Abstract
Metabolism, the continuous conversion between structural molecules and energy, is life in essence. Size, metabolic rate, and maximum life span appear to be inextricably interconnected in all biological organisms and almost follow a “universal” law. The notion of metabolic rate as the natural “rate of living” filled most of the academic discussion on aging in the early 20th century to be later replaced by the free-radical theory of aging. We argue that the rate of living theory was discarded too quickly and that studying factors affecting resting metabolic rate during the aging process may provide great insight into the core mechanisms explaining differential longevity between individuals, and possibly the process leading to frailty. We predict that measures of resting metabolic rate will be introduced in geriatric clinical practice to gather information on the degree of multisystem dysregulation, exhaustion of energy reserve, and risk of irreversible frailty.
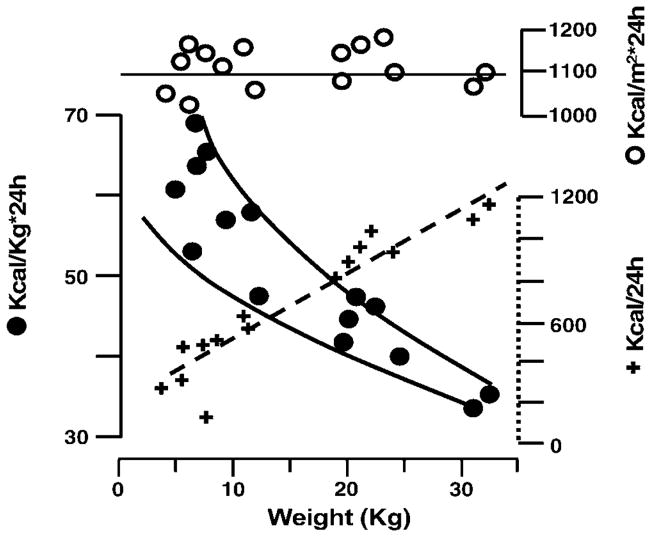
By virtue of metabolism, biological organisms acquire raw materials from the environment to produce and transform energy. The continuous flow of these processes is the essence of life (1). The idea that aging is linked to energy production and expenditure is intuitively attractive. At the beginning of the 20th century, Rubner (2) compared the energy metabolism and body mass of different animals (Figure 1), and Pearl proposed the “rate of living” theory, which formally started the gerontological debate on energetic expenditure and longevity (3).
Figure 1.
Rubner’s diagram (modified)2 based on data on resting metabolic rate (RMR) collected on 15 dogs of different sizes (from 3 kg to 30 kg). + = RMR in kcal/24 h; ●= kcal/kg body weight per 24 h; ○ kcal/m2*24 h.
According to Pearl’s theory, organisms are endowed with an “inherent vitality” that is depleted with a speed proportional to the rate of growth. The hypothesis was based on the observation that animals living at “imaginal temperature” tend to have shorter life span, probably due to higher activity and mass-specific metabolic rate (3).
The overall idea that metabolic rate may be the natural clock of biological organism remained seeded in the mind of many scientists and was further fueled by the publication of the “mouse–elephant curve” (4) suggesting that smaller animals had consistently higher metabolic rate and lower life span than larger animals (Figure 2).
Figure 2.
Data on resting metabolic rate (RMR), body mass, and maximum life span are plotted from 241 (153 terrestrial and 88 avian) animals. Data are from the AnAge Database (http://genomics.senescence.info/species/query.php).
A heated debate was started on the existence of a “universal mathematical law,” which links body size, metabolic rate, life span, and longevity (5,6). The discussion of the “universal equation” is still alive although it has evolved from a simple mathematical formulation to complex fractal models (7). Not surprisingly, the complexity of nature cannot be formally expressed by simple formulas such as the scaling laws (5–10), and the idea that little animals burn up quickly and die young turned out to be a rough oversimplification (11).
A critical stage of the discussion about the relationship between “body size, metabolic rate, longevity” is the formal presentation of the “free-radical theory of aging,” which, for the first time, provided a potential explanatory mechanism of the “rate of living.” Organisms with high metabolic rates may quickly accumulate reactive oxygen species (ROS), which consequently damage different macromolecules, and hasten senescence and death (12,13). Despite the connections and reciprocal support, the “free-radical” theory has grown in stature, whereas the “rate of living” theory has fallen into disrepute (13). We argue that the “rate of living” theory was discarded too quickly and that studying factors affecting resting metabolic rate (RMR) during the aging process may provide great insight into the core mechanisms explaining differential longevity between individuals, and possibly the process leading to frailty.
