Abstract
Background
Dehydroepiandrosterone sulfate (DHEAS) is an endogenously produced sex steroid that has been hypothesized to have anti-aging effects. Low DHEAS levels are associated with mortality in older men, but the relationship between DHEAS levels and mortality in women is not clearly defined.
Methods
The relationship between serum DHEAS level and 5-year mortality was analyzed in a cohort of 539 disabled women aged 65–100 years enrolled in the Women’s Health and Aging Study I (WHAS I). Using Cox proportional hazard models, we calculated multivariate-adjusted mortality risks by DHEAS quartiles and by DHEAS continuously, allowing for a nonlinear relationship. We also examined cause-specific mortality.
Results
We found a U-shaped relationship between DHEAS level and mortality. After adjusting for multiple covariates, women in the top and bottom DHEAS quartiles had a more than 2-fold higher 5-year mortality than did those in the middle quartiles (hazard ratio, 2.15; 95% confidence interval [CI], 1.17–3.98 for the top quartile and 2.05; 95% CI, 1.27–3.32 for the bottom quartile, each compared to the third quartile). Women with higher DHEAS levels tended to have greater cancer mortality, whereas those with lower DHEAS tended to have greater cardiovascular mortality.
Conclusion
Disabled older women with either low or high levels of DHEAS are at greater risk for death than are those with intermediate levels. More research is needed to determine if targeted dehydroepiandrosterone supplementation would provide clinical benefit to disabled older women.
Dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS) are the major sex steroids produced by the adrenal glands. As more than 99% of all DHEA is sulfated to DHEAS before secretion from the adrenal gland, DHEAS represents the primary circulating form of DHEA, with a long plasma half-life and little diurnal variation (1). DHEAS is the most abundant sex steroid, with concentrations more than 100-fold higher than any other sex steroid (2). DHEAS is thought to act principally as a prohormone via extra- and intracellular conversion to biologically active androgens and estrogens (2,3). Because of the widespread distribution of estrogen and androgen receptors, tissue-level conversion of DHEAS has the potential for pervasive systemic effects (4). Furthermore, due to differences in the underlying sex steroid and enzymatic milieu, sex-specific effects may arise from DHEAS conversion (2,5).
Circulating DHEAS levels decline significantly with age, resulting in mean levels at age 65 that are less than one fifth of mean levels at age 20 (6,7). The implications of age-associated DHEAS decline and differences in DHEAS levels between older individuals are poorly understood. Low DHEAS has been associated with increased all-cause and cardiovascular mortality in older men (8–11). The relationship between DHEAS and mortality is not clearly defined in healthy women, and not defined at all in disabled women (8,10–14), however.
Understanding the role of endogenous DHEAS is critical to understanding the biology of aging, potentially preventable outcomes such as frailty or premature mortality, and whether there are appropriate targets for DHEA supplementation. Although mean DHEAS levels are low in old age, DHEAS levels vary widely in both sexes (5,15). Despite insufficient data to support DHEA therapy in old age (16), the Dietary Supplement Health and Education Act of 1994 allows its over-the-counter purchase (17), and many older individuals take DHEA supplements, regardless of their DHEAS levels.
We sought to evaluate the relationship between underlying DHEAS levels and mortality in a large population-based sample of disabled older women. Due to evidence suggesting adverse effects in women from either high or low DHEAS levels (18–20), we hypothesized a nonlinear relationship between DHEAS and mortality, and a cause-specific mortality difference between those women with low and those with high DHEAS levels.
Methods
Participants
The Women’s Health and Aging Study I (WHAS I) is a study of the causes and course of disability among community-dwelling moderately to severely disabled older women. The study design is described in detail elsewhere (21,22). Briefly, a random sample of 6521 community-dwelling women ≥ 65 years old was selected from the Health Care Financing Administration’s (HCFA) Medicare Eligibility list for Baltimore, Maryland. Women who reported difficulty with ≥ 1 task in ≥ 2 of 4 domains of functioning (mobility/exercise tolerance, upper extremity activities, basic self-care, and household management tasks) and who had a Mini Mental State Examination (MMSE) (23) score of ≥ 18 were eligible for the study. Seventy-one percent (1002) of those eligible (1409) agreed to participate. The Johns Hopkins University Institutional Review Board approved the study, and all participants gave written informed consent.
Questionnaires were administered and examinations were performed in the participant’s home at 6-month intervals over 3 years beginning in 1992. Baseline blood samples were obtained within 90 days of one of the first examinations in 628 participants, processed, and stored at −80°C. Sufficient serum was available for DHEAS analysis in 539 women. Participants without blood samples were older and had more disability in activities of daily living, but were not different in body mass index (BMI) or number of chronic diseases. None of the women in WHAS I were taking DHEA-containing supplements, as determined from examination of all prescription and over-the-counter medication containers.
