Abstract
The human interleukin IL-6 was originally cloned in 1986. In 1993, William Ershler, in his article “IL-6: A Cytokine for Gerontologists,” indicated IL-6 as one of the main signaling pathways modulating the complex relationship between aging and chronic morbidity. Over the last 12 years, our understanding of the role of IL-6 in human physiology and pathology has substantially grown, although some of the questions originally posed by Ershler are still debated. In this review, we will focus on IL-6 structure, IL-6 signaling, and trans signaling pathways, and the role of IL-6 in geriatric syndromes and chronic disease. In the final section of this review, we dissect the critical elements of the IL-6 signaling pathway and point out targets for intervention that are targeted by emerging drugs, some still on the horizon and others already being tested in clinical trials.
Premises and a Brief History of Il-6
IN 1986, Kishimoto and collaborators cloned a DNA encoding a new human interleukin, BCDF/BSF-2, which was later named interleukin-6 (IL-6) (1). The extramembrane (IL-6r) and intramembrane (gp130) domain of the IL-6 receptor were characterized, respectively, in 1986 and 1988 (2,3). Since then, the interest in IL-6 in human physiology and pathology has increased exponentially. In 1993, William Ershler, in his article “IL-6: A Cytokine for Gerontologists,” indicated IL-6 as one of the main signaling pathways implicated in aging and chronic morbidity (4). Ershler’s intuition turned out to be prophetic.
Over the last 12 years, our understanding of the production and biological activity of IL-6 has substantially improved, and the role of IL-6 in aging and age-related conditions is now clearly established. However, some of Ershler’s questions are still in search of an answer.
Here, we review what is known about IL-6 structure, mechanism of action, and role in human pathology, focusing on what has been learned since the publication of the landmark Ershler article. Inflammation as a cardiovascular risk factor will not be addressed because this topic has been discussed in many other reviews (5). Specifically, we touch on trans signaling, the controversial debate on the proinflammatory versus antiinflammatory role of IL-6, and the role of IL-6 in several geriatric syndromes as well as in chronic inflammatory diseases. Finally, while discussing the critical elements of the IL-6 signaling pathway, we point out potential targets for intervention for emerging drugs, some already being tested in clinical trials and others still in the stages of development.
Structure and Signaling Pathways of Il-6: A Wonderful Complexity
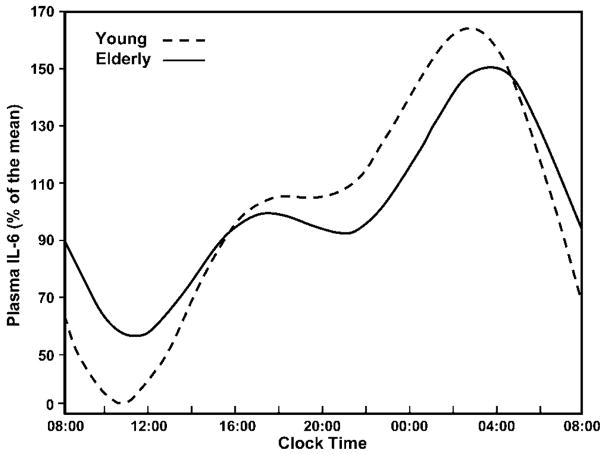
IL-6 is a helical glycoprotein with a molecular weight from 20 to 30 kD (1). Under physiologic conditions, the main source of IL-6 are cells of the immune system, vascular endothelial cells, and adipocytes. The concentration of IL-6 in the serum follows a circadian rhythm (6–9) (Figure 1).
Figure 1.
Interleukin (IL)-6 circadian rhythm. In both young and older persons, the secretion of IL-6 follows a circadian rhythm with two nadirs at about 8.00 and 21.00, and two zeniths at about 19.00 and 5.00.
IL-6 Production
IL-6 expression is mainly modulated by the nuclear factor kappa B (NF-KB). NF-KB proteins are maintained in the cytoplasm by their binding with inhibitory proteins (IKBs). A number of different stimuli, including cytokines, infections, and toxins, induce the phosphorylation, ubiquitinization, and subsequent degradation of the IKB protein by the proteasome. The degradation of IKB allows NF-KB to translocate to the nucleus and bind cognate DNA-binding sites to regulate the transcription of a large number of genes, including inflammatory cytokines (7,10–13).
The IL-6 receptor consists of two glycoprotein subunits: an 80 Kd cognate receptor subunit (IL-6r, CD126), which specifically recognizes IL-6, and a 130-Kd signal-transducing element (gp130), which is the ubiquitously expressed signaling receptor molecule for the IL-6 family. The binding of IL-6 to IL-6r is followed by the homodimerization of gp130 and activation of two distinct signaling pathways: 1) the gp130-associated cytoplasmatic Janus and tyrosine kinases (JAK1, JAK2, and TYK2) signal transducers and activators of transcription (STATs, particularly STAT 1 and 3), and 2) the Src homology 2-containing tyrosine phosphatase (SHP-2)/extracellular signal-regulated kinase (ERK)/mitogen-activated protein kinase (MAPK) pathways (14–16) (Figure 2).
Figure 2.
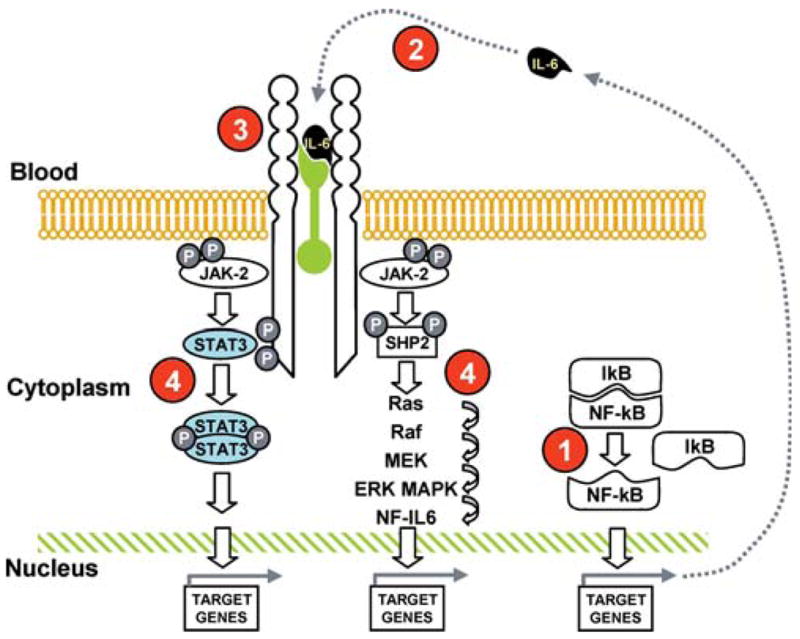
Interleukin (IL)-6-signaling pathways with potential drug targets. Two different pathways: phosphorylation of Janus kinase 2 (JAK2), phosphorylation and dimerization of signal transducer and activator of transcription 3 (STAT3) with successive translocation of STAT3 to the nucleus, and transactivation of target genes (STATs remain in the cytoplasm until they are activated by JAK); activation of the Ras-Raf pathway, which regulates phosphorylation of mitogen-activated protein kinase (MAPK), whose substrate is nuclear factor IL-6 (NF-IL-6). Note that two different IL-6-responsive elements exist in the promoter region of the acute-phase protein genes targeted by NF-IL-6 and STAT3 (13–15). The numbers in red indicate the potential drug targets and are discussed in the text.
IL-6 Trans Signaling and Actions
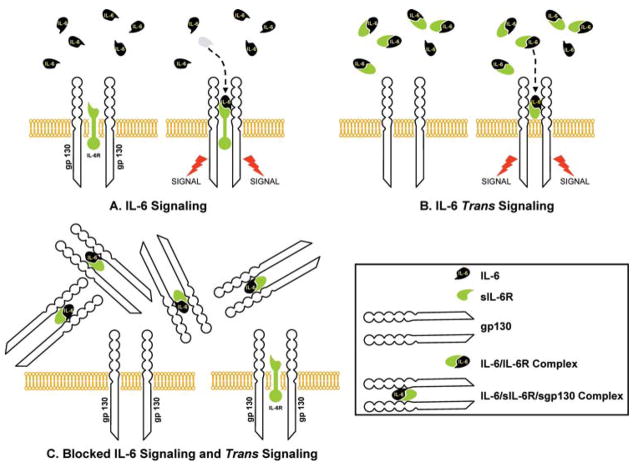
While the cognate IL-6r (CD126) is only expressed by hepatocytes, neutrophils, monocytes/macrophages, and specific lymphocyte subpopulations (17), IL-6 affects many more cell types. This is possible because IL-6r exists in a soluble form (sIL-6r), which can bind IL-6 and form a circulating IL-6/sIL-6r complex that can cause the dimerization of the ubiquitous gp130, even in cells that do not have IL-6r. Soluble IL-6r is formed by proteolytic shedding from the surface of neutrophils and monocytes or splicing during the transcription of the IL-6r gene (18,19).
The shedding process is stimulated by several factors including C-reactive protein (20) (which through this mechanism amplifies the IL-6 signaling), IL-8 (21), and cellular cholesterol depletion (22), and blocked by a metalloprotease inhibitor and tumor necrosis factor (TNF)-alpha protease inhibitor (TAPI) (23).
Factors that stimulate the production of the spliced form are still not well understood. The activation of the IL-6 pathway by the IL-6/sIL-6r, known as “trans signaling,” accounts for most of IL-6 biological activity and is a unique example of a cytokine-soluble receptor with agonistic instead of antagonistic properties (24,25).
