Abstract
Background
Although consumption of 3 meals/d is the most common pattern of eating in industrialized countries, a scientific rationale for this meal frequency with respect to optimal health is lacking. A diet with less meal frequency can improve the health and extend the lifespan of laboratory animals, but its effect on humans has never been tested.
Objective
A pilot study was conducted to establish the effects of a reduced-meal-frequency diet on health indicators in healthy, normal-weight adults.
Design
The study was a randomized crossover design with two 8-wk treatment periods. During the treatment periods, subjects consumed all of the calories needed for weight maintenance in either 3 meals/d or 1 meal/d.
Results
Subjects who completed the study maintained their body weight within 2 kg of their initial weight throughout the 6-mo period. There were no significant effects of meal frequency on heart rate, body temperature, or most of the blood variables measured. However, when consuming 1 meal/d, subjects had a significant increase in hunger; a significant modification of body composition, including reductions in fat mass; significant increases in blood pressure and in total, LDL-, and HDL-cholesterol concentrations; and a significant decrease in concentrations of cortisol.
Conclusions
Normal-weight subjects are able to comply with a 1 meal/d diet. When meal frequency is decreased without a reduction in overall calorie intake, modest changes occur in body composition, some cardiovascular disease risk factors, and hematologic variables. Diurnal variations may affect outcomes.
Keywords: Caloric restriction, meal frequency, intermittent fasting, cholesterol metabolism, blood pressure, controlled diet, normal-weight adults
INTRODUCTION
Overeating is a major cause of morbidity and mortality in humans, and, accordingly, caloric restriction has multiple health benefits for the obese (1, 2). Caloric restriction may also improve the health of persons who are not considered overweight (3). Whereas nutrient-dense, low-calorie diets have numerous health benefits, the influence of meal frequency on health has not been established. However, studies of various laboratory animals (4–6) and specifically of rodents (5, 7–9) have shown that dietary restriction (caloric restriction or intermittent fasting—long-term intermittent fasting in the case of rodents) can increase lifespan and protect against or suppress disease processes responsible for cardiovascular disease (CVD), cancer, diabetes, and neurodegenerative disorders. The latter studies (5, 7–9) showed beneficial effects of intermittent fasting on blood pressure, glucose metabolism, and vulnerability of cardiac and brain cells to injury. Despite a general perception among the public at large that it is important to eat ≥3 meals/d, no controlled studies have directly compared the effects of different meal frequencies on human health. This knowledge gap has been identified by the 2005 Dietary Guidelines Advisory Committee Report as a future research direction (10, 11).
Studies of rodents and monkeys have led to several hypotheses concerning the cellular and molecular mechanisms whereby dietary restriction extends lifespan and protects against disease. The oxidative stress hypothesis proposes that aging and age-related diseases result from cumulative oxidative damage to proteins, lipids, and nucleic acids; by decreasing the amount of oxyradicals produced in mitochondria, dietary restriction retards aging and disease (12). A second hypothesis is that dietary restriction is beneficial primarily because of its effects on energy metabolism; ie, it increases insulin sensitivity (13). A third hypothesis, which may have a particular relation to the beneficial effects of reduced meal frequency/intermittent fasting, is that dietary restriction induces a mild cellular stress response in which cells up-regulate the expression of genes that enable them to cope with severe stress (12). Several physiologic variables have been shown to change in animals maintained on caloric restriction or intermittent fasting regimens (or both), including decreased plasma insulin and glucose concentrations, decreased blood pressure and heart rate, and enhanced immune function (8, 14–16)
The current pilot study was conducted to determine the feasibility of controlled meal frequency in normal-weight, middle-aged men and women. Several physiologic outcomes and biomarkers of health were also investigated.
SUBJECTS AND METHODS
Subjects
Healthy men and women aged 40–50 y were recruited by newspaper advertisement from the greater Washington, DC, metropolitan area. Inclusion in the study was based on a body mass index (BMI; in kg/m2) between 18 and 25 and a usual eating pattern of 3 meals/d. Subjects were excluded if they reported tobacco use, recent pregnancy or lactation, history of CVD or medication use for CVD, hypertension, diabetes, psychiatric condition, cancer, or employment in high-risk occupations (this last exclusion criterion was due to the potential for dizziness or weakness during the meal-skipping phase). Study entry was approved by a physician on the basis of medical history, blood and urine test screening results, and a physical examination.
All subjects gave written informed consent. The experimental protocol was approved by the Johns Hopkins University Bloomberg School of Public Health Committee on Human Research and the MedStar Research Institute Institutional Review Board. The subjects were compensated for their participation in the study.
Study design
This study was a randomized crossover design with two 8-wk treatment periods. During the treatment periods, subjects consumed all of their calories for weight maintenance distributed in either 3 meals/d (control diet) or 1 meal/d (experimental diet). An 11-wk washout period was included between treatments. The control diet consisted of 3 meals/d (breakfast, lunch, and dinner) and the experimental “meal-skipping” (or 1 meal/d) diet consisted of the same daily allotment of food eaten within a 4-h period in the early evening. The subjects were fed at an energy intake that would maintain body weight so that meal frequency would be the only major change in their diet during the course of the study. The study was a collaborative effort between the US Department of Agriculture, Beltsville Human Nutrition Research Center (BHNRC; Beltsville, MD), and the National Institute on Aging (NIA; Baltimore, MD).
