Abstract
The National Institute on Aging (NIA) Geriatrics and Clinical Gerontology (GCG) Program convened an interdisciplinary Task Force on Comorbidity to foster the development of a research agenda on the multiple concurrent health problems that often occur in older persons. This report summarizes Task Force discussions held in Bethesda, Maryland (October 21–22, 2003; July 20–21, 2004) and serves as an introduction to the following three articles that address specific issues such as the nosological classification of impairment for the construction of comorbidity measures, staging and classification of disease severity, and methodological and analytical issues.
The risk of developing concomitant chronic illnesses and physiological limitations escalates with aging. Diabetes, respiratory diseases, cancer, cardiovascular problems, arthritis, hypertension, and certain other chronic conditions are more common in older than in younger persons. As a consequence, a new diagnosis of any common chronic health condition is likely to be made in the context of preexisting health problems.
Introduction
Advancing age is associated with increased vulnerability to chronic health problems. In later years of life, physiological decrements, chronic diseases, and other health problems tend to accumulate and complicate individuals’ health status and quality of life. New diagnoses of common problems add complexity to an older person’s health status, which is usually characterized by preexisting health problems.
Some changes involving natural and pathologic processes of aging affect almost everyone who lives long enough. Arthritis, hypertension, cancer, diabetes, osteoporosis, and Alzheimer’s disease occur primarily in older persons. Diseases of the cardiovascular, cerebrovascular, and pulmonary systems are other health conditions notably encountered in older individuals. Functional limitations, impairments, and geriatric syndromes (e.g., incontinence, falls, disability) are commonly associated with aging.
Certain pathological states are clinically evident and have a clear nosological definition; others remain subclinical (e.g., restricted reserve in organ systems) at least to a superficial investigation. In addition, a number of conditions typical of older persons clearly impact their health status (e.g., sarcopenia, anemia, chronic inflammation), but are not yet considered in the traditional disease nosology. Coexistence of these factors and the possibility of different interactions between or among them make evaluation of an older person’s overall health status extremely challenging.
The health care needs and comorbid health problems of older Americans call for a special focus. Thus, the NIA GCG Program convened a task force of leaders in research on aging from different disciplines and professions (e.g., geriatrics, gerontology, social science, nursing, medical specialties, and epidemiology) to explore conceptual and methodological complexities of comorbidity and its assessment. Task Force on Comorbidity members and consultants are listed in the Appendix.
William R. Hazzard, MD, chaired Task Force Meeting I. Harvey Jay Cohen, MD, chaired Task Force Meeting II. William Ershler, MD, and William Satariano, PhD, chaired the two break-out groups convened within each Task Force on Comorbidity meeting. The groups were charged with two related tasks: to lay the groundwork for research on medical treatment in the context of multiple health conditions, and to identify the steps needed to translate the concept of comorbidity into clinical practice.
Task Force Objectives
Identify research opportunities regarding important interactive health problems affecting elderly persons commonly faced by practitioners.
Propose research priorities to close the considerable knowledge gap on age-related comorbidity as it affects treatment efficacy and tolerance in older persons.
Identify research topics to improve diagnosis, prognosis, treatment, and prevention methods in older persons affected by comorbidity.
A synthesis of various perspectives expressed in the two Task Force meetings is provided in this report. Three NIA-commissioned background articles for Task Force discussions appear in this issue of the Journal.
Concept Exploration
What should be included under the term “comorbidity” is the first question.
Does the term include all geriatric assessment domains or is it restricted to biomedical problems?
Can priorities and hierarchical order be established for health problems?
What is the “natural history” of comorbidity when associated with an index condition?
In existing models, comorbidity is often considered as modulating the effect on health of an index condition and influencing diagnosis, treatment, and/or prognosis. Since this approach does not cover all situations, another alternative is emerging—multimorbidity.
What is the relationship between comorbidity and risk factors that involve pathological problems relevant across multiple diseases (e.g., inflammation, oxidative stress, and obesity)?
There was consensus that more focus on comorbidity is needed both in geriatric care and in clinical research on aging. The development of specific assessment tools is necessary to drive research and clinical care aimed at improving prognosis, diagnosis, treatment, and care of older persons with multiple health conditions. Yet the need for advancing conceptual and theoretical aspects of comorbidity should not be hampered by excessive simplification and operationalization—otherwise, there is a risk of losing sight of the real and practical issues.
It Depends on the Question
Given the complexity and heterogeneity involved in comorbidity, however, no single definition or measure would serve all research and clinical purposes. Rather, definition and measurement of comorbidity approaches may vary depending on practice or research objectives (e.g., clinical, epidemiological, health services) and outcomes of interest (i.e., patient physical function, public health needs, mortality). For example, the two terms “comorbidity” and “multimorbidity” (the latter term used more frequently in European studies and publications) are often used interchangeably but have different meanings.