The enthusiasm for studying the energetic cost of life has been rejuvenated by studies demonstrating the positive effects of caloric restriction on longevity in animal models. Caloric restriction is the only intervention that clearly improves longevity and reduces RMR (14,16). If a high RMR may facilitate ROS accumulation, caloric restriction (by reducing metabolic rate) may prevent ROS production and oxidative-mediated damage, therefore increasing life span.
Measuring Metabolic Rate in Humans
Measures of metabolism in homeothermic animals are usually operationalized as RMR, which reflects the minimum energy requirement in a fasting and resting state under thermoneutral conditions, and as maximum metabolic rate (MMR) achieved when most locomotor muscles are working at their sustainable maximum.
RMR accounts for 60%–70% of daily energy expenditure, whereas physical activity contributes 15%–30% (Figure 3) (17–19). Because of the difficulty in defining “resting state,” the measure of RMR is never exactly the standard minimum metabolism on which life depends (20). In fact, such a minimum rate of energy expenditure depends on many different factors, including nutritional state, health status, body temperature (an increase of 1°C in body temperature is associated with a 13% increase in RMR), menstrual cycle, and degree of wakefulness (21).
Figure 3.
Graphic representation of the hypothetical link between resting metabolic rate (RMR) and health. RMR accounts for 60%–70% of daily energy expenditure which is used to preserve the integrity and functionality of the “body machinery.” RMR is a measure of the speed of ATP + H2O and ADP + Pi cycling transformation, and is equivalent to the minimal speed of this reaction that is required to maintain the body homeostasis at rest. When an intrinsic (i.e., chronic inflammation, cancer) or extrinsic (i.e., infection, traumatic injury) noxa occurs, a certain degree of extra energy is required to maintain the equilibrium (homeostenosis). We propose that this extra energy is shifted from energy available for physical–cognitive activities to the compartment of RMR. This energetic shift increases the susceptibility of elderly persons to minor stress, and is maintained until recovery, which in frail individuals, may never be completed. This condition can be detected as an abnormally high RMR for the age, body composition, and level of physical activity of the individual. The chronic stealing of energy doomed to the physical–cognitive department produces physical and cognitive frailty, and, eventually, disability.
RMR is usually expressed as energy/body mass/time. Direct calorimetry is considered the gold standard measurement for RMR because of its precision, however, indirect calorimetry is a valid and considerably less expensive alternative (22,23). The main limits of all methods are that they provide a weighted average of the RMR for individual cells, tissues, and organs estimated over a different range of time. Statistical adjustments of RMR for body weight, body surface, body cell mass, fat-free mass/fat mass (FFM/FM) ratio, or creatinine levels partially, but not exhaustively, account for between-individual variability (24).
Correlates of RMR
Age and Sex
Data from cross-sectional studies suggest that RMR tends to decline progressively over the life span (25). The first rapid decline occurs from birth up to the end of the third decade, and the second much slower decline from adulthood until death (26). It is interesting that, between the ages of 25 and 30, the human body reaches its maximum level of complexity and maturity including the greatest expansion of alveolar area, the best reproductive boost, and maximum physical performance (27,28). Starting at birth and during the first year of life, RMR steeply declines, and at the age of 30 years, it is only approximately 70% of its value at the first birthday. Across the life span, women tend to have a lower RMR than do men. Probably, body composition and different hormonal patterns, account, at least in part, for such differences.
Neuroendocrine System
Thyroid hormones upregulate RMR, although the mechanism is still controversial (19,29). Testosterone and dehydroepiandrosterone stimulate RMR, in part, directly and, in part, through their effects on body composition (30). A significant depression of RMR has been observed in women with a history of amenorrhea and poor nutritional habits (31). Whether the age-related decline of RMR may be partially attributed to hormonal changes, i.e., reduced growth hormone, testosterone, and insulin resistance, or why hormonal changes that occur at puberty have little influence on the age-associated decline in RMR remain unclear.
Recent data suggest that the age-related decline of RMR may be due to the progressive removal of β-adrenergic receptors, the slowdown of the autonomic nervous system reflexes (i.e., release of norepinephrine, direct vasoconstriction reflex, increased muscle tone, and shivering) (32,33), and inappropriate hormone secretion (i.e., stimulation of thyroid axis, glucocorticoid feedback) (33,34). Supporting this hypothesis, older persons who habitually perform aerobic endurance exercise have an augmented β-adrenergic receptor and higher RMR than do their sedentary peers (35,36).