Study diets
Each day, subjects consumed dinner at the BHNRC Human Study Facility under the supervision of a registered dietitian. At the end of dinner, subject meal trays were inspected to ensure complete consumption of the food. All breakfasts and lunches were packed for carry-out. Only foods provided by the Human Study Facility were allowed to be consumed during the study. A 7-d menu cycle of typical American foods was formulated by using NUTRITIONIST PRO software (version 1.3; Axxya Systems, Stafford TX).
During the first 2 wk of the study, subjects randomly assigned to the 1 meal/d diet were fed 2 meals/d (lunch and dinner); for the next 6 wk, all food was consumed between 1700 and 2100, which created a minimum fast of 20 h/d. While maintaining the same macronutrient distribution between experimental and control diets, breakfast and lunch food items were substituted for traditional evening meal items. Energy-dense foods were chosen to assist in reducing the volume of food to be consumed.
Subjects were allowed unlimited amounts of calorie-free foods such as water, coffee (without sugar or milk), diet soft drinks, salt, and pepper. A 2-d emergency supply of food that met the study protocol was provided to each subject for use during any inclement weather. Subjects were required to consume all of the foods and only the foods provided by the Human Study Facility at specified times during the controlled feeding periods.
Body weight was measured every day before the evening meal, when subjects arrived at the facility. So that subjects could maintain constant body weight during the study, energy intake was adjusted in 200-kcal increments. Energy requirements for weight maintenance were calculated by using the Harris-Benedict formula, which estimates basal energy expenditure, and multiplied by an activity factor of 1.3–1.5. This formula has proven successful in estimating weight-maintenance energy requirements at our facility. Subjects completed a daily questionnaire regarding their general health; any consumption of prescription or over-the-counter medications; factors related to dietary compliance; and exercise performed; the questionnaire also gave subjects the opportunity to write in questions of their own about the diet. Subjects were encouraged to maintain their normal exercise routine throughout the study.
Physiologic measurements
Physiologic variables measured were blood pressure, heart rate, body temperature, and body composition. These measurements were collected at baseline, 4 wk, and at the end of each of the 2 treatment periods. Briefly, subjects were seated in a quiet room for ≈5 min, and blood pressure and heart rate were measured 3 times with a Dinamap Compact Monitor (Model TS; Critikon, Tampa, FL). Body temperature was measured on either a Dinamap or a portable oral digital thermometer (ADtempIII; American Diagnostic Corp, Hauppauge, NY). Body composition was measured by using bioelectrical impedance analysis (BIA) (model TBF-300A; Tanita, Arlington Heights, IL). Subjects fasted and refrained from heavy exercise before these measurements. Subjective satiety and hunger were assessed daily before consumption of the evening meal, in both the experimental and control diets, by using 4 visual analogue scales (VASs) that described hunger, desire to eat, the amount of food that could be eaten, and stomach fullness. The VASs were all 100-mm long, and they were anchored at either end with terms indicating opposite descriptors.
Biological sample collection and analysis
Blood was collected at baseline, 4 wk, and the end of each of the 2 treatment periods after a minimum of 12 h of fasting. All of the baseline samples were collected in the morning. The 4-wk and end-of-treatment samples were collected in the morning from subjects following the control diet and in the evening (before dinner) from subjects following the 1 meal/d diet. In addition, as a measure of compliance, blood samples were collected at unannounced times on 3 occasions from the subjects when they were consuming 1 meal/d and were analyzed for fasting blood glucose and triacylglycerol concentrations. The collected blood samples were used to prepare 0.8–2.0-mL aliquots of plasma, serum, and red blood cells that were stored at −80 °C in cryovials. Sample analyses included a lipid profile, a comprehensive metabolic panel, complete blood count (CBC), and cortisol concentration. Analyses were performed at the Core Laboratory of the NIA, National Institutes of Health (Baltimore, MD) and at Fairfax Medical Laboratory (Chantilly, VA) by using standard procedures and quality-control measures from the Clinical Laboratory Improvement Amendments [(CLIA) Internet: http://www.cms.hhs.gov/clia (accessed 1 January 2006)]. Plasma total cholesterol, HDL cholesterol, and triacyglycerol were measured enzymatically with commercial kits (Johnson & Johnson/Ortho Clinical Diagnostics, Raritan, NJ) on a Vitros 250 analyzer (Johnson & Johnson/Ortho Clinical Diagnostics). LDL-cholesterol concentrations were calculated by using the equation of Friedewald et al (17). Serum cortisol concentrations were analyzed on a chemiluminescence immunoassay analyzer (IMMULITE 2000; Diagnostic Products Corp, Los Angeles, CA) with intraassay and interassay CVs of 5.3% and 7.2%, respectively.
Physical activity assessment
Physical activity monitoring (PAM) was assessed with the use of the Actigraph accelerometer (MTI AM 7164–1.2; Manufacturing Technology Inc, Fort Walton Beach, FL) over the course of 7 d to obtain the average daily and weekly activity counts. Measurements were obtained during week 2 (baseline) and week 7 (end of treatment) of each treatment period. Subjects were instructed to wear the activity monitor a long as possible every day. The activity monitor, worn as a snugly fitting belt around the waist with the manufacturer’s “notch” facing upward, was set to read the data in 1-min segments. Subjects were asked to wear the monitor on the right hip, unless they reported being unable to do so. Regardless of the activity monitor placement, each subject wore the monitor on the same side and at the same location throughout the study. In addition to wearing the monitors, subjects maintained a small daily log to detail the times when the monitor was worn, the activities that were carried out when the monitor was not worn (ie, sleeping or showering), and any exercise performed (whether the monitor was worn or not). In each of the treatment periods, subjects were asked to wear the monitor for 9 d, with the intention of obtaining 7 full days of data. If a subject reported not wearing the activity monitor for a given day, he or she was asked to wear the monitor an extra day. Physical activity data obtained from the Actigraph accelerometer were processed by using a procedure developed in our facility. Briefly, most subjects typically remove the monitors periodically during the day or at night for sleep (or both). Analyses from our laboratory indicate that these missing data points can have a detrimental effect on the prediction of physical activity, so we developed a procedure that treats each monitoring day as a 24-h day no matter how long the monitor was worn on any day. Monitor files are scanned by a program that estimates the time spent sleeping and imputes a constant value for those times. Other missing strings of data >20 min long are “filled in” by imputation, on the basis of the monitor removal times reported in the log. These data processing procedures dramatically reduce the variability inherent with activity monitor data.
