Abstract
Objectives
To estimate in a community-dwelling elderly population the magnitude of renal function misclassification, occurring when persons with normal serum creatinine have reduced glomerular filtration rate (GFR), and to describe the participant characteristics related to misclassification.
Design
Cross-sectional.
Setting
Population-based study of older Italian people.
Participants
Six hundred sixty participants aged 65 to 92 with normal serum creatinine.
Measurements
GFR was estimated using the Cockcroft–Gault equation and creatinine clearance (CrCl) calculated from 24-hour urine collection.
Results
In participants with normal serum creatinine, 39% and 25% had moderate renal function impairment (GFR<60 mL/min) according to the Cockcroft–Gault equation and CrCl calculation, respectively. Prevalence of moderate renal impairment in those aged 65 to 74, 75 to 84, and 85 and older was 18.6%, 58.3%, and 96.8%, respectively (P for trend <.001) according to the Cockcroft–Gault equation, and 15%, 35.7%, and 58.7%, respectively (P for trend <.001) based on the CrCl calculation. In addition, female sex (P<.001) and normal or underweight (P<.05) were factors associated with high risk of misclassification.
Conclusion
Serum creatinine alone is one of the most widely used methods of assessing renal function in clinical practice despite its well-known poor correlation with GFR. A large proportion of older persons with impaired renal function are not diagnosed if clinicians rely solely on normal serum creatinine as evidence of normal renal function. Opportunities may be missed for slowing progression of kidney disease, managing comorbidities and complications related to renal impairment, and adjusting drug dosage for renal function.
Keywords: GFR, creatinine, misclassification bias, elderly
Kidney function is assessed in clinical practice to screen for kidney disease, to adapt dosage of medications for renal clearance, and to follow the evolution of known kidney disease. Because renal disease has different clinical presentations and patients are often asymptomatic, it is important to assess kidney function as accurately as possible, especially in older adults, since there is a progressive decline in renal function with age.
Glomerular filtration rate (GFR) is the best quantification of kidney function,1 but because it cannot be measured directly, it is estimated from serum concentration or urinary clearance of a filtration marker. Exogenous filtration markers such as inulin, which is the criterion standard filtration marker, give accurate estimates of GFR,2 but determining them is complex and expensive, has potential complications, and is inappropriate for general use.
Currently, serum creatinine is the most widely used method of assessing renal function in clinical practice, although it has been well established that serum creatinine alone may not be well correlated with true GFR.3,4 Indeed, as renal function declines, tubular creatinine secretion increases, leading to a blunted and delayed rise in serum creatinine concentration, which reaches the abnormal range only when more than half of the total filtration rate is lost.3 Furthermore, because creatinine is derived from the metabolism of creatine in the muscle and from dietary meat intake, factors related to reduced muscle mass, a potential problem in old age, and low dietary meat intake have a strong effect on serum creatinine concentration.4 To account for muscle mass, different estimation equations of GFR adjust serum creatinine concentration for different demographic characteristics and body composition variables. These include the Cockcroft–Gault equation5 or the Modification of Diet in Renal Disease (MDRD) Study equation.6
Despite the fact that serum creatinine is well known to be poorly correlated with GFR, and despite availability of different estimation equations, many clinicians continue to rely solely on serum creatinine as a measure of renal function and interpret a normal serum creatinine as indicating normal renal function. But how many patients with impaired kidney function are missed when relying on serum creatinine alone? Do women have the same risk of misclassification as men? Does this risk change according to body mass index (BMI)? The purpose of this article is to estimate the magnitude of renal function misclassification in a community-dwelling elderly population with normal serum creatinine values and to describe the participant characteristics related to misclassification.
Methods
Study Design and Population
This study used baseline cross-sectional data from the Invecchiare in Chianti Study (InCHIANTI; Aging in the Chianti area), a prospective population-based study of people living in the Chianti geographic area (Tuscany, Italy) that the Laboratory of Clinical Epidemiology of the Italian National Research Council on Aging (INRCA, Florence, Italy) planned in collaboration with the Laboratory of Epidemiology, Demography, and Biometry at the National Institute on Aging (NIA). Baseline data were collected between September 1998 and March 2000. A detailed description of the population sample and data collection has been previously published.7 The study met criteria outlined in the Declaration of Helsinki, and the INRCA ethics committee approved the InCHIANTI Study protocol. All subjects agreed to participate in the study and provided written informed consent.
The InCHIANTI Study population consisted of 1,155 participants aged 65 to 102 randomly selected using a multistage stratified sampling method. Exclusion criteria for these analyses were urine collection of less than 22 hours and missing values for BMI and serum creatinine. In addition, to examine persons with normal serum creatinine, the analyses excluded individuals outside the normal range as previously established for adults8 (men: 0.8–1.3 mg/dL (70.7–114.9μmol/L); women: 0.6–1.0 mg/dL (53.0–88.4 mol/L)). A total of 198 men and 297 women were excluded from the analyses, leading to a sample size of 660 people. Excluded men and women were older and had poorer physical and cognitive function than those included in the analyses.
