gating.
Figure 4.
Effects of Ro 25-6981 on sensory functions. Ro 25-6981 did not alter the startle response (A), prepulse inhibition of startle (B) (n=10–12/dose) or hotplate nociception (C) (n=7–10/dose). PP=prepulse intensity level above 65 dB background noise.
Hot plate nociception
Ro 25-6981 treatment had no effect on latency to show a pain response in the hot plate test (Figure 4C), demonstrating that treatment did not affect this measure of nociception.
Discussion
The NR2B subunit has emerged as a potential therapeutic target for a variety of neuropsychiatric and neurological conditions, including Alzheimer’s and Huntingdon’s disease, schizophrenia, and mood and anxiety disorders (Cryan and Dev, 2008; Danysz and Parsons, 2002; Gogas, 2006). The main findings of the present study were that systemic administration of the selective NR2B antagonist, Ro 25-6981, impaired the acquisition of fear memory in mice, and that this effect was modified by ageing.
The potential effects of systemically administered Ro 25-6981 on behaviors associated with NMDAR function, including locomotor exploration, anxiety-like behavior and sensorimotor gating, have not been well characterized in either rats or mice. At the dose range currently tested, Ro 25-6981 treatment had minimal effects on spontaneous locomotor exploration/activity (consonant with data in rats obtained by Kosowski and Liljequist, 2004) or anxiety-like behavior in the elevated plus-maze. Ro 25-6981 treatment also failed to alter responses on the hot plate assay, suggesting that anti-nociceptive actions were unlikely to account for drug effects on fear conditioning. In addition, we observed no effects of Ro 25-6981 treatment on acoustic startle reactivity or prepulse inhibition of the startle response, which is consistent with a previous study in rats which found that another NR2B selective antagonist, Ro 63-1908, failed to alter acoustic startle reactivity or sensorimotor gating at doses that impaired cognition (Higgins et al., 2003). The only significant effect currently observed was a decrease in center exploration in a novel open field at the highest dose tested, which may be indicative of an anxiety-like response to the drug (Cryan and Holmes, 2005) However, as no effect was seen in a second test for anxiety-related behavior, the elevated plus-maze, any effect on anxiety-like behavior does not appear to be robust. Indeed certain other NR2B-selective antagonists, including ifenprodil, have also failed to produce anxiety-related activity in mice (Dere et al., 2003). This provides an interesting contrast with the anxiolytic-like profile of subunit non-selective NMDAR antagonists (reviewed in Cryan and Dev, 2007) and gene knockout of the NR2A subunit (Boyce-Rustay and Holmes, 2006). Nonetheless, further studies will be needed to fully characterize the effects of Ro 25-6981 for potential anxiety-related effects, as well as sensorimotor gating and nociception, for example by using alternate assays or testing intracerebral region-specific injections. Notwithstanding, in the context of the present study, the absence of systemic Ro 25-6981 activity on these behaviors serves to exclude some of the potentially confounding effects on fear learning.
On a standard multi-trial delay cued fear conditioning paradigm, pre-training Ro 25-6981 treatment produced a significant deficit in tone recall measured twenty-four hours later. While a pre-training treatment experimental design does not dissociate drug effects on acquisition versus post-conditioning consolidation, the finding that Ro 25-6981 reduced freezing to the final tone presentation during conditioning is consistent with an impairment of fear acquisition rather than consolidation. Further supporting a selective effect of NR2B antagonism on fear acquisition, pre-training but not pre-recall intra-amygdala injection of the selective antagonist CP101,606 impaired subsequent fear recall in rats (Walker and Davis, 2008), while another study in rats found that systemic Ro 25-6981 injection prior to fear recall disrupted within-session extinction but also failed to affect fear recall (Dalton et al., 2007).
