Abstract
Aging is associated with increased incidence and prevalence of anemia that is associated with a number of adverse health outcomes. These include death, functional dependence, increased risk of therapeutic complications, falls, and dementia. In approximately 30% of cases, anemia in older individuals is due to either relative or absolute erythropoietin deficiency. Absolute erythropoietin deficiency may be primary or secondary to declining renal function. Relative erythropoietin deficiency is due to an age-related pro-inflammatory status that reduces the sensitivity of erythropoietic precursors to erythropoietin.
Despite this condition of erythropoietin deficiency, the management of anemia of aging with erythropoiesis stimulating factors (ESA) is controversial, unless the anemia is due to renal insufficiency. The main concern related to this treatment arises from eight studies of ESAs in cancer, suggesting that ESAs may reduce patient survival, in addition to increasing the risk of deep vein thrombosis. The results of these studies contrast with a host of other trials showing the safety of ESA. The discrepancy may be explained in part by the fact that, in the trials suggesting a detrimental effect of ESAs, the goal was to obtain hemoglobin levels higher than 12g/dl. Because of this concern, correction of anemia in elderly individuals with relative erythropoietin insufficiency should not be attempted outside clinical trials.
The incidence and prevalence of anemia increase with age 1. Anemia in the older aged person is associated with a number of unfavorable outcomes that include death 2, functional dependence 3, dementia 4, falls 5, and cardiovascular diseases 6, 7. Thus, it is important to establish whether the reversal of anemia may prevent or delay these complications.
In approximately 50% of cases, anemia of the elderly is due to reversible causes, including iron and cobalamin deficiency and chronic renal insufficiency 1, 8, 9. In approximately 30% of cases the cause of anemia appears due to relative or absolute erythropoietin insufficiency 8, 10, 11. While this form of anemia improves with the administration of erythropoiesis stimulating agents (ESA), the benefits of this treatment approach are controversial. Recent studies in cancer patients have raised concern that the complications of treatment with ESAs may hasten rather than delay the patient’s death 12–19. More research is clearly needed in this area.
In this article we briefly review some aspects of the physiology of aging that are relevant to the pathogenesis of anemia and the causes of anemia in older persons, with particular focus on the anemia of inflammation and anemia that develops in the context of cancer. Finally, we explore the controversial evidence that treatment with ESA is beneficial.
Biology of aging and its clinical implications for anemia
Aging may be defined as a progressive loss of entropy and fractality 20. Loss of entropy implies reduced energetic reserve, loss of fractality a reduced ability to perform complex activities, such as walking. Many scientists currently believe that most effects of the aging process are attributable to a progressive dysregulation of the signaling network that maintains a stable homeostasis. Although the elements of this network have not been fully defined, an emerging hypothesis focuses on the energetic homeostasis 20, 21. Nutrition and breathing (oxygen) are the raw materials used by living creatures to generate the energy required to maintain the integrity of the biological environment (homeostasis), both at rest and during stressful conditions such as physical exercise, cognitive effort and diseases. The complex signaling network that regulates the generation and distribution in the various physiological districts and tissues includes hormones, immuno-regulatory mechanisms and the oxidative stress/antioxidants equilibrium. Alteration of any one of these mechanisms can cause anemia. Once anemia is established it affects the distribution of oxygen, one of the clinical elements for energy generation, therefore causing further dysregulation and activating a vicious circle that subsequently leads to frailty and disability 22, 23. There is some evidence that changes in the circulating levels of thyroid hormones and testosterone that often occur with normal aging make older individuals more susceptible to anemia 24–26. The role of other hormonal axes in the anemia of aging remains unclear.