Biochemical Measurements
DHEAS was measured in duplicate by enzyme-linked immunosorbent assay (ELISA; American Laboratory Products Company, Windham, NH) from frozen specimens. A previous study has shown the stability of DHEAS levels in samples stored for up to 15 years (6). The interassay coefficient of variation was 3.3%; assay sensitivity was 2 μg/dL (0.05 μmol/L). Data from two studies show low within-person variability in DHEAS levels over time, supporting its use as an indicator of long-term DHEAS status (7,24).
Mortality Follow-up
Vital status was obtained through follow-up interviews with proxies, obituaries, and matching with the National Death Index during a mean follow-up of 50 months (median 60 months). Death certificates were obtained for all but six of the women who died. Cause-specific mortality was based on underlying cause of death coded according to the International Classification of Diseases.
Covariates
Seventeen chronic diseases were ascertained at baseline with disease-specific standardized algorithms (22). Disease categories used in analysis included congestive heart failure, diabetes mellitus, peripheral arterial disease, stroke, coronary heart disease (angina or myocardial infarction), chronic obstructive pulmonary disease, hip fracture, osteoarthritis, rheumatoid arthritis, and malignant neoplasms (excluding basal cell cancer).
Sociodemographic characteristics included age, race, education, smoking status, and menopause type (natural vs surgical). BMI (kg/m2), computed from objective measures, was categorized as <18.5, 18.5–24.9, 25–29.9, or ≥30.
Statistical Analysis
To explore a potential nonlinear association between DHEAS levels and mortality, analysis by DHEAS quartile was performed using two sets of DHEAS quartile cutoffs. The first cutoff values were derived from the quartiles of the 539 women aged 65–101 years enrolled in WHAS I; the second, from 599 women aged 70–79 years enrolled in the combined WHAS I and II (25). Together, participants in both studies represent the full spectrum of function in community-dwelling women. The relationship between DHEAS and mortality was similar using both cutoffs. Therefore, we used those data derived from both WHAS I and II to provide more meaningful reference values. Baseline characteristics were compared by DHEAS quartile, using the chi-square test for binary outcomes and analysis of variance (ANOVA) for continuous outcomes. Kaplan–Meier analysis was used to study survival for the DHEAS quartiles across the 5-year follow-up. The log-rank test was used to compare survival curves. Cox proportional hazard regression models were used to compare mortality risk across DHEAS categories (26). All of the regression models included adjustment for age, education, race, smoking status, BMI, oral estrogen use, oral corticosteroid use, and baseline adjudicated chronic conditions (coronary heart disease, congestive heart failure, peripheral arterial disease, hip fracture, osteoarthritis, rheumatoid arthritis, diabetes mellitus, cancer, stroke, and chronic obstructive pulmonary disease). The third quartile is displayed as the reference group for greater ease of interpretation. To justify our selection of DHEAS quartile cutoffs, we subsequently fit a multivariate-adjusted Cox model with log DHEAS modeled as a continuous covariate. To accommodate a hypothesized U-shaped relationship between DHEAS and mortality, we used two models: a polynomial regression including a linear and a quadratic term for log DHEAS, and a two-piece linear spline fit that allowed different slopes for log DHEAS above versus below a given threshold value of log DHEAS using a partial likelihood approach (27). The threshold value was selected by fitting a series of Cox models by varying log DHEAS from −0.4 to 0.6 log (μmol/L) in 0.01 increments; the model with the best fit (smallest deviance) was selected. Either a significant quadratic term in the polynomial model or a significant difference between the slopes above versus below the threshold value would support a nonlinear relationship. Compared to the polynomial model, the spline model resulted in a better fit based on goodness-of-fit statistics; therefore, we chose the spline fit as our final model. Analyses were performed using SPLUS (version 2000; Insightful Inc., Seattle, WA).
Results
Baseline characteristics of the study population are summarized in Table 1. The mean age of participants was 77.6 years (range, 65–100 years). Women with the lowest DHEAS levels were older, more likely to be white, and taking oral estrogens or oral corticosteroids. Furthermore, they were more likely to have coronary heart disease or rheumatoid arthritis. DHEAS levels were not related to reproductive variables, such as menopause type or prior bilateral oophorectomy.
Table 1.
