Abstract
Background
Low dietary intake and low serum concentrations of vitamin B6 and/or folate are associated with increased risk of vascular events, possibly because of their association with inflammation, which plays a crucial role in the pathogenesis of cardiovascular diseases.
Methods
Using data from 1320 participants in the population-based InCHIANTI study (586 men and 734 women; median age, 69 years; range, 21–102 years) for whom complete data on folate, vitamin B6, inflammatory markers, 5,10-methylenetetrahydrofolate reductase (MTHFR) C677T sequence variant, and important covariates were available, we evaluated the association of inflammatory markers with circulating concentrations of vitamin B6 and folate, independently of dietary vitamin intake, circulating vitamin concentrations, and MTHFR C677T sequence variant.
Results
According to multiple linear regression analysis, C-reactive protein and interleukin-6 receptor were strongly and negatively associated with circulating vitamin B6 but not with folate concentrations, independent of age, sex, serum creatinine, serum albumin, total energy intake, smoking history, dietary nutrient intake, and circulating homocysteine and vitamin concentrations. Serum folate concentrations were related to MTHFR 677 TT genotype in persons with folate intake in the lowest tertile (<221.2 μg/day). Vitamin C and retinol intakes were strongly and positively associated with serum folate concentrations independent of age, sex, serum creatinine, serum albumin, total energy intake, smoking history, homocysteine plasma concentrations, dietary nutrient intakes, serum vitamin B6 and vitamin B12 concentrations, and MTHFR C677T sequence variant.
Conclusions
Low serum vitamin B6, but not serum folate, concentrations are independent correlates of the proinflammatory state, and both are influenced by antioxidant reserves.
Prospective and cross-sectional studies have consistently demonstrated that low dietary intake and low serum concentrations of folate and/or vitamin B6 (1–7) are independent risk factors for vascular events. However, the mechanism of this association is unknown.
Evidence has accumulated on the crucial role of inflammation in the pathogenesis of atherosclerosis (8, 9). High C-reactive protein (CRP)7 concentrations are widely recognized as a strong independent risk factor for future cardiovascular events (10–12). Longitudinal studies have demonstrated that high interleukin-6 (IL-6) serum concentrations are associated with clinical progression of unstable angina and increased risk of myocardial infarction (13–15) and other cardiovascular events. IL-6 has greater predictive value than CRP as a cardiovascular risk factor (16).
Using data from the Framingham Heart Study, Friso et al. (17) demonstrated a strong relationship between impaired vitamin B6 status and a proinflammatory state, as indicated by CRP concentrations independent of homocysteine (Hcy) concentrations. These findings suggested that vitamin B6 may be implicated in some antiinflammatory mechanism, perhaps acting as a cofactor. In animal models, activated macrophages overexpress high-affinity folate receptors (18), suggesting that during an inflammatory state vitamin B6 may be mobilized from the liver and peripheral tissues to inflammation sites (19) and raising the interesting hypothesis that a prolonged proinflammatory state may lead to vitamin depletion, which perhaps contributes to a sustained chronic inflammatory response. Thus, relatively low concentrations of folate and vitamin B6 in older persons may be caused by the chronic mild proinflammatory state that characteristically affects this age group.
Using data from a large representative sample enrolled in the InCHIANTI study, we tested the hypothesis that the inflammatory state is associated with low vitamin B6 and folate concentrations independent of dietary intake and other potential confounders, including 5,10-methyl-enetetrahydrofolate reductase (MTHFR)8 C677T sequence variant.
Materials and Methods
Data are from the population-based InCHIANTI study, an epidemiologic study to evaluate factors affecting mobility in older persons living in the Chianti geographic area (Tuscany, Italy). The details of the study have been described elsewhere (20). Briefly, in August 1998, 1616 persons 21–102 years of age were selected from the population registry of Greve in Chianti (a rural area; 11 709 inhabitants, with 19.3% of the population 65 years or older) and Bagno a Ripoli (Antella village, near Florence; 4704 inhabitants, with 20.3% of the population 65 years or older). The participation rate was 90% (1453 of 1616).
All participants gave informed consent, and the study was approved by the Ethics Committee of the Italian National Institute of Research and Care of Aging. Blood samples were collected in the morning after the participants had been fasting for at least 8 h and sitting for at least 15 min. The analysis reported here is based on 1320 participants for whom complete data on folate, vitamin B6, inflammatory markers, MTHFR C677T sequence variant, and important covariates were available.
Assays
We used commercial enzymatic tests (Roche Diagnostics, GmbH) to measure serum total cholesterol, HDL-cholesterol, and triglyceride concentrations. Serum LDL-cholesterol concentrations were calculated by the Friedewald formula. The interassay CVs were <3.8% for total cholesterol, <5% for HDL-cholesterol, and <2.5% for triglycerides. Lipid values were expressed according to the risk categories suggested by the National Heart, Lung, and Blood Institute Expert Panel on Detection, Evaluation. and Treatment of High Blood Cholesterol in Adults (21).
Folate and vitamins B6 and B12 were measured in serum samples obtained from blood collected in evacuated tubes without anticoagulant, centrifuged at 2000g for 10 min, and stored at −80 °C.