The trans signaling mechanism is regulated by a soluble form of gp130 (sgp130), which can bind and completely inactivate the IL-6/sIL-6r complex (26,27) (Figure 3). IL-6/sIL-6r complex can also up-regulate the gp130 expression in certain cells, such as smooth muscle cells (28).
Figure 3.
Interleukin (IL)-6 signaling and trans signaling. The IL-6 receptor consists of two glycoprotein subunits: a cognate receptor subunit (IL-6r) and a 130-kD signal-transducing element (gp130). IL-6 binds the IL-6 receptor and activates the signal (A). The IL-6r can be shed from the membrane of the IL-6-sensitive cell and becomes soluble IL-6r. Soluble IL-6r (sIL-6r) binds IL-6 and forms IL-6/sIL-6r complex, which is able to activate the IL-6 signal. This process is known as “trans signaling” (B). A soluble form of gp130 binds and completely inactivates the IL-6 complex. As a consequence, cells with transmembrane gp130 remain insensitive to IL-6 (C).
IL-6 serum concentration, which is usually in the low pg/ml range, may increase 1000 fold, beyond 5 ng/ml (29). Conversely, levels of sIL-6R and sgp130 fluctuate much less (30). When IL-6 concentration rises, the percentage of IL-6/sIL-6r bound to gp130 progressively declines and the biological activity of IL-6 increases (27,30,31). Although the relevance of the modulator mechanism mentioned above in human physiology and pathology is still under investigation, it is already clear that a base measure of IL-6 provides little information on the degree of activation of the IL-6 pathway.
In animal models of chronic inflammatory disease of the gastrointestinal tract, the blockade of the IL-6 trans signaling caused T-cell apoptosis, indicating that the IL-6/sIL-6r contributes to the perpetuation of chronic intestinal inflammation (32). Accordingly, in a randomized controlled trial, the administration of a human anti-sIL-6r monoclonal antibody in patients with Crohn’s disease reduced the circulating levels of acute-phase reactants and resulted in slight clinical benefit (33). These findings are probably not disease specific and are consistent with the current view that the early phases of inflammation (neutrophil response, acute phase reactants) is mediated by CD126 signaling, and the transition to cell-mediated immunity is mediated by IL-6 trans signaling, perhaps through the prevention of T-cell apoptosis (32).
Il-6 As an Inflammatory Mediator
The physiological role of IL-6 has been mostly studied in the context of the acute phase response, although there is growing evidence that IL-6 also plays a central role in the pathogenesis of chronic disease (17). In acute inflammation, IL-6 promotes the expansion and activation of T cells and differentiation of B cells, and modulates the synthesis of positive (such as C-reactive protein and fibrinogen) and negative (such as albumin) acute phase reactants (34–37). Other typical manifestations of acute inflammation, such as fever, activation of the hypothalamic–pituitary–adrenal axis, anorexia, and lethargy are similarly induced by IL-6 (34). Interestingly, recent studies also suggest that one of the main functions of IL-6 is self-limiting the inflammatory response by suppressing the production of TNF-alpha and IL-1 beta (38,39) and increasing the synthesis of IL-1 receptor antagonist (IL-1 Ra) and soluble TNF receptor p55 (40). In addition, IL-6 confines neutrophil recruitment, favoring their replacement by mononuclear cells (41). In animal models, this effect is present during septic shock (42). Thus, IL-6 concomitantly regulates proinflammatory and antiinflammatory activities and contributes to both the development and the resolution of the acute inflammatory response. The switch from the inflammatory burst that follows an inflammatory stimulus to the chronic elevation of IL-6 typical of immune-mediated diseases and encountered in many older persons is much less understood. As mentioned above, there is evidence that, at least in some specific pathological conditions, the transition to a chronic inflammatory state is sustained by IL-6 trans signaling. However, a fundamental question remains of whether elevated levels of IL-6 are aimed at resolving an inflammatory response that is inappropriately long or whether a primary dysregulation of IL-6 production is responsible for a chronic proinflammatory state, which has a negative impact on health status. Future studies that evaluate the effects of blocking the IL-6 signaling in older persons affected by a chronic proinflammatory state and different patterns of comorbidity may shed light on this question.
Age-Related Changes in Il-6 and Il-6 Receptors
There is strong evidence that IL-6 serum concentration increases with age (43–50). We reported that IL-6 mean values ranged from 1.4 pg/ml (men) and 1.1 pg/ml (women) in the 65–74 years age group to 3.5 pg/ml (men) and 2.1 pg/ml (women) in persons 85 years and older, and that the age trend is partially independent of major confounders (50). Age-related increments in IL-6 are not explained by differential prevalence of IL-6 gene polymorphisms (51), while there is evidence that the excessive production or reduced clearance of oxygen free radicals, which stimulate IL-6 production, may be important (52). Giuliani and colleagues observed a significant increase of sIL-6r up to the seventh decade followed by a gradual decline (53). However, because of inconsistent findings, understanding the effect of age on circulating levels of sgp130 and sIL-6r requires further investigation.
Il-6, Adipose Tissue, Muscle, and Insulin Resistance
IL-6 is produced in skeletal muscle. Physical exercise produces a 10-fold increase in serum IL-6, mostly released from skeletal muscle and perhaps aimed at potentiating the insulin stimulation of glycogen synthesis in muscle cells (54,55). Body composition is also another important correlate of IL-6, especially the percentage of visceral fat. IL-6 produced by omental adipose tissue accounts for 10% to 35% of the body’s basal circulating IL-6 level (56,57). Because of the relationship with adiposity, it has been hypothesized that IL-6 and other proinflammatory cytokines are the main cause of insulin resistance. Interestingly, while the epidemiological evidence supporting this hypothesis is compelling, experimental studies failed to demonstrate the existence of such a mechanism (58).
It has even been suggested that IL-6 attenuates the insulin signal in energy supply tissues (liver, fat), whereas it enhances insulin action in energy-utilizing tissues (skeletal muscle) (54). In vivo and in vitro studies provide evidence for this hypothesis, suggesting that IL-6 signaling can induce a rapid and transient phosphorylation of insulin receptor substrate (IRS-1) in cultured skeletal muscle cells and in muscle tissue, but not in the liver. Of note, two inhibitory mechanisms of IL-6 on insulin action (phosphorylation of the inhibitory Ser-307 residue of IRS-1 and induction of SOCS-3 [suppressor of cytokine signaling 3] expression) have been found in liver, but not in muscle of IL-6-treated mice (59).
Effects of Diet and Exercise on Il-6 Secretion
Diet may affect IL-6 secretion both acutely and chronically. A high-fat meal, but not a high-carbohydrate meal, increases plasma levels of IL-6, and the magnitude of the increment is attenuated by a premeal ingestion of vitamin E and ascorbic acid (60). Circulating levels of polyunsaturated fatty acids, especially total n-3 fatty acids, are independently associated with lower levels of proinflammatory markers, including IL-6. Moreover, consuming a Mediterranean diet is associated with significantly lower IL-6 (61,62).
Plasma IL-6, but not TNF-alpha, increases during acute exercise in proportion to intensity, duration, and level of fitness. It has been suggested that IL-6 is important for the process of muscle repair and cell turnover, as well as for some of the beneficial health effects of exercise, especially on lipids (63). In contrast to these acute effects, persons who are chronically physically active tend to have lower levels of IL-6 and other inflammatory markers (64). A combined intervention of low-calorie diet and exercise significantly reduced circulating and tissue (adipose tissue) IL-6 levels (65).
Il-6 and Osteoporosis
IL-6 has potent antiapoptotic properties on osteoblasts (66) and may affect osteoclast development (67), both of which could lead to osteoporosis. In addition, IL-6 induces bone resorption by multiple mechanisms (68,69). In animal models, estrogens inhibit IL-6 gene expression, and blocking estrogens increases IL-6 and bone resorption (70). Interestingly, transgenic mice over-expressing IL-6 do not have osteoporosis (71). However, human studies show that IL-6 and sIL-6r levels are negatively associated with bone mineral density, and the IL-6 gene is an independent predictor of bone mineral density and peak bone mass (53,72,73).
Il-6 and Sex Hormones
Recent studies suggest that declines in the production and circulating levels of steroid hormones contribute to the mild proinflammatory state typical of older persons. For example, dehydroepiandrosterone (DHEA) and DHEA sulphate are negatively correlated with serum IL-6 and inhibit IL-6 secretion from human mononuclear cells (74). An effect of estrogens on IL-6 is also suggested by the detection of increased levels of IL-6, sIL-6r, and sgp130 after natural and surgical menopause (7,75–78) and increased postovariectomy sIL-6r levels (35%), which can be prevented by estrogen replacement (76). Additionally, low-dose estrogen treatment in postmenopausal women causes a reduction in IL-6 and sIL-6r circulating levels (76,79,80), which, in some cases, is paralleled by improvement of hip and lumbar spine bone mineral density (81).
Although studies examining the effect of testosterone on IL-6 have produced conflicting results, the addition of testosterone to cell cultures down-regulates IL-6 production in human macrophages (82) and in murine (83) and human cultured osteoblasts (84). In male participants of the InCHIANTI study population living in Tuscany (Italy), we found a significant, inverse independent association of bioavailable and total testosterone with sIL-6r, but not IL-6 and other inflammatory markers (85). Similarly, Khosla and coauthors found that antiandrogen treatment in men increased IL-6 and sIL-6r levels, and testosterone partially reverses this effect (86). The administration of testosterone partially prevents the rise in IL-6 that occurs after a coronary stenting (87). Surprisingly, two recent studies did not find any significant reduction in IL-6 serum levels after testosterone administration in older and postpubertal hypogonadal men (88,89).
The mechanism whereby DHEA, estrogens, or testosterone inhibit IL-6 gene expression is not understood. In fact, there are no estrogen or androgen response elements in the putative promoter region of the IL-6 gene. A clue for the underlying mechanism comes from recent data suggesting that androgens inhibit the transmigration of NF-KB from cytoplasm to nucleus (90,91).