Comorbidity indicates the co-occurrence of preexisting age-related health conditions (e.g., disability, anemia, impairments, urinary incontinence) or diseases (e.g., diabetes, heart disease, hypertension) in reference to an index disease (e.g., cancer, Parkinson’s disease, diabetes). On the other hand, multimorbidity is the co-occurrence of two or more diseases or active health conditions (e.g., aggregate of coequals) that may or may not be linked by a causal relationship or with no consistent dominant index disorder.
The full scope of assessing multimorbidity is currently a matter of debate. It could include a range of complex health problems and also embrace conditions such as impairment, disability, and physiological levels, alone or in some combination. Combining a mixture of diseases and conditions of variable severity, functional processes, and biological processes under a single term is quite complicated. Therefore, measuring multimorbidity may be intrinsically more difficult than concentrating on comorbidity.
Research Approaches/Frameworks
What is meant by comorbidity and/or multimorbidity varies according to whether the objective is research, diagnosis, prognosis, treatment, care, prevention, and etiology, alone or in some combination. At this stage, it is strategic to formulate specific questions, and then to develop the most adequate measurement tools to address such questions.
Second Conditions
Approaches that account for multiple pathways among conditions, mechanisms, and outcomes were highlighted. In clinical practice, thresholds for biological markers are often used for diagnostic purposes. For example, a hemoglobin level below 13 g/dL in men and below 12 g/dL in women is a diagnostic criterion for anemia.
There is a clear trade-off between the need for a standard, dichotomous definition for disease and the progressive and interacting nature of diseases. The clear-cut criteria play an important role in guiding clinicians’ ability to cure patients affected by single diseases and to predict effects of specific interventions. In the geriatric experience, however, it is becoming clear that the health status of older individuals is affected by the accumulation of biological dysfunctions in multiple systems. Each one may contribute to the clinical picture, even those whose severity is not yet over a diagnostic threshold for the standard definition of a disease. For example, borderline value of blood markers may become important predictors of outcome in older persons with substantial comorbidity.
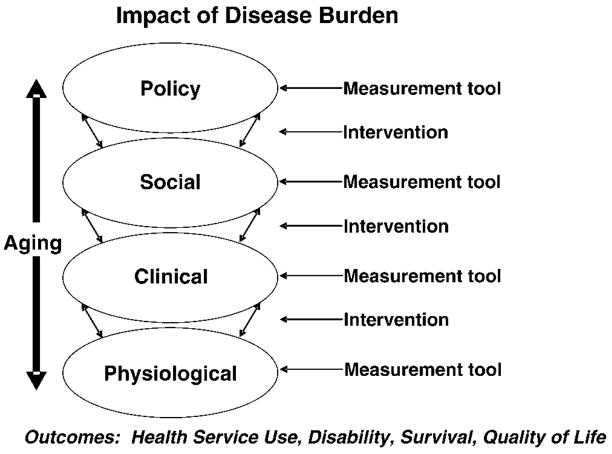
Dr. Jeanne Mandelblatt (Figure 1) proposed that research on comorbidity should consider subclinical and mechanistic approaches as two separate, but complementary pathways. In parallel, comorbidity indices should be developed to address specific outcomes (e.g., mortality, quality of life, disability, health care utilization). In this framework, one may look at comorbidity in each of the different areas to identify appropriate tools to measure overall aspects of health status that are relevant domains. Dr. Mandelblatt’s schema provides an overview of research complexity and issues. For example, on a continuum from risk factor to preclinical disease to overt disease, elements essential in a measure of multiple morbidities depend upon the nature of the research question.
Figure 1.
Impact of disease burden.
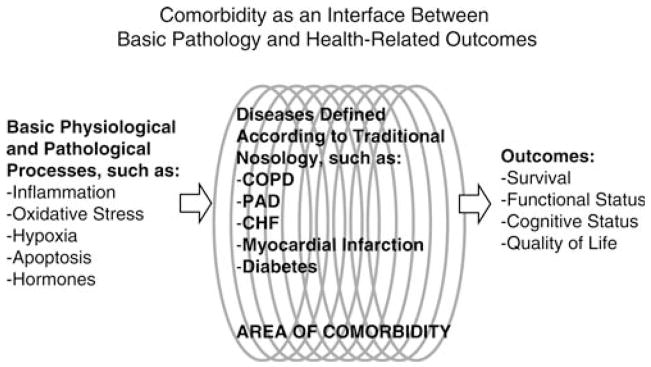
In Figure 2, designed by Dr. Luigi Ferrucci, comorbidity is conceptualized as an intermediate factor between physiological processes and final health outcomes (e.g., functional status, vital status).
Figure 2.
Comorbidity as an intermediate factor.