We measured vitamin B6 (pyridoxal-5-phosphate) with a commercial HPLC assay with fluorescence detection (Immun Diagnostik) and serum folate and vitamin B12 with a radioligand-binding assay (SimulTrac-SNB Radio-assay; ICN Pharmaceuticals). The minimum detectable concentrations were 0.2 μg/L for vitamin B6, 0.6 μg/L for folate, and 75 ng/L for vitamin B12. The intraassay CVs were 2.8% for vitamin B6, 4.1% for folate, and 11% for vitamin B12, and the interassay CVs were 4.1% for vitamin B6, 7.1% for folate, and 12% for vitamin B12.
We measured circulating concentrations of inflammatory markers with commercial assays as described previously (22). Plasma Hcy concentrations were measured by a fluorometric polarized immunoassay method (IMX; Abbott Laboratories).
MTHFR C677T Sequence Variant Analysis
We extracted genomic DNA from peripheral blood samples with a commercial reagent set (FlexiGene DNA reagent set; Qiagen GmbH). Screening for C677T MTHFR sequence variants was carried out on genomic DNA by microelectronic array technology (Nanochip Molecular Biology Workstation; Nanogen) (23). Briefly, genomic DNA was amplified with forward and reverse PCR primers (forward, 5′-biotin-TGA AGG AGA AGG TGT CTG CGG GA-3′; reverse, 5′-AGG ACG GTG CGG TGA GAG TG-3′). In addition, we designed 2 reporter oligonucleotides—one labeled with Cy3, specific for the wild-type nucleotide, and the other, labeled with Cy5, specific for the mutant nucleotide T (wild-type reporter, 5′-Cy3-TGA TGA AAT CGG-3′; mutant reporter 5′-Cy5-ATG ATG AAA TCGA-3′)—and 1 stabilizer oligonucleotide (5′-CTC CCG CAG ACA CCT TCT CCT TCA-3′). PCR products were desalted with the NucleoFast (96-well PCR plates; Macherey-Nagel) and electronically addressed to specific pads on the chip (NanoChip cartridge). After the stabilizer and reporter oligonucleotides were hybridized to the chip, the cartridge was placed in the reader, and the temperature was increased to 36 °C for discrimination. A fluorescence scan was performed, and the software directly assigned a genotype to each sample.
Covariates
Average daily intakes of energy (kcal), alcohol (<30 vs >30 g/day), and vitamins were estimated by administration of the European Prospective Investigation into Cancer and Nutrition (EPIC) food frequency questionnaire. The EPIC food frequency questionnaire provides a detailed assessment of food consumption during the previous year through a large number of structured and precoded questions. Originally, the questionnaire was designed to be self-administered. However, in a pilot study we discovered that this method of administration provides ambiguous results for older adults because they are likely to misunderstand the questions. Thus, in the InCHIANTI study, the EPIC questionnaire was administered by the interviewers. The information provided by the questionnaire was transformed to average daily intake of macro- and micronutrients, including vitamins, by custom software that uses the table of food composition for epidemiologic study in Italy, edited by the European Institute of Oncology in 1998 (24, 25), for reference.
Smoking status was assessed by self-reported data. Pack-years, a measure of smoking exposure that combines intensity and duration, was calculated as (packs smoked per day) × (years of smoking).
Statistical Analysis
We performed all analyses with the SPSS statistical package, Ver. 11.5. P values <0.05 were considered statistically significant.
Log-transformed values for serum vitamin B6, folate, serum inflammatory markers, nutrient intake, and the square root of alcohol intake were used in the analyses and back-transformed for data presentation.
We calculated the MTHFR C677T genotype distribution across serum folate tertiles (≤4.99, 5.0–8.16, and >8.16 nmol/L). Differences among serum folate tertiles were tested by general linear models adjusted for sex, age, serum creatinine, serum albumin, total energy intake, and smoking history.
Adjusted mean folate values according to MTHFR C677T genotype were estimated from a general linear model adjusted for age, sex, serum creatinine, serum albumin, smoking history, and total energy intake. We used multiple linear regression models to test the independent associations of inflammatory markers, nutrient intake, and other vitamins with vitamin B6 and folate. The model predicting folate also included the MTHFR genotypes as potential predictors. Variables found not to be associated with the outcome were removed from the final, most rigorous, regression model through a backward selection algorithm.
We studied the role of Hcy in the relationship between inflammation and vitamin B6 and serum folate concentrations by adding Hcy as a covariate in the final, most rigorous model predicting serum vitamin B6 and folate. The b coefficient estimated in the linear regression analysis indicates the expected mean change in logarithmically transformed vitamin B6 or folate associated with 1-unit changes in the independent variables. Therefore, for independent variables that are logarithmically transformed, a 1% change corresponds to a b% change in the dependent variable.
Results
Characteristics of the Study Group
The characteristics of the InCHIANTI participants are shown in Table 1. The study population in the analysis presented here included 734 females (55.6%) and 586 males (44.4%).
Table 1.