Il-6: Fuel for Frailty and Disability
The possible causal role of IL-6 in frailty, sarcopenia, and disability is a fascinating but still unsolved research question (92). Frail study participants have higher levels of IL-6 than nonfrail, age-matched individuals (93). Elevated serum IL-6 is positively associated with markers of physical frailty such as low walking speed, poor muscle strength (94), poor lower extremity performance (95), and anemia (96), even after adjustment for confounders.
IL-6 levels are cross-sectionally associated with disability in community-dwelling elderly persons (48) and are predictive of future disability in nondisabled older persons (97). We showed that most of the relationship between IL-6 and disability is accounted for by the detrimental effect of IL-6 on muscle strength (97). IL-6 may contribute to sarcopenia through different mechanisms, including a direct interference with insulin signal transduction and inhibition of the production and biological activity of insulin-like growth factor-1 (IGF-1) (98,99). Interestingly, low IGF-1 and IL-6 levels were independent and almost synergistic risk factors for disability both in the Women’s Health and Aging Study I and in the InCHIANTI Study (99,100).
In animal studies, IL-6 infusion results in muscle atrophy characterized by a preferential loss of myofibrillar protein (−17%), perhaps secondary to SOCS activation and to increased STAT phosphorylation in favor of a more catabolic profile (101).
Finally, it has been suggested that the core mechanism leading to age-associated frailty is excessive and unopposed oxidative stress, and, therefore, high IL-6 in frail older persons is only a marker of this phenomenon with little causal pathophysiologic role. The direct testing of this hypothesis is difficult because biomarkers of oxidative stress have limited reliability and validity (102).
Il-6 in Chronic Disease
IL-6 and Cancer
IL-6 is a growth/survival factor for a variety of tumor types. Activation of STAT3 by IL-6 is correlated with increased antiapoptotic (the bcl family), cell cycle gene expression and activation of androgen receptor genes. An extensive literature search suggests that IL-6 has a role in multiple myeloma and prostate and ovarian cancers. In addition, there is evidence that the excessive production of proinflammatory cytokines, including IL-6, accounts for tumor-related constitutional symptoms and associated cachexia (103–107).
Uncommon Conditions Where IL-6 Plays a Special Role
Three specific medical conditions (cardiac myxoma, Castleman’s disease, and multiple myeloma) warrant special examination in the context of IL-6 and disease pathogenesis. Cardiac myxoma, a benign atrial heart tumor, produces large quantities of IL-6, which cause autoantibody production, fever, joint pain, and anemia (108). Described by Dr. Benjamin Castleman in 1956, Castelman’disease is a relatively rare lymphoproliferative disorder caused by human herpes virus 8, whose genome encodes a viral IL-6, which causes most of the associated symptoms (109). Elevated levels of IL-6 in multiple myeloma protect tumor cells from glucocorticoid-induced death (105) by activating STAT 3-dependent antiapoptotic genes (110). Confirming the importance of trans signaling, sIL-6r is significantly elevated in myeloma patients and correlated with the disease severity (36,37,111–114).
IL-6 and Diabetes
The role of IL-6 on beta cell survival and type 1 diabetes is probably small (115–119). On the contrary, observational studies show that IL-6 is a risk factor for type 2 diabetes (120–122). Whether increased IL-6 is causal or merely reflects an attempt by the body to counteract low-grade inflammation, mirrored by elevation of other inflammatory markers, resulting from diabetes is still an open question.
IL-6 and Hypoproliferative Anemia
IL-6 in concert with other proinflammatory cytokines is the primary cause of anemia of inflammation, formerly defined as the anemia of chronic disease (123). Recent preclinical studies suggest that IL-6 stimulates hepatocytes to produce hepcidin, a polypeptide that inhibits intestinal absorption and reticuloendothelial release of iron (124). However, there is evidence that IL-6 may cause anemia through other mechanisms, including rapid hemodilution, down-regulation of the membrane-bound erythropoietin receptor, and impairment of erythroid proliferation and maturation (125–127).
IL-6 and Crohn’s Disease
In Crohn’s disease (CD), serum and intestinal levels of IL-6 and sIL-6r are higher than in controls (128,129) and correlate with the severity of inflammation (128,130). Accordingly, the IL-6-dependent STAT3 is highly phosphorylated (131), and lymphocyte gene expression of IL-6r and gp130 are enhanced (132).
Interestingly, IL-6 trans signaling protects mucosal T cells against apoptosis contributing to the perpetuation of chronic intestinal inflammation. Blockade of IL-6 trans signaling causes T-cell apoptosis and symptom improvement (32).
IL-6 and Rheumatoid Arthritis
SKG mice that spontaneously develop rheumatoid arthritis (RA) are completely protected from the disease if the IL-6 gene is knocked out, suggesting that IL-6 is critical to the pathophysiology of RA (133). Accordingly, sIL-6r and IL-6 levels are increased in the synovial fluid and the serum of RA patients and are highly correlated with disease activity (134–136).
IL-6 and Alzheimer’s Disease
In vitro, endogenously produced cytokines could influence beta amyloid peptide accumulation and tangle formation. Conversely, beta amyloid and tau proteins stimulate microglia, astrocytes, and oligodendrocytes to overproduce inflammatory mediators, therefore generating a “vicious cycle” leading to irreversible synaptic loss (137). In spite of this rationale, observational studies looking at specific patterns of elevated cytokines, such as IL-6, sIL-6r, and sgp130, in serum and in cerebrospinal fluid of AD patients have produced conflicting results (138,139).
From Bench to Bedside
Therapeutic Strategy Targeting IL-6-Signaling Pathways
In Figure 2, we outlined the elements of the IL-6 production and signaling pathways. Pharmacological intervention can modulate the IL-6 signaling pathways by interfering with different critical steps.
1. Proteasome and NF-KB
The proteasome, a multi-catalytic proteinase complex, is responsible for the majority of intracellular protein degradation. Proteasome inhibitors block the degradation of IKB, thereby sequestering NF-KB in the cytoplasm and preventing its protranscriptional activity (140,141). Bortezomib, the first proteasome inhibitor to undergo clinical testing, has significant efficacy against multiple myeloma and non-Hodgkin’s lymphoma (142).
Thalidomide and thalidomide analogues also down-regulate NF-KB activity, together with inhibiting the P13K/AKT and JAK/STAT (143). This therapeutic approach is currently been tested in a pilot project at the National Institute on Aging targeting frail participants.
2. Targeting IL-6
IL-6-neutralizing monoclonal antibodies (BE-8 and CNTO) are currently being tested in six early stage clinical trials performed in B-cell lymphoproliferative disorders, primary central nervous system lymphoma (primary cerebral lymphoma), multiple myeloma, and renal cell carcinoma. In preliminary studies, these compounds have shown dramatic reduction of C-reactive protein and improvements of cancer-related symptoms (fever, cachexia, and pain) (103). A novel series of IL-6 antagonists called Sants (super antagonists) antagonize receptor binding without activating the IL-6 signaling cascade (144) and induce cell cycle arrest in primary myeloma cells (145,146). These molecules are still in an early development stage and have not been tested in human studies.
3. Targeting IL-6 receptor
Tocilizumab, a humanized anti-IL-6 receptor antibody, is the most-studied intervention targeting sIL-6r in chronic inflammatory diseases (147,148). Pilot and full-scale randomized clinical trials are currently being conducted for the treatment of Crohn’s disease (33), rheumatoid arthritis (149), moderately active systemic lupus erythematosus (150), and Castelman’s disease (151,152).
Overall, tocilizumab administered for 4 to 60 weeks, at a daily dose of 2–8 mg/kg, was well tolerated and significantly improved inflammatory parameters in all diseases in which it was employed. The major side effects were an increase in lipid levels, which returned to normal values after treatment was withdrawn. The long-term safety of tocilizumab is still under investigation.
Recent observational and preliminary intervention studies suggest that the administration of soluble glycoprotein 130 may significantly reduce the severity of arthritis (153,154), probably by inactivating the IL-6/sIL-6r circulating complex.
4. Targeting JAK-STAT and JAK-MAPK
AG-490 (Tyrphostin) and Cucurbitacin I (JSI-124) are active against the JAK, the latter showing moderate selectivity for JAK2 (155,156). These molecules have not been tested in clinical trials. Studies on the pathway leading to apoptosis in myeloma cells showed that the Farnesyl transferase inhibitors R11 5777 inhibit MAPK, PI3K-AKT, and JAK/STAT pathways through inhibition of STAT3 and ERK1/2 (157,158).
Statins, curcumin, and cucurbitacin Q have been shown to decrease IL-6-induced CRP expression in human hepatocytes at the transcriptional level by reducing phosphorylation of STAT3 (159–161). No studies, with the exception of statins, in humans are available.
Lys685 acetylation is critical for STAT3 to form stable dimers required for cytokine-stimulated DNA binding and transcriptional regulation, to enhance transcription of cell growth-related genes, and to promote cell cycle progression. The identification of compounds that affect Lys685 acetylation is currently under development. There is in vitro evidence that Paclitaxel, a microtubule stabilizer, selectively inhibits STAT3 translocation (162). New short peptides called aptamers interact with STAT3 dimerization domain and inhibit DNA binding and transactivation (163). However, aptamers specific for IL-6 are not currently available. Gene transcription is another potential target of IL-6 modulation, and compounds such as transcription factor decoy, SiRNA, and antisense are under investigation.
Opening Questions for a Research Agenda
Since Ershler’s landmark article, our understanding of the IL-6-signaling pathway and its role in the development of a late-life, mild proinflammatory state, chronic morbidity, and frailty has grown tremendously. However, studying IL-6 has been similar to opening a Pandora’s box, we have generated many more new questions than definitive answers.