Statistical analysis
An analysis of variance (ANOVA) appropriate for a 2-period crossover study with repeated measures within period was used to evaluate meal frequency effects on outcome variables using the MIXED procedure in SAS software (version 9.0; SAS Institute, Cary NC). The statistical model included sequence, meal frequency, period, time within period, and time × meal frequency interaction as fixed effects. Period and time were modeled as repeated measures. The factor subject nested in sequence was included in the model as a random effect. The first observation within a period was included as a covariate. When the time × meal frequency interaction was significant (P < 0.05), within-time meal frequency effects were evaluated by using repeated-measures ANOVA. If this interaction was not significant, the main effect of meal frequency was evaluated. Data are presented as means ± SEMs.
RESULTS
Subject characteristics
Sixty-nine persons attended the study information meeting. Thirty-five gave written informed consent, and 32 completed the screening process. Twenty-one subjects (14 women, 7 men) ultimately were randomly assigned to the treatments. Fifteen subjects (10 women, 5 men) completed the feeding phase of the study. Complete data were analyzed and are presented for 15 subjects. In the 3 meal/d diet arm, 1 subject withdrew because of food dislikes. During the 1 meal/d diet, 5 subjects withdrew because of scheduling conflicts and health problems unrelated to the study. Only 1 of the 5 subjects withdrew specifically because of an unwillingness to consume the 1 meal/d diet. The mean BMI indicated that subjects were within the normal range. The physical characteristics of the 15 subjects at baseline are presented in Table 1.
TABLE 1.
Subject characteristics before the start of the controlled feeding1
| Value | |
|---|---|
| Age (y) | 45.0 ± 0.7 |
| Height (cm) | 168.0 ± 3.1 |
| Weight (kg) | 66.5 ± 3.1 |
| BMI (kg/m2) | 23.4 ± 0.5 |
| Fat mass (kg) | 16.2 ± 1.2 |
| Fat-free mass (kg) | 50.1 ± 2.9 |
| Total body water (kg) | 36.7 ± 2.1 |
| Total cholesterol (mg/dL) | 182.0 ± 8.5 |
| LDL cholesterol (mg/dL) | 109.1 ± 8.6 |
| HDL cholesterol (mg/dL) | 53.5 ± 3.9 |
| Triacylglycerol (mg/dL) | 97.1 ± 8.6 |
| Systolic blood pressure (mm Hg) | 115.6 ± 4.2 |
| Diastolic blood pressure (mm Hg) | 68.1 ± 2.6 |
| Heart rate (beats/min) | 65.6 ± 2.3 |
| Body temperature (°C) | 36.1 ± 0.2 |
All values are x̄ ± SEM; n = 15 (10 F, 5 M).
Diets
The composition of the 2 diets is shown in Table 2. Subject adherence to the controlled diets was judged to be excellent on the basis of observed consumption of the meals in the facility and review of the responses on the daily questionnaires. The random fasting triacylglycerol and glucose concentrations indicated that compliance with the 1 meal/d diet was acceptable. The mean triacylglycerol and glucose concentrations were 64.4 ± 5.7 and 79.7 ± 1.9 mg/dL, respectively. Thirty of 1650 evening meals (1.8%) provided during the entire study were packed for consumption away from the facility. The average daily energy intake across treatments was 2364 kcal in the 1 meal/d diet and 2429 kcal in the 3 meals/d diet. No significant differences were found in the percentages of macronutrients, fatty acids, cholesterol, and fiber between the 2 controlled diets.
TABLE 2.
Composition of the controlled diets1
| 1 Meal/d | 3 Meals/d | |
|---|---|---|
| Protein (% of energy) | 14.5 | 14.7 |
| Fat (% of energy) | 35.6 | 36.4 |
| Carbohydrate (% of energy) | 49.8 | 48.9 |
| PUFA:MUFA:SFA | 0.5:0.8:1.1 | 0.7:0.8:1.1 |
| Cholesterol (mg/1000 kcal) | 140 | 148 |
| Dietary fiber (mg/1000 kcal) | 7.4 | 8.9 |
Average daily intake for each subject in either diet was 2396.5 kcal. PUFA, polyunsaturated fatty acids; MUFA, monounsaturated fatty acids; SFA, saturated fatty acids.
Blood pressure
Systolic and diastolic blood pressures were significantly lowered by ≈6% during the period when subjects were consuming 3 meals/d than when they were consuming 1 meal/d. No significant effect of time (measurements taken at week 4 and week 8) or of treatment sequence on blood pressure was seen. No significant differences in heart rate and body temperature were observed between the 2 diet regimens (Table 3).
TABLE 3.