Characteristics of the study population (n = 1320).
| Mean (SD) age, years | 68.9 (15.6) |
| Age distribution, n (%) | |
| <65 years | 281 (21.3) |
| 65–74 years | 567 (43.0) |
| 75–84 years | 333 (25.2) |
| ≥85 years | 139 (10.5) |
| Sex, M/F | 586/734 |
| Mean (SD) serum creatinine, mg/L | 9.1 (1.9) |
| Smoking habits, n (%) | |
| Current smoker | 201 (15.2) |
| Former smoker | 363 (27.5) |
| Never smoked | 756 (57.3) |
| Mediana daily nutrient intake | |
| Energy intake, kcal/day | 1903 (1104–3121) |
| Alcohol, g/day | 7.95 (0.0–54.5) |
| Vitamin B6, mg/day | 1.65 (0.97–2.63) |
| Folate, μg/day | 254.4 (142.7–408.2) |
| Thiamine, mg/day | 0.90 (0.53–1.37) |
| Riboflavin, mg/day | 1.26 (0.76–2.04) |
| Niacin, mg/day | 16.46 (9.26–26.10) |
| Retinol, μg/day | 696.6 (300.0–2007.4) |
| β-Carotene, mg/day | 1.97 (0.83–4.77) |
| Vitamin C, mg/day | 107.1 (44.9–206.7) |
| Vitamin E, mg/day | 6.17 (3.34–10.29) |
| Mediana Hcy, μmol/L | 13.8 (8.7–27.3) |
| Mediana circulating serum vitamin concentrations | |
| Vitamin B6, nmol/L | 25.22 (7.22–65.39) |
| Folate, nmol/L | 6.58 (2.72–15.44) |
| Vitamin B12, pmol/L | 284.9 (121.8–794.3) |
| α-Tocopherol, μmol/L | 29.01 (18.03–43.70) |
| γ-Tocopherol, μmol/L | 1.29 (0.63–2.63) |
| Mediana concentrations of inflammatory markers | |
| CRP, mg/L | 2.38 (0.42–16.70) |
| IL-6, ng/L | 1.27 (0.36–6.43) |
| IL-6r, μg/L | 91.9 (33.8–212.0) |
| IL-1 receptor agonist, ng/L | 130.4 (56.1–309.5) |
| Leukocyte count, 106 cells/μL | 5.99 (4.10–9.03) |
| MTHFR C677T sequence variant, n (%) | |
| CC | 308 (23.33) |
| CT | 711 (53.86) |
| TT | 301 (22.81) |
Values in parentheses are the 5th–95th percentile.
Correlates of Serum Vitamin B6 Concentrations
In the multiple linear regression analysis, CRP and IL-6 receptor (IL-6r) were strongly and negatively associated with circulating vitamin B6 concentrations independent of age, sex, serum creatinine, serum albumin, total energy intake, smoking habits, dietary nutrient intakes, and circulating serum vitamin concentrations (Table 2, model 1). After the inclusion of Hcy in the model (model 2), CRP, and soluble IL-6r (sIL-6r) remained significantly associated with vitamin B6 concentrations as well as age, alcohol and β-carotene intakes, and serum folate, vitamin B12. and albumin concentrations (Table 2, model 2).
Table 2.
Multiple linear regression models relating vitamin intake and markers of inflammation to circulating vitamin B6 concentrations.a
| Log (vitamin B6 in nmol/L) |
||||
|---|---|---|---|---|
| Model 1b |
Model 2c |
|||
| Independent variables | b (SE) | P | b (SE) | P |
| Age (years × 10) | −0.042 (0.006) | <0.0001 | −0.036 (0.006) | <0.0001 |
| Square root of alcohol intake | 0.012 (0.004) | 0.001 | 0.013 (0.004) | 0.001 |
| Log(β-carotene intake in μg/day) | 0.194 (0.048) | <0.0001 | 0.191 (0.048) | <0.0001 |
| Log(vitamin B6 intake in mg/day) | 0.256 (0.126) | 0.042 | 0.220 (0.126) | 0.079 |
| Log(α-tocopherol in μmol/L) | 0.296 (0.071) | <0.0001 | 0.258 (0.071) | <0.0001 |
| Log(folate in nmol/L) | 0.132 (0.036) | <0.0001 | 0.108 (0.037) | 0.004 |
| Log(vitamin B12 in pmol/L) | 0.109 (0.035) | 0.002 | 0.083 (0.036) | 0.022 |
| Albumin (mg/dL) | 0.180 (0.029) | <0.0001 | 0.179 (0.029) | <0.0001 |
| Log(CRP in mg/L) | −0.116 (0.020) | <0.0001 | −0.123 (0.018) | <0.0001 |
| Log(IL-6r in ng/L) | −0.119 (0.036) | 0.001 | −0.117 (0.036) | 0.001 |
In the multiple linear regression analysis, circulating vitamin B6 (serum concentrations) was used as the dependent variable and age, sex, serum creatinine, serum albumin, smoking habits, total energy intake, inflammatory markers, nutrient intakes, plasma Hcy concentrations, and serum vitamin concentrations were used as covariates. In the final regression model, all variables not significantly and independently associated with serum vitamin B6 concentrations were removed through backward selection method.