Data Collection
Participants underwent a first assessment at home, in which they were interviewed, taught the procedure for the 24-hour urine collection, and given the 24-hour urine collection container. They were asked to note the beginning and end time of the urine collection in a diary to allow for computation of length of collection. In a second assessment, those who had fasted for at least 8 hours came to the study clinic in the morning to undergo a peripheral blood collection. They brought with them their entire 24-hour urine collection, whose volume was measured. Weight and height were measured at the time of the clinical examination performed during a third visit, with participants wearing light clothes and no shoes. BMI was computed as (weight (kg)/(height (m)),2) and categorized into four intervals (<22.0, 22.0–24.9, 25.0–29.9, and ≥30.0 kg/m2) using the World Health Organization cutoffs9 for the three highest categories, based on the relationship of BMI with disease and death. The cutoff of 22 kg/m2 was chosen for the low BMI group because of limited numbers of participants with lower BMI.
Laboratory Measures and Kidney Function Assessment
Serum creatinine and urinary creatinine from the 24-hour urine collection were measured using a modified Jaffe method and used to calculate and estimate creatinine clearance (CrCl).
GFR was estimated from the calculated and estimated CrCl.
Because a criterion standard test, such as the inulin test, was not available, two GFR estimation approaches whose results would bracket the true GFR were chosen. CrCl, using 24-hour urine collection, has been shown to consistently overestimate true GFR,10–12 whereas the Cockcroft–Gault estimation equation consistently underestimates true GFR in an older population.10–15 The MDRD Study equation was not deemed acceptable for this study's purpose, because it has been shown to overestimate11,12,14 and underestimate10,13,15 GFR in older population.
Calculated CrCl was computed from 24-hour urine collection using this formula:
Estimated CrCl was computed using the Cockcroft–Gault formula, with a 15% reduction for women due to their lower muscle mass:
Three different categories of renal function were created according to stages of chronic kidney disease established by the Kidney Disease Outcome Quality Initiative of the National Kidney Foundation1 (NKF-K/DOQI) and were used to describe the results of renal function assessed with both the Cockcroft–Gault equation and CrCl calculated using 24-hour urine collection: normal renal function (GFR ≥90 mL/min) or Stage 1 according to NKF-K/DOQI guidelines, mild renal function impairment (GFR 60–89 mL/min) or Stage 2, and moderate renal function impairment (GFR 30–59 mL/min) or Stage 3. NKF-K/DOQI guidelines define chronic kidney disease as GFR less than 60 mL/min or the presence of kidney damage regardless of the cause, for 3 months or more.1
Other Measures
Education assessment was based on the number of years that the subject attended school.
Chronic disease status was assessed through a medical examination completed by a trained geriatrician. The presence and the severity of specific medical conditions found during the examination were verified using standard algorithms16 based on medical history, drug treatments, symptoms and signs, medical documents, and hospital discharge records. Cognitive function was assessed using the Mini-Mental State Examination (MMSE)17 and physical function using the Short Physical Performance Battery (SPPB).18
Statistical Analyses
Men were compared with women according to demographic characteristics, anthropometrics, chronic disease status, and physical and cognitive function using t tests for continuous data and chi-square tests for categorical data (Table 1). The study population was first stratified into three categories of renal function (GFR 30–59, 60–89, and ≥90 mL/min) using the Cockcroft–Gault equation and CrCl calculated from 24-hour urine collection (Figure 1). Then further stratification was done to assess the influence of age, sex, and BMI (Figures 2 and 3). Age was categorized into three intervals (65–74, 75–84, and ≥85), while BMI was categorized into four groups (<22, 22–24.9, 25–29.9, ≥30.0 kg/m2). In addition, for each method of renal function assessment (Cockcroft–Gault equation and CrCl calculation), logistic-regression was used to determine the association between moderate renal impairment (GFR<60 mL/min) and age, sex, and BMI (Table 2). Indicator variables were used to generate odds ratios and 95% confidence intervals, whereas the significance of trends by age and BMI were determined using ordinal variables. All statistical analyses were performed using Stata version 9.0 (StataCorp, College Station, TX).
Table 1. Population Characteristics.