From a gerontological perspective, we believe that the most burning of the many possible questions are the following:
To clarify how the complex interaction between the relative concentrations of IL-6, sIL-6r, and gp130 affect the degree of activation of the IL-6 pathway in different cell types.
To understand the mechanisms that cause the switch from an acute inflammatory response to a chronic inflammatory state, particularly in reference to the mild proinflammatory state of aging.
To sort out the proinflammatory and antiinflammatory properties of IL-6 and how they are affected by patterns of concentration of biomarkers involved in the regulation of biological homeostasis.
To understand the relationship between health-related behaviors, in particular nutrition and physical activity, and the production of IL-6, sIL-6r, gp-130, and other inflammatory markers.
To test the hypothesis that a dysregulation in the IL-6 production and biological activity is central to the pathogenesis of frailty and, ultimately, whether treatment aimed at modulating the IL-6 pathway may prevent frailty or have positive effects in older frail patients.
To verify whether the mild proinflammatory state that often affects older persons is due to a primary dysregulation of the immune system or rather a reactive response to excessive and not adequately opposed oxidative stress.
Conclusion
The IL-6 pathway appears to be profoundly implicated in the pathophysiology of physical function decline and chronic diseases that often affect older persons. Because of this activity across multiple physiological systems, it is conceivable that a primary IL-6 dysregulation is the first step in the development of frailty. If IL-6 dysregulation is demonstrated to be a direct cause of physiologic decline with aging, rather than an innocent bystander reflecting some other process, then modulation of IL-6 production or effects could offer a major breakthrough in prevention and treatment of people at advanced old age.
References
- 1.Hirano T, Yasukawa K, Harada H, et al. Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature. 1986;324:73–76. doi: 10.1038/324073a0. [DOI] [PubMed] [Google Scholar]
- 2.Yamasaki K, Taga T, Hirata Y, et al. Cloning and expression of the human interleukin-6 (BSF-2/IFN beta 2) receptor. Science. 1988;241:825–828. doi: 10.1126/science.3136546. [DOI] [PubMed] [Google Scholar]
- 3.Hibi M, Murakami M, Saito M, Hirano T, Taga T, Kishimoto T. Molecular cloning and expression of an IL-6 signal transducer, gp130. Cell. 1990;63:1149–1157. doi: 10.1016/0092-8674(90)90411-7. [DOI] [PubMed] [Google Scholar]
- 4.Ershler WB. Interleukin-6: a cytokine for gerontologists. J Am Geriatr Soc. 1993;41:176–181. doi: 10.1111/j.1532-5415.1993.tb02054.x. [DOI] [PubMed] [Google Scholar]
- 5.Kritchevsky SB, Cesari M, Pahor M. Inflammatory markers and cardiovascular health in older adults. Cardiovasc Res. 2005;66:265–275. doi: 10.1016/j.cardiores.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 6.Akira S, Taga T, Kishimoto T. Interleukin-6 in biology and medicine. Adv Immunol. 1993;54:1–78. doi: 10.1016/s0065-2776(08)60532-5. [DOI] [PubMed] [Google Scholar]
- 7.Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu Rev Med. 2000;51:245–270. doi: 10.1146/annurev.med.51.1.245. [DOI] [PubMed] [Google Scholar]
- 8.Hirano T, Matsuda T, Nakajima K. Signal transduction through gp130 that is shared among the receptors for the interleukin 6 related cytokine subfamily. Stem Cells. 1994;12:262–277. doi: 10.1002/stem.5530120303. [DOI] [PubMed] [Google Scholar]
- 9.Vgontzas AN, Bixler EO, Lin HM, Prolo P, Trakada G, Chrousos GP. IL-6 and its circadian secretion in humans. Neuroimmunomodulation. 2005;12:131–140. doi: 10.1159/000084844. [DOI] [PubMed] [Google Scholar]
- 10.Yan SF, Tritto I, Pinsky D, et al. Induction of interleukin 6 (IL-6) by hypoxia in vascular cells. Central role of the binding site for nuclear factor-IL-6. J Biol Chem. 1995;270:11463–11471. doi: 10.1074/jbc.270.19.11463. [DOI] [PubMed] [Google Scholar]
- 11.Klausen T, Olsen NV, Poulsen TD, Richalet JP, Pedersen BK. Hypoxemia increases serum interleukin-6 in humans. Eur J Appl Physiol Occup Physiol. 1997;76:480–482. doi: 10.1007/s004210050278. [DOI] [PubMed] [Google Scholar]
- 12.Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol. 2002;2:725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- 13.Saito M, Yoshida K, Hibi M, Taga T, Kishimoto T. Molecular cloning of a murine IL-6 receptor-associated signal transducer, gp130, and its regulated expression in vivo. J Immunol. 1992;148:4066–4071. [PubMed] [Google Scholar]
- 14.Nakajima T, Kinoshita S, Sasagawa T, et al. Phosphorylation at threonine-235 by a ras-dependent mitogen-activated protein kinase cascade is essential for transcription factor NF-IL6. Proc Natl Acad Sci U S A. 1993;90:2207–2211. doi: 10.1073/pnas.90.6.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heinrich PC, Behrmann I, Muller-Newen G, Schaper F, Graeve L. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem J. 1998;334:297–314. doi: 10.1042/bj3340297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kishimoto T, Akira S, Narazaki M, Taga T. Interleukin-6 family of cytokines and gp130. Blood. 1995;86:1243–1254. [PubMed] [Google Scholar]
- 17.Bauer J, Bauer TM, Kalb T, et al. Regulation of interleukin 6 receptor expression in human monocytes and monocyte-derived macrophages. Comparison with the expression in human hepatocytes. J Exp Med. 1989;170:1537–1549. doi: 10.1084/jem.170.5.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mullberg J, Schooltink H, Stoyan T, et al. The soluble interleukin-6 receptor is generated by shedding. Eur J Immunol. 1993;23:473–480. doi: 10.1002/eji.1830230226. [DOI] [PubMed] [Google Scholar]
- 19.Desgeorges A, Gabay C, Silacci P, et al. Concentrations and origins of soluble interleukin 6 receptor-alpha in serum and synovial fluid. J Rheumatol. 1997;24:1510–1516. [PubMed] [Google Scholar]
- 20.Jones SA, Novick D, Horiuchi S, Yamamoto N, Szalai AJ, Fuller GM. C-reactive protein: a physiological activator of interleukin 6 receptor shedding. J Exp Med. 1999;189:599–604. doi: 10.1084/jem.189.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marin V, Montero-Julian F, Gres S, Bongrand P, Farnarier C, Kaplanski G. Chemotactic agents induce IL-6R alpha shedding from polymorphonuclear cells: involvement of a metalloproteinase of the TNF-alpha-converting enzyme (TACE) type. Eur J Immunol. 2002;32:2965–2970. doi: 10.1002/1521-4141(2002010)32:10<2965::AID-IMMU2965>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 22.Matthews V, Schuster B, Schutze S, et al. Cellular cholesterol depletion triggers shedding of the human interleukin-6 receptor by ADAM10 and ADAM17 (TACE) J Biol Chem. 2003;278:38829–38839. doi: 10.1074/jbc.M210584200. [DOI] [PubMed] [Google Scholar]
- 23.Mullberg J, Durie FH, Otten-Evans C, et al. A metalloprotease inhibitor blocks shedding of the IL-6 receptor and the p60 TNF receptor. J Immunol. 1995;155:5198–5205. [PubMed] [Google Scholar]
- 24.Jones SA, Rose-John S. The role of soluble receptors in cytokine biology: the agonistic properties of the sIL-6R/IL-6 complex. Biochim Biophys Acta. 2002;1592:251–263. doi: 10.1016/s0167-4889(02)00319-1. [DOI] [PubMed] [Google Scholar]
- 25.Narazaki M, Yasukawa K, Saito T, et al. Soluble forms of the interleukin-6 signal-transducing receptor component gp130 in human serum possessing a potential to inhibit signals through membrane-anchored gp130. Blood. 1993;82:1120–1126. [PubMed] [Google Scholar]
- 26.Jostock T, Mullberg J, Ozbek S, et al. Soluble gp130 is the natural inhibitor of soluble interleukin-6 receptor transsignaling responses. Eur J Biochem. 2001;268:160–167. doi: 10.1046/j.1432-1327.2001.01867.x. [DOI] [PubMed] [Google Scholar]
- 27.Klein B, Zhang XG, Lu ZY, Bataille R. Interleukin-6 in human multiple myeloma. Blood. 1995;85:863–872. [PubMed] [Google Scholar]
- 28.Klouche M, Bhakdi S, Hemmes M, Rose-John S. Novel path to activation of vascular smooth muscle cells: up-regulation of gp130 creates an autocrine activation loop by IL-6 and its soluble receptor. J Immunol. 1999;163:4583–4589. [PubMed] [Google Scholar]
- 29.Montero-Julian FA. The soluble IL-6 receptors: serum levels and biological function. Cell Mol Biol. 2001;47:583–597. [PubMed] [Google Scholar]