Baseline Characteristics by Reference Population–Based DHEAS Quartiles in 539 Women in WHAS I
| Characteristic | 1st Quartile (<22 μg/dL) N = 163 | 2nd Quartile (22–40 μg/dL) N = 147 | 3rd Quartile (40–68 μg/dL) N = 145 | 4th Quartile (>68 μg/dL) N = 84 | p Value* |
|---|---|---|---|---|---|
| Age, y† | 80.0 (7.6) | 76.9 (7.5) | 77.0 (8.1) | 75.4 (7.6) | <.001 |
| Black, % | 19.0 | 30.6 | 29.7 | 40.5 | <.01 |
| Education, y | 9.9 | 9.6 | 9.8 | 9.4 | .73 |
| Current smoking status, % | 9.2 | 12.2 | 12.4 | 15.5 | .53 |
| Body mass index, kg/m2 | 27.7 | 28.7 | 29.0 | 29.8 | .13 |
| Oral estrogen use, % | 12.9 | 6.8 | 4.1 | 4.8 | .02 |
| Oral corticosteroid use, % | 11.7 | 2.0 | 2.1 | 1.2 | <.001 |
| Natural menopause, % | 65.5 | 65.3 | 66.2 | 60.7 | .85 |
| Bilateral oophorectomy, % | 20.0 | 18.6 | 19.0 | 19.5 | .99 |
| Chronic diseases, % | |||||
| Osteoarthritis | 46.6 | 56.5 | 47.6 | 50.0 | .32 |
| Coronary heart disease | 38.0 | 35.4 | 29.0 | 21.4 | .04 |
| Chronic obstructive pulmonary disease | 31.3 | 32.0 | 24.8 | 25.0 | .40 |
| Peripheral arterial disease | 22.1 | 24.5 | 17.2 | 14.3 | .20 |
| Diabetes mellitus | 18.4 | 15.7 | 18.6 | 15.5 | .85 |
| Congestive heart failure | 12.9 | 10.2 | 11.7 | 3.6 | .14 |
| Cancer | 10.4 | 10.9 | 9.0 | 16.7 | .34 |
| Stroke | 7.4 | 6.8 | 4.1 | 4.8 | .61 |
| Rheumatoid arthritis | 6.8 | 0.7 | 2.8 | 1.2 | .01 |
| Hip fracture | 6.1 | 6.8 | 9.0 | 2.4 | .28 |
| Activities of daily living, %‡ | .95 | ||||
| 0 | 35.0 | 36.1 | 35.9 | 40.5 | |
| 1 | 28.8 | 29.3 | 29.7 | 25.0 | |
| 2 | 12.9 | 15.0 | 13.1 | 17.9 | |
| ≥3 | 23.3 | 19.7 | 21.4 | 16.7 | |
Notes: Multiply by .02714 to convert from conventional (μg/dL) to SI units (μmol/L).
Based on analysis of variance test for continuous outcomes and chi-square test for categorical outcomes.
Expressed as mean (standard deviation).
Number of activities of daily living tasks (bathing, getting in/out of bed, using toilet, dressing, eating) with any difficulty.
DHEAS = dehydroepiandrosterone sulfate; WHAS I = Women’s Health and Aging Study I.
After 5 years, 150 of 539 women (28%) had died. The Kaplan–Meier survival functions over the 5-year period, stratified by DHEAS level, are shown in Figure 1. Women in the bottom quartile had the highest mortality, followed by women in the top quartile, with women in the middle quartiles demonstrating the lowest mortality (p = .009).
Figure 1.
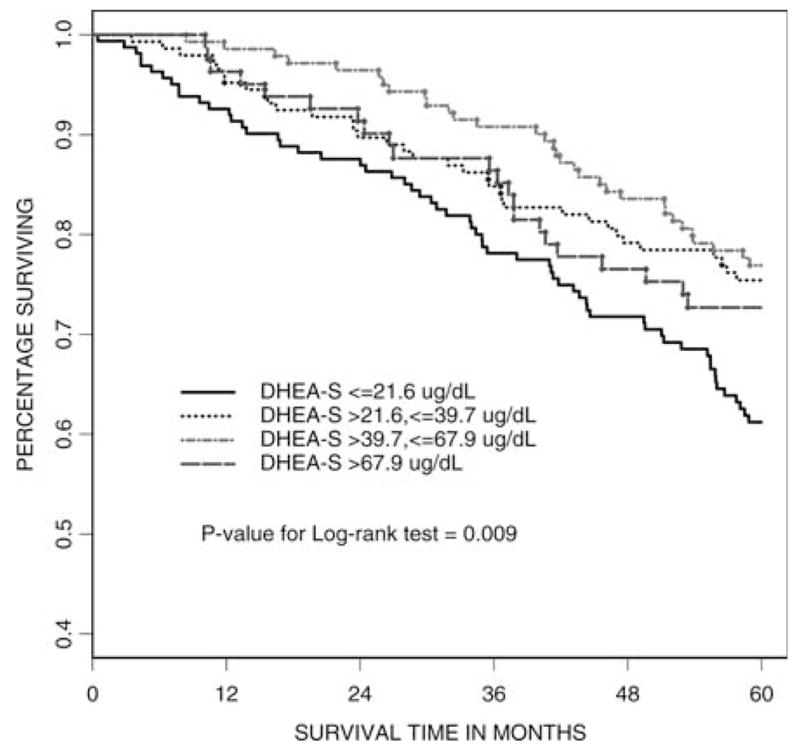
Five-year survival categorized by dehydroepiandrosterone sulfate (DHEAS) level in 539 women.