Inflammation is perhaps the most studied element of the homeostatic network dysregulation, particularly in the context of anemia. Aging is characterized by a progressive mild pro-inflammatory state which is revealed by rising levels of pro-inflammatory markers (Figure 1) 27. Although this phenomenon can be partially explained by increasing prevalence of chronic disease and other risk factors with age, even when the effect of these factors is taken into account and even when “extremely healthy” individuals are selected, older individuals still have higher levels of Interleukin-6 (IL-6) and other pro-inflammatory cytokines and acute phase reactive proteins. Evidence is accumulating that such a pro-inflammatory state is a strong independent risk factor for a number of negative health-related outcomes, including cardiovascular disease, disability, cognitive impairment and short-term mortality 28–32. The mechanisms underlying this association are still under investigation.
Figure 1.
It is has long been known that patients with chronic inflammatory diseases, typically cancer, infectious diseases or autoimmune diseases, tend to develop anemia, usually of mild severity but sometimes severe and refractory to treatment 33. Based on the physiology of erythropoiesis and the results of pre-clinical and clinical studies, researchers have suggested four main mechanisms by which inflammation may affect anemia: 1) inflammation makes erythropoiesis ineffective by inhibiting proliferation and differentiation of erythroid precursors and/or down-regulation of the biological response to EPO (EPO resistance), perhaps by down-regulation of the EPO receptor. Accordingly, in patients with chronic kidney disease, the dose of EPO required to maintain a certain hemoglobin level is, on average, 30–70% higher in patients with CRP>20mg/L compared with those having lower CRP 34, 35; 2) inflammation reduces the amount of EPO production that is normally determined by anemia-induced relative hypoxia 34, 36; 3) inflammation causes the up-regulation of hepcidin synthesis that, by enhancing the proteolysis of ferroportin, reduces the intestinal absorption and recycling of iron and creates a functional form of iron deficiency 37, 38; 4) inflammation negatively affects erythrocyte survival, which is not fully compensated by increased erythropoiesis 39.
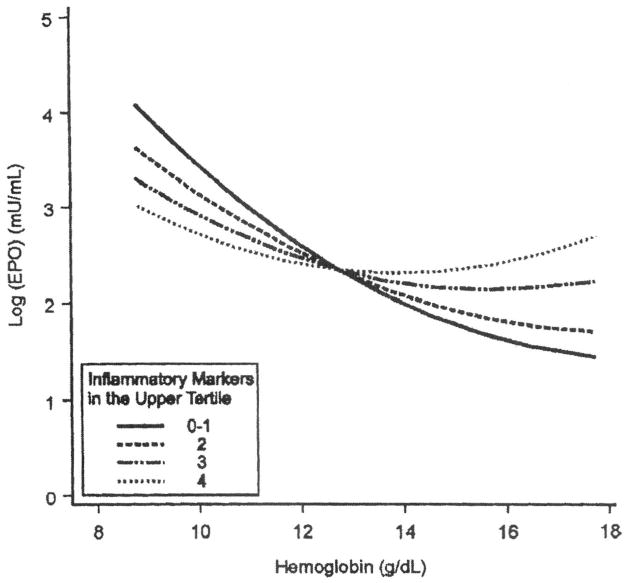
To explore the first two hypotheses, Ferrucci et al. studied how inflammation affects the relationship between hemoglobin (Hb) and erythropoietin 11. Unexpectedly, higher inflammation was associated with an EPO level higher than expected in participants free of anemia, and lower than expected in anemic participants (Figure 2). These findings suggest that inflammation hampers the response to EPO (because of either EPO signal jamming or intrinsic bone marrow dysfunction). In the early stage of anemia development, this defect is fully compensated by increased EPO production. However, subsequently, when the ability to over-produce EPO is exhausted, anemia becomes clinically manifest. Ongoing studies are testing the hypothesis that subjects with high EPO and high levels of inflammatory markers (especially IL-6 and CRP) have high risk of developing anemia. Accordingly, there is preliminary evidence that older subjects with high levels of inflammatory markers and high EPO are at high risk of developing anemia. If confirmed, these findings will have important clinical implications because they indicate that a condition of high inflammation and “inappropriately” high EPO levels mark a condition of “preclinical anemia.” If these subjects at high risk of developing overt anemia after hemopoietic stress could be identified, preventive or supportive strategies could be implemented at an early stage.