- 30.Grotzinger J, Kernebeck T, Kallen KJ, Rose-John S. IL-6 type cytokine receptor complexes: hexamer, tetramer or both? Biol Chem. 1999;380:803–813. doi: 10.1515/BC.1999.100. [DOI] [PubMed] [Google Scholar]
- 31.Rose-John S, Neurath MF. IL-6 trans-signaling: the heat is on. Immunity. 2004;20:2–4. doi: 10.1016/s1074-7613(04)00003-2. [DOI] [PubMed] [Google Scholar]
- 32.Atreya R, Mudter J, Finotto S, et al. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in Crohn disease and experimental colitis in vivo. Nat Med. 2000;6:583–588. doi: 10.1038/75068. [DOI] [PubMed] [Google Scholar]
- 33.Ito H, Takazoe M, Fukuda Y, et al. A pilot randomized trial of a human anti-interleukin-6 receptor monoclonal antibody in active Crohn’s disease. Gastroenterology. 2004;126:989–996. doi: 10.1053/j.gastro.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 34.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 35.Heinrich PC, Castell JV, Andus T. Interleukin-6 and the acute phase response. Biochem J. 1990;265:621–636. doi: 10.1042/bj2650621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jones SA, Horiuchi S, Topley N, Yamamoto N, Fuller GM. The soluble interleukin 6 receptor: mechanisms of production and implications in disease. FASEB J. 2001;15:43–58. doi: 10.1096/fj.99-1003rev. [DOI] [PubMed] [Google Scholar]
- 37.Kallen KJ. The role of transsignalling via the agonistic soluble IL-6 receptor in human diseases. Biochim Biophys Acta. 2002;1592:323–343. doi: 10.1016/s0167-4889(02)00325-7. [DOI] [PubMed] [Google Scholar]
- 38.Xing Z, Gauldie J, Cox G, et al. IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J Clin Invest. 1998;101:311–320. doi: 10.1172/JCI1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schindler R, Mancilla J, Endres S, Ghorbani R, Clark SC, Dinarello CA. Correlations and interactions in the production of interleukin-6 (IL-6), IL-1, and tumor necrosis factor (TNF) in human blood mononuclear cells: IL-6 suppresses IL-1 and TNF. Blood. 1990;75:40–47. [PubMed] [Google Scholar]
- 40.Tilg H, Trehu E, Atkins MB, Dinarello CA, Mier JW. Interleukin-6 (IL-6) as an anti-inflammatory cytokine: induction of circulating IL-1 receptor antagonist and soluble tumor necrosis factor receptor p55. Blood. 1994;83:113–118. [PubMed] [Google Scholar]
- 41.Jones SA. Directing transition from innate to acquired immunity: defining a role for IL-6. J Immunol. 2005;175:3463–3468. doi: 10.4049/jimmunol.175.6.3463. [DOI] [PubMed] [Google Scholar]
- 42.Barton BE, Jackson JV. Protective role of interleukin 6 in the lipo-polysaccharide-galactosamine septic shock model. Infect Immun. 1993;61:1496–1499. doi: 10.1128/iai.61.4.1496-1499.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wei J, Xu H, Davies JL, Hemmings GP. Increase of plasma IL-6 concentration with age in healthy subjects. Life Sci. 1992;51:1953–1956. doi: 10.1016/0024-3205(92)90112-3. [DOI] [PubMed] [Google Scholar]
- 44.Ershler WB, Sun WH, Binkley N, et al. Interleukin-6 and aging: blood levels and mononuclear cell production increase with advancing age and in vitro production is modifiable by dietary restriction. Lymphokine Cytokine Res. 1993;12:225–230. [PubMed] [Google Scholar]
- 45.Hager K, Machein U, Krieger S, Platt D, Seefried G, Bauer J. Interleukin-6 and selected plasma proteins in healthy persons of different ages. Neurobiol Aging. 1994;15:771–772. doi: 10.1016/0197-4580(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 46.McKane WR, Khosla S, Peterson JM, Egan K, Riggs BL. Circulating levels of cytokines that modulate bone resorption: effects of age and menopause in women. J Bone Miner Res. 1994;9:1313–1318. doi: 10.1002/jbmr.5650090821. [DOI] [PubMed] [Google Scholar]
- 47.Kania DM, Binkley N, Checovich M, Havighurst T, Schilling M, Ershler WB. Elevated plasma levels of interleukin-6 in postmenopausal women do not correlate with bone density. J Am Geriatr Soc. 1995;43:236–239. doi: 10.1111/j.1532-5415.1995.tb07328.x. [DOI] [PubMed] [Google Scholar]
- 48.Cohen HJ, Pieper CF, Harris T, Rao KM, Currie MS. The association of plasma IL-6 levels with functional disability in community-dwelling elderly. J Gerontol A Biol Sci Med Sci. 1997;52A:M201–M208. doi: 10.1093/gerona/52a.4.m201. [DOI] [PubMed] [Google Scholar]
- 49.Harris TB, Ferrucci L, Tracy RP, et al. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 1999;106:506–512. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- 50.Ferrucci L, Corsi A, Lauretani F, et al. The origins of age-related proinflammatory state. Blood. 2005;105:2294–2299. doi: 10.1182/blood-2004-07-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Walston J, Arking DE, Fallin D, et al. IL-6 gene variation is not associated with increased serum levels of IL-6, muscle, weakness, or frailty in older women. Exp Gerontol. 2005;40:344–352. doi: 10.1016/j.exger.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 52.Sarkar D, Fisher P. Molecular mechanisms of aging-associated inflammation. Cancer Lett. 2005 Jun 21; doi: 10.1016/j.canlet.2005.04.009. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 53.Giuliani N, Sansoni P, Girasole G, et al. Serum interleukin-6, soluble interleukin-6 receptor and soluble gp130 exhibit different patterns of age- and menopause-related changes. Exp Gerontol. 2001;36:547–557. doi: 10.1016/s0531-5565(00)00220-5. [DOI] [PubMed] [Google Scholar]
- 54.Weigert C, Hennige AM, Brodbeck K, Haring HU, Schleicher ED. Interleukin-6 acts as insulin sensitizer on glycogen synthesis in human skeletal muscle cells by phosphorylation of Ser473 of Akt. Am J Physiol Endocrinol Metab. 2005;289:E251–E257. doi: 10.1152/ajpendo.00448.2004. [DOI] [PubMed] [Google Scholar]
- 55.Weigert C, Hennige AM, Lehmann R, et al. Direct cross-talk of interleukin-6 and insulin signal transduction via insulin receptor substrate-1 in skeletal muscle cells. Biol Chem. 2006 Jan 17; doi: 10.1074/jbc.M509782200. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 56.Mohamed-Ali V, Goodrick S, Rawesh A, et al. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J Clin Endocrinol Metab. 1997;82:4196–4200. doi: 10.1210/jcem.82.12.4450. [DOI] [PubMed] [Google Scholar]
- 57.Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab. 1998;83:847–850. doi: 10.1210/jcem.83.3.4660. [DOI] [PubMed] [Google Scholar]
- 58.Abbatecola A, Ferrucci L, Grella R, et al. Diverse effect of inflammatory markers on insulin resistance and insulin-resistance syndrome in the elderly. J Am Geriatr Soc. 2004;52:399–404. doi: 10.1111/j.1532-5415.2004.52112.x. [DOI] [PubMed] [Google Scholar]
- 59.Senn JJ, Klover PJ, Nowak IA, et al. Suppressor of cytokine signaling-3 (SOCS-3), a potential mediator of interleukin-6-dependentbinsulin resistance in hepatocytes. J Biol Chem. 2003;278:13740–13746. doi: 10.1074/jbc.M210689200. [DOI] [PubMed] [Google Scholar]
- 60.Nappo F, Esposito K, Cioffi M, et al. Postprandial endothelial activation in healthy subjects and in type 2 diabetic patients: role of fat and carbohydrate meals. J Am Coll Cardiol. 2002;39:1145–1150. doi: 10.1016/s0735-1097(02)01741-2. [DOI] [PubMed] [Google Scholar]
- 61.Ferrucci L, Guralnik JM, Woodman RC, et al. Proinflammatory state and circulating erythropoietin in persons with and without anemia. Am J Med. 2005;118:1288. doi: 10.1016/j.amjmed.2005.06.039. [DOI] [PubMed] [Google Scholar]
- 62.Esposito K, Marfella R, Ciotola M, et al. Effect of a Mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA. 2004;292:1440–1446. doi: 10.1001/jama.292.12.1440. [DOI] [PubMed] [Google Scholar]
- 63.Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol. 2005;98:1154–1662. doi: 10.1152/japplphysiol.00164.2004. [DOI] [PubMed] [Google Scholar]
- 64.Elosua R, Bartali B, Ordovas JM, Corsi AM, Lauretani F, Ferrucci L InCHIANTI Investigators. Association between physical activity, physical performance, and inflammatory biomarkers in an elderly population: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2005;60A:760–767. doi: 10.1093/gerona/60.6.760. [DOI] [PubMed] [Google Scholar]
- 65.Bruun JM, Helge JW, Richelsen B, Stallknecht B. Diet and Exercise Reduce Low-grade Inflammation and Macrophage Infiltration in Adipose Tissue but not in Skeletal Muscle in Severely Obese Subjects. Am J Physiol Endocrinol Metab. 2005 Dec 13; doi: 10.1152/ajpendo.00506.2005. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 66.Heymann D, Rousselle AV. gp130 Cytokine family and bone cells. Cytokine. 2000;12:1455–1468. doi: 10.1006/cyto.2000.0747. [DOI] [PubMed] [Google Scholar]
