Abstract
Background
Our objectives were to determine whether obesity is associated with a greater functional decline compared with the ideal body mass index (BMI) among persons with peripheral arterial disease (PAD) and to determine the associations between weight gain and loss and functional declines in PAD. We hypothesized that baseline obesity and weight gain during follow-up would each be associated with functional declines in persons with PAD.
Methods
The design was a prospective cohort study. The subjects were 389 men and women with PAD (mean ankle-brachial index, 0.65 ± 0.14) who were followed up prospectively for a median of 48 months. The main outcome measures were functional assessments (6-minute walk, usual- and rapid-paced 4-m walking speed, and summary performance score). Weight and height were measured at baseline and annually. Results were adjusted for age, sex, race, comorbidities, ankle-brachial index, education, leg symptoms, exercise status, depressive symptoms, pack-years of cigarette smoking, prior-year functioning, and patterns of missing data.
Results
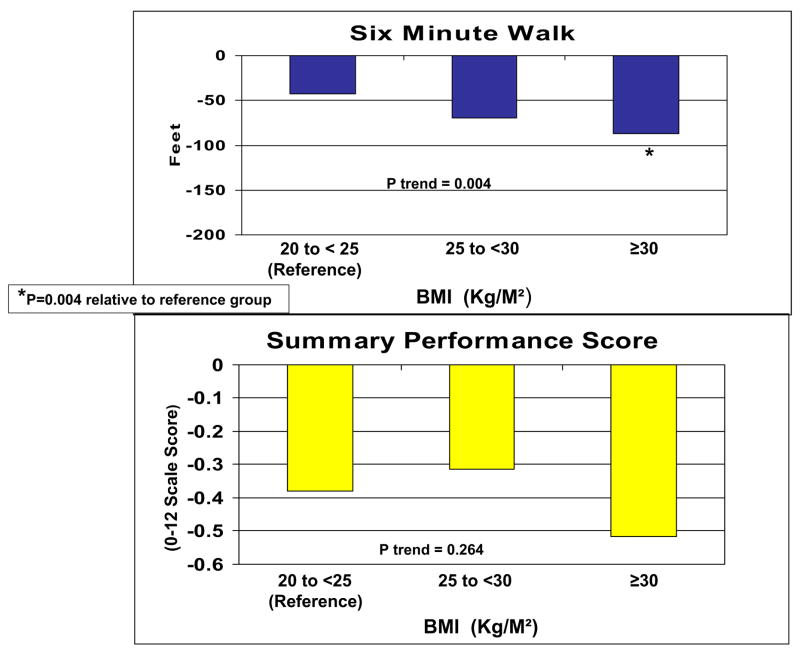
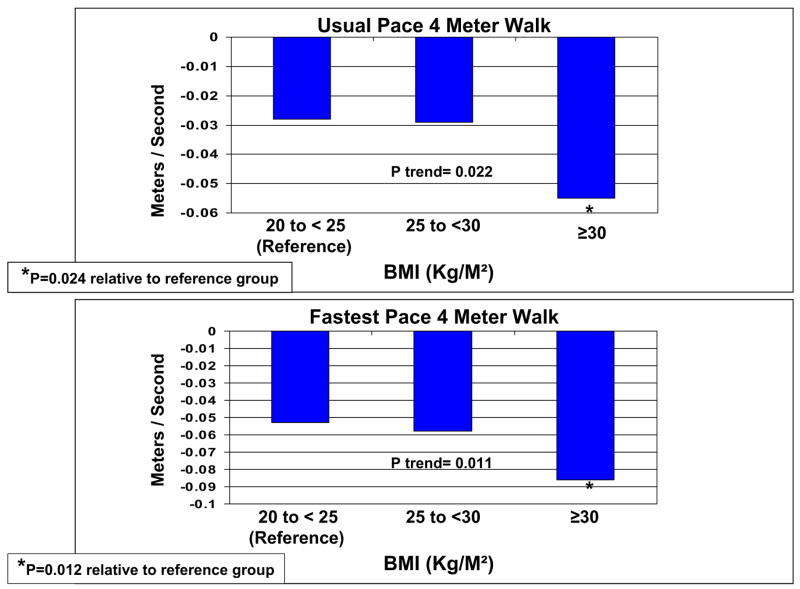
Compared with those with a baseline BMI between 20 and 25 kg/m2, PAD participants with baseline BMI greater than 30 kg/m2 had a significantly greater average annual decline in 6-minute walk performance (−13.1 vs −26.5 m/y; P = .004), usual-paced 4-m walking velocity (−0.028 vs −0.055 m/s per year; P = .024), and fast-paced 4-m walking velocity (−0.053 vs −0.086 m/s per year; P = .012). Persons with weight gain between 5 and 10 pounds after baseline who walked for exercise regularly had significantly less decline in the 6-minute walk than persons without significant weight change who did not walk for exercise (P = .04).