| Population Studied | |||
|---|---|---|---|
| Characteristic | Men (n = 302) | Women (n = 358) | P-value |
| Age, mean ± SD | 73.9 ± 6.5 | 74.4 ± 6.6 | .37 |
| Body mass index, kg/m2, mean ± SD | 27.1 ± 3.3 | 27.8 ± 4.4 | .03 |
| Weight, kg, mean ± SD | 74.5 ± 11.5 | 65.0 ± 11.2 | <.001 |
| Height, cm, mean ± SD | 165.5 ± 7.3 | 152.9 ± 6.6 | <.001 |
| Education, years of school, mean ± SD | 6.2 ± 3.5 | 4.7 ± 2.5 | <.001 |
| Mini-Mental State Examination score, mean ± SD | 25.7 ± 3.4 | 24.8 ± 3.8 | .002 |
| Short Physical Performance Battery score, mean ± SD | 10.9 ± 2.1 | 9.9 ± 2.6 | <.001 |
| Comorbidities, number, mean ± SD | 1.9 ± 1.4 | 1.9 ± 1.2 | .80 |
| Diabetes mellitus, n (%) | 40 (13.2) | 40 (11.2) | .42 |
| Hypertension, n (%) | 189 (62.6) | 239 (66.8) | .26 |
| Stroke, n (%) | 23 (7.6) | 16 (4.5) | .09 |
| Ischemic cardiomyopathy, n (%) | 42 (13.9) | 36 (10.1) | .13 |
| Congestive cardiomyopathy, n (%) | 73 (24.2) | 87 (24.3) | .97 |
| Peripheral arterial disease, n (%) | 55 (18.2) | 38 (10.6) | .005 |
| Chronic obstructive pulmonary disease, n (%) | 59 (19.5) | 15 (4.2) | <.001 |
| Arthritis, n (%) | 61 (20.2) | 154 (43.0) | <.001 |
| Cancer, n (%) | 12 (4.0) | 23 (6.4) | .16 |
| Parkinson's disease, n (%) | 6 (2.0) | 7 (2.0) | .94 |
SD = standard deviation.
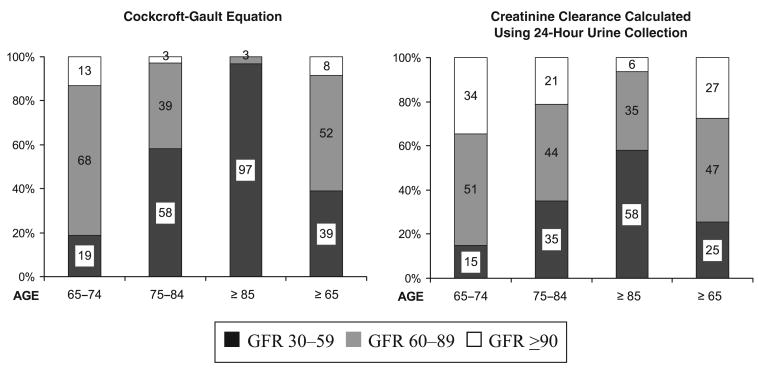
Figure 1.
Distribution of three levels of glomerular filtration rate (GFR) (mL/min), estimated with both the Cockcroft–Gault equation and creatinine clearance calculated using 24-hour urine collection in a population of people aged 65 and older with normal serum creatinine values, with and without stratification according to age.
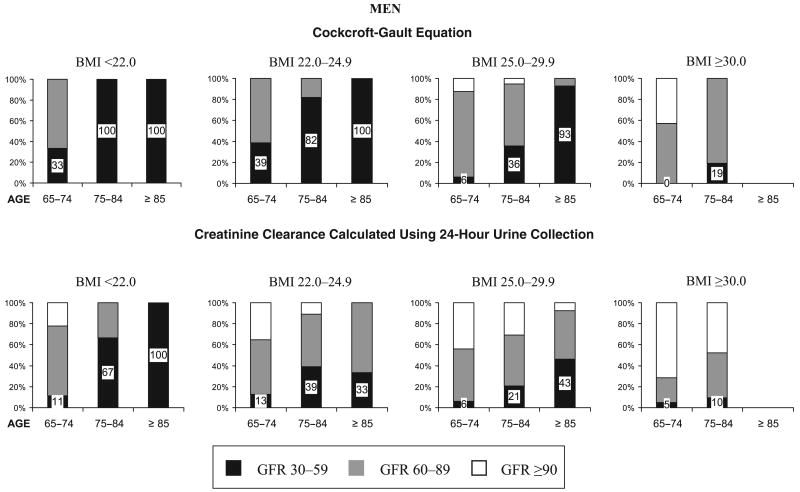
Figure 2.
Distribution according to body mass index (BMI) (kg/m2) and age of three levels of glomerular filtration rate (GFR) (mL/min) estimated with both Cockcroft–Gault equation and creatinine clearance calculated using 24-hour urine collection in a population of men aged 65 and older with normal serum creatinine values. No men aged 85 and older with a BMI of 30.0 and higher had complete data for serum creatinine, BMI, and calculated creatinine clearance.
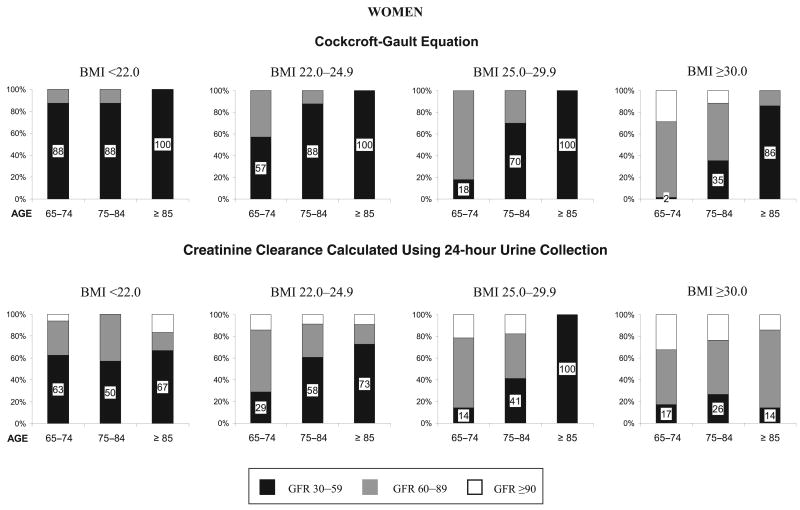
Figure 3.