- 67.Udagawa N, Takahashi N, Katagiri T, et al. Interleukin (IL)-6 induction of osteoclast differentiation depends on IL-6 receptors expressed on osteoblastic cells but not on osteoclast progenitors. J Exp Med. 1995;182:1461–1468. doi: 10.1084/jem.182.5.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bertolini DR, Votta B, Hoffman S, Strassmann G. Interleukin 6 production in fetal rat long bone cultures is correlated with PGE2 release and does not correlate with the extent of bone resorption. Cytokine. 1994;6:368–375. doi: 10.1016/1043-4666(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 69.Ishimi Y, Miyaura C, Jin CH, et al. IL-6 is produced by osteoblasts and induces bone resorption. J Immunol. 1990;145:3297–3303. [PubMed] [Google Scholar]
- 70.Miyaura C, Kusano K, Masuzawa T, et al. Endogenous bone-resorbing factors in estrogen deficiency: cooperative effects of IL-1 and IL-6. J Bone Miner Res. 1995;10:1365–1373. doi: 10.1002/jbmr.5650100914. [DOI] [PubMed] [Google Scholar]
- 71.Pacifici R. Aging and cytokine production. Calcif Tissue Int. 1999;65:345–351. doi: 10.1007/s002239900710. [DOI] [PubMed] [Google Scholar]
- 72.Ferrari SL, Karasik D, Liu J, et al. Interactions of interleukin-6 promoter polymorphisms with dietary and lifestyle factors and their association with bone mass in men and women from the Framingham Osteoporosis Study. J Bone Miner Res. 2004;19:552–559. doi: 10.1359/JBMR.040103. [DOI] [PubMed] [Google Scholar]
- 73.Moffett SP, Zmuda JM, Cauley JA, et al. SOF Research Group. Association of the G-174C variant in the interleukin-6 promoter region with bone loss and fracture risk in older women. J Bone Miner Res. 2004;19:1612–1618. doi: 10.1359/JBMR.040707. [DOI] [PubMed] [Google Scholar]
- 74.Straub RH, Konecna L, Hrach S, et al. Serum dehydroepiandrosterone (DHEA) and DHEA sulfate are negatively correlated with serum interleukin-6 (IL-6), and DHEA inhibits IL-6 secretion from mononuclear cells in man in vitro: possible link between endocrinosenescence and immunosenescence. J Clin Endocrinol Metab. 1998;83:2012–2017. doi: 10.1210/jcem.83.6.4876. [DOI] [PubMed] [Google Scholar]
- 75.Pfeilschifter J, Koditz R, Pfohl M, Schatz H. Changes in proinflammatory cytokine activity after menopause. Endocr Rev. 2002;23:90–119. doi: 10.1210/edrv.23.1.0456. [DOI] [PubMed] [Google Scholar]
- 76.Girasole G, Giuliani N, Modena AB, Passeri G, Pedrazzoni M. Oestrogens prevent the increase of human serum soluble interleukin-6 receptor induced by ovariectomy in vivo and decrease its release in human osteoblastic cells in vitro. Clin Endocrinol (Oxf) 1999;51:801–807. doi: 10.1046/j.1365-2265.1999.00896.x. [DOI] [PubMed] [Google Scholar]
- 77.Jilka RL, Passeri G, Girasole G, et al. Estrogen loss upregulates hematopoiesis in the mouse: a mediating role of IL-6. Exp Hematol. 1995;23:500–506. [PubMed] [Google Scholar]
- 78.Manolagas SC, Jilka RL. Bone marrow, cytokines, and bone remodeling. Emerging insights into the pathophysiology of osteoporosis. N Engl J Med. 1995;332:305–311. doi: 10.1056/NEJM199502023320506. [DOI] [PubMed] [Google Scholar]
- 79.Berg G, Ekerfelt C, Hammar M, Lindgren R, Matthiesen L, Ernerudh J. Cytokine changes in postmenopausal women treated with estrogens: a placebo-controlled study. Am J Reprod Immunol. 2002;48:63–69. doi: 10.1034/j.1600-0897.2002.01061.x. [DOI] [PubMed] [Google Scholar]
- 80.Prestwood KM, Unson C, Kulldorff M, Cushman M. The effect of different doses of micronized 17beta-estradiol on C-reactive protein, interleukin-6, and lipids in older women. J Gerontol A Biol Sci Med Sci. 2004;59A:827–832. doi: 10.1093/gerona/59.8.m827. [DOI] [PubMed] [Google Scholar]
- 81.D’Elia HF, Mattsson LA, Ohlsson C, Nordborg E, Carlsten H. Hormone replacement therapy in rheumatoid arthritis is associated with lower serum levels of soluble IL-6 receptor and higher insulin-like growth factor 1. Arthritis Res. 2003;5:R202–R209. doi: 10.1186/ar761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.D’Agostino P, Milano S, Barbera C, et al. Sex hormones modulate inflammatory mediators produced by macrophages. Ann N Y AcadSci. 1999;876:426–429. doi: 10.1111/j.1749-6632.1999.tb07667.x. [DOI] [PubMed] [Google Scholar]
- 83.Bellido T, Jilka RL, Boyce BF, et al. Regulation of interleukin-6, osteoclastogenesis, and bone mass by androgens. The role of the androgen receptor. J Clin Invest. 1995;95:2886–2895. doi: 10.1172/JCI117995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hofbauer LC, Ten RM, Khosla S. The anti-androgen hydroxyflutamide and androgens inhibit interleukin-6 production by an androgen-responsive human osteoblastic cell line. J Bone Miner Res. 1999;14:1330–1337. doi: 10.1359/jbmr.1999.14.8.1330. [DOI] [PubMed] [Google Scholar]
- 85.Maggio M, Basaria S, Ble A, et al. Correlation between testosterone and the inflammatory marker soluble interleukin-6 receptor in older men. J Clin Endocrinol Metab. 2006;91:345–347. doi: 10.1210/jc.2005-1097. [DOI] [PubMed] [Google Scholar]
- 86.Khosla S, Atkinson EJ, Dunstan CR, O’Fallon WM. Effect of estrogen versus testosterone on circulating osteoprotegerin and other cytokine levels in normal elderly men. J Clin Endocrinol Metab. 2002;87:1550–1554. doi: 10.1210/jcem.87.4.8397. [DOI] [PubMed] [Google Scholar]
- 87.Guler N, Batyraliev T, Dulger H, et al. The effects of short term (3 weeks) testosterone treatment on serum inflammatory markers in men undergoing coronary artery stenting. Int J Cardiol. 2005 doi: 10.1016/j.ijcard.2005.06.027. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 88.Lambert CP, Sullivan DH, Evans WJ. Effects of testosterone replacement and/or resistance training on interleukin-6, tumor necrosis factor alpha, and leptin in elderly men ingesting megestrol acetate: a randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2003;58A:165–170. doi: 10.1093/gerona/58.2.m165. [DOI] [PubMed] [Google Scholar]
- 89.Malkin CJ, Pugh PJ, Jones RD, Kapoor D, Channer KS, Jones TH. The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. J Clin Endocrinol Metab. 2004;89:3313–3318. doi: 10.1210/jc.2003-031069. [DOI] [PubMed] [Google Scholar]
- 90.Keller ET, Chang C, Ershler WB. Inhibition of NFkappaB activity through maintenance of I kappa B alpha levels contributes to dihydrotestosterone-mediated repression of the interleukin-6 promoter. J Biol Chem. 1996;271:26267–26275. doi: 10.1074/jbc.271.42.26267. [DOI] [PubMed] [Google Scholar]
- 91.Iwasaki Y, Asai M, Yoshida M, Nigawara T, Kambayashi M, Nakashima N. Dehydroepiandrosterone-sulfate inhibits nuclear factor-kappaB-dependent transcription in hepatocytes, possibly through antioxidant effect. J Clin Endocrinol Metab. 2004;89:3449–3454. doi: 10.1210/jc.2003-031441. [DOI] [PubMed] [Google Scholar]
- 92.Morley JE, Baumgartner RM. Cytokine-related aging process. J Gerontol A Biol Sci Med Sci. 2004;59A:M924–M929. doi: 10.1093/gerona/59.9.m924. [DOI] [PubMed] [Google Scholar]
- 93.Leng SX, Cappola AR, Andersen RE, et al. Serum levels of insulin-like growth factor-I (IGF-I) and dehydroepiandrosterone sulfate (DHEA-S), and their relationships with serum interleukin-6, in the geriatric syndrome of frailty. Aging Clin Exp Res. 2004;16:153–157. doi: 10.1007/BF03324545. [DOI] [PubMed] [Google Scholar]
- 94.Taaffe DR, Harris TB, Ferrucci L, Rowe J, Seeman TE. Cross-sectional and prospective relationships of interleukin-6 and C-reactive protein with physical performance in elderly persons: MacArthur studies of successful aging. J Gerontol A Biol Sci Med Sci. 2000;55A:M709–M715. doi: 10.1093/gerona/55.12.m709. [DOI] [PubMed] [Google Scholar]
- 95.Cesari M, Penninx BW, Pahor M, et al. Inflammatory markers and physical performance in older persons: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2004;59A:242–248. doi: 10.1093/gerona/59.3.m242. [DOI] [PubMed] [Google Scholar]
- 96.Leng S, Chaves P, Koenig K, Walston J. Serum interleukin-6 and hemoglobin as physiological correlates in the geriatric syndrome of frailty: a pilot study. J Am Geriatr Soc. 2002;50:1268–1271. doi: 10.1046/j.1532-5415.2002.50315.x. [DOI] [PubMed] [Google Scholar]