Conclusions
Obesity is associated with functional decline in persons with PAD. Walking exercise may protect against functional decline in PAD persons with modest weight gain.
Lower extremity peripheral arterial disease (PAD) affects 8 to 12 million men and women in the United States.1 Persons with PAD have increased functional decline compared with persons without PAD.2 Identifying modifiable risk factors associated with functional decline in PAD may ultimately lead to therapies that improve functioning and prevent functional decline in PAD.
Obesity and overweight are increasingly prevalent in the United States.3 However, to our knowledge, no prior studies have described associations between obesity or weight change and functional decline in persons with PAD. This study assessed the associations between baseline weight and weight change and functional decline over a 4-year follow-up in persons with PAD. We hypothesized that a higher baseline body mass index (BMI) would be associated with greater functional decline in persons with PAD. We further hypothesized that, as compared with PAD persons with stable weight, those who gained significant weight after baseline would have greater functional decline, whereas persons who lost significant weight would have less functional decline.
METHODS
Methods for this longitudinal observational study of men and women with and without PAD have been described.2 The protocol was institutional review board approved by Northwestern University and Catholic Health Partners Hospital. Participants gave informed consent.
Participant identification
Participants were aged 55 years and older at baseline. Participants were identified consecutively from patients with PAD identified in three Chicago-area noninvasive vascular laboratories. A small number were identified from among patients in a large general internal medicine practice who were screened for PAD with the ankle-brachial index (ABI). Baseline visits occurred between October 1998 and January 2000. Follow-up visits were scheduled annually. PAD was defined as ABI less than 0.90 at the baseline visit.
Exclusion criteria
Exclusion criteria have been reported.2 Patients with dementia, recent major surgery, or foot or leg amputations were excluded. Nursing home residents and wheelchair-bound patients were excluded. Non–English-speaking patients were excluded because investigators were not fluent in non-English languages. Participants who underwent lower extremity revascularization were excluded after the date of their revascularization. Participants with missing BMI data at baseline were excluded (n = 9). Because of small numbers, participants with baseline BMI values of 20 kg/m2 or less were excluded (n = 19).
ABI measurement
By using established methods, a handheld Doppler probe (Vascular Pocket Dop II; Nicolet, Golden, Colo) was used to obtain systolic pressures in the right and left brachial, dorsalis pedis, and posterior tibial arteries.2,4 Each pressure was measured twice. On the basis of prior study, the ABI was calculated in each leg by dividing the mean of the dorsalis pedis and posterior tibial pressures by the mean of the four brachial pressures.4 Average brachial pressures in the arm with highest pressure were used when one brachial pressure was higher than the opposite brachial pressure in both measurement sets and the two brachial pressures differed by 10 mm Hg or more in at least one measurement set, because in such cases subclavian stenosis was possible. The ABI for the leg with the lowest ABI was used in the analyses.
Leg symptom groups
Leg symptoms were classified into one of five groups by using the San Diego Claudication Questionnaire, according to previous study.2,5 Leg symptom groups were as follows: (1) intermittent claudication (exertional calf pain that does not begin at rest, causes the participant to stop walking, and resolves within 10 minutes of rest), (2) leg pain on exertion and rest, (3) atypical exertional leg pain/carry on, (4) atypical exertional leg pain/stop, or (5) asymptomatic (no exertional pain in either leg or buttock on walking).
Comorbidities
Algorithms developed for the Women’s Health and Aging Study were used to document the following comorbidities: angina, diabetes mellitus, myocardial infarction, stroke, heart failure, pulmonary disease, spinal stenosis, disk disease, and hip fracture.6 American College of Rheumatology criteria were used to diagnose knee and hip osteoarthritis.7,8
Functional measures
Objective functional measures in this study have been described previously.2
Six-minute walk
In accordance with a standardized protocol, participants walked up and down a 30.5 m hallway for 6 minutes after instructions to cover as much distance as possible. The test-retest reliability of the 6-minute walk was 0.87 (P < .001) in our laboratory among 81 PAD participants who completed the test approximately 1 to 2 weeks apart.