Body Size
Across species, RMR increases with body size, whereas RMR per unit of body mass is inversely related to body size. For instance, in a 20 g mouse, the mass-specific RMR is about six times higher than that in a human. It has been proposed that the relationship between RMR and body size scales according to the power law RMR = aMbb, where a is a constant related to the characteristics of the organism, Mb is the body size, and b is the universal exponent that fits all living organisms. The search for the true value of the b exponent in this “universal” formula has been the object of a lively debate. According to Rubner’s “surface law,” the value of b is ⅔, whereas Kleiber (37) suggested a value close to ¾. Because RMR is strongly related to the production of heat and oxygen consumption (38), Hemmingsen (39) suggested that the b value depends mainly on the balance between two factors. On one hand, there is a tendency for metabolism to increase in direct proportion to mass as species increase in size. On the other hand, there are surface limitations constraining the acquisition of nutrients/gases and the elimination of wastes/heat.
Body Composition
Lean body mass is usually regarded as the main determinant of RMR (39–41), although interindividual differences in body composition explain only 20% of the variance in RMR (41,42). Accordingly, the fat body mass/lean body mass ratio accounts for more than 60% of the variance in resting oxygen consumption (VO2), whereas age only accounts for about 14% (43). As we mentioned above, age-related changes in FFM percentage only partly explain changes in RMR over the life span (44–48).
Physical Activity
Although measured at rest, RMR is strongly influenced by physical activity because the increased metabolic rate during exercise is partially carried over even when exercise stops. Therefore, persons who are physically active maintain higher RMR and less body fat than do sedentary controls (49,50). However, strength training may slow down the age-related RMR decline independently of its effect on body composition (50). Whether prolonged and intensive exercise may totally counteract the age-related decline in RMR is uncertain (51–54).
Because maximum physical performance depends on the mechanisms that produce and deliver energy, it is possible that age-related decline in metabolic rate contributes to the development of progressive mobility disability often observed in elderly people. However, the energetic aspect of disability in older persons has been substantially neglected, despite the fact that fat mass (not muscle mass) is a powerful predictor of disability and that additional energy is required to activate compensatory strategies or defensive mechanisms such as limited and acquired immunity and anti-apoptotic mechanisms.
Dietary Factors
High energy intake stimulates whereas caloric restriction reduces RMR (53,55). Food thermogenesis corresponds to approximately 10% of daily energy expenditure. During excessive eating, RMR substantially increases and remains high even after digestion, therefore partially counteracting the effect of excessive energy intake. Diet-induced thermogenesis is greater on a high protein and carbohydrate diet than on a fat diet, and it is significantly enhanced by sympathetic nervous system (SNS) activity and β-adrenergic stimulation (56). During aging, total dietary intake, total energy expenditure, and β-adrenergic tone all decline and account for part of the age-related decline in RMR (25).
Health or Disease Status
RMR is strongly affected by disease status, drugs, and emotional stress. Increased RMR in persons with chronic diseases (such as chronic heart failure and cancer) contributes to negative energy balance and cachexia (57,58). The relationship between high RMR and poor health has been explained as increased myocardial oxygen consumption, metabolic cost of breathing, increased sympathetic tone (59), and higher circulating levels of tumor necrosis factor and other proinflammatory cytokines (20). However, from a geriatric perspective, increased RMR that accompanies poor health status can be interpreted as the need of additional energy required to counteract the impending breakdown of the homeostatic equilibrium that is characteristic of chronic morbidity in old age.
This interpretation is consistent with the evolutionistic hypothesis that species have developed metabolic pathways that minimize the amount of energy required to maintain a stable homeostasis (60). We have previously suggested that morbidity in old age is the consequence of impaired complexity and thinning of the homeostatic potential lead to stereotype responses and loss of energetic efficiency (61). The final consequence of this energy switch is an increased energy wasting, which in turn results from an additional reactive increment of RMR (Figure 4). Within this framework, we argue that in older persons RMR may be interpreted as an index of the energetic cost of life, which is strongly related to health status (Figure 3).
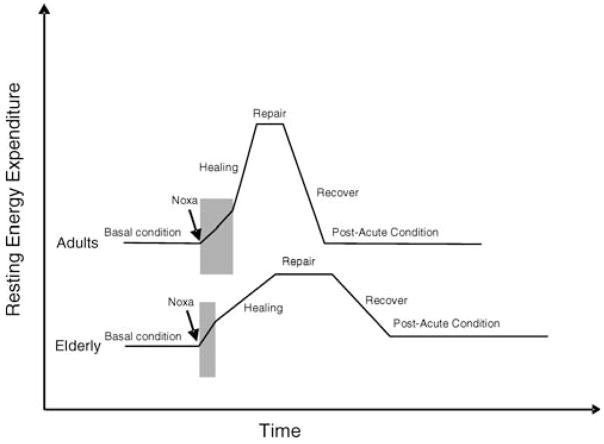
Figure 4.