It was notable that Ro 25-6981’s impairing effects on multi-trial conditioning were quite modest. It does not however appear that this is due to a failure to overcome a strong fear response produced by repeated tone × high-shock pairings in a multi-trial paradigm. This is because similarly modest effects were observed in the putatively less fear intensive low-shock multi-trial paradigm and 1-trial cued fear conditioning paradigms; both of which generally produced lower levels of fear than the multi-trial high-shock paradigm. Thus, the magnitude of the fear learning deficit produced by Ro 25-6981 is similar across strong and weak fear learning conditions, and thereby argues against a differential recruitment of NR2B as a function of conditioning strength. The alternative, and currently most parsimonious, interpretation is that selective blockade of NR2B-containing NMDARs does not disrupt fear learning to the same extent as more widespread blockade of NMDARs (at least when NR2B antagonists are delivered systemically). Another possibility is that because NR2B antagonists are more potent at diheteromeric than triheteromeric NR2B-containing NMDARs (Hatton and Paoletti, 2005; Kash and Winder, 2007), a proportion of NR2B-containing NMDARs could be relatively resistant to the memory impairing effects of Ro 25-6981.
The lateral amygdala is the most likely site of the fear impairing memory impairing effects of systemically administered Ro 25-6981. The lateral amygdala is the principle brain region mediating cued fear acquisition (Fanselow and Poulos, 2005; LeDoux, 2000; Maren and Quirk, 2004), and NR2B is expressed on the majority of thalamo-amygdala dendritic spines (Radley et al., 2007). Antagonism of NR2B-containing NMDARs via ifenprodil or genetic disruption of NR2B tyrosine phosphorylation disrupts synaptic plasticity in the basolateral amygdala (Bauer et al., 2002; Li et al., 1995; Nakazawa et al., 2006; Weisskopf and LeDoux, 1999). Furthermore, intra-amygdala injection of the NR2B antagonists ifenprodil or CP101,606 is sufficient to impair fear conditioning in rats while, in contrast, pre-training siRNA knockdown or selective pharmacological blockade of NR2B with Ro 25-6981 in the anterior cingulate impairs the acquisition of context (but not cued) fear memory and intra-hippocampal infusion of Ro 25-6981 impairs trace but not delay cued fear conditioning (Blair et al., 2005; Rodrigues et al., 2001; Sotres-Bayon et al., 2007; Valenzuela-Harrington et al., 2007; Walker and Davis, 2008; Zhao et al., 2005). However, given the modest fear memory impairing effects of Ro 25-6981 in the current study, it is noteworthy that the aforementioned memory impairing effects of pre-training intra-amygdala CP101,606 which, as with Ro 25-6981, is more 2B-selective than ifenprodil (Kash and Winder, 2007), were only evident within a narrow dose range (Walker and Davis, 2008).
Nonetheless, present data provide novel evidence that a NR2B-mediated component of fear learning may be compromised with ageing. The specific finding was that twelve month old C57BL/6Tac mice showed good basal fear, similar to that of three month old C57BL/6Tac counterparts but, unlike the younger mice, were resistant to the fear memory impairing effects of Ro 25-6981. We chose to examine the memory impairing effects of Ro 25-6981 at twelve months because rodents are not yet considered ‘aged’ and likely to exhibit global cognitive deficits (Barnes et al., 1997; Clayton et al., 2002; Magnusson et al., 2007). Rather twelve months perhaps more closely approximate to ‘middle age’ in humans when more subtle age-related changes begin to manifest. It should be made clear that for reasons of availability C57BL/6Tac mice were used for this ageing experiment rather than the C57BL/6J line used in the other experiments. However, vehicle-treated mice from the two lines showed very similar levels of fear (i.e., forty-eight percent freezing for C57BL/6Tac, forty-nine percent freezing for C57BL/6J). In addition, the three month old mice in this experiment were also C57BL/6Tac and showed a clear fear recall deficit at the highest dose of Ro 25-6981, replicating the effect of this dose in C57BL/6J tested across conditioning paradigms. Thus, the loss of the drug’s efficacy in the twelve month old C57BL/6Tac mice appears to be a genuine effect of ageing rather than an idiosyncrasy of this line of C57BL/6 mice. Nonetheless, a final point to bear in mind is that our data do not demonstrate that ageing is only associated with a loss of the fear impairing effects of Ro 25-6981 and cannot not exclude the possibility that other positive (and as yet to determined) effects of Ro 25-6981 are not also diminished in older mice.