Model 1 included age; sex; serum creatinine and albumin; total energy intake; smoking habits; folate, vitamin B6, retinol, β-carotene, vitamin C, vitamin E, and alcohol intakes; circulating concentrations of vitamin B12, folate, α-tocopherol, γ-tocopherol, CRP, IL-6, IL-1 receptor agonist, and IL-6r; and leukocyte count as covariates.
Model 2 included the same covariates as model 1 plus plasma Hcy concentrations.
Correlates of Serum Folate Concentrations
When we adjusted the model for age, sex, serum creatinine, serum albumin, total energy intake, and smoking habits, the prevalence of the MTHFR TT genotype was significantly (P < 0.001) higher in the lowest than in the highest tertile of circulating serum folate (Table 3). Serum folate concentrations were lower in participants carrying the MTHFR 677 TT genotype [median (5th–95th percentiles), 5.71 (5.35–6.10) nmol/L] than in participants with the CT [6.59 (6.32–6.87) nmol/L] or CC [6.95 (6.52–7.41) nmol/L] genotype. The percentage of participants with low serum folate concentrations (<5 nmol/L) was significantly higher among those carrying the TT genotype (42.9%) than among those carrying the CC or CT genotype (28.9% and 31.7%, respectively; P < 0.01).
Table 3.
MTHFR C677T genotype distribution according to tertiles of circulating folate.a
|
MTHFR C677T genotype, % (95% confidence interval) |
|||
|---|---|---|---|
| Folate, nmol/L | CC | CT | TTb |
| ≤4.99 (n = 430) | 19.7 (19.4–19.9) | 51.1 (50.9–51.4) | 29.2 (29.0–29.4) |
| 5.0–8.16 (n = 439) | 23.9 (23.7–24.1) | 53.0 (52.8–53.2) | 23.1 (22.9–23.3) |
| 38.16 (n = 451) | 25.5 (25.2–25.8) | 58.0 (57.7–58.3) | 16.5 (16.4–16.6) |
Percentages, 95% confidence intervals, and P values are adjusted for age, sex, serum creatinine, serum albumin, smoking habits, and total energy intake.
Prevalence of the MTHFR TT genotype significantly (P < 0.001) higher in the lowest than in the highest tertile of circulating serum folate.
Serum folate concentrations were related to MTHFR C677T sequence variant in participants with folate intake in the lowest tertile (<221.2 μg/day), but not in participants with folate intake in the highest tertiles (221.2–292.2 and >292.2 μg/day). In particular, in participants in the lowest tertile of folate intake and carrying the TT genotype, serum folate concentrations were significantly lower than those found in participants with the CT or CC genotype (Fig. 1).
Fig. 1. Serum folate concentration according to MTHFR C677T genotype and tertiles of folate intake.
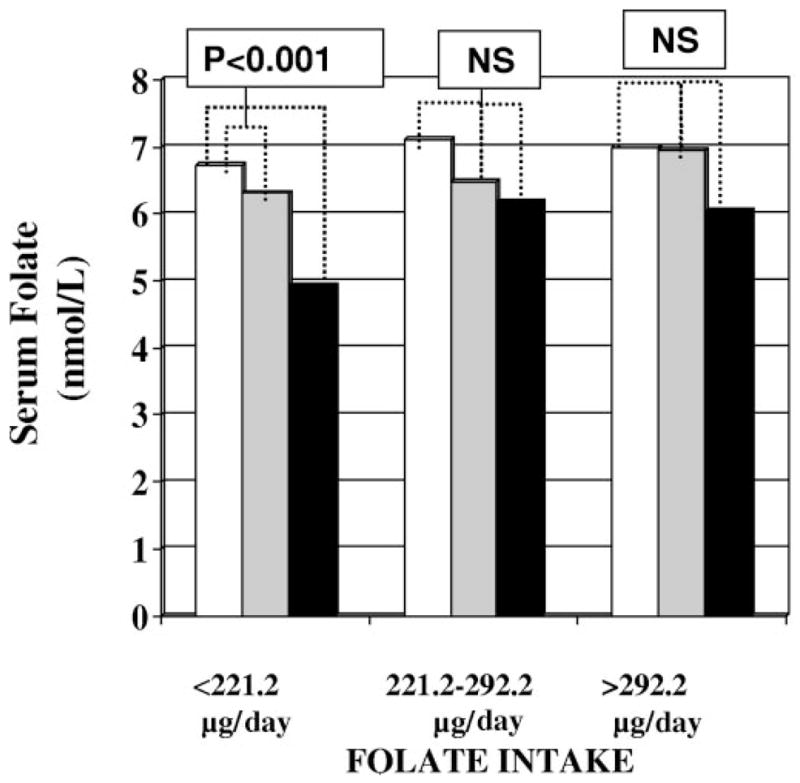
Adjusted mean folate values according to MTHFR C677T genotype were estimated from linear models adjusted for age, sex, serum creatinine, serum albumin, smoking habits, and total energy intake. MTHFR genotypes: □, CC;  , CT; ■, TT. NS, not significant.
, CT; ■, TT. NS, not significant.