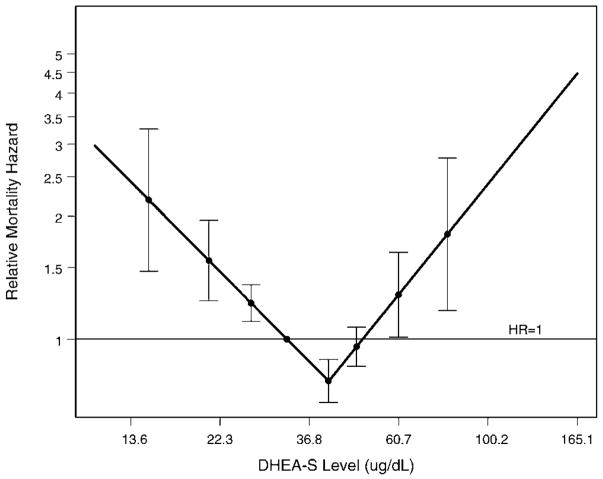
In Cox proportional hazard models, this U-shaped relationship between DHEAS levels and mortality was confirmed (Table 2). In multivariate-adjusted models, women in the top and bottom DHEAS quartiles had a more than 2-fold higher 5-year mortality than those in the middle quartiles (hazard ratio, 2.15; 95% confidence interval [CI], 1.17–3.98; p = .004 for the top quartile and 2.05; 95% CI, 1.27–3.32, p = .014 for the bottom quartile, each compared to the third quartile). The chronic disease covariates are the major contributors to the increase in magnitude in the top quartile estimate in the multivariate-adjusted analysis. We subsequently explored the dependency of the DHEAS–mortality relationship on our selected cutoff points. In Figure 2, the spline model of the multivariate-adjusted relative hazard of 5-year mortality is shown, further supporting increased risk at either extreme of DHEAS level. The DHEAS level associated with the lowest mortality risk in our cohort was determined to be 41 μg/dL. When we fit age and fully adjusted Cox proportional hazard models with log DHEAS as a continuous linear covariate, the regression coefficient for log DHEAS was not statistically significant (p = .07 and p = .11, respectively).
Table 2.
Adjusted Risk for 5-Year Mortality by DHEAS Quartile
| Hazard Ratio | 1st Quartile | 2nd Quartile | 3rd Quartile | 4th Quartile |
|---|---|---|---|---|
| Age-adjusted (95% CI) | 1.82 (1.16–2.87)* | 1.13 (0.67–1.90) | 1.0 (Reference) | 1.58 (0.88–2.84) |
| Multivariate-adjusted (95% CI)‡ | 2.05 (1.27–3.32)* | 1.18 (0.69–2.02) | 1.0 (Reference) | 2.15 (1.17–3.98)† |
Notes: p < .01 vs reference group.
p < .05 vs reference group.
Adjusted for age, education, race, smoking status, body mass index, estrogen use, corticosteroid use, chronic conditions including coronary heart disease, congestive heart failure, peripheral artery disease, hip fracture, osteoarthritis, rheumatoid arthritis, diabetes mellitus, cancer, stroke, and chronic obstructive pulmonary disease.
DHEAS = dehydroepiandrosterone sulfate; CI = confidence interval.
Figure 2.
Multivariate-adjusted relative hazard for 5-year mortality as a continuous function of log (dehydroepiandrosterone sulfate) (DHEAS) with a spline, standardized to the relative mortality hazard of the median DHEAS level. The lowest mortality risk is achieved when DHEAS = 41 μg/dL. The points represent deciles of DHEAS levels, excluding those with undetectable levels (bottom decile), and the error bars represent point-wise 95% confidence intervals.