Magnusson and colleagues’ work demonstrating that loss of NR2B at the protein expression level does not manifest until fifteen months in the frontal cortex of C57BL/6Nia mice and even later in hippocampus (Magnusson, 2000, 2001; Magnusson et al., 2002; Magnusson et al., 2007; Ontl et al., 2004). Consistent with their data, we found no evidence of reduced NR2B protein levels in the amygdala, dorsal hippocampus or medial prefrontal cortex of the twelve month old C57BL/6Tac mice. This suggests that the loss of sensitivity to Ro 25-6981 reflects functional alterations preceding the loss of protein itself, perhaps involving improper synaptic targeting or coupling to downstream signaling mechanisms (Malenka and Bear, 2004). Another, and not necessarily exclusive, possibility is that the ratio of NR2B to NR2A, at the protein and/or functional level, changes with ageing having the effect of reducing the importance of NR2B relative to NR2A for fear memory formation. These mechanisms await elucidation but could provide insight into how NMDAR-mediated learning processes are dynamically regulated with ageing.
In summary, the present study provides further evidence that selective blockade of NR2B-containing NMDARs is sufficient to impair the acquisition of conditioned fear behavior. This effect was demonstrated using various conditioning procedures, but was overall quite modest. The same dose range of Ro 25-6981 had minimal effects on locomotor exploration, anxiety-like behavior, nociception, or sensorimotor gating. Ro 25-6981’s learning impairing effects were also absent in twelve month old mice. These data further support a role for NR2B-containing NMDARs in fear learning and suggest that this role may diminish with ageing. Further studies along these lines could ultimately have implications for understanding the contribution of NMDAR to the pathophysiology and treatment not only of fear-related neuropsychiatric conditions such as post-traumatic stress disorder, but also other disease states in which NMDARs are implicated including age-related cognitive dysfunction and Alzheimer’s disease.
Acknowledgments
Research supported by the Intramural Research Program of the National Institute of Alcohol Abuse and Alcoholism (Z01-AA000411).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bannerman DM, Niewoehner B, Lyon L, Romberg C, Schmitt WB, Taylor A, Sanderson DJ, Cottam J, Sprengel R, Seeburg PH, Kohr G, Rawlins JN. NMDA Receptor Subunit NR2A Is Required for Rapidly Acquired Spatial Working Memory But Not Incremental Spatial Reference Memory. J Neurosci. 2008;28:3623–3630. doi: 10.1523/JNEUROSCI.3639-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannerman DM, Rawlins JN, Good MA. The drugs don’t work-or do they? Pharmacological and transgenic studies of the contribution of NMDA and GluR-A-containing AMPA receptors to hippocampal-dependent memory. Psychopharmacology (Berl) 2006;188:552–566. doi: 10.1007/s00213-006-0403-6. [DOI] [PubMed] [Google Scholar]
- Barnes CA, Rao G, Shen J. Age-related decrease in the N-methyl-D-aspartateR-mediated excitatory postsynaptic potential in hippocampal region CA1. Neurobiol Aging. 1997;18:445–452. doi: 10.1016/s0197-4580(97)00044-4. [DOI] [PubMed] [Google Scholar]