Stress-response trajectories in adult compared to older persons. Figure shows the hypothetical trajectories of change in resting energy expenditure that occur after a noxa/stress injury in a young and in an old, frail individual. Note that the duration of each phase of the trajectory is shorter in adults than in elderly persons, and that when maximum recovery is accomplished older persons maintain resting energy expenditure higher than before the stress. The model, therefore, lays the foundation for stress accumulation. Older persons consistently take more time to recover from a stress. Moreover, in postacute conditions, they spend more energy for the “housekeeping functions” and have fewer resources available for functional activities and work (i.e., physical activity).
RMR and the Clock of Aging
Most of the evidence that “the higher metabolic rate or velocity of life, the shorter is its duration” has been collected by comparing different animal species. Whether what we know about animals can be applied to humans is unknown. Studies conducted on Caenorhabditis elegans, Musca comune, and Drosophila melanogaster found that, as environmental temperature increases, animals become more active and have a shorter life span, perhaps due to higher metabolic rate (3,6,62). The first series of studies that addressed this hypothesis by performing comparisons between or within species (6,10,63) were later replaced by transgenic and natural mutant animal models (64–66) and more recently by caloric restriction models (67).
In discussing RMR, the current debate on caloric restriction is particularly relevant. Caloric restriction is associated with a longer life span in rodents as well in other short-lived species, although its effect on human and primate longevity is highly uncertain (14,16). It has been suggested that caloric restriction boosts longevity by slowing down metabolism and reducing ROS synthesis, which in turn offsets the damage to redox-sensitive transcription factors, inhibits the activation of proinflammatory pathways, and downregulates the synthesis of hypoxia-inducible factors (68).
Unfortunately, this fascinating hypothesis is only minimally supported by empirical data in humans. For instance, women have lower RMR but similar levels of oxidative stress markers and circulating antioxidants than do men (69). Even the notion that low RMR is a marker of health is in contrast with evidence that exercise, which probably is the most powerful intervention that increases health and functional status, causes a substantial increment of RMR and total energy expenditure.
In childhood and youth, oxygen consumption, heart and breath rate, body temperature, mental and physical activity, as well as energy expenditure, are high because the steady somatic growth and accelerated protein synthesis require constant amounts of high energy (70,71). With aging, the reduction of maximum oxygen consumption (MVO2) (72) and peripheral muscle mass (28) reduces the demand for energy, at least during resting conditions. When a chronic morbidity occurs in adulthood or late life, RMR increases because the amount of energy required to avoid the breakdown of the biological homeostasis increases tremendously. In addition, more energy is required to fight the diseases and counteract the wear and tear of the systems that result from the derangement of the metabolic network and the declined efficiency of the “body machinery.”
In this framework, differences in RMR between adulthood and old age may be interpreted as a progressive reduction of the energy required by an organism that is losing complexity but still maintaining a certain degree of homeostatic equilibrium. On the contrary, differences between individuals may reveal different levels of stability of health status and the degree of utilization of functional reserves. In other words, measuring RMR in older individuals may provide information on their risk of developing functional decline (Figure 4). If this hypothesis can be supported by empirical data, measuring RMR in geriatric patients may provide substantial information on the degree of instability of energy metabolism, current use of energetic reserve, and risk of frailty development. Studies currently in the field are actively testing this hypothesis.
Acknowledgments
This editorial was supported by the Intramural Research Program of the National Institutes of Health, National Institute on Aging.