Distribution according to body mass index (BMI) (kg/m2) and age of three levels of glomerular filtration rate (GFR) (mL/min) estimated with both the Cockcroft–Gault equation and creatinine clearance calculated using 24-hour urine collection in a population of women aged 65 and older with normal serum creatinine values.
Table 2. Odds Ratios for Glomerular Filtration Rate (GFR) Less than 60 mL/min, According to Age Group, Sex, and Body Mass Index (BMI) Status in a Population of People Aged 65 and Older with Normal Serum Creatinine Values.
| GFR Estimation with Cockcroft-Gault Equation | GFR Estimation with Creatinine Clearance Calculated Using 24-Hour Urine Collection | |||||
|---|---|---|---|---|---|---|
| Characteristic | Cases/Total (%) | OR (95% CI) | P-value | Cases/Total (%) | OR (95% CI) | P-value |
| Age | ||||||
| 65–74 | 71/381 (18.6) | 1.00* | 57/381 (15.0) | 1.00* | ||
| 75–84 | 126/216 (58.3) | 10.32 (6.36–16.75) | <.001 | 77/216 (35.7) | 3.25 (2.14–4.93) | <.001 |
| ≥85 | 61/63 (96.8) | 216.15 (48.80–957.50)† | <.001 | 37/63 (58.7) | 7.48 (4.05–13.8)† | <.001 |
| Sex | ||||||
| Male | 95/302 (31.5) | 1.00* | 51/302 (16.9) | 1.00* | ||
| Female | 163/358 (45.5) | 3.13 (1.97–4.96) | <.001 | 120/358 (33.5) | 2.90 (1.92–4.39) | <.001 |
| BMI, kg/m2 | ||||||
| <22.0 | 38/47 (80.9) | 15.66 (6.40–38.28) | <.001 | 26/47 (55.3) | 4.20 (2.10–8.44) | <.001 |
| 22.0–24.9 | 99/141 (70.2) | 7.05 (4.05–12.26) | <.001 | 52/141 (36.9) | 1.85 (1.15–2.97) | .01 |
| 25.0–29.9 | 98/302 (34.5) | 1.00* | 67/302 (22.2) | 1.00* | ||
| ≥30.0 | 23/170 (13.5) | 0.22 (0.11–0.40)† | <.001 | 26/170 (15.3) | 0.58 (0.34–0.98)† | .04 |
Reference group.
P<.001 for trend.
OR = odds ratio; CI = confidence interval.
Results
Overall, 82.8% of participants who had serum creatinine tested were within the normal range. Characteristics of the population are shown in Table 1. The study population consisted of 302 men and 358 women, with mean ages of 73.9 and 74.4 years, respectively. Women had significantly higher mean BMI, fewer years of school, and lower MMSE and SPPB scores than men. Men and women had the same number of comorbidities, but women experienced significantly less peripheral arterial disease and chronic obstructive pulmonary disease and more arthritis than men.
Figure 1 describes the distribution of three different levels of GFR estimated with both the Cockcroft–Gault equation and CrCl calculated using 24-hour urine collection in a population of people aged 65 and older, stratified into three age groups, who had normal serum creatinine values. Using the Cockcroft–Gault equation, only 8% of the population aged 65 and older had normal renal function (GFR≥90), and more than one-third (39%) had moderate renal function impairment (GFR 30–59). When GFR was estimated with CrCl calculated using 24-hour urine collection, the proportion of those aged 65 and older with normal GFR was higher (27%), but 25% had a GFR below 60 mL/min despite a normal serum creatinine concentration. Both estimation methods showed that the proportion of persons with moderately impaired renal function increased with age. To assess potential bias resulting from limiting the study population to people with complete urine collections, analyses were repeated without excluding persons with incomplete urine collection. In this sample of 863 people aged 65 and older with normal serum creatinine values, no significant differences were found in the distribution of the three levels of GFR assessed with Cockcroft–Gault estimation equation (GFR 30–59 mL/min, 41%; GFR 60–89 mL/min, 51%; GFR≥90 mL/min, 8%).
To evaluate the extent of misclassification by age, sex, and BMI, further stratification was done. Figures 2 and 3 display, according to age and BMI groups, the proportion of men and women with normal serum creatinine who had an estimated GFR of 30 to 59, 60 to 89, and 90 mL/min or greater. GFR estimated with the Cockcroft–Gault equation is shown at the top of the figures, and CrCl calculated using 24-hour urine collection is shown at the bottom of the figures.