In the multiple linear regression analysis, after adjustment for several confounders (age, sex, serum creatinine, serum albumin, total energy intake, smoking habits, dietary nutrient intakes, and circulating vitamin concentrations), vitamin C and retinol intakes and circulating serum concentrations of α-tocopherol were significantly and positively associated with circulating serum folate concentrations (Table 4, model 1). Smoking history as well as circulating γ-tocopherol concentrations and homozygosity for the MTHFR C677T sequence variant (TT genotype vs CT + CC genotypes) were significantly and inversely associated with circulating folate concentrations (Table 4, model 1). Serum concentrations of CRP, IL-6, IL-6r, IL-1 receptor antagonist, and albumin and the leukocyte count were not significantly associated with serum folate concentrations [b (SE) log(CRP), 0.028 (0.035); log(IL-6), −0.011 (0.022); log(IL-6r), −0.019 (0.030); log(IL-1 receptor agonist), 0.019 (0.030); albumin, −0.034 (0.024); log(leukocyte count), −0.087 (0.069)]. After the inclusion of plasma Hcy concentrations in the model (model 2), vitamin C and retinol intakes, circulating serum concentrations of vitamin B6 and B12, sex, and smoking habits remained significant predictors of serum folate concentrations (Table 4, model 2).
Table 4.
Multiple linear regression models relating vitamin intake and inflammation to circulating folate concentrations.a
| Log(folate in nmol/L) |
||||
|---|---|---|---|---|
| Model 1b |
Model 2c |
|||
| Independent variables | b (SE) | P | b (SE) | P |
| Sex (women vs men) | 0.038 (0.014) | 0.005 | 0.014 (0.0054) | 0.007 |
| Smoking habits (current smoker vs ex-smoker and never smoked) | −0.060 (0.018) | 0.001 | −0.049 (0.018) | 0.007 |
| Log(retinol in μg/day) | 0.031 (0.018) | 0.001 | 0.077 (0.034) | 0.021 |
| Log(vitamin C intake in mg/day) | 0.244 (0.048) | <0.0001 | 0.255 (0.053) | <0.0001 |
| Log(α-tocopherol in μmol/L) | 0.169 (0.062) | 0.006 | 0.088 (0.060) | 0.144 |
| Log(γ-tocopherol in μmol/L) | −0.079 (0.038) | 0.035 | −0.038 (0.033) | 0.246 |
| Log(vitamin B6 in nmol/L) | 0.074 (0.021) | <0.0001 | 0.053 (0.021) | <0.013 |
| Log(vitamin B12 in pmol/L) | 0.195 (0.027) | <0.0001 | 0.130 (0.028) | <0.0001 |
| MTHFR (TT vs CT+TT) | −0.041 (0.016) | 0.009 | −0.024 (0.015) | 0.116 |
| Log(Hcy in μmol/L) | −0.519 (0.052) | <0.0001 | ||
In the multiple linear regression analysis, circulating folate (serum concentrations) was used as the dependent variable and age, sex, serum creatinine, serum albumin, smoking habits, total energy intake, inflammatory markers, nutrient intakes, Hcy, and circulating vitamin concentrations were used as covariates. In the final regression model, all variables not significantly and independently associated with circulating folate were removed through backward selection method.
Model 1 included age; sex; serum creatinine; total energy intake; smoking habits; folate, vitamin B6, thiamine, retinol, β-carotene, vitamin C, and vitamin E intakes; circulating concentrations of vitamin B6, vitamin B12, and α- and γ-tocopherol; and MTHFR sequence variant (TT genotype vs CT plus CC genotypes) as the covariates.
Model 2 included the same covariates as model 1 plus plasma Hcy concentrations.
Discussion
Our results demonstrate that in the elderly, inflammatory markers such as CRP, IL-6r, and serum antioxidant vitamin concentrations are correlates of serum vitamin B6 concentrations independent of dietary intake, smoking history, serum albumin, and plasma Hcy concentrations and that antioxidant vitamin intakes, but not inflammatory markers, are significant correlates of serum folate concentrations independent of relevant covariates, including folate intake and smoking history. Furthermore, this large study extends, in elderly adults, the observation that the TT genotype of the MTHFR C677T sequence variant is significantly and independently associated with low concentrations of serum folate, particularly in participants with low folate intake.
Our findings support the notion already suggested by the Framingham study of a relationship between vitamin B6 and inflammation, and provide an original contribution to this literature by showing that such a relationship is not limited to CRP but also involves the sIL-6r. In addition, our data are in accordance with recent evidence of a link between inflammation and vitamin B6 not only in patients affected by inflammatory diseases such as rheumatoid arthritis and inflammatory bowel disease (19, 26), but also in stroke patients and in controls (27). Moreover, our findings are at variance with those observed in a subset of the ARIC study, in which leukocyte count but not CRP was a correlate of serum vitamin B6 (28). Changes in sIL-6r concentrations have been determined in numerous clinical disorders (29), but the biological significance of sIL-6r within these pathologies remains unclear.