- Bartlett TE, Bannister NJ, Collett VJ, Dargan SL, Massey PV, Bortolotto ZA, Fitzjohn SM, Bashir ZI, Collingridge GL, Lodge D. Differential roles of NR2A and NR2B-containing NMDA receptors in LTP and LTD in the CA1 region of two-week old rat hippocampus. Neuropharmacology. 2007;52:60–70. doi: 10.1016/j.neuropharm.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Bauer EP, Schafe GE, LeDoux JE. NMDA receptors and L-type voltage-gated calcium channels contribute to long-term potentiation and different components of fear memory formation in the lateral amygdala. J Neurosci. 2002;22:5239–5249. doi: 10.1523/JNEUROSCI.22-12-05239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair HT, Sotres-Bayon F, Moita MA, Ledoux JE. The lateral amygdala processes the value of conditioned and unconditioned aversive stimuli. Neuroscience. 2005;133:561–569. doi: 10.1016/j.neuroscience.2005.02.043. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Boyce-Rustay JM, Holmes A. Genetic inactivation of the NMDA receptor NR2A subunit has anxiolytic- and antidepressant-like effects in mice. Neuropsychopharmacology. 2006;31:2405–2414. doi: 10.1038/sj.npp.1301039. [DOI] [PubMed] [Google Scholar]
- Brigman JL, Feyder M, Saksida LM, Bussey TJ, Mishina M, Holmes A. Impaired discrimination learning in mice lacking the NMDA receptor NR2A subunit. Learn Mem. 2008;15:50–54. doi: 10.1101/lm.777308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Cui Z, Feng R, Tang YP, Qin J, Mei B, Tsien JZ. Maintenance of superior learning and memory function in NR2B transgenic mice during ageing. Eur J Neurosci. 2007;25:1815–1822. doi: 10.1111/j.1460-9568.2007.05431.x. [DOI] [PubMed] [Google Scholar]
- Carmignoto G, Vicini S. Activity-dependent decrease in NMDA receptor responses during development of the visual cortex. Science. 1992;258:1007–1011. doi: 10.1126/science.1279803. [DOI] [PubMed] [Google Scholar]
- Clayton DA, Mesches MH, Alvarez E, Bickford PC, Browning MD. A hippocampal NR2B deficit can mimic age-related changes in long-term potentiation and spatial learning in the Fischer 344 rat. J Neurosci. 2002;22:3628–3637. doi: 10.1523/JNEUROSCI.22-09-03628.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravens CJ, Vargas-Pinto N, Christian KM, Nakazawa K. CA3 NMDA receptors are crucial for rapid and automatic representation of context memory. Eur J Neurosci. 2006;24:1771–1780. doi: 10.1111/j.1460-9568.2006.05044.x. [DOI] [PubMed] [Google Scholar]
- Cryan CF, Dev KK. In: The glutamatergic system as a potential therapeutic target for the treatment of anxiety disorders. Blanchard RJ, Blanchard DC, Nutt DJ, editors. 2007. [Google Scholar]
- Cryan JF, Dev KK. Role of glutamate in anxiety. In: Blanchard DC, Blanchard RM, editors. Handbook of fear and anxiety. London: Elsevier; 2008. pp. 269–301. [Google Scholar]
- Cryan JF, Holmes A. The ascent of mouse: advances in modelling human depression and anxiety. Nat Rev Drug Discov. 2005;4:775–790. doi: 10.1038/nrd1825. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol. 2001;11:327–335. doi: 10.1016/s0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- Dalton GL, Wang YT, Floresco SB, Phillips AG. Disruption of AMPA Receptor Endocytosis Impairs the Extinction, but not Acquisition of Learned Fear. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301642. [DOI] [PubMed] [Google Scholar]
- Danysz W, Parsons CG. Neuroprotective potential of ionotropic glutamate receptor antagonists. Neurotox Res. 2002;4:119–126. doi: 10.1080/10298420290015872. [DOI] [PubMed] [Google Scholar]
- Dere E, Topic B, De Souza Silva MA, Fink H, Buddenberg T, Huston JP. NMDA-receptor antagonism via dextromethorphan and ifenprodil modulates graded anxiety test performance of C57BL/6 mice. Behav Pharmacol. 2003;14:245–249. doi: 10.1097/00008877-200305000-00009. [DOI] [PubMed] [Google Scholar]