Blood pressure and body composition of subjects after consumption of either 1 meal/d or 3 meals/d1
| 1 Meal/d | 3 Meals/d | P2 | |
|---|---|---|---|
| Systolic blood pressure (mm Hg) | 116.1 ± 1.93 | 109.5 ± 1.9 | 0.02 |
| Diastolic blood pressure (mm Hg) | 69.8 ± 1.3 | 66.0 ± 1.3 | 0.04 |
| Heart rate (beats/min) | 70.3 ± 1.6 | 67.8 ± 1.7 | 0.30 |
| Body temperature (°C) | 36.3 ± 0.2 | 35.9 ± 0.2 | 0.53 |
| Body weight (kg) | 65.9 ± 3.2 | 67.3 ± 3.2 | 0.01 |
| Fat mass (kg) | 14.2 ± 1.0 | 16.3 ± 1.0 | 0.001 |
| Fat-free mass (kg) | 50.9 ± 0.4 | 49.4 ± 0.4 | 0.06 |
| Total body water (kg) | 37.2 ± 0.4 | 36.1 ± 0.4 | 0.05 |
n = 15 (10 F, 5 M).
Within-period repeated-measures ANOVA.
Least-squares x̄ ± SEM (all such values).
Visual analogue scales
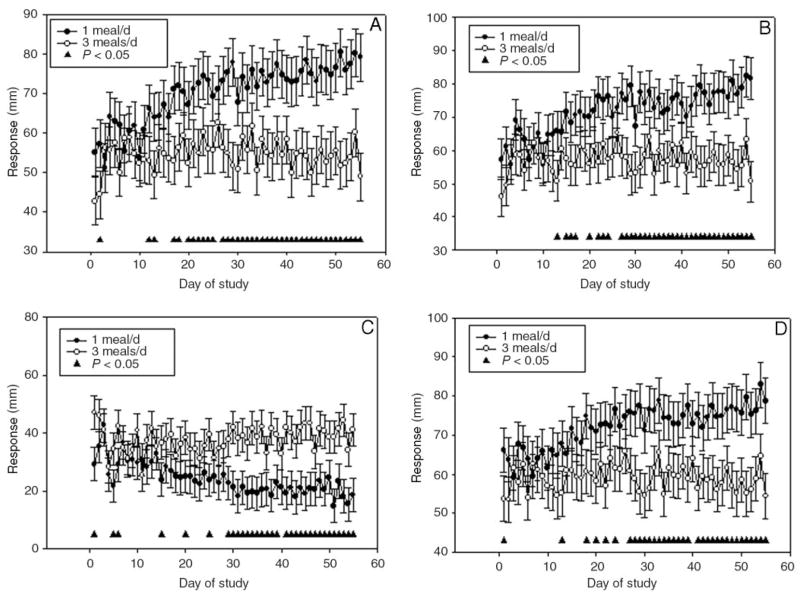
There was a significant treatment effect between the 2 diets on ratings of hunger, desire to eat, fullness, and prospective consumption (ie, the amount of food subjects thought they could eat). The 1 meal/d diet was significantly higher for hunger (P = 0.003), desire to eat (P = 0.004), and prospective consumption (P = 0.006) than was the 3 meals/d diet. Feelings of fullness were significantly (P = 0.001) lower in the 1 meal/d than in the 3 meals/d diet. In addition to the significant treatment effect by diet, a significant time effect (day of study) was observed for hunger, desire to eat, fullness, and prospective consumption. Over time, hunger, desire to eat, and prospective consumption were significantly higher in the 1 meal/d than in the 3 meals/d diet. Fullness was significantly lower over time in the 1 meal/d diet than in the 3 meals/d diet (Figure 1).
FIGURE 1.
Least-squares x̄ (± SEM) values for subjective ratings of hunger and satiety (A, hunger; B, desire to eat; C, fullness; and D, prospective meal consumption) measured daily by using visual analogue scales (100 mm) during each study period. n = 15 (10 F, 5 M). There was a significant treatment effect between the 1 meal/d and the 3 meals/d diets for all 4 ratings, P < 0.05 (from within-period repeated-measures ANOVA). Significant time × treatment interaction (▲), P < 0.05.
Body weight and composition
Subjects’ weight and body fat mass were lowered (1.4 and 2.1 kg, respectively) after consumption of the 1 meal/d diet but not after consumption of the 3 meals/d diet. No significant differences in fat-free mass and total body water were observed between the diet groups (Table 3). Even with an 11-wk washout period between the 2 diet protocols, no significant differences from baseline were seen in body weight, fat mass, fat-free mass, or total body water in either period of the study.
Physical activity
No evidence was found of a significant difference in physical activity after consumption of the 1 meal/d or the 3 meals/d diet.
Biological samples
Consumption of 1 meal/d lowered blood urea nitrogen by 13.4%. The serum liver enzymes alkaline phosphatase, serum glutamic pyruvic transaminase, and serum glutamic oxaloacetic transaminase were higher 4.6%, 17.5%, and 16.0% higher, respectively, when subjects consumed 1 meal/d than when they consumed 3 meals/d. Serum albumin was 4.5% higher and cortisol concentrations were 48.9% lower after consumption of 1 meal/d than after consumption of 3 meals/d. Total, LDL, and HDL cholesterol were 11.7%, 16.8%, and 8.4% higher, respectively, in subjects consuming 1 meal/d than in those consuming 3 meals/d. The hematologic variables that differed significantly between the diet groups were those of hemoglobin, hematocrit and red blood cells. Serum concentrations of creatinine, glucose, total protein, uric acid, and all other metabolic variables were not significantly affected by the diets (Table 4).