This paradigm emphasizes the interactions between conventional medical nosology and the geriatric approach of comprehensive assessment. In modern medicine, a detailed nosological classification of disease is used to accumulate experience about response to certain treatments and to future patients. However, in the geriatric experience, more emphasis is given to the accumulation of biologic dysfunctions in multiple systems that contribute to the overall clinical picture and have functional consequences. The basic biological dysfunctions that cause pathology are few (e.g., oxidative stress, inflammation), but their accumulation in different organ systems translates into different diseases and impairments. Ultimately, how physiological impairments and disease should be combined in comorbidity indices depends on their relationships with outcomes, which in geriatrics are mostly of a functional nature.
An inclusive biomedical framework of age-related issues that a practitioner or clinical researcher faces was presented by Dr. Evan Hadley (Table 1).
Table 1.
Biomedical Framework Presented by Dr. Evan Hadley
| Pathophysiology and Prognosis |
|
| Diagnosis |
|
| Treatment |
|
| Etiology |
|
Note: Dr. Hadley’s framework was used in NIA RFA-AG-05-007, Developing Interventions for Multiple Morbidities, issued December 14, 2004; receipt date January 13, 2005; Dr. Susan Nayfield, NIA, GCG, Program Contact.
Conditions relevant to comorbidity include disease, disabilities, and/or impairments, the interactions of which may affect etiology, pathophysiology, prognosis, diagnosis, and treatment. Two queries provided examples.
Does coexistence of frailty in older persons with cardiovascular disease increase cardiovascular mortality?
Does a combination of osteoarthritis with vision impairments have synergistic effects on mobility or disability?
Measures of Comorbidity and Multimorbidity Assessment
The technical and practical aspects of measurement and assessment were not addressed per se in the two Task Force meetings. There was concurrence, however, that assessment of physical and cognitive functioning and limitations should be incorporated into all research and clinical care involving older persons afflicted with chronic disease(s). To capture an overall health status of a patient, one needs to understand frailty, function, and disease. Diseases cannot be left out. We already know so much about their response to treatment. Diseases represent the interface between traditional medicine and geriatrics.
Selection of measurement tools for health appraisal varies according to the setting, population, and research questions. A challenging aspect in using summary measures of comorbidity concerns how to categorize and determine influence of particular combinations of conditions. Indeed, summary measures may impede progress in understanding issues of sequencing, the impact of sequencing, and nonlinearity. Current measures and methods, diverse in content and approaches, have different outcome goals and are limited in prognostic perspective. They include interviews, self-reports, medical record reviews, death certificates, administrative and medical record databases, summary indices derived from presence of selected conditions, and, less frequently, observations of physical functioning of patients. Combined effects of the comorbid conditions are not often determined. Indices that do approach the relationship between and among conditions are limited.
Relative to comorbidity and multimorbidity indicators, three measurement issues were cited: 1) Limitations in functional status. The degree of independence or difficulty in basic and instrumental activities of daily living should be considered for inclusion in all comorbidity evaluations. 2) Severity levels of comorbidity and multimorbidity should address additive and multiplicative relationships. 3) Although biologic and physical responses within individuals are major foci of treatment and care, they are not disconnected from social and psychological events and changes occurring in older patients’ lives.
Summary of Central Issues
Various comorbid conditions overlap and contribute to patient complexity. The minimal array of health and medical conditions (i.e., diseases, age-related problems, behaviors) require evaluation and inclusion into an index that is sensitive and adequate to address one or more research questions.
Physical disability in older patients has certain medical consequences not present in older patients with no disability. For example, a physically impaired individual will likely have greater health care needs (e.g., increased risk for falls and acute illnesses, assistance with activities of daily living).
Synergism of disease pairs and association of comorbidity and disability warrant explicit attention to special severity criteria.
Health problems arising due to the index disease itself and/or its treatment must be considered.
Temporal relationship of disease severity and overall comorbidity burden must be addressed.
Effects of number and types of drugs, alone or in combination, on expected responses and adverse events must be examined.
The critical question is how best to utilize and apply the multilayered systems of information (e.g., on diseases, other health conditions, impairments, functioning, or disabilities) on behalf of older persons. Table 2 outlines issues that warrant further conceptual clarification and progress to improve prevention, earlier detection, prognosis benefit, and treatment decisions of health conditions typical of olderpatients.
Table 2.
Research Questions
| Physiologic and Biologic Interactions |
|
| Multiplicative Effects of Specific Sets of Diseases and Conditions |
|
| Biological Markers |
|
| Impairment |
|
| Diseases and Health Conditions |
|
| Animal Models |
|
Information derived from the NIA Task Force members’ expertise and discussions will enhance geriatric research program planning efforts. This report shares the excellent conceptual and substantive contributions of all Task Force members and consultants who enthusiastically engaged in an exchange of scientific insights in the discussions directed at the important focus on comorbidity and multiple morbidities in older persons.