The mechanism by which inflammation is associated with low vitamin B6 concentrations has not been completely elucidated. It has been demonstrated that during a chronic inflammatory state, circulating and hepatic concentrations of vitamin B6 tend to decrease (19). Because vitamin B6 is involved in the repair and synthesis of nucleic acid and proteins, low vitamin B6 concentrations may reflect the increased consumption of this coenzyme in the accelerated synthesis of cytokines and antibodies and in accelerated cell proliferation.
Interestingly, we found that low vitamin B6 concentrations were independently associated with low β-carotene intake and α-tocopherol concentrations, suggesting that an imbalance in the oxidative stress and antioxidant mechanisms may contribute to the decreases in vitamin B6 concentrations in inflammatory states.
We previously demonstrated that inflammation is strongly related to Hcy (22), and it is well known that vitamin B6 affects the serum concentration of Hcy. Thus, it was reasonable to hypothesize that the relationship between vitamin B6 and inflammatory state could be mediated by Hcy. However, the inclusion of Hcy in the linear regression model did not substantially attenuate the relationship between inflammatory markers and vitamin B6.
In our study, vitamin C intake and α-tocopherol concentrations were strongly associated with serum folate concentrations, independent of age, sex, smoking history, and folate intake, suggesting that noncompensated oxidative stress may contribute to the decrease in folate as well as serum vitamin B6 concentrations. The antioxidant molecule vitamin C is known to influence folate synthesis by enhancing formation of the polyglutamate derivatives from tetrahydrofolic acid and enhancing the transformation of folic acid into tetrahydrofolic acid (30). Interestingly, in Italian blood donors who were smokers, supplementation with folate plus vitamin C led to greater increases in serum and erythrocyte folate than did supplementation with folate alone (31).
By showing that MTHFR C677T sequence variant is an independent predictor of serum folate in older persons, especially those with low folate intake, our study confirms similar observations obtained in middle-aged European and North American populations (32–34).
In addition to the well-known influence on Hcy concentrations, folate may influence genomic DNA methylation reactions, particularly in participants with low serum folate concentrations and persons homozygous for the MTHFR C677T TT sequence variant (33).
The finding of the present investigation, demonstrating that low vitamin B6 is an independent correlate of proinflammatory state, may appear not to be consistent with the results of recent clinical trials showing no effect of folic acid, vitamin B6, and vitamin B12 supplementation in reducing cardiovascular events (35, 36). Nevertheless, all of the interventional trials confirmed the role of Hcy as marker of an increased risk of vascular events.
A limitation of this study is represented by the nature of our study (cross-sectional study), which did not allow us to determine the causative role of inflammation in inducing low circulating vitamin B6 concentrations.
In conclusion, we demonstrated that CRP and sIL-6r, 2 important biomarkers of inflammation, are independent correlates of vitamin B6 concentrations. Antioxidant vitamins, but not inflammatory markers, were independent correlates of serum folate. The authors of future studies looking at vitamins as modifiable risk factors for health outcomes must consider inflammation related to vitamin B6 and oxidative stress related to vitamin B6 and folate as potential confounders in the analyses.
Acknowledgments
The InCHIANTI study was supported as a “targeted project” (ICS 110.1/RS97.71) by the Italian Ministry of Health and in part by the US National Institute on Aging (Contracts N01-AG-916413 and N01-AG-821336), and by the Intramural Research Program of the US National Institute on Aging (Contracts 263 MD 9164 13 and 263 MD 821336). None of the sponsoring institutions interfered with the collection, analysis, presentation, or interpretation of the data reported here.
Footnotes
Nonstandard abbreviations: CRP, C-reactive protein; IL, interleukin; Hcy, homocysteine; EPIC, European Prospective Investigation into Cancer and Nutrition; and (s)IL-6r, (soluble) interleukin-6 receptor.
Human gene: MTHFR, 5,10-methylenetetrahydrofolate reductase (NADPH).