- 97.Ferrucci L, Harris TB, Guralnik JM, et al. Serum IL-6 level and the development of disability in older persons. J Am Geriatr Soc. 1999;47:639–646. doi: 10.1111/j.1532-5415.1999.tb01583.x. [DOI] [PubMed] [Google Scholar]
- 98.Cappola AR, Bandeen-Roche K, Wand GS, Volpato S, Fried LP. Association of IGF-I levels with muscle strength and mobility in older women. J Clin Endocrinol Metab. 2001;86:4139–4146. doi: 10.1210/jcem.86.9.7868. [DOI] [PubMed] [Google Scholar]
- 99.Barbieri M, Ferrucci L, Ragno E, et al. Chronic inflammation and the effect of IGF-I on muscle strength and power in older persons. Am J Physiol Endocrinol Metab. 2003;284:E481–E487. doi: 10.1152/ajpendo.00319.2002. [DOI] [PubMed] [Google Scholar]
- 100.Cappola AR, Xue QL, Ferrucci L, Guralnik JM, Volpato S, Fried LP. Insulin-like growth factor I and interleukin-6 contribute synergistically to disability and mortality in older women. J Clin Endocrinol Metab. 2003;88:2019–2025. doi: 10.1210/jc.2002-021694. [DOI] [PubMed] [Google Scholar]
- 101.Haddad F, Zaldivar F, Cooper DM, Adams GR. IL-6-induced skeletal muscle atrophy. J Appl Physiol. 2005;98:911–917. doi: 10.1152/japplphysiol.01026.2004. [DOI] [PubMed] [Google Scholar]
- 102.Ferrucci L, Ble A, Bandinelli S, Windham BG, Simonsick EM. Inflammation: the fire of frailty? Longevity and Frailty. In: Carey JR, Robine J-M, Michel JP, Christen Y, editors. Research and Perspectives in Longevity. XII. New York: Springer; 2005. pp. 91–98. [Google Scholar]
- 103.Barton BE. Interleukin-6 and new strategies for the treatment of cancer, hyperproliferative diseases and paraneoplastic syndromes. Expert Opin Ther Targets. 2005;9:737–752. doi: 10.1517/14728222.9.4.737. [DOI] [PubMed] [Google Scholar]
- 104.Petrucci MT, Ricciardi MR, Ariola C, et al. Cell cycle regulation and induction of apoptosis by IL-6 variants on the multiple myeloma cell line XG-1. Ann Hematol. 1999;78:13–18. doi: 10.1007/s002770050465. [DOI] [PubMed] [Google Scholar]
- 105.Brocke-Heidrich K, Kretzschmar AK, Pfeifer G, et al. Interleukin-6-dependent gene expression profiles in multiple myeloma INA-6 cells reveal a Bcl-2 family-independent survival pathway closely associated with Stat3 activation. Blood. 2004;103:242–251. doi: 10.1182/blood-2003-04-1048. [DOI] [PubMed] [Google Scholar]
- 106.Shariat SF, Andrews B, Kattan MW, Kim J, Wheeler TM, Slawin KM. Plasma levels of interleukin-6 and its soluble receptor are associated with prostate cancer progression and metastasis. Urology. 2001;58:1008–1015. doi: 10.1016/s0090-4295(01)01405-4. [DOI] [PubMed] [Google Scholar]
- 107.Trikha M, Corringham R, Klein B, Rossi JF. Targeted anti-interleukin-6 monoclonal antibody therapy for cancer: a review of the rationale and clinical evidence. Clin Cancer Res. 2003;9:4653–4665. [PMC free article] [PubMed] [Google Scholar]
- 108.Kishimoto T. Interleukin-6: from basic science to medicine—40 years in immunology. Annu Rev Immunol. 2005;23:1–21. doi: 10.1146/annurev.immunol.23.021704.115806. [DOI] [PubMed] [Google Scholar]
- 109.Soulier J, Grollet L, Oksenhendler E, et al. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman’s disease. Blood. 1995;86:1276–1280. [PubMed] [Google Scholar]
- 110.Kopantzev Y, Heller M, Swaminathan N, Rudikoff S. IL-6 mediated activation of STAT3 bypasses Janus kinases in terminally differentiated B lineage cells. Oncogene. 2002;21:6791–6800. doi: 10.1038/sj.onc.1205815. [DOI] [PubMed] [Google Scholar]
- 111.Pulkki K, Pelliniemi TT, Rajamaki A, Tienhaara A, Laakso M, Lahtinen R. Soluble interleukin-6 receptor as a prognostic factor in multiple myeloma. Finnish Leukaemia Group. Br J Haematol. 1996;92:370–374. doi: 10.1046/j.1365-2141.1996.d01-1470.x. [DOI] [PubMed] [Google Scholar]
- 112.Kyrtsonis MC, Dedoussis G, Zervas C, et al. Soluble interleukin-6 receptor (sIL-6R), a new prognostic factor in multiple myeloma. Br J Haematol. 1996;93:398–400. doi: 10.1046/j.1365-2141.1996.4721018.x. [DOI] [PubMed] [Google Scholar]
- 113.Kyriakou D, Papadaki H, Eliopoulos AG, Foudoulakis A, Alexandrakis M, Eliopoulos GD. Serum soluble IL-6 receptor concentrations correlate with stages of multiple myeloma defined by serum beta 2-microglobulin and C-reactive protein. Int J Hematol. 1997;66:367–371. doi: 10.1016/s0925-5710(97)00055-8. [DOI] [PubMed] [Google Scholar]
- 114.Jones SA, Richards PJ, Scheller J, Rose-John S. IL-6 transsignaling: the in vivo consequences. J Interferon Cytokine Res. 2005;25:241–253. doi: 10.1089/jir.2005.25.241. [DOI] [PubMed] [Google Scholar]
- 115.Wadt KA, Larsen CM, Andersen HU, Nielsen K, Karlsen AE, Mandrup-Poulsen T. Ciliary neurotrophic factor potentiates the beta-cell inhibitory effect of IL-1beta in rat pancreatic islets associated with increased nitric oxide synthesis and increased expression of inducible nitric oxide synthase. Diabetes. 1998;47:1602–1608. doi: 10.2337/diabetes.47.10.1602. [DOI] [PubMed] [Google Scholar]
- 116.Rabinovitch A, Suarez-Pinzon WL. Role of cytokines in the pathogenesis of autoimmune diabetes mellitus. Rev Endocr Metab Disord. 2003;4:291–299. doi: 10.1023/a:1025160614313. [DOI] [PubMed] [Google Scholar]
- 117.Choi SE, Choi KM, Yoon IH, et al. IL-6 protects pancreatic islet beta cells from pro-inflammatory cytokines-induced cell death and functional impairment in vitro and in vivo. Transpl Immunol. 2004;13:43–53. doi: 10.1016/j.trim.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 118.Pilstrom B, Bjork L, Bohme J. Demonstration of a TH1 cytokine profile in the late phase of NOD insulitis. Cytokine. 1995;7:806–814. doi: 10.1006/cyto.1995.0097. [DOI] [PubMed] [Google Scholar]
- 119.Kristiansen OP, Mandrup-Poulsen T. Interleukin-6 and diabetes: the good, the bad, or the indifferent? Diabetes. 2005;54(Suppl 2):S114–S124. doi: 10.2337/diabetes.54.suppl_2.s114. [DOI] [PubMed] [Google Scholar]
- 120.Hu FB, Meigs JB, Li TY, Rifai N, Manson JE. Inflammatory markers and risk of developing type 2 diabetes in women. Diabetes. 2004;53:693–700. doi: 10.2337/diabetes.53.3.693. [DOI] [PubMed] [Google Scholar]
- 121.Spranger J, Kroke A, Mohlig M, et al. Inflammatory cytokines and the risk to develop type 2 diabetes: results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes. 2003;52:812–817. doi: 10.2337/diabetes.52.3.812. [DOI] [PubMed] [Google Scholar]
- 122.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 123.Baraldi-Junkins CA, Beck AC, Rothstein G. Hematopoiesis and cytokines. Relevance to cancer and aging. Hematol Oncol Clin North Am. 2000;14:45–61. doi: 10.1016/s0889-8588(05)70277-x. [DOI] [PubMed] [Google Scholar]
- 124.Nemeth E, Rivera S, Gabayan V, et al. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113:1271–1276. doi: 10.1172/JCI20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Means RT., Jr Advances in the anemia of chronic disease. Int J Hematol. 1999;70:7–12. [PubMed] [Google Scholar]
- 126.Schooley JC, Kullgren B, Allison AC. Inhibition by interleukin-1 of the action of erythropoietin on erythroid precursors and its possible role in the pathogenesis of hypoplastic anaemias. Br J Haematol. 1987;67:11–17. doi: 10.1111/j.1365-2141.1987.tb02289.x. [DOI] [PubMed] [Google Scholar]
- 127.Zucker S, Friedman S, Lysik RM. Bone marrow erythropoiesis in the anemia of infection, inflammation, and malignancy. J Clin Invest. 1974;53:1132–1138. doi: 10.1172/JCI107651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mitsuyama K, Toyonaga A, Sasaki E, et al. Soluble interleukin-6 receptors in inflammatory bowel disease: relation to circulating interleukin-6. Gut. 1995;36:45–49. doi: 10.1136/gut.36.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gross V, Andus T, Caesar I, Roth M, Scholmerich J. Evidence for continuous stimulation of interleukin-6 production in Crohn’s disease. Gastroenterology. 1992;102:514–519. doi: 10.1016/0016-5085(92)90098-j. [DOI] [PubMed] [Google Scholar]
- 130.Reinisch W, Gasche C, Tillinger W, et al. Clinical relevance of serum interleukin-6 in Crohn’s disease: single point measurements, therapy monitoring, and prediction of clinical relapse. Am J Gastroenterol. 1999;94:2156–2164. doi: 10.1111/j.1572-0241.1999.01288.x. [DOI] [PubMed] [Google Scholar]
- 131.Suzuki A, Hanada T, Mitsuyama K, et al. CIS3/SOCS3/SSI3 plays a negative regulatory role in STAT3 activation and intestinal inflammation. J Exp Med. 2001;193:471–481. doi: 10.1084/jem.193.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Holub MC, Mako E, Devay T, et al. Increased interleukin-6 levels, interleukin-6 receptor and gp130 expression in peripheral lymphocytes of patients with inflammatory bowel disease. Scand J Gastroenterol Suppl. 1998;228:47–50. [PubMed] [Google Scholar]