References
- 1.Gillooly JF, Brown JH, West GB, Savage VM, Charnov EL. Effects of size and temperature on metabolic rate. Science. 2001;293:2248–2251. doi: 10.1126/science.1061967. [DOI] [PubMed] [Google Scholar]
- 2.Rubner M. Über den Einfluss der Körpergrösse auf Stoff und Kraftwechsel. Z Biol. 1883;19:535–562. [Google Scholar]
- 3.Lints FA. The rate of living theory revisited. Gerontology. 1989;35:36–57. doi: 10.1159/000212998. [DOI] [PubMed] [Google Scholar]
- 4.Benedict FG. Vital Energetics: A Study in Comparative Basal Metabolism. Washington, DC: Carnegie Institution of Washington; 1938. [Google Scholar]
- 5.White CR, Seymour RS. Sample size and mass range effects on the allometric exponent of basal metabolic rate. Comp Biochem Physiol A Mol Integr Physiol. 2005;142:74–78. doi: 10.1016/j.cbpa.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 6.Speakman JR. Body size, energy metabolism and lifespan. J Exp Biol. 2005;208:1717–1730. doi: 10.1242/jeb.01556. [DOI] [PubMed] [Google Scholar]
- 7.West GB, Brown JH. The origin of allometric scaling laws in biology from genomes to ecosystems: towards a quantitative unifying theory of biological structure and organization. J Exp Biol. 2005;208:1575–1592. doi: 10.1242/jeb.01589. [DOI] [PubMed] [Google Scholar]
- 8.Suarez RK, Darveau CA. Multi-level regulation and metabolic scaling. J Exp Biol. 2005;208:1627–1634. doi: 10.1242/jeb.01503. [DOI] [PubMed] [Google Scholar]
- 9.West GB, Brown JH, Enquist BJ. The fourth dimension of life: fractal geometry and allometric scaling of organisms. Science. 1999;284:1677–1679. doi: 10.1126/science.284.5420.1677. [DOI] [PubMed] [Google Scholar]
- 10.Lovegrove BG. The zoogeography of mammalian basal metabolic rate. Am Nat. 2000;156:201–219. doi: 10.1086/303383. [DOI] [PubMed] [Google Scholar]
- 11.Miller R, Austad S. Large animals in the fast lane. Science. 1999;285:199. doi: 10.1126/science.285.5425.199b. [DOI] [PubMed] [Google Scholar]
- 12.Sinclair DA. Toward a unified theory of caloric restriction and longevity regulation. Mech Ageing Dev. 2005;126:987–1002. doi: 10.1016/j.mad.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 13.Beckman KB, Ames BN. The free radical theory of aging matures. Physiol Rev. 1998;78:547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- 14.Lane MA, Mattison JA, Roth GS, Brant LJ, Ingram DK. Effects of long-term diet restriction on aging and longevity in primates remain uncertain. J Gerontol A Biol Sci Med Sci. 2004;59A:405–407. doi: 10.1093/gerona/59.5.b405. [DOI] [PubMed] [Google Scholar]
- 15.Speakman JR, Krol E, Johnson MS. The functional significance of individual variation in basal metabolic rate. Physiol Biochem Zool. 2004;77:900–915. doi: 10.1086/427059. [DOI] [PubMed] [Google Scholar]
- 16.Mattson MP, Wan R. Beneficial effects of intermittent fasting and caloric restriction on the cardiovascular and cerebrovascular systems. J Nutr Biochem. 2005;16:129–137. doi: 10.1016/j.jnutbio.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 17.Rolfe DF, Brown GC. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol Rev. 1997;77:731–758. doi: 10.1152/physrev.1997.77.3.731. [DOI] [PubMed] [Google Scholar]
- 18.Hochachka PW, Beatty CL. Patterns of control of maximum metabolic rate in humans. Comp Biochem Physiol A Mol Integr Physiol. 2003;136:215–225. doi: 10.1016/s1095-6433(03)00195-8. [DOI] [PubMed] [Google Scholar]
- 19.Hulbert AJ, Else PL. Basal metabolic rate: history, composition, regulation, and usefulness. Physiol Biochem Zool. 2004;77:869–876. doi: 10.1086/422768. [DOI] [PubMed] [Google Scholar]
- 20.Levine TB, Levine AB. Regional blood flow supply and demand in heart failure. Am Heart J. 1990;120:1547–1551. doi: 10.1016/0002-8703(90)90057-5. [DOI] [PubMed] [Google Scholar]