Figure 2.
The mediatory role of hepcidin, a peptide hormone produced by hepatocytes, in the pathogenesis of anemia of inflammation is suggested by a number of studies40. By interacting with the unique iron transporter ferroportin, hepcidin controls iron intestinal absorption and mobilization from hepatocytes and macrophages, thereby causing low serum iron and ineffective erythropoiesis. Hepcidin levels rise in the iron replete state and decline in response to anemia, active erythropoiesis and hypoxia. Hepcidin synthesis is also stimulated by inflammatory mediators, a mechanism that could explain iron segregation in inflammatory conditions. In vitro treatment of primary hepatocytes with IL-6, LPS or LPS-stimulated macrophages increased hepcidin mRNA expression, and this induction is blocked by treatment with anti-IL-6 antibodies. In humans, acute and/or severe increase in inflammatory markers in diseases characterized by a strong inflammatory response (such as rheumatoid arthritis, abscess and sepsis) is associated with increased hepcidin urinary excretion.41. However, it is still unclear whether chronic inflammation, and especially the mild-pro-inflammatory state of aging, causes hepcidin up-regulation and contributes to anemia of chronic disease. Patients with acute and chronic infections have lower serum iron, lower transferrin saturation and higher ferritin concentration than persons without apparent inflammation. However, there is still no definitive proof that the iron lowering effect of inflammation is mediated by hepcidin. The scarcity of specific studies of hepcidin in human is explainable, at least in part, by methodological limitations in the available assays. Finally, while there is some evidence that inflammation is associated with reduced erythrocyte half-life, no large study has fully tested the hypothesis that the mild-pro-inflammatory state of aging causes altered erythrocyte half-life. In fact, there is a paucity of studies on the effect of aging on erythrocyte survival.
As reported above, several epidemiological studies have found that as much as 1/3 of the anemia in the older population is unexplained, even when extensive clinical information is available, with the exception of a direct examination of the bone marrow. Contrary to the hypothesis that the pro-inflammatory state of aging is at the root of all the “unexplained” anemias of aging, recent findings from the InCHIANTI study suggest that “unexplained” anemia of aging may occur in the context of a hypoergic state characterized by levels of inflammatory markers significantly lower than in the control, non-anemic population 10. In this condition an absolute erythropoietin deficiency is present.
In conclusion, the aging process is accompanied by physiological modifications that reflect a state of increased dysfunction in the mechanisms that maintain the energetic homeostasis. Several of these modifications make older individuals more susceptible to the development of anemia, and probably also play a causal role in the pathogenesis of anemia in the elderly. Translation of this recent evidence into clinical practice is approaching, although further research is needed. Approximately one third of anemia in older individuals is due to relative or absolute erythropoietin deficiency and, at least theoretically, may be reversed by ESA treatment. The benefit of this treatment is questionable, however, as illustrated by the case of cancer.
At least in theory, anemia may contribute to the physical function decline often observed in many older individuals by interfering with oxygen delivery to the brain, heart and muscles 22. Accordingly, anemia is more common in institutionalized elderly patients and is strongly associated with low muscle strength, poor lower extremity performance and mobility disability 2, 22, 42–44. However, an alternative explanation is that anemia may be proxy for the underlying comorbid diseases and frailty that cause disability. Addressing this question is critical for geriatric research. Since prevention and management of disability is a major goal of geriatric medicine, the possibility that anemia is one of the few potentially reversible causes of disability in older persons is particularly appealing. However, there is currently little information on whether correcting mild anemia in older persons can prevent or reverse physical impairment and/or disability, or increase life expectancy in patients with multiple chronic morbidities.
Anemia of cancer in the elderly
The incidence and prevalence of cancer increase with age 45. Anemia is present in more than 60% of cancer patients and the risk of anemia is higher with more advanced stages of cancer 46. The most common mechanisms of anemia in cancer patents are chronic inflammation and myelotoxic treatment such as chemotherapy and radiation therapy. As age is a risk factor for myelosuppression, older individuals may be more susceptible than young ones to chemotherapy-induced anemia. Unrecognized renal insufficiency may also play a role in the pathogenesis of anemia in elderly patients with cancer.