- 133.Hata H, Sakaguchi N, Yoshitomi H, et al. Distinct contribution of IL-6, TNF-alpha, IL-1, and IL-10 to T cell-mediated spontaneous autoimmune arthritis in mice. J Clin Invest. 2004;114:582–588. doi: 10.1172/JCI21795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Houssiau FA, Devogelaer JP, Van Damme J, de Deuxchaisnes CN, VanSnick J. Interleukin-6 in synovial fluid and serum of patients with rheumatoid arthritis and other inflammatory arthritides. Arthritis Rheum. 1988;31:784–788. doi: 10.1002/art.1780310614. [DOI] [PubMed] [Google Scholar]
- 135.Kotake S, Sato K, Kim KJ, et al. Interleukin-6 and soluble interleukin-6 receptors in the synovial fluids from rheumatoid arthritis patients are responsible for osteoclast-like cell formation. J Bone Miner Res. 1996;11:88–95. doi: 10.1002/jbmr.5650110113. [DOI] [PubMed] [Google Scholar]
- 136.Madhok R, Crilly A, Watson J, Capell HA. Serum interleukin 6 levels in rheumatoid arthritis: correlations with clinical and laboratory indices of disease activity. Ann Rheum Dis. 1993;52:232–234. doi: 10.1136/ard.52.3.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cacquevel M, Lebeurrier N, Cheenne S, Vivien D. Cytokines in neuroinflammation and Alzheimer’s disease. Curr Drug Targets. 2004;5:529–534. doi: 10.2174/1389450043345308. [DOI] [PubMed] [Google Scholar]
- 138.Marz P, Heese K, Hock C, et al. Interleukin-6 (IL-6) and soluble forms of IL-6 receptors are not altered in cerebrospinal fluid of Alzheimer’s disease patients. Neurosci Lett. 1997;239:29–32. doi: 10.1016/s0304-3940(97)00886-0. [DOI] [PubMed] [Google Scholar]
- 139.Hampel H, Sunderland T, Kotter HU, et al. Decreased soluble inter-leukin-6 receptor in cerebrospinal fluid of patients with Alzheimer’s disease. Brain Res. 1998;780:356–359. doi: 10.1016/s0006-8993(97)01355-3. [DOI] [PubMed] [Google Scholar]
- 140.Traenckner EB, Wilk S, Baeuerle PA. A proteasome inhibitor prevents activation of NF-kappa B and stabilizes a newly phosphorylated form of I kappa B-alpha that is still bound to NF-kappa B. Embo J. 1994;13:5433–5441. doi: 10.1002/j.1460-2075.1994.tb06878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Palombella VJ, Conner EM, Fuseler JW, et al. Role of the proteasome and NF-kappa B in streptococcal cell wall-induced polyarthritis. Proc Natl Acad Sci U S A. 1998;95:15671–15676. doi: 10.1073/pnas.95.26.15671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Voorhees PM, Orlowski RZ. The proteasome and proteasome inhibitors in cancer therapy. Annu Rev Pharmacol Toxicol. 2006;46:189–213. doi: 10.1146/annurev.pharmtox.46.120604.141300. [DOI] [PubMed] [Google Scholar]
- 143.Catley L, Tai YT, Chauhan D, Anderson KC. Perspectives for combination therapy to overcome drug-resistant multiple myeloma. Drug Resist Updat. 2005;8:205–218. doi: 10.1016/j.drup.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 144.Sporeno E, Savino R, Ciapponi L, et al. Human interleukin-6 receptor super-antagonists with high potency and wide spectrum on multiple myeloma cells. Blood. 1996;87:4510–4519. [PubMed] [Google Scholar]
- 145.Petrucci MT, Ricciardi MR, Ariola C, et al. Cell cycle regulation and induction of apoptosis by IL-6 variants on the multiple myeloma cell line XG-1. Ann Hematol. 1999;78:13–18. doi: 10.1007/s002770050465. [DOI] [PubMed] [Google Scholar]
- 146.Hodge DR, Peng B, Cherry JC, et al. Interleukin 6 supports the maintenance of p53 tumor suppressor gene promoter methylation. Cancer Res. 2005;65:4673–4682. doi: 10.1158/0008-5472.CAN-04-3589. [DOI] [PubMed] [Google Scholar]
- 147.Sato K, Tsuchiya M, Saldanha J, et al. Humanization of a mouse anti-human interleukin-6 receptor antibody comparing two methods for selecting human framework regions. Mol Immunol. 1994;31:371–381. doi: 10.1016/0161-5890(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 148.Mihara M, Nishimoto N, Ohsugi Y. The therapy of autoimmune diseases by anti-interleukin-6 receptor antibody. Expert Opin Biol Ther. 2005;5:683–690. doi: 10.1517/14712598.5.5.683. [DOI] [PubMed] [Google Scholar]
- 149.Nishimoto N, Yoshizaki K, Miyasaka N, et al. Treatment of rheumatoid arthritis with humanized anti-interleukin-6 receptor antibody: a multicenter, double-blind, placebo-controlled trial. Arthritis Rheum. 2004;50:1761–1769. doi: 10.1002/art.20303. [DOI] [PubMed] [Google Scholar]
- 150.Tackey E, Lipsky PE, Illei GG. Rationale for interleukin-6 blockade in systemic lupus erythematosus. Lupus. 2004;13:339–343. doi: 10.1191/0961203304lu1023oa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Nishimoto N, Sasai M, Shima Y, et al. Improvement in Castleman’s disease by humanized anti-interleukin-6 receptor antibody therapy. Blood. 2000;95:56–61. [PubMed] [Google Scholar]
- 152.Nishimoto N, Kanakura Y, Aozasa K, et al. Humanized anti-interleukin-6 receptor antibody treatment of multicentric Castleman disease. Blood. 2005;106:2627–2632. doi: 10.1182/blood-2004-12-4602. [DOI] [PubMed] [Google Scholar]
- 153.Nowell MA, Richards PJ, Horiuchi S, et al. Soluble IL-6 receptor governs IL-6 activity in experimental arthritis: blockade of arthritis severity by soluble glycoprotein 130. J Immunol. 2003;171:3202–3209. doi: 10.4049/jimmunol.171.6.3202. [DOI] [PubMed] [Google Scholar]
- 154.Mitsuyama K, Tomiyasu N, Suzuki A, et al. A form of circulating interleukin-6 receptor component soluble gp130 as a potential interleukin-6 inhibitor in inflammatory bowel disease. Clin Exp Immunol. 2006;143:125–131. doi: 10.1111/j.1365-2249.2005.02960.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wang LH, Kirken RA, Erwin RA, Yu CR, Farrar WL. JAK3, STAT, and MAPK signalling pathways as novel molecular targets for the tyrphostin AG-490 regulation of IL-2-mediated T cell response. J Immunol. 1999;162:3897–3904. [PubMed] [Google Scholar]
- 156.Blaskovich MA, Sun J, Cantor A, Turkson J, Jove R, Sebti SM. Discovery of JSI-124 (cucurbitacin I), a selective Janus kinase/signal transducer and activator of transcription 3 signaling pathway inhibitor with potent antitumor activity against human and murine cancer cells in mice. Cancer Res. 2003;63:1270–1279. [PubMed] [Google Scholar]
- 157.Le Gouill S, Pellat-Deceunynck C, Harousseau JL, et al. Farnesyl transferase inhibitor R115777 induces apoptosis of human myeloma cells. Leukemia. 2002;16:1664–1667. doi: 10.1038/sj.leu.2402629. [DOI] [PubMed] [Google Scholar]
- 158.David E, Sun SY, Waller EK, Chen J, Khuri FR, Lonial S. The combination of the farnesyl transferase inhibitor lonafarnib and the proteasome inhibitor bortezomib induces synergistic apoptosis in human myeloma cells that is associated with down-regulation of p-AKT. Blood. 2005;106:4322–4329. doi: 10.1182/blood-2005-06-2584. [DOI] [PubMed] [Google Scholar]
- 159.Arnaud C, Burger F, Steffens S, et al. Statins reduce interleukin-6-induced C-reactive protein in human hepatocytes: new evidence for direct antiinflammatory effects of statins. Arterioscler Thromb Vasc Biol. 2005;25:1231–1236. doi: 10.1161/01.ATV.0000163840.63685.0c. [DOI] [PubMed] [Google Scholar]
- 160.Bharti AC, Donato N, Aggarwal BB. Curcumin (diferuloylmethane) inhibits constitutive and IL-6-inducible STAT3 phosphorylation in human multiple myeloma cells. J Immunol. 2003;171:3863–3871. doi: 10.4049/jimmunol.171.7.3863. [DOI] [PubMed] [Google Scholar]
- 161.Sun J, Blaskovich MA, Jove R, Livingston SK, Coppola D, Sebti SM. Cucurbitacin Q: a selective STAT3 activation inhibitor with potent antitumor activity. Oncogene. 2005;24:3236–3245. doi: 10.1038/sj.onc.1208470. [DOI] [PubMed] [Google Scholar]
- 162.Gleason EL, Hogan JC, Stephens JM. Stabilization, not polymerization, of microtubules inhibits the nuclear translocation of STATs in adipocytes. Biochem Biophys Res Commun. 2004;325:716–718. doi: 10.1016/j.bbrc.2004.10.081. [DOI] [PubMed] [Google Scholar]
- 163.Nagel-Wolfrum K, Buerger C, Wittig I, Butz K, Hoppe-Seyler F, Groner B. The interaction of specific peptide aptamers with the DNA binding domain and the dimerization domain of the transcription factor Stat3 inhibits transactivation and induces apoptosis in tumor cells. Mol Cancer Res. 2004;2:170–182. [PubMed] [Google Scholar]