- Duffy S, Labrie V, Roder JC. D-Serine Augments NMDA-NR2B Receptor-Dependent Hippocampal Long-Term Depression and Spatial Reversal Learning. Neuropsychopharmacology. 2008;33:1004–1018. doi: 10.1038/sj.npp.1301486. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Kim JJ. Acquisition of contextual Pavlovian fear conditioning is blocked by application of an NMDA receptor antagonist D,L-2-amino-5-phosphonovaleric acid to the basolateral amygdala. Behav Neurosci. 1994;108:210–212. doi: 10.1037//0735-7044.108.1.210. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Poulos AM. The neuroscience of mammalian associative learning. Annu Rev Psychol. 2005;56:207–234. doi: 10.1146/annurev.psych.56.091103.070213. [DOI] [PubMed] [Google Scholar]
- Feyder M, Wiedholz L, Sprengel R, Holmes A. Impaired associative fear learning in mice with complete loss or haploinsufficiency of AMPA GluR1 receptors. Frontiers in Behavioral Neuroscience. 2008;1:4. doi: 10.3389/neuro.08.004.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewirtz JC, Davis M. Second-order fear conditioning prevented by blocking NMDA receptors in amygdala. Nature. 1997;388:471–474. doi: 10.1038/41325. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology (Berl) 2001;156:117–154. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- Gogas KR. Glutamate-based therapeutic approaches: NR2B receptor antagonists. Curr Opin Pharmacol. 2006;6:68–74. doi: 10.1016/j.coph.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Handley SL, Mithani S. Effects of alpha-adrenoceptor agonists and antagonists in a maze-exploration model of ‘fear’-motivated behaviour. Naunyn Schmiedebergs Arch Pharmacol. 1984;327:1–5. doi: 10.1007/BF00504983. [DOI] [PubMed] [Google Scholar]
- Hatton CJ, Paoletti P. Modulation of triheteromeric NMDA receptors by N-terminal domain ligands. Neuron. 2005;46:261–274. doi: 10.1016/j.neuron.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Hefner K, Holmes A. Ontogeny of fear-, anxiety- and depression-related behavior across adolescence in C57BL/6J mice. Behav Brain Res. 2006 doi: 10.1016/j.bbr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hestrin S. Developmental regulation of NMDA receptor-mediated synaptic currents at a central synapse. Nature. 1992;357:686–689. doi: 10.1038/357686a0. [DOI] [PubMed] [Google Scholar]
- Higgins GA, Ballard TM, Huwyler J, Kemp JA, Gill R. Evaluation of the NR2B-selective NMDA receptor antagonist Ro 63-1908 on rodent behaviour: evidence for an involvement of NR2B NMDA receptors in response inhibition. Neuropharmacology. 2003;44:324–341. doi: 10.1016/s0028-3908(02)00402-1. [DOI] [PubMed] [Google Scholar]
- Holmes A, Parmigiani S, Ferrari PF, Palanza P, Rodgers RJ. Behavioral profile of wild mice in the elevated plus-maze test for anxiety. Physiol Behav. 2000;71:509–516. doi: 10.1016/s0031-9384(00)00373-5. [DOI] [PubMed] [Google Scholar]
- Kash T, Winder DG. NMDAR LTP and LTD induction: 2B or Not 2B...is that the question? Debates in Neuroscience. 2007;1:79–84. [Google Scholar]
- Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- Kirkwood A, Rioult MC, Bear MF. Experience-dependent modification of synaptic plasticity in visual cortex. Nature. 1996;381:526–528. doi: 10.1038/381526a0. [DOI] [PubMed] [Google Scholar]