In cancer patients, anemia has been associated with lower survival, reduced response to radiation therapy 46–48, and increased risk of chemotherapy-induced neutropenia 49, 50. In addition, anemia is a major cause of fatigue, that has been associated with functional dependence in the elderly 46, 51, 52. In a retrospective study by Grogan et al, reversal of anemia with blood transfusions was associated with improved response of cervical cancer to radiation therapy 48. The authors demonstrated that the chance of response was inversely related to nadir hemoglobin levels, irrespective of whether or not the patients had received blood transfusions.
The advent of ESAs has presented the opportunity to study the benefits of reversing anemia without blood transfusions. A number of studies, summarized in a recent systematic review and meta-analysis 53 involving patients treated with chemotherapy, have shown that
ESAs increased the hemoglobin of anemic cancer patients in approximately 50% of cases;
improvement in hemoglobin was associated with improved energy levels and quality of life and reduced fatigue.
The steepest improvement of energy levels was reached when the hemoglobin levels were raised from 11 to 13 gm/dl 54.
A number of randomized controlled studies demonstrated the ESAs improved the hemoglobin and with it the energy level of patients receiving chemotherapy for a variety of diseases, without affecting overall survival 55–57. Intravenous iron supplementation increased the response to ESAs by 50%, whereas oral iron was not effective 58. This finding indicated that patients with anemia of chronic inflammation have a reduced capacity to mobilize iron from the tissue stores and to absorb iron. Presumably both effects are mediated by hepcidin 59.
A 2006 Cochran meta-analysis 60 of all published studies of ESAs in cancer concluded that the use of ESAs:
resulted in a 30% reduction in blood transfusions in cancer patients;
was not associated with an appreciable change in survival;
was associated with increased risk of thromboembolic complications.
The enthusiasm for the use of ESAs in cancer patients has been mitigated by the reports of eight randomized and controlled studies which show that these medications may shorten the survival of cancer patients, irrespective of whether or not they receive antineoplastic treatment (Table 1). All the studies reporting an adverse effect of the ESAs aimed at obtaining hemoglobin levels > 13 gm/dl or higher. This is an important difference compared with previous randomized controlled studies that had target hemoglobin levels of 12 gm/dl or lower. These different treatment goals may account at least in part for the unfavorable outcome. It is very possible that when hemoglobin levels are raised above 13 gm/dl, the risk of thromboembolic complications, including death, may be increased. It is also possible that somehow tumor growth is stimulated by a “refeeding” phenomenon. In other words, the improved oxygenation of the tissues may lead to improved general nutrition that is associated with accelerated tumor growth. It should be underlined however that increased tumor growth as a result of ESAs has never been conclusively demonstrated.[f1] The cause of death in patients experiencing shorter survival when treated with ESAs was reported as “cancer”, but such a diagnosis does not allow us to identify the mechanism of death. This may be related to increased thromboembolism rather than to cancer-induced end organ failure.
Table 1.