To investigate differences in cause of death by DHEAS quartile, we next examined cause-specific mortality. Data from death certificates were available for 144 of the 150 deaths. As shown in Table 3, women with lower DHEAS levels appeared modestly more likely to die of cardiovascular causes, whereas women with higher levels appeared possibly more likely to die of cancer, though these findings were not statistically significant.
Table 3.
Cause-Specific Mortality by DHEAS Quartile
| Cause of Death | 1st Quartile | 2nd Quartile | 3rd Quartile | 4th Quartile | p Value* |
|---|---|---|---|---|---|
| Cardiovascular, % (N) | 47 (28) | 46 (15) | 43 (13) | 41 (9) | 25 |
| Cancer, % (N) | 19 (11) | 18 (6) | 27 (8) | 23 (5) | .77 |
| Other, % (N) | 34 (20) | 36 (12) | 30 (9) | 36 (8) | .33 |
Notes: Data are expressed as percentages, with number with each cause in parentheses.
Based on age-adjusted Cox proportional hazards model.
DHEAS = dehydroepiandrosterone sulfate.
Discussion
Disabled older women with low or high levels of DHEAS were at greater risk for death over a 5-year period than were women with intermediate levels, even after accounting for multiple other factors associated with mortality. This U-shaped relationship between hormone level and adverse effects is a common paradigm in endocrinology; examples of pairs of disorders representing deficiency and excess include hyperthyroidism, hypothyroidism, Cushing’s syndrome and adrenal insufficiency.
The role of DHEAS in aging has been investigated primarily in terms of the impact of low DHEAS levels, due to their overall gradual decline after puberty. Several studies (18,20,28) have linked low baseline DHEAS levels with cardiovascular disease, muscle weakness, and depressed mood. However, longitudinal studies of individuals older than 65 years have confirmed that 30% of them do not experience a decline in levels (11,15,29). Furthermore, high DHEAS levels have been associated with breast cancer risk (19); the latter finding, in particular, led us to examine mortality risk associated with both high and low DHEAS levels.
Several studies have examined the relationship between DHEAS levels and mortality in healthier older women. Results from 1171 women aged 65–76 years who participated in the Cambridge General Practice Health Study suggested a U shape (8). These results were not statistically significant, possibly due to fewer deaths in this younger, healthier population, which had only 10% mortality over the follow-up period, compared to 28% in our study. The Helsinki Aging Study (14) showed no difference in 5-year mortality rate by age-stratified DHEAS quartiles, though mortality rates in 75-year-old women displayed a U shape. The PAQUID (Personnes Agée QUID) study, which did not allow for a U-shaped relationship, did not show an association between DHEAS levels and mortality (10,11). In a preliminary report from the Rancho Bernardo Study (12), a U-shaped relationship was seen between DHEAS and cardiovascular mortality, though not all-cause mortality. Subsequent analyses with longer follow-up have shown no relationship between DHEAS and cardiovascular mortality, though models allowing for a nonlinear relationship were not used (13,30). The use of models allowing for a nonlinear relationship is critical in analyzing hormonal data. As illustrated in previous studies and ours, a link between DHEAS and mortality was not present in models using DHEAS as a linear variable, but became apparent when nonlinear associations were permitted. Our study, in contrast to the previous studies, does not ignore the underlying biologic paradigm, allowing our models to represent most accurately the distribution of DHEAS data.
Our results suggest two interpretations: Deficiency or excess of DHEAS mediates adverse effects that increase mortality risk, or DHEAS is a marker of health status, without any pathogenic effects of its own. Mechanistic data support a potential physiologic role of DHEAS in mortality risk. DHEAS may have anti-inflammatory properties, decreasing cardiovascular risk; immune regulatory activity, improving autoimmunity; or it may be converted to estrogen, promoting hormone-responsive cancers (4,31,32). Our cause-specific mortality data, though preliminary, support these specific pathways of DHEAS effects. However, examples also exist of hormonal deficiencies in response to coexistent illness, such as the nonthyroidal illness (euthyroid sick) syndrome, correction of which has not consistently resulted in beneficial outcomes (33). Indeed, in a small study of younger individuals undergoing cholecystectomy, DHEAS levels declined significantly within 1 week of this acute stress, though longer follow-up was not available (34). Conversely, adrenocorticotropic hormone-dependent stimulation of adrenal androgen synthesis is present, though blunted, with age (35); thus, it is possible that high DHEAS levels are a marker of physiologic stress. This possibility is supported by data from a small study of older patients in whom DHEAS levels rose immediately following cardiac surgery and returned to baseline levels within 30 days (36). Although we adjusted for multiple potential confounders, including 10 different comorbid diseases, we cannot exclude the possibility that DHEAS levels represent indicators of health status. Studies of disabled women that assess the impact of altering DHEAS levels toward an optimal level are required to distinguish between these possibilities and to clarify the role of DHEAS in disability and old age.