The greatest magnitude of misclassification (GFR<60 mL/min with normal serum creatinine) was found in normal and underweight persons with both GFR estimation assessments (Figures 2 and 3). The magnitude of misclassification increased markedly with age in nearly all BMI subgroups and was larger in women than in men.
For example, results of GFR estimated using the Cockcroft–Gault equation showed that at least 33% of men (Figure 2) and 57% of women (Figure 3) aged 65 and older who were under- or normal weight (BMI <22 and 22–24.9 kg/m2) had a GFR below 60 mL/min. The percentages increased considerably with age, including nearly all persons aged 75 to 84 and the entire population aged 85 and older. Results for GFR estimation with CrCl calculated using 24-hour urine collection showed lower rates of moderate renal function impairment (GFR<60 mL/min) than using the Cockcroft–Gault equation, although it included at least one-third of normal or underweight men aged 75 and older (Figure 2) and half or more of women meeting the same criteria (Figure 3). The magnitude of misclassification was lower at higher BMI levels but showed a similar pattern distribution across age and sex for both estimation assessments.
The percentage of participants with normal (GFR≥90) and mildly impaired (GFR 60–89) renal function was greater in the lower age and higher BMI categories. Overall, as age increased, the proportion of persons with normal and mildly impaired renal function decreased as the proportion of moderate renal function impairment (GFR 30–59) increased, reflecting the progressive and continuous aspect of decline in renal function in older age.
Table 2 shows the results of multiple logistic regression performed to estimate the association of age, sex, and BMI with having a GFR less than 60 mL/min despite a normal serum creatinine. For both methods of estimating GFR, older age, female sex, and lower BMI were associated with greater odds of having a GFR of less than 60 mL/min.
Discussion
Although serum creatinine is known to be an inaccurate assessment of true renal function,3 the magnitude of misclassification of renal function in persons with normal serum creatinine has generally not been appreciated, especially in older individuals. Therefore, the aim of this study was to describe the extent of this misclassification specifically in a population of people aged 65 and older with normal serum creatinine and to highlight the characteristics of the subset of this population at greatest risk of being misdiagnosed.
Analyses presented here show that depending on normal serum creatinine alone as evidence of normal renal function will result in missing moderate impairment of kidney function in a substantial proportion of the older population. In older people with normal serum creatinine, from one quarter to one third had abnormal kidney function, according to CrCl calculation and the Cockcroft–Gault equation, respectively. Persons with GFR less than 60 mL/min would meet the criteria of chronic kidney disease if this impairment lasted 3 months or longer.1 Women and, in general, normal- and underweight people had the highest rates of being misclassified. Furthermore, stratification by age shows that risk of misclassification increased considerably with age. Overall, these results indicated that the great majority of older adults with renal impairment had normal serum creatinine values. Indeed, of the 465 cases of renal impairment (<60 mL/min using Cockcroft–Gault equation) in the total InCHIANTI Study sample aged 65 and older, 355 (76.3%) had normal serum creatinine.
In addition to serum creatinine, two additional methods were used to assess renal function, allowing for comparison and emphasis of their limits. Because CrCl assesses the total amount (filtered and secreted) of creatinine found in the urine, it overestimates GFR, especially with impairment in kidney function, and represents the upper limit of what the true GFR may be. On the contrary, the Cockcroft–Gault equation has been shown to underestimate true GFR in very old people,13 probably because of the large effect of high age on the estimating equation. Taking into account these considerations, true GFR as assessed using a criterion-standard method is likely to be between these two estimates.
This study focused on the percentage of older adults with normal serum creatinine who were estimated to have abnormal kidney function. The authors are unaware of other published results in which a representative population has been examined in this way. An observational study assessing the efficacy of serum creatinine and albuminuria to detect chronic kidney disease (CKD), defined as a GFR less than 60 mL/min per 1.72 m2, using the MDRD Study formula in a population of 7,596 adults with diabetes mellitus aged 62.6 ± 14.8 in Salford, United Kingdom, corroborates these findings. In that study, 54.7% of the population with diabetes mellitus and CKD had a normal serum creatinine (≤120 μmol/L (1.4 mg/dL)), with a greater proportion of women than men having normal serum creatinine.19 In a study of a nonrepresentative population of patients referred for laboratory testing by their physicians, 47.3% of those aged 70 and older with normal serum creatinine, defined using a higher cutpoint (130 μmol/L (1.5 mg/dL)) than used in the current study were found to have low GFR (≤50 mL/min using the Cockcroft–Gault equation).20 This study did not assess CrCl and did not examine misclassification of abnormal renal function according to sex or BMI.
The strengths of the current study are that it uses data from a representative sample of community-dwelling older adults, which allows for generalizing the findings, and presents and compares results from the most widely used clinical kidney function assessments. Having a complete 24-hour urine collection in a high proportion of subjects is an additional strength of the study. Furthermore, the cohort is well characterized in terms of disease status and body composition, data that are not available in studies that use collections of laboratory data that are not linked to a standardized evaluation. A limitation of the study is that there is no criterion standard measure of GFR, making it necessary to rely on an endogenous filtration marker, creatinine, rather than an ideal exogenous filtration marker.