References
- 1.Voutilainen S, Lakka TA, Porkkala-Sarataho E, Rissanen T, Kaplan GA, Salonen JT. Low serum folate concentrations are associated with an excess incidence of acute coronary events: the Kuopio Ischaemic Heart Disease Risk Factor Study. Eur J Clin Nutr. 2000;54:424–8. doi: 10.1038/sj.ejcn.1600991. [DOI] [PubMed] [Google Scholar]
- 2.Booth GL, Wang EEL. Preventive health care, 2000 update: screening and management of hyperhomocysteinemia for the prevention of coronary artery disease events. The Canadian Task Force on Preventive Health Care. CMAJ. 2000;163:21–9. [PMC free article] [PubMed] [Google Scholar]
- 3.Voutilainen S, Rissanen TH, Virtanen J, Lakka TA, Salonen JT. Kuopio Ischemic Heart Disease Risk Factor Study: low dietary folate intake is associated with an excess incidence of acute coronary events: The Kuopio Ischemic Heart Disease Risk Factor Study. Circulation. 2001;103:2674–80. doi: 10.1161/01.cir.103.22.2674. [DOI] [PubMed] [Google Scholar]
- 4.Hu FB, Willett WC. Diet and coronary heart disease: findings from the Nurses’ Health Study and Health Professionals’ Follow-up Study. J Nutr Health Aging. 2001;5:132–8. [PubMed] [Google Scholar]
- 5.Bazzano LA, He J, Ogden LG, Loria C, Vupputuri S, Myers L, et al. Dietary intake of folate and risk of stroke in US men and women: NHANES I Epidemiologic Follow-up Study. National Health and Nutrition Examination Survey. Stroke. 2002;33:1183–8. doi: 10.1161/01.str.0000014607.90464.88. [DOI] [PubMed] [Google Scholar]
- 6.Voutilainen S, Virtanen JK, Rissanen TH, Alfthan G, Laukkanen J, Nyyssonen K, et al. Serum folate and homocysteine and the incidence of acute coronary events: the Kuopio Ischaemic Heart Disease Risk Factor Study. Am J Clin Nutr. 2004;80:317–23. doi: 10.1093/ajcn/80.2.317. [DOI] [PubMed] [Google Scholar]
- 7.Friso S, Girelli D, Martinelli N, Olivieri O, Lotto V, Bozzini C, et al. Low plasma vitamin B-6 concentrations and modulation of coronary artery disease risk. Am J Clin Nutr. 2004;79:992–8. doi: 10.1093/ajcn/79.6.992. [DOI] [PubMed] [Google Scholar]
- 8.Blake GJ, Ridker PM. Inflammatory bio-markers and cardiovascular risk prediction. J Intern Med. 2002;252:283–94. doi: 10.1046/j.1365-2796.2002.01019.x. [DOI] [PubMed] [Google Scholar]
- 9.Libby P, Ridker PM. Inflammation and atherosclerosis: role of C-reactive protein in risk assessment. Am J Med. 2004;111:9S–16S. doi: 10.1016/j.amjmed.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Ridker PM. High-sensitivity C-reactive protein and cardiovascular risk: rationale for screening and primary prevention. Am J Cardiol. 2003;92:17K–22K. doi: 10.1016/s0002-9149(03)00774-4. [DOI] [PubMed] [Google Scholar]
- 11.Blake GJ, Ridker PM. C-reactive protein and other inflammatory risk markers in acute coronary syndromes. J Am Coll Cardiol. 2003;41:37S–42S. doi: 10.1016/s0735-1097(02)02953-4. [DOI] [PubMed] [Google Scholar]
- 12.Willerson JT, Ridker PM. Inflammation as a cardiovascular risk factor. Circulation. 2004;109:2–10. doi: 10.1161/01.CIR.0000129535.04194.38. [DOI] [PubMed] [Google Scholar]
- 13.Biasucci LM, Liuzzo G, Fantuzzi G, Caligiuri G, Rebuzzi AG, Ginnetti F, et al. Increasing concentrations of interleukin (IL)-1Ra and IL-6 during the first 2 days of hospitalization in unstable angina are associated with increased risk of in-hospital coronary events. Circulation. 1999;99:2079–84. doi: 10.1161/01.cir.99.16.2079. [DOI] [PubMed] [Google Scholar]
- 14.Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101:1767–72. doi: 10.1161/01.cir.101.15.1767. [DOI] [PubMed] [Google Scholar]
- 15.Lindmark E, Diderholm E, Wallentin L, Siegbahn A. Relationship between interleukin 6 and mortality in patients with unstable coronary artery disease: effects of an early invasive or non-invasive strategy. JAMA. 2001;286:2107–13. doi: 10.1001/jama.286.17.2107. [DOI] [PubMed] [Google Scholar]
- 16.Cesari M, Penninx BW, Newman AB, Kritchevsky SB, Nicklas BJ, Sutton-Tyrrell K, et al. Inflammatory markers and onset of cardiovascular events: results from the Health ABC study. Circulation. 2003;108:2317–22. doi: 10.1161/01.CIR.0000097109.90783.FC. [DOI] [PubMed] [Google Scholar]
- 17.Friso S, Jacques PF, Wilson PW, Rosenberg IH, Selhub J. Low circulating vitamin B(6) is associated with elevation of the inflammation marker C-reactive protein independently of plasma homocysteine concentrations. Circulation. 2001;103:2788–91. doi: 10.1161/01.cir.103.23.2788. [DOI] [PubMed] [Google Scholar]
- 18.Turk MJ, Breur GJ, Widmer WR, Paulos CM, Xu LC, Grote LA, et al. Folate-targeted imaging of activated macrophages in rats with adjuvant-induced arthritis. Arthritis Rheum. 2002;46:1947–55. doi: 10.1002/art.10405. [DOI] [PubMed] [Google Scholar]