- 21.Donahoo WT, Levine JA, Melanson EL. Variability in energy expenditure and its components. Curr Opin Clin Nutr Metab Care. 2004;7:599–605. doi: 10.1097/00075197-200411000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Schoeller DA. Recent advances from application of doubly labeled water to measurement of human energy expenditure. J Nutr. 1999;129:1765–1768. doi: 10.1093/jn/129.10.1765. [DOI] [PubMed] [Google Scholar]
- 23.Haugen HA, Melanson EL, Tran ZV, Kearney JT, Hill JO. Variability of measured resting metabolic rate. Am J Clin Nutr. 2003;78:1141–1145. doi: 10.1093/ajcn/78.6.1141. [DOI] [PubMed] [Google Scholar]
- 24.Heymsfield SB. Measurements of energy balance. Acta Diabetol. 2003;40:117–121. doi: 10.1007/s00592-003-0042-x. [DOI] [PubMed] [Google Scholar]
- 25.Wilson MM, Morley JE. Invited review: Aging and energy balance. J Appl Physiol. 2003;95:1728–1736. doi: 10.1152/japplphysiol.00313.2003. [DOI] [PubMed] [Google Scholar]
- 26.Henry CJ. Mechanisms of changes in basal metabolism during ageing. Eur J Clin Nutr. 2000;54:77–91. doi: 10.1038/sj.ejcn.1601029. [DOI] [PubMed] [Google Scholar]
- 27.Merkus PJ. Effects of childhood respiratory diseases on the anatomical and functional development of the respiratory system. Paediatr Respir Rev. 2003;4:28–39. doi: 10.1016/s1526-0542(02)00311-1. [DOI] [PubMed] [Google Scholar]
- 28.Janssens JP, Pache JC, Nicod LP. Physiological changes in respiratory function associated with ageing. Eur Respir J. 1999;13:197–205. doi: 10.1034/j.1399-3003.1999.13a36.x. [DOI] [PubMed] [Google Scholar]
- 29.Meunier N, Beattie JH, Ciarapica D, et al. Basal metabolic rate and thyroid hormones of late-middle-aged and older human subjects: the ZENITH study. Eur J Clin Nutr. 2005;59:53–57. doi: 10.1038/sj.ejcn.1602299. [DOI] [PubMed] [Google Scholar]
- 30.De Pergola G. The adipose tissue metabolism: role of testosterone and dehydroepiandrosterone. Int J Obes Relat Metab Disord. 2000;24:59–63. doi: 10.1038/sj.ijo.0801280. [DOI] [PubMed] [Google Scholar]
- 31.Kaufman BA, Warren MP, Dominguez JE, Wang J, Heymsfield SB, Pierson RN. Bone density and amenorrhea in ballet dancers are related to a decreased resting metabolic rate and lower leptin levels. J Clin Endocrinol Metab. 2002;87:2777–2783. doi: 10.1210/jcem.87.6.8565. [DOI] [PubMed] [Google Scholar]
- 32.Esler M, Hastings J, Lambert G, Kaye D, Jennings G, Seals D. The influence of aging on the human sympathetic nervous system and brain norepinephrine turnover. Am J Physiol. 2002;282:909–916. doi: 10.1152/ajpregu.00335.2001. [DOI] [PubMed] [Google Scholar]
- 33.Bell C, Day DS, Jones PP, Christou DD, Petitt DS, Osterberg K, Melby CL, Seals DR. High energy flux mediates the tonically augmented beta-adrenergic support of resting metabolic rate in habitually exercising older adults. J Clin Endocrinol Metab. 2004;89:3573–3578. doi: 10.1210/jc.2003-032146. [DOI] [PubMed] [Google Scholar]
- 34.Gruenewald DA, Matsumoto AM. Aging of the endocrine system and selected endocrine disorders. In: Hazzard WR, Blass JP, Halter JB, Ouslander JG, Tinetti ME, editors. Principles of Geriatric Medicine and Gerontology. 5. New York: McGraw-Hill; [Google Scholar]
- 35.Maeda T, Sugawara A, Fukushima T, Higuchi S, Ishibashi K. Effects of lifestyle, body composition, and physical fitness on cold tolerance in humans. J Physiol Anthropol Appl Human Sci. 2005;24:439–443. doi: 10.2114/jpa.24.439. [DOI] [PubMed] [Google Scholar]
- 36.Poehlman ET, Danforth E., Jr Endurance training increases metabolic rate and norepinephrine appearance rate in older individuals. Am J Physiol. 1991;261:233–239. doi: 10.1152/ajpendo.1991.261.2.E233. [DOI] [PubMed] [Google Scholar]
- 37.Kleiber M. Body size and metabolism. Hilgardia. 1932;6:315–353. [Google Scholar]
- 38.Hoppeler H, Weibel ER. Scaling functions to body size: theories and facts. J Exp Biol. 2005;208:1573–1574. doi: 10.1242/jeb.01630. [DOI] [PubMed] [Google Scholar]