Studies have been conducted to evaluate other endogenous markers with features that are close to an ideal filtration marker. Cystatin C, a nonglycosylated protein produced by all nucleated cells, may be a promising endogenous marker of GFR if other factors do not affect its production. Its clearance shows a pattern close to a criterion standard marker: free filtration by the glomerulus and re-absorption and catabolization by the tubular epithelial cells, with a small amount excreted in the urine.4 Recent studies have compared the accuracy of cystatin C and serum creatinine in relation to a standard method of GFR measurement and showed a significant superiority of cystatin C to serum creatinine as a marker of GFR.21 However, a cross-sectional study of 8,058 persons with mean age of 49 recently showed that not only renal function but also age, sex, C-reactive protein, cigarette smoking, weight, and height influenced cystatin C. After adjusting for these factors, serum cystatin C was no longer superior to serum creatinine as a marker of moderate kidney function impairment.22 Thus, further methodological work needs to be performed before using cystatin C as a substitute for serum creatinine.
In the recently published NKF-K/DOQI guidelines,1 the Cockcroft–Gault equation and the MDRD Study equation are both proposed as good estimates of GFR in an adult population, but the Cockcroft–Gault equation rather than the MDRD Study equation was chosen for the current study, because the MDRD Study equation has not yet been validated in older adults.23 Indeed, few studies based on older populations have compared the performance of the Cockcroft–Gault and MDRD Study equations in estimating true GFR as measured using an exogenous marker.10–15 Furthermore, the direction of bias (estimated GFR minus true GFR) found by these studies is inconsistent using the MDRD Study equation, with both underestimation10,13,15 and overestimation11,12,14 of true GFR. Not knowing the direction of bias is increasingly problematic for clinicians caring for older adults, because GFR estimation is now often reported automatically in patient clinical laboratory records using the MDRD Study equation. The Cockcroft–Gault equation, alternatively, consistently underestimates the true GFR.10–15 By combining Cockcroft–Gault estimates with 24-hour CrCl, which consistently overestimates true GFR, it was possible to bracket true GFR in the assessment of misclassification.
There are multiple reasons why it is imperative to accurately identify persons with CKD. Persons with early-stage CKD have been shown to have high morbidity (hypertension, diabetes mellitus and its complications, anemia, malnutrition, bone disease, calcium and phosphorus metabolism disorders, impairment of functioning and well-being, and cardiovascular disease including coronary heart disease, cerebrovascular disease, peripheral vascular disease, and heart failure).1 Greater healthcare utilization24–26 and greater risk of mortality, particularly from cardiovascular disease,26 have also been demonstrated in patients with early-stage CKD. Nevertheless, despite validated assessments that can detect earlier stages of CKD5,6,27 and therapeutic interventions effective in slowing or preventing the progression toward kidney failure and its complications (such as strict glucose control in diabetes mellitus, strict blood pressure control, angiotensin-converting enzyme inhibition, or angiotensin-2 receptor blockade),1,28–33 under-recognition of risk factors for CKD and early stages of CKD have been postulated as one of the explanations for the increased incidence and prevalence of kidney failure.26,34 Lack of screening,34–36 misinterpretation of serum creatinine concentration,37 and low awareness of kidney disease in affected people despite a high prevalence of CKD in the U.S. population38 have been described in the literature.
Normal serum creatinine with reduced GFR, which leads to underascertainment of kidney disease when only serum creatinine is used, may represent an early, transient state in the progression of CKD. For example, a retrospective study of patients identified by a laboratory as having abnormal serum creatinine and abnormal GFR found that 2 years before, 28% had normal serum creatinine, but 64% of these patients with normal serum creatinine had a GFR lower than 50 mL/min.20 The state of normal serum creatinine in the face of low GFR is related to age, sex, and BMI, as demonstrated in the current study, but is also a result of increased tubular creatinine secretion, which normally represents more than 15% of urinary CrCl, but may be as high as 92% of urinary CrCl when GFR is less then 40 mL/min.3 As GFR decreases, increases in tubular secretion may be large enough to keep serum creatinine in the normal range.3 In older persons, the combination of low muscle mass with reduced creatinine production and increased tubular secretion can explain the normal serum creatinine seen in persons with substantial GFR decline demonstrated in the current study.
In conclusion, this study demonstrates that appropriate methods of assessment can identify an important subset of the older population meeting criteria for CKD despite normal serum creatinine. Diagnosing these people may allow clinicians to introduce effective treatments to decrease disease progression, better manage comorbidities and their complications, and appropriately choose medications and adjust drug dosage, all components of high-quality medical care.
Acknowledgments
This study was supported as a “targeted project” (ICS 110.1\RS97.71) by the Italian Ministry of Health, and in part by the Intramural Research Program of the National Institute on Aging, NIH. Sandra V. Giannelli was supported by funds from the Department of Rehabilitation and Geriatrics, Geneva University Hospitals, Geneva, Switzerland.