- 19.Chiang EP, Bagley PJ, Selhub J, Nadeau M, Roubenoff R. Abnormal vitamin B(6) status is associated with severity of symptoms in patients with rheumatoid arthritis. Am J Med. 2003;114:283–7. doi: 10.1016/s0002-9343(02)01528-0. [DOI] [PubMed] [Google Scholar]
- 20.Ferrucci L, Bandinelli S, Benvenuti E, Di Iorio A, Macchi C, Harris TB, et al. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48:1618–25. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- 21.Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adult (Adult Treatment Panel III): final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 22.Gori AM, Corsi AM, Fedi S, Gazzini A, Sofi F, Bartali B, et al. A proinflammatory state is associated with hyperhomocysteinemia in the elderly population. Am J Clin Nutr. 2005;82:335–41. doi: 10.1093/ajcn.82.2.335. [DOI] [PubMed] [Google Scholar]
- 23.Frusconi S, Giusti B, Rossi L, Bernabini S, Poggi F, Giotti I, et al. Improvement of low-density microelectronic array technology to characterize 14 mutations/single-nucleotide polymorphisms from several human genes on a large scale. Clin Chem. 2004;50:775–7. doi: 10.1373/clinchem.2003.025197. [DOI] [PubMed] [Google Scholar]
- 24.Pisani P, Faggiano F, Krogh V, Palli D, Vineis P, Berrino F. Relative validity and reproducibility of a food frequency dietary questionnaire for use in the Italian EPIC centres. Int J Epidemiol. 1997;26:S152–60. doi: 10.1093/ije/26.suppl_1.s152. [DOI] [PubMed] [Google Scholar]
- 25.Salvini S. A food composition database for epidemiological studies in Italy. Cancer Lett. 1997;114:299–300. doi: 10.1016/s0304-3835(97)04686-7. [DOI] [PubMed] [Google Scholar]
- 26.Saibeni S, Cattaneo M, Vecchi M, Zighetti ML, Lecchi A, Lombardi R, et al. Low vitamin B(6) plasma levels, a risk factor for thrombosis, in inflammatory bowel disease: role of inflammation and correlation with acute phase reactants. Am J Gastroenterol. 2003;98:112–7. doi: 10.1111/j.1572-0241.2003.07160.x. [DOI] [PubMed] [Google Scholar]
- 27.Kelly PJ, Kistler JP, Shih VE, Mandell R, Atassi N, Barron M, et al. Inflammation, homocysteine, and vitamin B6 status after ischemic stroke. Stroke. 2004;35:12–5. doi: 10.1161/01.STR.0000106481.59944.2F. [DOI] [PubMed] [Google Scholar]
- 28.Folsom AR, Desvarieux M, Nieto FJ, Boland LL, Ballantyne CM, Chambless LE. B vitamin status and inflammatory markers. Atherosclerosis. 2003;169:169–74. doi: 10.1016/s0021-9150(03)00161-8. [DOI] [PubMed] [Google Scholar]
- 29.Jones SA, Horiuchi S, Topley N, Yamamoto N, Fuller GM. The soluble interleukin 6 receptor: mechanisms of production and implications in disease. FASEB J. 2001;15:43–8. doi: 10.1096/fj.99-1003rev. [DOI] [PubMed] [Google Scholar]
- 30.Anderson TW. Large-scale trials of vitamin C. Ann N Y Acad Sci. 1975;258:498–504. doi: 10.1111/j.1749-6632.1975.tb29308.x. [DOI] [PubMed] [Google Scholar]
- 31.Cafolla A, Dragoni F, Girelli G, Tosti ME, Costante A, De Luca AM, et al. Effect of folic acid and vitamin C supplementation on folate status and homocysteine level: a randomized controlled trial in Italian smoker-blood donors. Atherosclerosis. 2002;163:105–11. doi: 10.1016/s0021-9150(01)00745-6. [DOI] [PubMed] [Google Scholar]
- 32.Girelli D, Friso S, Trabetti E, Olivieri O, Russo C, Pessotto R, et al. Methylenetetrahydrofolate reductase C677T mutation, plasma homocysteine, and folate in subjects from northern Italy with or without angiographically documented severe coronary atherosclerotic disease: evidence for an important genetic-environmental interaction. Blood. 1998;91:4158–63. [PubMed] [Google Scholar]
- 33.Friso S, Choi SW, Girelli D, Mason JB, Dolnikowski GG, Bagley PJ, et al. A common mutation in the 5,10-methylenetetrahydrofolate reductase gene affects genomic DNA methylation through an interaction with folate status. Proc Natl Acad Sci U S A. 2002;99:5606–11. doi: 10.1073/pnas.062066299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Bree A, Verschuren WM, Bjorke-Monsen AL, van der Put NM, Heil SG, Trijbels FJ, et al. Effect of the methylenetetrahydrofolate reductase 677C→T mutation on the relations among folate intake and plasma folate and homocysteine concentrations in a general population sample. Am J Clin Nutr. 2003;77:687–93. doi: 10.1093/ajcn/77.3.687. [DOI] [PubMed] [Google Scholar]
- 35.Lonn E, Yusuf S, Arnold MJ, Sheridan P, Pogue J, Micks M, et al. Heart Outcomes Prevention Evaluation (HOPE) 2 Investigators. Homocysteine lowering with folic acid and B vitamins in vascular disease. N Engl J Med. 2006;354:1567–77. doi: 10.1056/NEJMoa060900. [DOI] [PubMed] [Google Scholar]
- 36.Bonaa KH, Njolstad I, Ueland PM, Schirmer H, Tverdal A, Steigen T, et al. NORVIT Trial investigators. Homocysteine lowering and cardiovascular events after acute myocardial infarction. N Engl J Med. 2006;354:1626–32. doi: 10.1056/NEJMoa055227. [DOI] [PubMed] [Google Scholar]