We identified a U-shaped relationship using several different modeling strategies and after adjustment for multiple factors. WHAS I, a cohort designed to represent the one-third most disabled women living in the community, comprised a group of women who are in need of beneficial interventions. The use of sampling from the HCFA database instead of a volunteer recruitment mechanism and home visits instead of travel to a study clinic enabled us to enroll and closely follow a representative sample of women who ordinarily exclude themselves or are excluded from research studies. Although DHEAS is the major circulating form of the hormone, DHEA is the formulation used in exogenous administration. Interventional studies of DHEA in adults with adrenal insufficiency have demonstrated clear benefits in lean body mass, bone mineral density, mood, and fatigue (4). Data in healthy older individuals, however, do not consistently support benefits of DHEA therapy (4,16). The largest randomized, placebo-controlled trial, the DHEAge Study, included 140 men and 140 women aged 60–79 years randomized to DHEA or placebo for 1 year. No benefits were found in muscle strength, muscle cross-sectional area, cognition, or well-being in healthy older men and women, but a slight improvement was seen in bone mineral density and libido in women > 70 years (35,37). Recent preliminary data from a randomized trial of 56 men and women treated with DHEA for 6 months showed decreases in visceral fat and improvements in insulin sensitivity, emphasizing the need for continued research in this area (38).
There are many unanswered questions regarding the appropriate target population for DHEA therapy. Classified as a dietary supplement, DHEA has not undergone the U.S. Food and Drug Administration (FDA)–mandated testing required of other hormones, despite widespread usage without medical supervision. Furthermore, it is not required to be manufactured in compliance with the FDA’s Good Manufacturing Practices, with one study showing that only 7 of 16 tested preparations had DHEA content within 90%–110% of the labeled claim (39). No trials have continued for more than 1 year, included hard clinical endpoints, or studied the relevant population of older people with disability, raising questions regarding its long-term risk/benefit ratio, particularly given the potential harm from raising tissue levels of estrogen and testosterone. Our data do not suggest that older women with higher endogenous DHEAS levels have greater longevity. Rather, the disabled older women studied here with higher levels had higher mortality, as did those with the lowest levels. Although this was an observational study, these data suggest that administration of additional DHEA to women with higher DHEAS levels through supplements would not be beneficial, and would be potentially harmful. More research is needed to determine if targeted DHEA supplementation would provide clinical benefit to disabled older women. At present, there is insufficient evidence from our study and others to recommend DHEA supplementation to improve the health status of older individuals.
Acknowledgments
This research was supported by National Institute on Aging (NIA) grant K23 AG19161, an American Federation for Aging Research (AFAR)/Pfizer Research Grant, NIA Contract N01-AG-1-2112, NIA grants R01-AG11703 and R37-AG19905, and the Johns Hopkins Hospital and Johns Hopkins Bayview Medical Center General Clinical Research Centers (GCRCs).