Acknowledgments
Although the articles included in this section were supported by the NIA, opinions, findings, conclusions, or recommendations expressed in the articles belong to the authors, and do not necessarily reflect the view of the NIA.
The NIA is grateful for the outstanding leadership provided by Dr. William Hazzard and Dr. Harvey J. Cohen, who chaired Task Force Meetings I and II, respectively. The resourceful assistance of Dr. William Ershler and Dr. William Satariano is appreciated for their leadership and effective guidance as chairpersons of the two Task Force break-out groups in both meetings.
The authors are pleased to acknowledge Dr. Jeanne Mandelblatt’s comprehensive conceptual contribution displayed in Figure 1, Dr. Luigi Ferrucci’s rendition of “a common sense” paradigm in Figure 2, and Dr. Evan Hadley’s biomedical framework of age-related issues in Table 1.
The Task Force research recommendations in Table 2 and potential lines of inquiry in Table 3 emphasize interdisciplinary approaches reflecting the make-up of the NIA Task Force on comorbidity itself. We are grateful for the many thoughtful suggestions and issues raised by the participants. Special thanks to the Study Team efforts that resulted in the three background articles included in this issue.
Table 3.
Potential Lines of Inquiry
|
Appendix
National Institute on Aging Comorbidity Task Force Members
Harvey Jay Cohen, MD
Duke University Medical Center, Durham, NC
William Ershler, MD
NIA Intramural Program, Baltimore MD
Institute for Advanced Studies in Aging and Geriatric Medicine, Washington, DC
Martine Extermann, MD
University of South Florida, Tampa, FL
Luigi Ferrucci, MD
NIA Intramural Program, Baltimore, MD
Linda Fried, MD
Cynthia Boyd, MD (Consultant)
Johns Hopkins Medical Institutions, Baltimore, MD
Jerry H. Gurwitz, MD
Meyers Primary Care Institute, Worcester, MA
Jeffrey Halter, MD
University of Michigan, Ann Arbor, MI
William R. Hazzard, MD
VA Puget Sound Health Care Systems, Seattle, WA
Carrie Klabunde, PhD
National Cancer Institute, NIH, Bethesda, MD
Jeanne S. Mandelblatt, MD, MPH
Georgetown University Medical Center, Washington
Vincent Mor, PhD
Brown University, Providence, RI
Marco Pahor, MD
University of Florida, Gainesville, FL
David Reuben, MD
Arun Karlamangla, MD (Consultant)
University of California, Los Angeles, CA
William Satariano, PhD
University of California at Berkeley, CA
Rebecca Silliman, MD, PhD
Timothy Lash, DPH (Consultant)
Boston Medical Center, Boston, MA
Stephanie Studenski, MD
University of Pittsburgh, Pittsburgh, PA
Mary Tinetti, MD
Yale University School of Medicine, New Haven, CT
Claudette G. Varricchio, DSN, RN
National Institute of Nursing Research, NIH
Terrie Wetle, PhD
Brown University, Providence, RI
Darryl Wieland, PhD, MPH
Palmetto Richland Memorial Hospital, Columbia, SC
National Institute on Aging Staff
Jack Guralnik, MD, PhD (NIA Intramural Program)
Evan Hadley, MD
J. Taylor Harden, PhD, RN
Susan Molchan, MD
Susan Nayfield, MD
Rosemary Yancik, PhD
National Institutes of Health, Bethesda, MD
References
- 1.van den Akker M, Buntinx F, Roos S, Knottnerus JA. Problems in determining occurrence rates of multimorbidity. J Clin Epidemiol. 2001;54:675–679. doi: 10.1016/s0895-4356(00)00358-9. [DOI] [PubMed] [Google Scholar]
- 2.Karlamangla A, Tinetti M, Guralnik J, Studenski S, Wetle T, Reuben D. Comorbidity in older adults: nosology of impairment, diseases, and conditions. J Gerontol A Biol Sci Med Sci. 2007;62A:296–300. doi: 10.1093/gerona/62.3.296. [DOI] [PubMed] [Google Scholar]
- 3.Lash TL, Mor V, Wieland D, Ferrucci L, Satariano W, Silliman RA. Methodology, design, and analytic techniques to address measurement of comorbid disease. J Gerontol A Biol Sci Med Sci. 2007;62A:281–285. doi: 10.1093/gerona/62.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyd CM, Weiss CO, Halter J, Han C, Ershler WB, Fried LF. Framework for evaluating disease severity measures in older adults with comorbidity. J Gerontol A Biol Sci Med Sci. 2007;62A:286–295. doi: 10.1093/gerona/62.3.286. [DOI] [PubMed] [Google Scholar]