Footnotes
Author Contributions: Sandra V. Giannelli: study concept and design, analysis and interpretation of data, preparation of the manuscript. Kushang V. Patel: analysis and interpretation of data, preparation of the manuscript. B. Gwen Windham and Francesco Pizzarelli: analysis and interpretation of data. Luigi Ferrucci: study concept and design, acquisition of subjects and data, analysis and interpretation of data. Jack M. Guralnik: study concept and design, acquisition of subjects and data, analysis and interpretation of data, preparation of the manuscript.
Sponsor's Role: The granting institutions named above did not interfere in any way with the design, methods, subjects recruitment, data collection, or analysis and preparation of the manuscript.
References
- 1.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease. Evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–S266. [PubMed] [Google Scholar]
- 2.Rahn KH, Heidenreich S, Bruckner D. How to assess glomerular function and damage in humans. J Hypertens. 1999;17:309–317. doi: 10.1097/00004872-199917030-00002. [DOI] [PubMed] [Google Scholar]
- 3.Shemesh O, Golbetz H, Kriss JP, et al. Limitations of creatinine as a filtration marker in glomerulopathic patients. Kidney Int. 1985;28:830–838. doi: 10.1038/ki.1985.205. [DOI] [PubMed] [Google Scholar]
- 4.Stevens LA, Coresh J, Greene T, et al. Assessing kidney function—measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473–2483. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- 5.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 6.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 7.Ferrucci L, Bandinelli S, Benvenuti E, et al. Subsystems contributing to the decline in ability to walk: Bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48:1618–1625. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- 8.Doolan PD, Alpen EL, Theil GB. A clinical appraisal of the plasma concentration and endogenous clearance of creatinine. Am J Med. 1962;32:65–79. doi: 10.1016/0002-9343(62)90183-3. [DOI] [PubMed] [Google Scholar]
- 9.Physical status. The use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ Tech Rep Series. 1995;854:1–452. [PubMed] [Google Scholar]
- 10.Kang YS, Han KH, Han SY, et al. Characteristics of population with normal serum creatinine impaired renal function and: The validation of a MDRD formula in a healthy general population. Clin Nephrol. 2005;63:258–266. doi: 10.5414/cnp63258. [DOI] [PubMed] [Google Scholar]
- 11.Lamb EJ, Webb MC, Simpson DE, et al. Estimation of glomerular filtration rate in older patients with chronic renal insufficiency: Is the modification of diet in renal disease formula an improvement? J Am Geriatr Soc. 2003;51:1012–1017. doi: 10.1046/j.1365-2389.2003.51330.x. [DOI] [PubMed] [Google Scholar]
- 12.Van Den Noortgate NJ, Janssens WH, Delanghe JR, et al. Serum cystatin C concentration compared with other markers of glomerular filtration rate in the old old. J Am Geriatr Soc. 2002;50:1278–1282. doi: 10.1046/j.1532-5415.2002.50317.x. [DOI] [PubMed] [Google Scholar]
- 13.Froissart M, Rossert J, Jacquot C, et al. Predictive performance of the modification of diet in renal disease and Cockcroft-Gault equations for estimating renal function. J Am Soc Nephrol. 2005;16:763–773. doi: 10.1681/ASN.2004070549. [DOI] [PubMed] [Google Scholar]
- 14.Lamb EJ, Wood J, Stowe HJ, et al. Susceptibility of glomerular filtration rate estimations to variations in creatinine methodology: A study in older patients. Ann Clin Biochem. 2005;42:11–18. doi: 10.1258/0004563053026899. [DOI] [PubMed] [Google Scholar]
- 15.Verhave JC, Fesler P, Ribstein J, et al. Estimation of renal function in subjects with normal serum creatinine levels: Influence of age and body mass index. Am J Kidney Dis. 2005;46:233–241. doi: 10.1053/j.ajkd.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 16.Guralnik JM, Fried LP, Simonsik EM, et al. The Women's Health and Aging Study Health and Social Characteristics of Older Women with Disability. Bethesda, MD: National Institute on Aging; 1995. [Google Scholar]
- 17.Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 18.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 19.Middleton RJ, Foley RN, Hegarty J, et al. The unrecognized prevalence of chronic kidney disease in diabetes. Nephrol Dial Transplant. 2006;21:88–92. doi: 10.1093/ndt/gfi163. [DOI] [PubMed] [Google Scholar]
- 20.Duncan L, Heathcote J, Djurdjev O, et al. Screening for renal disease using serum creatinine: Who are we missing? Nephrol Dial Transplant. 2001;16:1042–1046. doi: 10.1093/ndt/16.5.1042. [DOI] [PubMed] [Google Scholar]