TABLE 4.
Metabolic panel, lipids, and hematologic variables after consumption of 1 meal/d or 3 meals/d1
| 1 Meal/d | 3 Meals/d | P2 | |
|---|---|---|---|
| Sodium (mEq/L) | 143.0 ± 0.83 | 142.0 ± 0.8 | 0.29 |
| Potassium (mEq/L) | 4.2 ± 0.1 | 4.4 ± 0.1 | 0.19 |
| Chloride (mEq/L) | 104.4 ± 0.5 | 104.0 ± 0.5 | 0.27 |
| Carbon dioxide content (mEq/L) | 27.5 ± 0.4 | 27.5 ± 0.4 | 0.91 |
| Anion gap | 11.2 ± 0.3 | 10.8 ± 0.3 | 0.29 |
| Blood urea nitrogen (mg/dL) | 12.9 ± 0.4 | 14.9 ± 0.4 | < 0.0001 |
| Creatinine (mg/dL) | 0.9 ± 0.02 | 0.9 ± 0.02 | 0.35 |
| Blood urea nitrogen:creatinine | 15.3 ± 0.7 | 17.0 ± 0.7 | 0.01 |
| Glucose (mg/dL) | 85.9 ± 1.5 | 89.4 ± 1.5 | 0.14 |
| Calcium (mg/dL) | 9.9 ± 0.1 | 9.8 ± 0.1 | 0.17 |
| Phosphorus (g/dL) | 3.5 ± 0.1 | 3.6 ± 0.1 | 0.40 |
| Total protein (g/dL) | 7.3 ± 0.1 | 7.1 ± 0.1 | 0.05 |
| Albumin (g/dL) | 4.4 ± 0.1 | 4.2 ± 0.1 | 0.01 |
| Globulin (g/dL) | 2.9 ± 0.1 | 2.9 ± 0.1 | 0.50 |
| Albumin:globulin | 1.5 ± 0.03 | 1.5 ± 0.03 | 0.28 |
| Alkaline phosphatase (IU/L) | 69.5 ± 2.8 | 66.3 ± 2.8 | 0.05 |
| Serum glutamic pyruvic transaminase (IU/L) | 40.0 ± 4.8 | 33.0 ± 4.8 | 0.01 |
| Serum glutamic oxaloacetic transaminase (IU/L) | 28.7 ± 2.1 | 24.1 ± 2.1 | 0.01 |
| Total bilirubin (mg/dL) | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.43 |
| Triacyglycerol (mg/dL) | 93.2 ± 7.7 | 102.2 ± 7.7 | 0.08 |
| Cholesterol (mg/dL) | 216.5 ± 5.3 | 191.0 ± 5.3 | 0.001 |
| HDL cholesterol (mg/dL) | 61.9 ± 1.8 | 56.7 ± 1.8 | 0.01 |
| LDL cholesterol (mg/dL) | 136.2 ± 4.0 | 113.3 ± 4.0 | 0.001 |
| Cortisol (μg/dL) | 7.2 ± 0.9 | 14.1 ± 0.9 | <0.0001 |
| Uric acid (mg/dL) | 4.6 ± 0.1 | 4.5 ± 0.1 | 0.75 |
| Red blood cell (×102/L) | 4.3 ± 0.1 | 4.5 ± 0.1 | 0.001 |
| Hemoglobin (g/dL) | 13.2 ± 0.2 | 13.6 ± 0.2 | <0.0001 |
| Hematocrit (%) | 37.3 ± 1.2 | 40.1 ± 1.1 | 0.01 |
| Mean cell volume (fL) | 89.1 ± 0.3 | 89.2 ± 0.3 | 0.65 |
n = 15 (10 F, 5 M).
Within-period repeated-measures ANOVA.
Least-squares x̄ ± SEM (all such values).
DISCUSSION
This study is among the first controlled randomized clinical trials to evaluate the effects of controlled meal frequency on normal-weight, middle-aged adults. We found that the consumption of a meal-skipping diet (ie, 1 meal/d), rather than the traditional 3 meals/d diet, is feasible for a short duration.
Our study withdrawal rate was 28.6%. Typical rates of withdrawal from human feeding studies at our facility are ≈4–7% (18–20). We can hypothesize that subject withdrawals increased because the subjects were asked to consume all food for the day in 1 meal; however, only 1 subject specifically stated this reason for withdrawing. Most subjects were able to consume all calories in the 1 meal/d diet. Study withdrawals were reported to be due to subject scheduling conflicts and health problems that were unrelated to the study.
Only a few experimental studies have tested the effect of meal frequency on satiety measures. The results of the VASs suggest that subjects did not become habituated to the 1 meal/d diet. Over time, hunger, desire to eat, and prospective consumption increased, whereas feelings of fullness decreased. Similarly, subjects who followed an alternate-day-fast diet for 3 wk had a significant increase in hunger and desire to eat on their fasting days than at baseline, but they did not become habituated to the alternate-day-fast diet, and they were just as hungry on their first day of fasting as on the last day (21). Although subjective hunger and satiety assessments were not made after the evening meal, in comments during consumption of the 1 meal/d diet, most subjects reported extreme fullness after the meal and had difficulty finishing their food in the allotted time. Further research is required to gain a better understanding of subjective satiety on meal frequency.