- Kiyama Y, Manabe T, Sakimura K, Kawakami F, Mori H, Mishina M. Increased thresholds for long-term potentiation and contextual learning in mice lacking the NMDA-type glutamate receptor epsilon1 subunit. J Neurosci. 1998;18:6704–6712. doi: 10.1523/JNEUROSCI.18-17-06704.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosowski AR, Liljequist S. The NR2B-selective N-methyl-D-aspartate receptor antagonist Ro 25-6981 [(+/−)-(R*,S*)-alpha-(4-hydroxyphenyl)-beta-methyl-4-(phenylmethyl)-1-pipe ridine propanol] potentiates the effect of nicotine on locomotor activity and dopamine release in the nucleus accumbens. J Pharmacol Exp Ther. 2004;311:560–567. doi: 10.1124/jpet.104.070235. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laube B, Kuhse J, Betz H. Evidence for a tetrameric structure of recombinant NMDA receptors. J Neurosci. 1998;18:2954–2961. doi: 10.1523/JNEUROSCI.18-08-02954.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lee H, Kim JJ. Amygdalar NMDA receptors are critical for new fear learning in previously fear-conditioned rats. J Neurosci. 1998;18:8444–8454. doi: 10.1523/JNEUROSCI.18-20-08444.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XF, Phillips R, LeDoux JE. NMDA and non-NMDA receptors contribute to synaptic transmission between the medial geniculate body and the lateral nucleus of the amygdala. Exp Brain Res. 1995;105:87–100. doi: 10.1007/BF00242185. [DOI] [PubMed] [Google Scholar]
- Liu XB, Murray KD, Jones EG. Switching of NMDA receptor 2A and 2B subunits at thalamic and cortical synapses during early postnatal development. J Neurosci. 2004;24:8885–8895. doi: 10.1523/JNEUROSCI.2476-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftis JM, Janowsky A. The N-methyl-D-aspartate receptor subunit NR2B: localization, functional properties, regulation, and clinical implications. Pharmacol Ther. 2003;97:55–85. doi: 10.1016/s0163-7258(02)00302-9. [DOI] [PubMed] [Google Scholar]
- Lopez de Armentia M, Sah P. Development and subunit composition of synaptic NMDA receptors in the amygdala: NR2B synapses in the adult central amygdala. J Neurosci. 2003;23:6876–6883. doi: 10.1523/JNEUROSCI.23-17-06876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson KR. The aging of the NMDA receptor complex. Front Biosci. 1998;3:e70–80. doi: 10.2741/a368. [DOI] [PubMed] [Google Scholar]
- Magnusson KR. Declines in mRNA expression of different subunits may account for differential effects of aging on agonist and antagonist binding to the NMDA receptor. J Neurosci. 2000;20:1666–1674. doi: 10.1523/JNEUROSCI.20-05-01666.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson KR. Influence of diet restriction on NMDA receptor subunits and learning during aging. Neurobiol Aging. 2001;22:613–627. doi: 10.1016/s0197-4580(00)00258-x. [DOI] [PubMed] [Google Scholar]
- Magnusson KR, Nelson SE, Young AB. Age-related changes in the protein expression of subunits of the NMDA receptor. Brain Res Mol Brain Res. 2002;99:40–45. doi: 10.1016/s0169-328x(01)00344-8. [DOI] [PubMed] [Google Scholar]
- Magnusson KR, Scruggs B, Zhao X, Hammersmark R. Age-related declines in a two-day reference memory task are associated with changes in NMDA receptor subunits in mice. BMC Neurosci. 2007;8:43. doi: 10.1186/1471-2202-8-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Maragos WF, Chu DC, Young AB, D’Amato CJ, Penney JB., Jr Loss of hippocampal [3H]TCP binding in Alzheimer’s disease. Neurosci Lett. 1987;74:371–376. doi: 10.1016/0304-3940(87)90326-0. [DOI] [PubMed] [Google Scholar]
- Maren S, Quirk GJ. Neuronal signalling of fear memory. Nat Rev Neurosci. 2004;5:844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- Millstein RA, Ralph RJ, Yang RJ, Holmes A. Effects of repeated maternal separation on prepulse inhibition of startle across inbred mouse strains. Genes Brain Behav. 2006;5:346–354. doi: 10.1111/j.1601-183X.2005.00172.x. [DOI] [PubMed] [Google Scholar]