Repeated chair rises
Participants sat in a straight-backed chair with arms folded across the chest and stood five times consecutively as quickly as possible. The time to five completed chair rises was measured.9,10
Standing balance
Participants were asked to hold three increasingly difficult standing positions for 10 seconds each: standing with feet together side by side and parallel (side-by-side stand), standing with feet parallel with the toes of one foot adjacent to and touching the heel of the opposite foot (semitandem stand), and standing with one foot directly in front of the other (tandem stand).9,10
Four-meter walking velocity
Walking velocity was measured with a 4-meter walk performed at usual and fastest paces. Each walk was performed twice. The faster walk in each pair was used in analyses.9,10 The test-retest reliability was 0.82 (P < .001) in our laboratory among 84 PAD participants who completed the test approximately 1 to 2 weeks apart.
Summary performance score
The summary performance score combined data from the usual-paced 4-meter walking velocity, time to rise from a seated position five times, and standing balance. Individuals received a 0 for each task they were unable to complete. One to four scores were assigned for remaining tasks, based on quartiles of performance for more than 6000 participants in the Established Populations for the Epidemiologic Study of the Elderly.9,10 Scores were summed to obtain the summary performance score, ranging from 0 to 12. The test-retest reliability of the summary performance score was 0.69 (P < .001) in our laboratory among 77 PAD participants who completed the test approximately 1 to 2 weeks apart.
Depressive symptoms
Depressive symptoms were measured annually by using the Geriatric Depression Scale Short Form, a 15-item questionnaire assessing the number of depressive symptoms.11
Weight and weight change
Height and weight were measured at each visit. BMI was calculated as weight (kilograms) divided by height squared (meters). Overweight was defined as a BMI of 25 to less than 30 kg/m2, and obesity was defined as a BMI of 30 kg/m2 or higher.12 Normal weight was defined as a BMI of 20 to less than 25 kg/m2.
Blood collection and analyses
Blood was collected into ethylenediamine tetraacetic acid Vacutainer (Becton, Dickinson and Company, Franklin Lakes, NJ) tubes and immediately iced. Blood was stored at −70 °C until analyses were completed. High-sensitivity C-reactive protein (hsCRP) levels were determined with an immunotechnique on a Behring BN II analyzer (Dade Behring, Wilmington, Del).
Other measures
Cigarette smoking (pack-years) and patient-reported walking exercise frequency were assessed annually by patient report.13
Follow-up
As previously described, participants unable to complete functional measures at follow-up as a result of wheelchair confinement, exhaustion, or other significant symptoms were classified as too disabled to complete functional measures.2,13 When no information was provided for the reason a participant refused to complete functional tests, those who met at least two of the following criteria were considered too disabled to walk: (1) the participant reported walking fewer than five blocks during the previous week, (2) the score for repeated chair rises was 0 or 1, or (3) the score for the standing balance test was 0 or 1. The criteria were defined before data analyses.2 The minimum value for each test was equivalent to the poorest performance among those who completed testing at the corresponding visit.
Statistical analyses
Baseline characteristics across BMI groups were compared by using general linear regression models for continuous variables and logistic regression models for categorical variables. All participants included in analyses returned for at least one follow-up visit. In comparing changes in functioning (eg, 6-minute walk distance) across different patient groups, a longitudinal or repeated-measures analysis of covariance was performed by using the mixed-effects linear regression analysis.14 Dependent variables for each analysis were the successive annual differences in each functional measure. For example, for the 6-minute walk, the dependent variable was defined as the successive differences in 6-minute walk distances (ie, the difference in distance from baseline to the first follow-up visit [FV-1], the difference in distance from FV-1 to the second annual follow-up visit [FV-2], the difference in distance from FV-2 to the third annual follow-up visit [FV-3], and the difference in distance from FV-3 to the fourth annual follow-up visit [FV-4]).
A repeated-measures analysis of covariance adjusting for baseline covariates (sex, age, and race) and a time-dependent covariate representing functional performance at the immediately preceding visit was performed on these successive differences. Thus, differences in baseline functioning across the BMI groups were adjusted for in analyses. Mixed-effects models were used to account for the potential correlations among successive annual differences in each functional measure of the same participant. Analyses were repeated adjusting additionally for baseline comorbidities, ABI, leg symptoms, regular exercise, depressive symptoms, and pack-years of smoking (time-dependent covariate). Baseline BMI was included as the independent variable of primary interest. These analyses were repeated, including the annual change in ABI and newly diagnosed comorbidities as time-dependent covariates. Similar analyses were repeated by using time-dependent models, in which participants were classified according to change in weight during the prior year (weight gain ≥ 10 pounds, weight gain between 5 and 10 pounds, weight loss between 5 and 10 pounds, and weight loss ≥10 pounds) at each visit. In these analyses, participants with less than a 5-pound weight change served as the reference group. Finally, analyses were repeated in which participants in each of the weight change categories were further classified according to whether they were regular exercisers vs nonexercisers. Nonexercisers without a significant weight change served as the reference group. To determine whether there was a threshold effect for associations between higher BMI and greater functional decline, the BMI was also categorized into 10 equally sized groups, and differences in functional decline across these groups were estimated, adjusting for confounders.