- 39.Hemmingsen AM. Energy metabolism is related to body size and respiratory surfaces and its evolution. Rep Steno Mem Hosp Nord Insulin Lab. 1960;9:1–110. [Google Scholar]
- 40.Tzankoff SP, Norris AH. Longitudinal changes in basal metabolic rate in man. J Appl Physiol. 1978;33:536–539. doi: 10.1152/jappl.1978.45.4.536. [DOI] [PubMed] [Google Scholar]
- 41.Ravussin E, Swinburn BA. Metabolic predictors of obesity: cross-sectional versus longitudinal data. Int J Obes Relat Metab Disord. 1993;17:28–31. [PubMed] [Google Scholar]
- 42.Muller MJ, Bosy-Westphal A, Kutzner D, Heller M. Metabolically active components of fat-free mass and resting energy expenditure in humans: recent lessons from imaging technologies. Obes Rev. 2002;3:113–122. doi: 10.1046/j.1467-789x.2002.00057.x. [DOI] [PubMed] [Google Scholar]
- 43.Bader N, Bosy-Westphal A, Dilba B, Muller MJ. Intra- and interindividual variability of resting energy expenditure in healthy male subjects -biological and methodological variability of resting energy expenditure. Br J Nutr. 2005;94:843–849. doi: 10.1079/bjn20051551. [DOI] [PubMed] [Google Scholar]
- 44.Byrne NM, Hills AP, Hunter GR, Weinsier RL, Schutz Y. Metabolic equivalent: one size does not fit all. J Appl Physiol. 2005;99:1112–1119. doi: 10.1152/japplphysiol.00023.2004. [DOI] [PubMed] [Google Scholar]
- 45.Blanc S, Schoeller DA, Bauer D, et al. Energy requirements in the eighth decade of life. Am J Clin Nutr. 2004;79:303–310. doi: 10.1093/ajcn/79.2.303. [DOI] [PubMed] [Google Scholar]
- 46.Poehlman ET, Toth MJ, Webb GD. Na-K pump activity contributes to the age-related decline in RMR. J Clin Endocrinol Metab. 1993;76:1054–1059. doi: 10.1210/jcem.76.4.8386182. [DOI] [PubMed] [Google Scholar]
- 47.Krems C, Luhrmann PM, Strassburg A, Hartmann B, Neuhauser-Berthold M. Lower resting metabolic rate in the elderly may not be entirely due to changes in body composition. Eur J Clin Nutr. 2005;59:255–262. doi: 10.1038/sj.ejcn.1602066. [DOI] [PubMed] [Google Scholar]
- 48.Speakman JR, Selman C. Physical activity and resting metabolic rate. Proc Nutr Soc. 2003;62:621–634. doi: 10.1079/PNS2003282. [DOI] [PubMed] [Google Scholar]
- 49.Gilliat-Wimberly M, Manore MM, Woolf K, Swan PD, Carroll SS. Effects of habitual physical activity on the resting metabolic rates and body compositions of women aged 35 to 50 years. J Am Diet Assoc. 2001;101:1181–1188. doi: 10.1016/S0002-8223(01)00289-9. [DOI] [PubMed] [Google Scholar]
- 50.Lemmer JT, Ivey FM, Ryan AS, et al. Effect of strength training on resting metabolic rate and physical activity: age and gender comparisons. Med Sci Sports Exerc. 2001;33:532–541. doi: 10.1097/00005768-200104000-00005. [DOI] [PubMed] [Google Scholar]
- 51.Suominen H, Heikkinen E. Enzyme activities in muscle and connective tissue of M. vastus lateralis in habitually training and sedentary 33- to 70-year-old men. Eur J Appl Physiol Occup Physiol. 1975;34:249–254. doi: 10.1007/BF00999938. [DOI] [PubMed] [Google Scholar]
- 52.Pannemans DL, Westerterp KR. Energy expenditure, physical activity and basal metabolic rate of elderly subjects. Br J Nutr. 1995;73:571–581. doi: 10.1079/bjn19950059. [DOI] [PubMed] [Google Scholar]
- 53.Brochu M, Starling RD, Ades PA, Poehlman ET. Are aerobically fit older individuals more physically active in their free-living time? A doubly labeled water approach. J Clin Endocrinol Metab. 1999;84:3872–3876. doi: 10.1210/jcem.84.11.6096. [DOI] [PubMed] [Google Scholar]
- 54.van Pelt RE, Dinneno FA, Seals DR, Jones PP. Age-related decline in RMR in physically active men: relation to exercise volume and energy intake. Am J Physiol Endocrinol Metab. 2001;281:633–639. doi: 10.1152/ajpendo.2001.281.3.E633. [DOI] [PubMed] [Google Scholar]