Although within normal values, both systolic and diastolic blood pressures were higher than baseline during consumption of the 1 meal/d diet. Experimental data for normal-weight men and women on the effects of consumption of 1 meal/d rather than 3 meals/d on blood pressure have not previously been reported. Overweight men and women showed that consumption of 1 meal/d, with caloric restriction, improved blood pressure and heart rate after exercise (22). In animal models, intermittent fasting without caloric restriction has been shown to decrease blood pressure and heart rate (15). The observed increase in blood pressure in our subject population consuming 1 meal/d may be due to a circadian rhythm in blood pressure (23). Diurnal changes may have occurred, because blood pressure measurements were obtained in the late afternoon in the 1 meal/d diet versus early morning in the 3 meals/d.
It is interesting that body weight and body fat decreased in the 1 meal/d diet, which may be partially explained by a slight deficit of 65 kcal in daily energy intake. This change in body composition may also be influenced by the effect that eating patterns could have on metabolic activity. Rats that followed a nibbling diet and then a diet that consisting of 1 large meal developed an increase net flux of free fatty acids from fat deposits and an increase in gluconeogenesis (24, 25). Similar alterations in metabolism may have occurred in our subjects, which may have contributed to weight and fat mass loss. Gluconeogenesis typically begins 4–6 h after the last meal and becomes fully active as stores of liver glycogen are depleted. Free fatty acids and amino acids that are substrates for gluconeogenesis are used for the energy supply (26, 27).
Altered circulating lipid concentrations are recognized as risk factors for CVD (28). In the current study, we found both proatherogenic (increases in total and LDL cholesterol) and antiatherogenic (an increase in HDL cholesterol and a decrease in triacylglycerols) changes after consumption of the 1 meal/d diet. These changes appeared to be independent of the controlled diets, because dietary cholesterol and the ratio of fatty acids were held constant. Studies that have attempted to determine the effects of meal frequency on biomarkers of health, such as lipid concentrations, are inconsistent. In one experimental study, healthy men were fed either 3 meals/d or 17 small snacks/d for 2 wk; subjects consuming the 17-snack diet had reductions in total and LDL-cholesterol concentrations, whereas the concentrations did not change in the subjects consuming 3 meals/d (29). Two studies also showed that omitting breakfast has harmful effects on health outcomes related to CVD (30, 31), and another study showed that this omission may reduce risk factors for CVD (32).
Consumption of 1 meal/d increased albumin and liver enzymes and decreased blood urea nitrogen in our study subjects, although these values remained within normal reference ranges (33). Subjects consuming the 1 meal/d diet also had decreased cortisol concentrations. Although all blood collection occurred after 12 h of fasting, the timing of blood collection differed between the 2 diet groups. Blood was collected in the early morning, before breakfast (ie, after a 12-h fast), from subjects consuming 3 meals/d and in the late afternoon, before the evening meal (ie, after an 18-h fast), from those consuming l meal/d. Subjects consuming 1 meal/d had decreased cortisol concentrations, which were most likely due to diurnal variations in this hormone. Cortisol is typically elevated in the morning and decreases later in the afternoon (34, 35).
During Ramadan, the holy month during which Muslims fast from dawn to dusk, diurnal variations of nutrition biomarkers have been observed in practicing Muslims. Previous research has shown that, unlike in nonfasting periods, cortisol concentrations are biphasic during Ramadan fasting (36). These researchers reported an increase in serum cortisol starting at 1200 h that reaches a plateau between 1600 and 2000. During Ramadan fasting, diurnal variation in cortisol differs significantly from the normal diurnal variation (36). We found that subjects’ hemoglobin, hematocrit, and red blood cells were lower after consumption of the 1 meal/d diet, whereas the mean cell volume was considered to be of normal concentration. The latter results could be the result of an increase in blood volume or a change in the production of red blood cells. Previous research on Ramadan fasting has shown a suppression in red blood cell production along with an increasing trend for anemia (37). The latter results were likely due to a decrease in the intakes of calories and of iron-containing foods during the fasting month of Ramadan (37). No major, clinically relevant, diet-related changes were seen in the comprehensive metabolic panel or CBC, which indicated that the 1 meal/d diet was well tolerated in that group of healthy men and women.
Several limitations of the design of the current study warrant consideration. Although this was a pilot study, the small sample size was particularly limiting. Blood, blood pressure, body temperature, and body-composition measurements were taken in the early morning from subjects consuming 3 meals/d and in the late afternoon from those consuming l meal/d; results may have differed if the latter measurements also were obtained in the early morning. BIA may also not be the best method for assessing body composition because of its tendency to overestimate fat mass in lean subjects (38). The subject population of the current study also was fairly homogenous; future research should include overweight and obese populations to allow determination of the effects of meal frequency in those groups.
Previous studies documented improvements in the health and longevity of rats and mice maintained on an intermittent-fasting regimen in which they were deprived of food for a 24-h period every other day (5–9, 15, 16); in these studies, the experimental diet resulted in overall reductions in calorie intake of up to 30%. However, in some studies, the amount of caloric restriction was small (5–10%) and the physiologic changes were relatively large, which suggests that the extended fasting period itself contributed to the benefits of the diet (8). The present findings suggest that, without a reduction in calorie intake, a reduced-meal-frequency diet does not afford major health benefits in humans. Improvements in glucose regulation and cardiovascular health in rodents occur during several months of intermittent fasting; the time during which the subjects were maintained on the 1 meal/d diet in the present study may therefore not be sufficient to achieve stable changes in physiology. A long-term reduced-meal-frequency diet that also includes a 20–30% reduction in calorie intake would more closely resemble the intermittent fasting regimen that is widely used in rodent studies.