Common reasons for a lack of complete follow-up data included death, nursing home placement, or severe disability that made follow-up visits difficult for study participants. Excluding participants without complete follow-up would introduce significant bias into our analyses, because the participants with greatest functional decline would be excluded from analyses. Thus, missing data were adjusted for in our statistical analyses.
Under this initial mixed-effects regression analysis, statistically valid inference is guaranteed, provided that missing data caused by patient dropout are unrelated to unobserved data (ie, any missing data are missing at random). As a safeguard against violations to this assumption that missing data were missing at random, we repeated the fully adjusted comparisons by using a repeated-measures pattern-mixture analysis of covariance model.14,15 In this model, patients may be classified into possible patterns of missing data. Because data were analyzed by using successive differences, there were four observed patterns of missing differences in our analyses. The patterns of missing data were included as binary indicator covariates (centered about their means). By including patterns of missing data in analyses as centered covariates and averaging over these patterns by using adjusted least-squares means, one can obtain an unbiased estimate of the marginal means, adjusting for covariates.16 Analyses were performed with SAS statistical software (version 9.1; SAS Institute Inc, Cary, NC).
RESULTS
Three hundred eighty-nine participants completed baseline testing and attended at least one follow-up visit. Overall, 164 (42.2%) of the 389 participants attended all follow-up visits. Rates of mortality or dropout at each follow-up visit among participants with a baseline BMI of 20 to less than 25 kg/m2 were 26% for FV-2, 22% for FV-3, and 35% for FV-4. These rates among participants with a baseline BMI of 25 to less than 30 kg/m2 were 24% for FV-2, 20% for FV-3, and 29% for FV-4. Rates of mortality or dropout for participants with a BMI of 30 kg/m2 or more were 17% for FV-2, 23% for FV-3, and 28% for FV-4. Compared with participants with complete follow-up, those who attended fewer than the four annual follow-up visits had higher baseline prevalences of heart failure (31.1% vs 22.0%; P = .045), stroke (14.7% vs 6.7%; P = .014), and pulmonary disease (37.8% vs 23.2%; P =.002). Participants who completed all follow-up visits had better baseline lower extremity performance than those who completed fewer follow-up visits (378.3 ± 108.2 vs 320.6 ± 118.6 m [P < .001] for the 6-minute walk; 10.2 ± 2.2 vs 9.3 ± 2.6 for the summary performance score [P < .001]).
Table I shows characteristics of the cohort according to baseline BMI levels. Higher baseline BMI levels were associated with younger age, higher ABI values, and a higher prevalence of diabetes mellitus (Table I). Higher baseline BMI was also associated with poorer baseline performance for the 6-minute walk and the usual-paced 4-m walk (Table II). The incidence of newly diagnosed cancer during follow-up was comparable across the three BMI groups. However, participants with a baseline BMI greater than 30 kg/m2 had a higher incidence of diabetes mellitus than the two other BMI groups (3.7% for BMI 20 to <25 kg/m2 vs 5.7% for BMI 25 to <30 kg/m2 vs 9.4% for BMI <30 kg/m2).
Table I.
Baseline characteristics of participants with peripheral arterial disease according to baseline body mass index (n = 389)
| Variable | 20 < BMI < 25 (n = 107) | 25 ≤ BMI < 30 (n = 176) | BMI ≥ 30 (n = 106) | P trend |
|---|---|---|---|---|
| Age (y) | 74.2 (8.8) | 71.8 (8.4) | 69.3 (7.5) | <.001 |
| Person-years of follow-up | 2.64 (1.30) | 2.72 (1.35) | 2.88 (1.27) | .198 |
| % Male | 56.1 | 63.6 | 65.1 | .176 |
| % Black | 14.0 | 14.2 | 19.8 | .247 |
| Ankle-brachial index | 0.627 (0.14) | 0.654 (0.14) | 0.682 (0.15) | .006 |
| Weight (pounds) | 142.4 (21.5) | 168.5 (21.7) | 207.3 (31.4) | <.001 |
| Cigarette smoking (pack-y) | 36.7 (33.3) | 34.4 (30.6) | 43.7 (37.7) | .131 |
| Angina (%) | 30.8 | 37.5 | 39.6 | .183 |
| Myocardial infarction (%) | 26.2 | 27.3 | 32.1 | .340 |
| Congestive heart failure (%) | 23.4 | 25.6 | 34.0 | .083 |
| Stroke (%) | 13.1 | 11.4 | 9.4 | .402 |
| Diabetes (%) | 24.3 | 31.3 | 43.4 | .003 |
| Lower extremity arthritis (%) | 43.0 | 38.6 | 43.4 | .955 |
| Pulmonary disease (%) | 24.3 | 34.7 | 34.0 | .129 |
BMI, Body mass index.