- 55.Mole PA. Impact of energy intake and exercise on resting metabolic rate. Sports Med. 1990;10:72–87. doi: 10.2165/00007256-199010020-00002. [DOI] [PubMed] [Google Scholar]
- 56.Jones PP, Van Pelt RE, Johnson DG, Seals DR. Role of sympathetic neural activation in age- and habitual exercise-related differences in the thermic effect of food. J Clin Endocrinol Metab. 2004;89:5138–5144. doi: 10.1210/jc.2004-0101. [DOI] [PubMed] [Google Scholar]
- 57.Westerterp KR, Wilson SA, Rolland V. Diet induced thermogenesis measured over 24 h in a respiration chamber: effect of diet composition. Int J Obes Relat Metab Disord. 1999;23:287–292. doi: 10.1038/sj.ijo.0800810. [DOI] [PubMed] [Google Scholar]
- 58.Poehlman ET, Scheffers J, Gottlieb SS, Fisher ML, Vaitekevicius P. Increased resting metabolic rate in patients with congestive heart failure. Ann Intern Med. 1994;121:860–862. doi: 10.7326/0003-4819-121-11-199412010-00006. [DOI] [PubMed] [Google Scholar]
- 59.Riley M, Elborn JS, McKane WR, Bell N, Stanford CF, Nicholls DP. Resting energy expenditure in chronic cardiac failure. Clin Sci (Lond) 1991;80:633–639. doi: 10.1042/cs0800633. [DOI] [PubMed] [Google Scholar]
- 60.Pittman JG, Cohen P. The pathogenesis of cardiac cachexia. N Engl J Med. 1964;271:453–460. doi: 10.1056/NEJM196408272710908. [DOI] [PubMed] [Google Scholar]
- 61.Hochachka PW, Somero GN. Mechanism and Process in Physiological Evolution. Oxford: Oxford University Press; 2002. Biochemical Adaptation. [Google Scholar]
- 62.Ferrucci L, Windham BG, Fried L. Frailty in older persons. Genus. 2005;61:39–53. [Google Scholar]
- 63.Ragland SS, Sohal RS. Ambient temperature, physical activity and aging in the housefly. Musca domestica. Exp Gerontol. 1975;10:279–289. doi: 10.1016/0531-5565(75)90005-4. [DOI] [PubMed] [Google Scholar]
- 64.Speakman JR, van Acker A, Harper EJ. Age-related changes in the metabolism and body composition of three dog breeds and their relationship to life expectancy. Aging Cell. 2003;2:265–275. doi: 10.1046/j.1474-9728.2003.00061.x. [DOI] [PubMed] [Google Scholar]
- 65.Shmookler Reis RJ, Ebert RH., II Genetics of aging: current animal models. Exp Gerontol. 1996;1:69–81. doi: 10.1016/0531-5565(95)00019-4. [DOI] [PubMed] [Google Scholar]
- 66.Morris BJ. A forkhead in the road to longevity: the molecular basis of lifespan becomes clearer. J Hypertens. 2005;23:1285–1309. doi: 10.1097/01.hjh.0000173509.45363.dd. [DOI] [PubMed] [Google Scholar]
- 67.Kenyon C. The plasticity of aging: insights from long-lived mutants. Cell. 2005;120:449–460. doi: 10.1016/j.cell.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 68.Lane MA, Ingram DK, Roth GS. Nutritional modulation of aging in nonhuman primates. J Nutr Health Aging. 1999;3:69–76. [PubMed] [Google Scholar]
- 69.Kim HJ, Jung KJ, Yu BP, Cho CG, Choi JS, Chung HY. Modulation of redox-sensitive transcription factors by calorie restriction during aging. Mech Ageing Dev. 2002;123:1589–1595. doi: 10.1016/s0047-6374(02)00094-5. [DOI] [PubMed] [Google Scholar]
- 70.Rush JW, Sandiford SD. Plasma glutathione peroxidase in healthy young adults: influence of gender and physical activity. Clin Biochem. 2003;36:345–351. doi: 10.1016/s0009-9120(03)00039-0. [DOI] [PubMed] [Google Scholar]
- 71.Ho SP, Chan-Yeung M, Chow KK, Ip MS, Mak JC. Antioxidant enzyme activities in healthy Chinese adults: influence of age, gender and smoking. Respirology. 2005;10:305–309. doi: 10.1111/j.1440-1843.2005.00702.x. [DOI] [PubMed] [Google Scholar]
- 72.Holliday MA. Metabolic rate and organ size during growth from infancy to maturity and during late gastation and early infancy. Pediatrics. 1971;47:169. [PubMed] [Google Scholar]
- 73.Shock NW, Yiengst MJ. Age changes in basal respiratory measurements and metabolism in males. J Gerontol. 1955;10:31–40. doi: 10.1093/geronj/10.1.31. [DOI] [PubMed] [Google Scholar]