References
- 1.Longcope C. Metabolism of dehydroepiandrosterone. Ann N Y Acad Sci. 1995;774:143–148. doi: 10.1111/j.1749-6632.1995.tb17378.x. [DOI] [PubMed] [Google Scholar]
- 2.Labrie F. Adrenal androgens and intracrinology. Semin Reprod Med. 2004;22:299–309. doi: 10.1055/s-2004-861547. [DOI] [PubMed] [Google Scholar]
- 3.Yen SS. Dehydroepiandrosterone sulfate and longevity: new clues for an old friend. Proc Natl Acad Sci U S A. 2001;98:8167–8169. doi: 10.1073/pnas.161278698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arlt W. Dehydroepiandrosterone and ageing. Best Pract Res Clin Endocrinol Metab. 2004;18:363–380. doi: 10.1016/j.beem.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Laughlin GA, Barrett-Connor E. Sexual dimorphism in the influence of advanced aging on adrenal hormone levels: the Rancho Bernardo Study. J Clin Endocrinol Metab. 2000;85:3561–3568. doi: 10.1210/jcem.85.10.6861. [DOI] [PubMed] [Google Scholar]
- 6.Orentreich N, Brind JL, Rizer RL, et al. Age changes and sex differences in serum dehydroepiandrosterone sulfate concentrations throughout adulthood. J Clin Endocrinol Metab. 1984;59:551–555. doi: 10.1210/jcem-59-3-551. [DOI] [PubMed] [Google Scholar]
- 7.Nafziger AN, Bowlin SJ, Jenkins PL, et al. Longitudinal changes in dehydroepiandrosterone concentrations in men and women [see comments] J Lab Clin Med. 1998;131:316–323. doi: 10.1016/s0022-2143(98)90181-0. [DOI] [PubMed] [Google Scholar]
- 8.Trivedi DP, Khaw KT. Dehydroepiandrosterone sulfate and mortality in elderly men and women. J Clin Endocrinol Metab. 2001;86:4171–4177. doi: 10.1210/jcem.86.9.7838. [DOI] [PubMed] [Google Scholar]
- 9.Barrett-Connor E, Khaw KT, Yen SS. A prospective study of dehydroepiandrosterone sulfate, mortality, and cardiovascular disease. N Engl J Med. 1986;315:1519–1524. doi: 10.1056/NEJM198612113152405. [DOI] [PubMed] [Google Scholar]
- 10.Berr C, Lafont S, Debuire B, et al. Relationships of dehydroepiandrosterone sulfate in the elderly with functional, psychological, and mental status, and short-term mortality: a French community-based study. Proc Natl Acad Sci U S A. 1996;93:13410–13415. doi: 10.1073/pnas.93.23.13410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mazat L, Lafont S, Berr C, et al. Prospective measurements of dehydroepiandrosterone sulfate in a cohort of elderly subjects: relationship to gender, subjective health, smoking habits, and 10-year mortality. Proc Natl Acad Sci U S A. 2001;98:8145–8150. doi: 10.1073/pnas.121177998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barrett-Connor E, Khaw KT. Absence of an inverse relation of dehydroepiandrosterone sulfate with cardiovascular mortality in post-menopausal women. N Engl J Med. 1987;317:711. doi: 10.1056/NEJM198709103171116. [DOI] [PubMed] [Google Scholar]
- 13.Barrett-Connor E, Edelstein SL. A prospective study of dehydroepiandrosterone sulfate and cognitive function in an older population: the Rancho Bernardo Study. J Am Geriatr Soc. 1994;42:420–423. doi: 10.1111/j.1532-5415.1994.tb07491.x. [DOI] [PubMed] [Google Scholar]
- 14.Tilvis RS, Kahonen M, Harkonen M. Dehydroepiandrosterone sulfate, diseases and mortality in a general aged population. Aging (Milano) 1999;11:30–34. [PubMed] [Google Scholar]
- 15.Orentreich N, Brind JL, Vogelman JH, et al. Long-term longitudinal measurements of plasma dehydroepiandrosterone sulfate in normal men. J Clin Endocrinol Metab. 1992;75:1002–1004. doi: 10.1210/jcem.75.4.1400863. [DOI] [PubMed] [Google Scholar]
- 16.Allolio B, Arlt W. DHEA treatment: myth or reality? Trends Endocrinol Metab. 2002;13:288–294. doi: 10.1016/s1043-2760(02)00617-3. [DOI] [PubMed] [Google Scholar]
- 17.Dietary Supplement Heath and Education Act of 1994. Public Law 103–417.
- 18.Tchernof A, Labrie F. Dehydroepiandrosterone, obesity and cardiovascular disease risk: a review of human studies. Eur J Endocrinol. 2004;151:1–14. doi: 10.1530/eje.0.1510001. [DOI] [PubMed] [Google Scholar]
- 19.The Endogenous Hormones and Breast Cancer Collaborative Group. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst. 2002;94:606–616. doi: 10.1093/jnci/94.8.606. [DOI] [PubMed] [Google Scholar]
- 20.Barrett-Connor E, von Muhlen D, Laughlin GA, et al. Endogenous levels of dehydroepiandrosterone sulfate, but not other sex hormones, are associated with depressed mood in older women: the Rancho Bernardo Study. J Am Geriatr Soc. 1999;47:685–691. doi: 10.1111/j.1532-5415.1999.tb01590.x. [DOI] [PubMed] [Google Scholar]