- Morris RG, Davis S, Butcher SP. Hippocampal synaptic plasticity and NMDA receptors: a role in information storage? Philos Trans R Soc Lond B Biol Sci. 1990;329:187–204. doi: 10.1098/rstb.1990.0164. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, McHugh TJ, Wilson MA, Tonegawa S. NMDA receptors, place cells and hippocampal spatial memory. Nat Rev Neurosci. 2004;5:361–372. doi: 10.1038/nrn1385. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, Sun LD, Quirk MC, Rondi-Reig L, Wilson MA, Tonegawa S. Hippocampal CA3 NMDA receptors are crucial for memory acquisition of one-time experience. Neuron. 2003;38:305–315. doi: 10.1016/s0896-6273(03)00165-x. [DOI] [PubMed] [Google Scholar]
- Nakazawa T, Komai S, Watabe AM, Kiyama Y, Fukaya M, Arima-Yoshida F, Horai R, Sudo K, Ebine K, Delawary M, Goto J, Umemori H, Tezuka T, Iwakura Y, Watanabe M, Yamamoto T, Manabe T. NR2B tyrosine phosphorylation modulates fear learning as well as amygdaloid synaptic plasticity. Embo J. 2006;25:2867–2877. doi: 10.1038/sj.emboj.7601156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyton J, Paoletti P. Relating NMDA receptor function to receptor subunit composition: limitations of the pharmacological approach. J Neurosci. 2006;26:1331–1333. doi: 10.1523/JNEUROSCI.5242-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ontl T, Xing Y, Bai L, Kennedy E, Nelson S, Wakeman M, Magnusson K. Development and aging of N-methyl-D-aspartate receptor expression in the prefrontal/frontal cortex of mice. Neuroscience. 2004;123:467–479. doi: 10.1016/j.neuroscience.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Perez-Otano I, Ehlers MD. Homeostatic plasticity and NMDA receptor trafficking. Trends Neurosci. 2005;28:229–238. doi: 10.1016/j.tins.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Quinlan EM, Lebel D, Brosh I, Barkai E. A molecular mechanism for stabilization of learning-induced synaptic modifications. Neuron. 2004;41:185–192. doi: 10.1016/s0896-6273(03)00874-2. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Farb CR, He Y, Janssen WG, Rodrigues SM, Johnson LR, Hof PR, LeDoux JE, Morrison JH. Distribution of NMDA and AMPA receptor subunits at thalamo-amygdaloid dendritic spines. Brain Res. 2007;1134:87–94. doi: 10.1016/j.brainres.2006.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues SM, Schafe GE, LeDoux JE. Intra-amygdala blockade of the NR2B subunit of the NMDA receptor disrupts the acquisition but not the expression of fear conditioning. J Neurosci. 2001;21:6889–6896. doi: 10.1523/JNEUROSCI.21-17-06889.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenmund C, Stern-Bach Y, Stevens CF. The tetrameric structure of a glutamate receptor channel. Science. 1998;280:1596–1599. doi: 10.1126/science.280.5369.1596. [DOI] [PubMed] [Google Scholar]
- Rosenzweig ES, Barnes CA. Impact of aging on hippocampal function: plasticity, network dynamics, and cognition. Prog Neurobiol. 2003;69:143–179. doi: 10.1016/s0301-0082(02)00126-0. [DOI] [PubMed] [Google Scholar]
- Sakimura K, Kutsuwada T, Ito I, Manabe T, Takayama C, Kushiya E, Yagi T, Aizawa S, Inoue Y, Sugiyama H, et al. Reduced hippocampal LTP and spatial learning in mice lacking NMDA receptor epsilon 1 subunit. Nature. 1995;373:151–155. doi: 10.1038/373151a0. [DOI] [PubMed] [Google Scholar]
- Schorge S, Colquhoun D. Studies of NMDA receptor function and stoichiometry with truncated and tandem subunits. J Neurosci. 2003;23:1151–1158. doi: 10.1523/JNEUROSCI.23-04-01151.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotres-Bayon F, Bush DE, LeDoux JE. Acquisition of fear extinction requires activation of NR2B-containing NMDA receptors in the lateral amygdala. Neuropsychopharmacology. 2007;32:1929–1940. doi: 10.1038/sj.npp.1301316. [DOI] [PubMed] [Google Scholar]