Values are expressed as mean (standard deviation) unless otherwise indicated.
Table II.
Baseline characteristics of participants with peripheral arterial disease according to baseline body mass index (n = 389)
| Age-adjusted functional performance | 20 < BMI ≤ 25 (n = 107) | 25 < BMI ≤ 30 (n = 176) | BMI > 30 (n = 106) | P trend |
|---|---|---|---|---|
| Six-minute walk distance (m) | 1167 (355.7) m | 1187 (361.8) m | 1003 (305.7) m | .002 |
| Four-meter normal walking velocity (m/s) | 0.934 | 0.895 | 0.829 | <.001 |
| Four-meter fast walking velocity (m/s) | 1.25 | 1.23 | 1.15 | .014 |
| Summary performance score | 10.1 | 9.7 | 9.3 | .014 |
BMI, Body mass index.
Figs 1 and 2 show associations between baseline BMI and average annual functional decline at the 4-year follow-up, adjusting for age, sex, race, ABI, leg symptoms, comorbid diseases, cigarette smoking, exercise level, depressive symptoms, prior-year performance, and patterns of missing data. In fully adjusted analyses, we observed significant associations between higher baseline BMI and greater functional decline for 6-minute walk performance (P trend = .004), normal-paced 4-m walking speed (P trend = .022), and fast-paced 4-m walking speed (P trend = .011; Figs 1 and 2). Compared with the reference group of participants with a baseline BMI between 20 and 25 kg/m2, those with a baseline BMI greater than 30 kg/m2 had a significantly greater average annual decline in 6-minute walk performance (P = .004), usual-paced 4-m walking velocity (P = .024), and fast-paced 4-m walking velocity (P = .012; Figs 1 and 2). The results shown in Figs 1 and 2 were similar at the 3-year follow-up. However, P trend values did not achieve statistical significance at 3-year follow-up. Results shown in Figs 1 and 2 were similar to results that adjusted only for age, sex, and prior-year functioning. Results shown in Figs 1 and 2 also did not change significantly after additional adjustment for the annual change in ABI or after additional adjustment for comorbid diseases diagnosed after the baseline visit. Furthermore, findings shown in Figs 1 and 2 were similar when analyses were repeated with the percentage change in each functional outcome as the dependent variable, and additional analyses did not reveal a threshold effect for the association between higher BMI and greater functional decline. Finally, after the analyses in Figs 1 and 2 were repeated with additional adjustment for hsCRP, the association between the baseline BMI and the decline in 6-minute walk performance was essentially unchanged. However, associations between baseline BMI and declines in the usual-paced and fast-paced 4-m walks were no longer statistically significant (P = .079 and P = .088, respectively).
Fig 1.
Baseline body mass index (BMI) and average annual functional decline at 4-year follow-up in men and women with lower extremity peripheral arterial disease (n = 389).
Fig 2.
Baseline body mass index (BMI) and average annual decline in walking speed at 4-year follow-up in men and women with lower extremity peripheral arterial disease (n = 389).
Between baseline and FV-1, 29 (8%) participants gained 10 pounds or more, and 32 (8.8%) participants lost 10 pounds or more. Compared with participants with no significant weight change, participants who gained 10 or more pounds in a year had no significant differences in functional decline (−12.39 m) for the 6-minute walk among participants who gained 10 or more pounds per year relative to those with no weight change [P = .070] and −0.029 m/s per year for the usual-paced 4-m walk among participants who gained 10 or more pounds per year relative to those with no weight change [P = .070]).