In conclusion, altered meal frequency is feasible in healthy, normal-weight, middle-aged men and women. Consumption of 1 meal/d resulted in weight loss and a decrease in fat mass with little modification in calorie consumption. It remains unclear whether altered meal frequency would lead to changes in weight and body composition in obese subjects.
Footnotes
Supported by the National Institute on Aging and the US Department of Agriculture.
DJB, WVR, SSN, LF, DKI, DLL, and MPM were responsible for the design of the study; KS and PS recruited subjects and coordinated the study; KSS, KS, and PS were responsible for data collection, and discussed the data and DRP and GKH collected the physical activity and physiologic data; KSS was responsible for data analysis; DJB, WVR, SSN, LF, DKI, DLL, and MPM evaluated the data analysis results; KSS wrote the draft of the manuscript; and DJB, WVR, SSN, LF, DKI, DLL, and MPM contributed to revisions of the manuscript. None of the authors had a personal or financial conflict of interest.
References
- 1.Haslam DW, James WP. Obesity. Lancet. 2005;366:1197–209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- 2.Wing RR. Use of very-low-calorie diets in the treatment of obese persons with non-insulin-dependent diabetes mellitus. J Am Diet Assoc. 1995;95:569–72. doi: 10.1016/S0002-8223(95)00155-7. [DOI] [PubMed] [Google Scholar]
- 3.Fontana L, Meyer TE, Klein S, Holloszy JO. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc Natl Acad Sci U S A. 2004;101:6659–63. doi: 10.1073/pnas.0308291101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mattison JA, Lane MA, Roth GS, Ingram DK. Calorie restriction in rhesus monkeys. Exp Gerontol. 2003;38:35–46. doi: 10.1016/s0531-5565(02)00146-8. [DOI] [PubMed] [Google Scholar]
- 5.Mattson MP, Wan R. Beneficial effects of intermittent fasting and caloric restriction on the cardiovascular and cerebrovascular systems. J Nutr Biochem. 2005;16:129–37. doi: 10.1016/j.jnutbio.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 6.Weindruch R, Sohal RS. Seminars in medicine of the Beth Israel Deaconess Medical Center. Caloric intake and aging. N Engl J Med. 1997;337:986–94. doi: 10.1056/NEJM199710023371407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmet I, Wan R, Mattson MP, Lakatta EG, Talan M. Cardioprotection by intermittent fasting in rats. Circulation. 2005;112:3115–21. doi: 10.1161/CIRCULATIONAHA.105.563817. [DOI] [PubMed] [Google Scholar]
- 8.Anson RM, Guo Z, de Cabo R, et al. Intermittent fasting dissociates beneficial effects of dietary restriction on glucose metabolism and neuronal resistance to injury from calorie intake. Proc Natl Acad Sci U S A. 2003;100:6216–20. doi: 10.1073/pnas.1035720100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berrigan D, Perkins SN, Haines DC, Hursting SD. Adult-onset calorie restriction and fasting delay spontaneous tumorigenesis in p53-deficient mice. Carcinogenesis. 2002;23:817–22. doi: 10.1093/carcin/23.5.817. [DOI] [PubMed] [Google Scholar]
- 10.Nutrition and your health: dietary guidelines for Americans. 2005 Dietary Guidelines Advisory Committee Report, Research Recommendations. [accessed 1 January 2006]; Internet: http://www.health.gov/dietaryguidelines/dga2005/report/HTML/F_ResearchRec.htm.
- 11.Mattson MP. The need for controlled studies of the effects of meal frequency on health. Lancet. 2005;365:1978–80. doi: 10.1016/S0140-6736(05)66667-6. [DOI] [PubMed] [Google Scholar]
- 12.Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Speakman JR, Selman C, McLaren JS, Harper EJ. Living fast, dying when? The link between aging and energetics. J Nutr. 2002;132(suppl):1583S–97S. doi: 10.1093/jn/132.6.1583S. [DOI] [PubMed] [Google Scholar]
- 14.Roth GS, Ingram DK, Lane MA. Caloric restriction in primates and relevance to humans. Ann N Y Acad Sci. 2001;928:305–15. doi: 10.1111/j.1749-6632.2001.tb05660.x. [DOI] [PubMed] [Google Scholar]
- 15.Wan R, Camandola S, Mattson MP. Intermittent food deprivation improves cardiovascular and neuroendocrine responses to stress in rats. J Nutr. 2003;133:1921–9. doi: 10.1093/jn/133.6.1921. [DOI] [PubMed] [Google Scholar]
- 16.Wan R, Camandola S, Mattson MP. Intermittent fasting and dietary supplementation with 2-deoxy-D-glucose improve functional and metabolic cardiovascular risk factors in rats. FASEB J. 2003;17:1133–4. doi: 10.1096/fj.02-0996fje. [DOI] [PubMed] [Google Scholar]
- 17.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 18.Baer DJ, Judd JT, Clevidence BA, Tracy RP. Dietary fatty acids affect plasma markers of inflammation in healthy men fed controlled diets: a randomized crossover study. Am J Clin Nutr. 2004;79:969–73. doi: 10.1093/ajcn/79.6.969. [DOI] [PubMed] [Google Scholar]