- Sprengel R, Suchanek B, Amico C, Brusa R, Burnashev N, Rozov A, Hvalby O, Jensen V, Paulsen O, Andersen P, Kim JJ, Thompson RF, Sun W, Webster LC, Grant SG, Eilers J, Konnerth A, Li J, McNamara JO, Seeburg PH. Importance of the intracellular domain of NR2 subunits for NMDA receptor function in vivo. Cell. 1998;92:279–289. doi: 10.1016/s0092-8674(00)80921-6. [DOI] [PubMed] [Google Scholar]
- Sze C, Bi H, Kleinschmidt-DeMasters BK, Filley CM, Martin LJ. N-Methyl-D-aspartate receptor subunit proteins and their phosphorylation status are altered selectively in Alzheimer’s disease. J Neurol Sci. 2001;182:151–159. doi: 10.1016/s0022-510x(00)00467-6. [DOI] [PubMed] [Google Scholar]
- Takehara K, Kawahara S, Munemoto Y, Kuriyama H, Mori H, Mishina M, Kirino Y. The N-methyl-D-aspartate (NMDA)-type glutamate receptor GluRepsilon2 is important for delay and trace eyeblink conditioning in mice. Neurosci Lett. 2004;364:43–47. doi: 10.1016/j.neulet.2004.04.024. [DOI] [PubMed] [Google Scholar]
- Tang YP, Shimizu E, Dube GR, Rampon C, Kerchner GA, Zhuo M, Liu G, Tsien JZ. Genetic enhancement of learning and memory in mice. Nature. 1999;401:63–69. doi: 10.1038/43432. [DOI] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela-Harrington M, Gruart A, Delgado-Garcia JM. Contribution of NMDA receptor NR2B subunit to synaptic plasticity during associative learning in behaving rats. Eur J Neurosci. 2007;25:830–836. doi: 10.1111/j.1460-9568.2007.05325.x. [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M. Amygdala infusions of an NR2B-selective or an NR2A-preferring NMDA receptor antagonist differentially influence fear conditioning and expression in the fear-potentiated startle test. Learn Mem. 2008;15:67–74. doi: 10.1101/lm.798908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Davis M. Involvement of NMDA receptors within the amygdala in short- versus long-term memory for fear conditioning as assessed with fear-potentiated startle. Behav Neurosci. 2000;114:1019–1033. [PubMed] [Google Scholar]
- Walker DL, Paschall GY, Davis M. Glutamate receptor antagonist infusions into the basolateral and medial amygdala reveal differential contributions to olfactory vs. context fear conditioning and expression. Learn Mem. 2005;12:120–129. doi: 10.1101/lm.87105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, TesFaye E, Yasuda RP, Mash DC, Armstrong DM, Wolfe BB. Effects of postmortem delay on subunits of ionotropic glutamate receptors in human brain. Brain Res Mol Brain Res. 2000;80:123–131. doi: 10.1016/s0169-328x(00)00111-x. [DOI] [PubMed] [Google Scholar]
- Weisskopf MG, LeDoux JE. Distinct populations of NMDA receptors at subcortical and cortical inputs to principal cells of the lateral amygdala. J Neurophysiol. 1999;81:930–934. doi: 10.1152/jn.1999.81.2.930. [DOI] [PubMed] [Google Scholar]
- Wiedholz LM, Owens WA, Horton RE, Feyder M, Karlsson RM, Hefner K, Sprengel R, Celikel T, Daws LC, Holmes A. Mice lacking the AMPA GluR1 receptor exhibit striatal hyperdopaminergia and ‘schizophrenia-related’ behaviors. Mol Psychiatry. 2008;13:631–640. doi: 10.1038/sj.mp.4002056. [DOI] [PubMed] [Google Scholar]
- Yang RJ, Mozhui K, Karlsson RM, Cameron HA, Williams RW, Holmes A. Variation in Mouse Basolateral Amygdala Volume is Associated With Differences in Stress Reactivity and Fear Learning. Neuropsychopharmacology. 2008 doi: 10.1038/sj.npp.1301665. [DOI] [PubMed] [Google Scholar]
- Zhao MG, Toyoda H, Lee YS, Wu LJ, Ko SW, Zhang XH, Jia Y, Shum F, Xu H, Li BM, Kaang BK, Zhuo M. Roles of NMDA NR2B subtype receptor in prefrontal long-term potentiation and contextual fear memory. Neuron. 2005;47:859–872. doi: 10.1016/j.neuron.2005.08.014. [DOI] [PubMed] [Google Scholar]