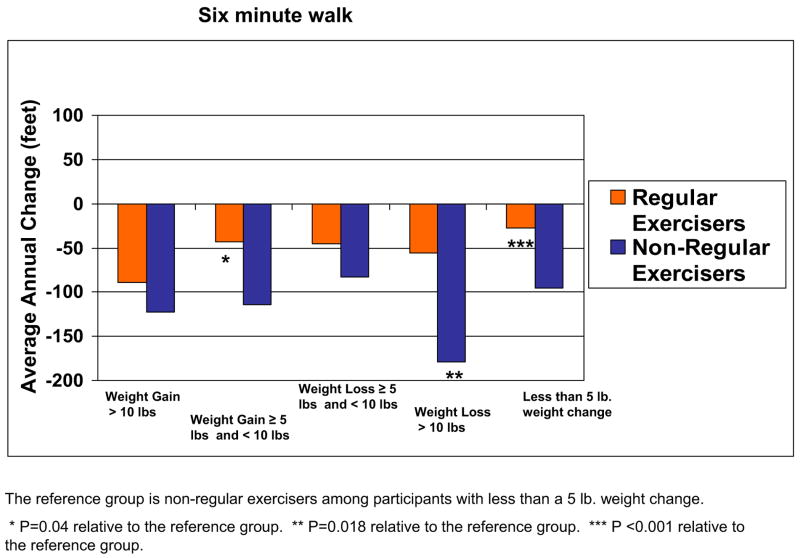
Fig 3 shows adjusted associations between changes in weight and declines in 6-minute walk performance, in which each category of weight change was stratified according to whether participants engaged in regular exercise. Analyses were adjusted for age, sex, race, baseline BMI, comorbidities, smoking, depression, ABI, leg symptoms, prior-year functioning, and patterns of missing data. In these analyses, baseline BMI was not associated with significant declines in 6-minute walk performance (P = .112). However, we observed an interaction effect for associations between weight change, exercise, and declines in 6-minute walk performance (P = .008). Compared with the reference group of nonexercisers with less than a 5-pound weight change, regular exercisers who gained between 5 and 10 pounds had significantly less decline in 6-minute walk performance (P = .04). Compared with nonexercisers with less than a 5-pound weight change, nonexercisers who lost more than 10 pounds had significantly greater decline in 6-minute walk performance (P = .018). For remaining functional outcomes, nonexercisers who lost more than 10 pounds had a significantly greater decline in the usual-paced 4-m walk speed (P = .008), fast-paced 4-m walk speed (P = .010), and summary performance score (P < .001) compared with the reference group of nonexercisers with less than a 5-pound weight change. There were no other consistent significant associations between the weight change/exercise categories and functional decline, and there were no other significant interactions for associations between weight change, exercise, and decline in functioning.
Fig 3.
Average annual change in 6-minute walk performance over 4-year follow-up among men and women with peripheral arterial disease according to annual weight change and exercise habits (n = 389).
DISCUSSION
In this study of 389 men and women with PAD, 27% had a BMI greater than 30 kg/m2 at baseline. To our knowledge, no prior studies have assessed associations between BMI or changes in body weight and functional decline among persons with PAD. This study showed that PAD persons with baseline BMI values greater than 30 kg/m2 had a significantly greater annual decline in 6-minute walk performance at 4-year follow-up compared with PAD persons with baseline BMI values of 20 to 25 kg/m2. Thus, our findings indicate that obesity, a modifiable risk factor, is associated with increased functional decline in persons with PAD.
PAD persons with baseline BMI levels greater than 30 kg/m2 also had a significantly greater annual decline in usual-paced and fast-paced 4-m walking velocity compared with PAD persons with an ideal baseline BMI. However, these associations were no longer statistically significant after additional adjustment for hsCRP. Markers of inflammation, such as hsCRP, are increased in obese individuals.17 Additionally, chronic inflammation has been proposed as a potential mechanism of functional decline in the elderly.18–20 For example, previous studies suggest that inflammation may contribute to age-related reductions in muscle strength and mass, in part by inhibiting muscle repair after injury. Thus, higher levels of inflammation among obese patients with PAD may contribute in part to the relationship between obesity and greater functional decline.
It is important to note that our results suggest that regular walking exercise may be an important deterrent of functional decline in PAD persons who gain modest amounts of weight. Among PAD persons with annual weight gain between 5 and 10 pounds, those who walked for exercise three times weekly had significantly less decline in 6-minute walk performance than participants without weight change. This suggests the possibility that regular walking exercise offsets the negative effects of modest weight gain on functional decline in persons with PAD.
PAD persons with an annual weight loss of 10 pounds or more who walked regularly for exercise also did not have increased rates of functional decline compared with nonexercisers without substantial weight change. In contrast, nonexercisers with 10 pounds or more of weight loss per year experienced significant decline in all functional outcome measures. Although regular walking exercise may protect against functional decline due to weight loss, it is even more likely that weight loss unaccompanied by exercise reflects unintentional weight loss. Participants with unintentional weight loss are likely to have declining health, skeletal muscle loss, or both. For these patients, declining health, declining lean body mass, or both may be responsible for the observed functional decline.