- 21.Dharnidharka VR, Kwon C, Stevens G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: A meta-analysis. Am J Kidney Dis. 2002;40:221–226. doi: 10.1053/ajkd.2002.34487. [DOI] [PubMed] [Google Scholar]
- 22.Knight EL, Verhave JC, Spiegelman D, et al. Factors influencing serum cystatin C levels other than renal function and the impact on renal function measurement. Kidney Int. 2004;65:1416–1421. doi: 10.1111/j.1523-1755.2004.00517.x. [DOI] [PubMed] [Google Scholar]
- 23.Myers GL, Miller WG, Coresh J, et al. Recommendations for improving serum creatinine measurement. A Report from the Laboratory Working Group of the National Kidney Disease Education Program. Clin Chem. 2006;52:5–18. doi: 10.1373/clinchem.2005.0525144. [DOI] [PubMed] [Google Scholar]
- 24.Nissenson AR, Collins AJ, Hurley J, et al. Opportunities for improving the care of patients with chronic renal insufficiency: Current practice patterns. J Am Soc Nephrol. 2001;12:1713–1720. doi: 10.1681/ASN.V1281713. [DOI] [PubMed] [Google Scholar]
- 25.Khan SS, Kazmi WH, Abichandani R, et al. Health care utilization among patients with chronic kidney disease. Kidney Int. 2002;62:229–236. doi: 10.1046/j.1523-1755.2002.00432.x. [DOI] [PubMed] [Google Scholar]
- 26.Obrador GT, Pereira BJ, Kausz AT. Chronic kidney disease in the United States: An underrecognized problem. Semin Nephrol. 2002;22:441–448. doi: 10.1053/snep.2002.2002.35962. [DOI] [PubMed] [Google Scholar]
- 27.Gault MH, Longerich LL, Harnett JD, et al. Predicting glomerular function from adjusted serum creatinine. Nephron. 1992;62:249–256. doi: 10.1159/000187054. [DOI] [PubMed] [Google Scholar]
- 28.ACE Inhibitors in Diabetic Neuropathy Trialist Group. Should all patients with type 1 diabetes mellitus and microalbuminuria receive angiotensin-converting enzyme inhibitors? A meta-analysis of individual patient data. Ann Intern Med. 2001;134:370–379. doi: 10.7326/0003-4819-134-5-200103060-00009. [DOI] [PubMed] [Google Scholar]
- 29.Giatras I, Lau J, Levey AS. Effect of angiotensin-converting enzyme inhibitors on the progression of nondiabetic renal disease: A meta-analysis of randomized trials. Angiotensin-Converting-Enzyme Inhibition and Progressive Renal Disease Study Group. Ann Intern Med. 1997;127:337–345. doi: 10.7326/0003-4819-127-5-199709010-00001. [DOI] [PubMed] [Google Scholar]
- 30.Jafar TH, Schmid CH, Landa M, et al. Angiotensin-converting enzyme inhibitors and progression of nondiabetic renal disease. A meta-analysis of patient-level data. Ann Intern Med. 2001;135:73–87. doi: 10.7326/0003-4819-135-2-200107170-00007. [DOI] [PubMed] [Google Scholar]
- 31.Kshirsagar AV, Joy MS, Hogan SL, et al. Effect of ACE inhibitors in diabetic and nondiabetic chronic renal disease: A systematic overview of randomized placebo-controlled trials. Am J Kidney Dis. 2000;35:695–707. doi: 10.1016/s0272-6386(00)70018-7. [DOI] [PubMed] [Google Scholar]
- 32.Laffel LM, McGill JB, Gans DJ. The beneficial effect of angiotensin-converting enzyme inhibition with captopril on diabetic nephropathy in normotensive IDDM patients with microalbuminuria. North American Microalbuminuria Study Group. Am J Med. 1995;99:497–504. doi: 10.1016/s0002-9343(99)80226-5. [DOI] [PubMed] [Google Scholar]
- 33.Remuzzi G, Bertani T. Pathophysiology of progressive nephropathies. N Engl J Med. 1998;339:1448–1456. doi: 10.1056/NEJM199811123392007. [DOI] [PubMed] [Google Scholar]
- 34.McClellan WM, Knight DF, Karp H, et al. Early detection and treatment of renal disease in hospitalized diabetic and hypertensive patients: Important differences between practice and published guidelines. Am J Kidney Dis. 1997;29:368–375. doi: 10.1016/s0272-6386(97)90197-9. [DOI] [PubMed] [Google Scholar]
- 35.Kissmeyer L, Kong C, Cohen J, et al. Community nephrology: Audit of screening for renal insufficiency in a high risk population. Nephrol Dial Transplant. 1999;14:2150–2155. doi: 10.1093/ndt/14.9.2150. [DOI] [PubMed] [Google Scholar]
- 36.Stevens LA, Fares G, Fleming J, et al. Low rates of testing and diagnostic codes usage in a commercial clinical laboratory: Evidence for lack of physician awareness of chronic kidney disease. J Am Soc Nephrol. 2005;16:2439–2448. doi: 10.1681/ASN.2005020192. [DOI] [PubMed] [Google Scholar]
- 37.Patel UD, Young EW, Ojo AO, et al. CKD progression and mortality among older patients with diabetes. Am J Kidney Dis. 2005;46:406–414. doi: 10.1053/j.ajkd.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 38.Coresh J, Byrd-Holt D, Astor BC, et al. Chronic kidney disease awareness, prevalence, and trends among U.S. adults, 1999–2000. J Am Soc Nephrol. 2005;16:180–188. doi: 10.1681/ASN.2004070539. [DOI] [PubMed] [Google Scholar]