- 19.Judd JT, Baer DJ, Chen SC, et al. Plant sterol esters lower plasma lipids and most carotenoids in mildly hypercholesterolemic adults. Lipids. 2002;37:33–42. doi: 10.1007/s11745-002-0861-y. [DOI] [PubMed] [Google Scholar]
- 20.Judd JT, Baer DJ, Clevidence BA, et al. Effects of margarine compared with those of butter on blood lipid profiles related to cardiovascular disease risk factors in normolipemic adults fed controlled diets. Am J Clin Nutr. 1998;68:768–77. doi: 10.1093/ajcn/68.4.768. [DOI] [PubMed] [Google Scholar]
- 21.Heilbronn LK, Smith SR, Martin CK, Anton SD, Ravussin E. Alternate-day fasting in nonobese subjects: effects on body weight, body composition, and energy metabolism. Am J Clin Nutr. 2005;81:69–73. doi: 10.1093/ajcn/81.1.69. [DOI] [PubMed] [Google Scholar]
- 22.Caviezel F, Margonato A, Slaviero G, et al. Early improvement of left ventricular function during caloric restriction in obesity. Int J Obes. 1986;10:421–6. [PubMed] [Google Scholar]
- 23.Profant J, Dimsdale JE. Race and diurnal blood pressure patterns. A review and meta-analysis. Hypertension. 1999;33:1099–104. doi: 10.1161/01.hyp.33.5.1099. [DOI] [PubMed] [Google Scholar]
- 24.Fabry P, Tepperman J. Meal frequency—a possible factor in human pathology. Am J Clin Nutr. 1970;23:1059–68. doi: 10.1093/ajcn/23.8.1059. [DOI] [PubMed] [Google Scholar]
- 25.Gwinup G, Byron RC, Roush WH, Kruger FA, Hamwi GJ. Effect of nibbling versus gorging on serum lipids in man. Am J Clin Nutr. 1963;13:209–13. doi: 10.1093/ajcn/13.4.209. [DOI] [PubMed] [Google Scholar]
- 26.Azzout B, Bois-Joyeux B, Chanez M, Peret J. Development of gluconeogenesis from various precursors in isolated rat hepatocytes during starvation or after feeding a high protein, carbohydrate-free diet. J Nutr. 1987;117:164–9. doi: 10.1093/jn/117.1.164. [DOI] [PubMed] [Google Scholar]
- 27.Kreisberg RA, Pennington LF, Boshell BR. Lactate turnover and gluconeogenesis in normal and obese humans. Effect of starvation. Diabetes. 1970;19:53–63. doi: 10.2337/diab.19.1.53. [DOI] [PubMed] [Google Scholar]
- 28.Executive Summary of the Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation And Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 29.Jenkins DJ, Wolever TM, Vuksan V, et al. Nibbling versus gorging: metabolic advantages of increased meal frequency. N Engl J Med. 1989;321:929–34. doi: 10.1056/NEJM198910053211403. [DOI] [PubMed] [Google Scholar]
- 30.Farshchi HR, Taylor MA, Macdonald IA. Deleterious effects of omitting breakfast on insulin sensitivity and fasting lipid profiles in healthy lean women. Am J Clin Nutr. 2005;81:388–96. doi: 10.1093/ajcn.81.2.388. [DOI] [PubMed] [Google Scholar]
- 31.Keski-Rahkonen A, Kaprio J, Rissanen A, Virkkunen M, Rose RJ. Breakfast skipping and health-compromising behaviors in adolescents and adults. Eur J Clin Nutr. 2003;57:842–53. doi: 10.1038/sj.ejcn.1601618. [DOI] [PubMed] [Google Scholar]
- 32.Martin A, Normand S, Sothier M, Peyrat J, Louche-Pelissier C, Laville M. Is advice for breakfast consumption justified? Results from a short-term dietary and metabolic experiment in young healthy men. Br J Nutr. 2000;84:337–44. doi: 10.1017/s0007114500001616. [DOI] [PubMed] [Google Scholar]
- 33.Young DS. Implementation of SI units for clinical laboratory data. Style specifications and conversion tables. Ann Intern Med. 1987;106:114–29. doi: 10.7326/0003-4819-106-1-114. [DOI] [PubMed] [Google Scholar]
- 34.Posener JA, Schildkraut JJ, Samson JA, Schatzberg AF. Diurnal variation of plasma cortisol and homovanillic acid in healthy subjects. Psychoneuroendocrinology. 1996;21:33–8. doi: 10.1016/0306-4530(95)00033-x. [DOI] [PubMed] [Google Scholar]
- 35.Posener JA, Schildkraut JJ, Williams GH, Schatzberg AF. Late feedback effects of hypothalamic-pituitary-adrenal axis hormones in healthy subjects. Psychoneuroendocrinology. 1998;23:371–83. doi: 10.1016/s0306-4530(98)00006-7. [DOI] [PubMed] [Google Scholar]
- 36.Bogdan A, Bouchareb B, Touitou Y. Ramadan fasting alters endocrine and neuroendocrine circadian patterns. Meal-time as a synchronizer in humans? Life Sci. 2001;68:1607–15. doi: 10.1016/s0024-3205(01)00966-3. [DOI] [PubMed] [Google Scholar]
- 37.Dewanti L, Watanabe C, Sulistiawati Ohtsuka R. Unexpected changes in blood pressure and hematological parameters among fasting and non-fasting workers during Ramadan in Indonesia. Eur J Clin Nutr. 2006;60:877–81. doi: 10.1038/sj.ejcn.1602393. [DOI] [PubMed] [Google Scholar]
- 38.Sun G, French CR, Martin GR, et al. Comparison of multifrequency bioelectrical impedance analysis with dual-energy X-ray absorptiometry for assessment of percentage body fat in a large, healthy population. Am J Clin Nutr. 2005;81:74–8. doi: 10.1093/ajcn/81.1.74. [DOI] [PubMed] [Google Scholar]