Our findings are consistent with previous studies of obesity and functional decline in community-dwelling men and women without PAD.21–23 Among participants aged 65 years and older in the Cardiovascular Health Study, men and women in the highest quintile of fat mass at baseline had a significantly increased risk of developing disability at the 3-year follow-up (odds ratio was 2.63 for women and 1.73 for men).22 In contrast, there was no significant association between weight change and the risk of disability. In the Chicago Heart Association Detection Project in Industry, significant linear associations were also observed between a higher baseline BMI and poorer health status at 26-year follow-up, as measured by the Health Status Questionnaire 12.23
There are at least two potential mechanisms for the observed associations between baseline obesity and functional decline. First, obesity is associated with an increased burden of comorbid diseases that could adversely influence functioning. Our analyses included statistical adjustment for these comorbidities. Second, obesity may lead to greater deconditioning over time if obese individuals with PAD are less physically active than those who are not obese. Consistent with this hypothesis, regular walking exercise seemed to protect individuals with modest weight gain against functional decline during follow-up.
Our study has weaknesses. First, because of the observational study design, associations reported here cannot be construed as causal. Second, it is possible that an unidentified confounder contributed to the observed associations. In particular, lower BMI may be indicative of healthy lifestyle behaviors that influence functional decline but were not measured in this study. Third, the sample size was insufficient to determine whether significant weight change was associated with differential levels of functional decline across the categories of BMI studied. Fourth, participants were not specifically questioned about whether weight loss was unintentional. Fifth, many participants did not participate in all four follow-up tests. However, our statistical methods should have minimized the effects of missing data on the results. In support of this assertion, results of analyses at the 3-year follow-up were similar to those at the 4-year follow-up, although there were more missing data at the 4-year follow-up time point. Sixth, we did not collect data on the distribution of lower extremity arterial disease (ie aortoiliac vs infrainguinal), and this might have modified the associations reported here. Finally, recent data suggest that the waist-hip ratio may be a more important predictor of cardiovascular events than the BMI. However, we did not collect data on the waist-hip ratio.24
This study identifies a modifiable risk factor associated with functional decline in persons with PAD. Obese patients with PAD experience significantly greater average annual functional decline compared with PAD patients with ideal BMI. Furthermore, regular exercise may be protective against a decline in walking endurance in PAD patients who gain modest amounts of weight. Further study is needed to determine whether interventions that target weight loss and promote walking exercise prevent functional decline in persons with PAD.
Acknowledgments
Supported by grants R01-HL58099, R01-HL64739, and R01-HL073351 from the National Heart, Lung, and Blood Institute and by grant RR-00048 from the National Center for Research Resources, National Institutes of Health. Supported in part by an Established Investigator Award from the American Heart Association (M.M.M.).
Footnotes
Competition of interest: none.
Presented at the Midwest Society of General Internal Medicine, Chicago, IL, Sept 30, 2005.
AUTHOR CONTRIBUTIONS
Conception and design: MMM, MHC, LF, JMG, KL, PG, JRS
Analysis and interpretation: MMM, MHC, LF, JMG, LT, KL, PG, JT, JRS
Data collection: MMM, JMG, KL, PG, EC, WHP
Writing the article: MMM, KL
Critical revision of the article: MMM, MHC, LF, JMG, PG, JRS
Final approval of the article: MMM, MHC, LF, JMG, LT, KL, PG, JT, JRS, EC, WHP
Statistical analysis: JMG, LT, KL, JT
Obtained funding: MMM, MHC, KL, PG
Overall responsibility: MMM
References
- 1.Hiatt WR. Medical treatment of peripheral arterial disease and claudication. N Engl J Med. 1992;326:381–6. doi: 10.1056/NEJM200105243442108. [DOI] [PubMed] [Google Scholar]
- 2.McDermott MM, Liu K, Greenland P, et al. Functional decline in peripheral arterial disease: associations with the ankle brachial index and leg symptoms. JAMA. 2004;292:453–61. doi: 10.1001/jama.292.4.453. [DOI] [PubMed] [Google Scholar]
- 3.US Department of Health and Human Services, Centers for Disease Control and Prevention. Prevalence of overweight and obesity among adults: United States; 1999. Hyattsville (MD): National Center for Health Statistics; 2000. [Google Scholar]