- 21.Kasper JD, Shapiro S, Guralnik JM, et al. Designing a community study of moderately to severely disabled older women: the Women’s Health and Aging Study. Ann Epidemiol. 1999;9:498–507. doi: 10.1016/s1047-2797(99)00026-5. [DOI] [PubMed] [Google Scholar]
- 22.The Women’s Health and Aging Study: Health and Social Characteristics of Older Women with Disability. Bethesda, MD: National Institute on Aging; 1995. [Google Scholar]
- 23.Folstein MF, Folstein SE, McHugh PR. Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 24.Thomas G, Frenoy N, Legrain S, et al. Serum dehydroepiandrosterone sulfate levels as an individual marker. J Clin Endocrinol Metab. 1994;79:1273–1276. doi: 10.1210/jcem.79.5.7962319. [DOI] [PubMed] [Google Scholar]
- 25.Cappola AR, Bandeen-Roche K, Wand GS, et al. Association of IGF-I levels with muscle strength and mobility in older women. J Clin Endocrinol Metab. 2001;86:4139–4146. doi: 10.1210/jcem.86.9.7868. [DOI] [PubMed] [Google Scholar]
- 26.Prentice RL, Gloeckler LA. Regression analysis of grouped survival data with application to breast cancer data. Biometrics. 1978;34:57–67. [PubMed] [Google Scholar]
- 27.Hall CB, Lipton RB, Sliwinski M, et al. A change point model for estimating the onset of cognitive decline in preclinical Alzheimer’s disease. Stat Med. 2000;19:1555–1566. doi: 10.1002/(sici)1097-0258(20000615/30)19:11/12<1555::aid-sim445>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 28.Valenti G, Denti L, Maggio M, et al. Effect of DHEAS on skeletal muscle over the life span: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2004;59A:466–472. doi: 10.1093/gerona/59.5.m466. [DOI] [PubMed] [Google Scholar]
- 29.Kahonen MH, Tilvis RS, Jolkkonen J, et al. Predictors and clinical significance of declining plasma dehydroepiandrosterone sulfate in old age. Aging (Milano) 2000;12:308–314. doi: 10.1007/BF03339852. [DOI] [PubMed] [Google Scholar]
- 30.Barrett-Connor E, Goodman-Gruen D. The epidemiology of DHEAS and cardiovascular disease. Ann N Y Acad Sci. 1995;774:259–270. doi: 10.1111/j.1749-6632.1995.tb17386.x-i1. [DOI] [PubMed] [Google Scholar]
- 31.Iwasaki Y, Asai M, Yoshida M, et al. Dehydroepiandrosterone-sulfate inhibits nuclear factor-kappaB-dependent transcription in hepatocytes, possibly through antioxidant effect. J Clin Endocrinol Metab. 2004;89:3449–3454. doi: 10.1210/jc.2003-031441. [DOI] [PubMed] [Google Scholar]
- 32.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 33.Stathatos N, Levetan C, Burman KD, et al. The controversy of the treatment of critically ill patients with thyroid hormone. Best Pract Res Clin Endocrinol Metab. 2001;15:465–478. doi: 10.1053/beem.2001.0164. [DOI] [PubMed] [Google Scholar]
- 34.Osorio A, Vara-Thorbeck R, Rosell J, et al. Dehydroepiandrosterone sulfate and growth axis hormones in patients after surgery. World J Surg. 2002;26:1079–1082. doi: 10.1007/s00268-002-6368-7. [DOI] [PubMed] [Google Scholar]
- 35.Baulieu EE, Thomas G, Legrain S, et al. Dehydroepiandrosterone (DHEA), DHEA sulfate, and aging: contribution of the DHEAge Study to a sociobiomedical issue. Proc Natl Acad Sci U S A. 2000;97:4279–4284. doi: 10.1073/pnas.97.8.4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maggio M, Ceda GP, De Cicco G, et al. Acute changes in circulating hormones in older patients with impaired ventricular function undergoing on-pump coronary artery bypass grafting. J Endocrinol Invest. 2005;28:711–719. doi: 10.1007/BF03347554. [DOI] [PubMed] [Google Scholar]
- 37.Percheron G, Hogrel JY, Denot-Ledunois S, et al. Effect of 1-year oral administration of dehydroepiandrosterone to 60- to 80-year-old individuals on muscle function and cross-sectional area: a double-blind placebo-controlled trial. Arch Intern Med. 2003;163:720–727. doi: 10.1001/archinte.163.6.720. [DOI] [PubMed] [Google Scholar]
- 38.Villareal DT, Holloszy JO. Effect of DHEA on abdominal fat and insulin action in elderly women and men: a randomized controlled trial. JAMA. 2004;292:2243–2248. doi: 10.1001/jama.292.18.2243. [DOI] [PubMed] [Google Scholar]
- 39.Parasrampuria J, Schwartz K, Petesch R. Quality control of dehydroepiandrosterone dietary supplement products. JAMA. 1998;280:1565. doi: 10.1001/jama.280.18.1565. [DOI] [PubMed] [Google Scholar]