- 4.McDermott MM, Criqui MH, Liu K, et al. The lower ankle brachial index calculated by averaging the dorsalis pedis and posterior tibial arterial pressures is most closely associated with leg functioning in peripheral arterial disease. J Vasc Surg. 2000;32:1164–71. doi: 10.1067/mva.2000.108640. [DOI] [PubMed] [Google Scholar]
- 5.Criqui MH, Denenberg JO, Bird CE, et al. The correlation between symptoms and non-invasive test results in patients referred for peripheral arterial disease testing. Vasc Med. 1996;1:65–71. doi: 10.1177/1358863X9600100112. [DOI] [PubMed] [Google Scholar]
- 6.Guralnik JM, Fried LP, Simonsick EM, et al. The Women’s Health and Aging Study: health and social characteristics of older women with disability (NIH publication No. 95-4009, Appendix E) Bethesda (MD): National Institute on Aging; 1995. [Google Scholar]
- 7.Altman R, Alarcon G, Appelrouth D, et al. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hip. Arthritis Rheum. 1991;34:505–14. doi: 10.1002/art.1780340502. [DOI] [PubMed] [Google Scholar]
- 8.Altman R, Asch E, Bloch D, et al. Development of criteria for the classification and reporting of osteoarthritis. Arthritis Rheum. 1986;29:1039–49. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 9.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 10.Guralnik JM, Ferrucci L, Simonsick E, Salive ME, Wallace RB. Lower extremity function in persons over 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–61. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lyness JM, Noel TK, Cox C, King DA, Conwell Y, Caine ED. Screening for depression in elderly primary care patients. A comparison of the Center for Epidemiologic Studies-Depression Scale and the Geriatric Depression Scale. Arch Intern Med. 1997;157:449–54. [PubMed] [Google Scholar]
- 12.Gregg EW, Cheng YJ, Cadwell BL, et al. Secular trends in cardiovascular disease risk factors according to body mass index in US adults. JAMA. 2005;293:1868–74. doi: 10.1001/jama.293.15.1868. [DOI] [PubMed] [Google Scholar]
- 13.McDermott MM, Liu K, Ferrucci L, et al. Self directed walking exercise and functional decline in peripheral arterial disease. Ann Intern Med. 2006;144:10–20. doi: 10.7326/0003-4819-144-1-200601030-00005. [DOI] [PubMed] [Google Scholar]
- 14.Laird N, Ware J. Random-effects models for longitudinal data. Biometrics. 1982;3844:963–74. [PubMed] [Google Scholar]
- 15.Little RJA. Modeling the drop-out mechanism in repeated-measures studies. J Am Stat Assoc. 1995;90:1112–21. [Google Scholar]
- 16.Fitzmaurice GM, Laird NM, Shneyer L. An alternative parameterization of the general linear mixture model for longitudinal data with non-ignorable drop-outs. Stat Med. 2001;20:1009–21. doi: 10.1002/sim.718. [DOI] [PubMed] [Google Scholar]
- 17.Panagiotakos DB, Pitsavos C, Yannakoulia M, Chrysohoou C, Stefanadis C. The implication of obesity and central fat on markers of chronic inflammation: the ATTICA study. Atherosclerosis. 2005;183:308–15. doi: 10.1016/j.atherosclerosis.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 18.Visser M, Pahor M, Taaffe DR, et al. Relationship of interleukin-6 and tumor necrosis factor-α with muscle mass and muscle strength in elderly men and women: the health ABC study. J Gerontol A Biol Sci Med Sci. 2002;57:M326–32. doi: 10.1093/gerona/57.5.m326. [DOI] [PubMed] [Google Scholar]
- 19.Mitch WE, Goldberg AL. Mechanism of muscle wasting: the role of ubiquitin-proteasome pathway. N Engl J Med. 1997;335:1897–905. doi: 10.1056/NEJM199612193352507. [DOI] [PubMed] [Google Scholar]
- 20.Ferrucci L, Penninx BW, Volpato S, et al. Change in muscle strength explains accelerated decline of physical function in older women with high interleukin-6 serum levels. J Am Geriatr Soc. 2002;12:1947–54. doi: 10.1046/j.1532-5415.2002.50605.x. [DOI] [PubMed] [Google Scholar]
- 21.Launer LJ, Harris T, Rumpel C, Madans J. Body mass index, weight change, and risk of mobility disability in middle-aged and older women. JAMA. 1994;271:1093–8. [PubMed] [Google Scholar]
- 22.Visser M, Langlois J, Guralnik JM, et al. High body fatness, but not low fat-free mass, predicts disability in older men and women: the Cardiovascular Health Study. Am J Clin Nutr. 1998;68:584–90. doi: 10.1093/ajcn/68.3.584. [DOI] [PubMed] [Google Scholar]
- 23.Daviglus ML, Liu K, Yan LL, et al. Body mass index in middle age and health-related quality of life in older age. Arch Intern Med. 2003;163:2448–55. doi: 10.1001/archinte.163.20.2448. [DOI] [PubMed] [Google Scholar]
- 24.Kragelund C, Hassager C, Hildebrandt P, Torp-Pedersen C, Kober L. Impact of obesity on long-term prognosis following acute myocardial infarction. Int J Cardiol. 2005;98:123–31. doi: 10.1016/j.ijcard.2004.03.042. [DOI] [PubMed] [Google Scholar]