Summary of studies showing a detrimental effect of ESA on tumor progression and/or survival
| Study | Neoplasm | Treatment | ESA | Hb goal |
|---|---|---|---|---|
| Henke et al 19 | Head and neck | Radiotherapy | Epoetin β | >14 (women) >15 (men) |
| Hedenus et al18 | Lymphomas | Chemotherapy | Darbepoetin α | >14 (women) >15 (men) |
| Leyland Jones et al (2005) 17 | Breast | Chemotherapy | Epoetin α | >14 |
| Wright et al 16 | Non small cell lung cancer | Radiotherapy | Epoetin α | >14 |
| Overgaard et al 15 | Head and neck | Radiotherapy | Darbepoetin α | >15.5 |
| PREPARE 14 | Breast | Chemotherapy | Darbepoetin α | >13 |
| Thomas et al 13 | Cervix | Chemoradiotherapy | Darbepoetin α | >14 |
| Smith et al 12 | Solid tumors | No antineoplastic treatment | Darbepoetin α | >13 |
Two of the studies deserve particular comments. Henke et al, reported that epoetin α appeared detrimental to patients whose tumor expressed epoetin receptors 61. This interpretation of the study findings was highly criticized however, because the technique employed did not appear specific for epoetin receptors 62. Perhaps the most consequential of these studies has been AMGEN Int 003 12. The main goal of AMGEN Int 003 was to demonstrate that darbepoetin α reduced the need of blood transfusions in patients with metastatic cancer not receiving chemotherapy. The secondary goal was to demonstrate improved quality of life. More than 900 individuals with hemoglobin levels lower than 12 gm/dl were randomized to receive darbepoetin or no treatment. Neither of the goals was achieved and darbepoetin appeared to shorten the survival of these individuals. A subset analysis of this study revealed that the majority of patients experiencing a shorter survival were those who had not responded to erythropoietin. The interpretation of this finding is puzzling. Another concern about this study is the fact that women accounted for the majority of patients in the placebo group, but were in the minority in the treatment group. This imbalance might have been responsible for the results, at least in part, as women have a better cancer prognosis than men.
Clearly, there are a number of important questions arising from the studies of ESAs in cancer which we will try to address:
Does the use of ESAs reduce the use of blood transfusions in cancer patients? The answer is yes. All studies, including the Cochran meta-analysis, were affirmative in relation to this outcome. The only outlier was the INT003 study in which approximately half the patients were from Eastern Europe, where the availability of blood is more limited than in Western Europe and North America. The failure to see a reduction in blood transfusion may simply reflect different criteria utilized to transfuse patients in different countries. When the North American and Western European criteria are adopted, ESAs do reduce the need for blood transfusions.
Does the use of ESAs reduce the incidence and severity of fatigue? The answer is yes. Virtually all studies exploring this outcome were affirmative. A major caveat, however, should be pointed out. None of the studies proved that improvement of fatigue led to improvement in performance status or, in the case of older patients, to preservation of functional independence. This issue should be addressed in future studies.
Does the use of ESAs increase the risk of thromboembolism? The answer is yes and age may be a risk factor 53, 60. This complication may offset the benefits of ESAs, at least in part. Future studies should establish how to minimize this risk. This could be achieved by prophylactic anticoagulation or with lower doses of ESAs or a lower level of hemoglobin targets.
Do ESAs affect the patient’s survival? The answer is controversial. It is clear that ESAs do not produce an improvement in survival. There is some concern that these compounds may hasten the death of some cancer patients. This appears to be more likely in patients not receiving antineoplastic treatment and in patients whose hemoglobin was increased above 12 gm/dl. The mechanism of this complication is not clear. It may involve undetected pulmonary embolism or other thromboembolic complications, increased tumor growth, or other as yet undiscovered effects of ESAs. The data on survival are very difficult to interpret for several reasons, however. First of all they may change with time. For example, the most recent analysis of the BEST study 17 failed to show increased mortality of patients treated with epoetin (John Glaspy, personal communication, San Diego Convention Center, April 14 2008, American Association of Cancer Research). Secondly, a patient-based meta-analysis of all studies 63 in lieu of a “trial-based” meta-analysis failed to show increased mortality due to ESA in any group of patients, including those in whom hemoglobin was increased above 12 gm/dl. Thirdly, a study of patients with non-small cell lung cancer 64, in which all patients received the same treatment, did not show that epoetin was detrimental to patient survival. This study is extremely important because it is the only trial in which patients were comparable in terms of disease, disease stage and treatment.
Do ESAs enhance cancer growth? The answer to this question is also uncertain. In experimental conditions there is no proof that ESAs enhance cancer growth. In some of the human studies 12–19 [f2]ESAs have been associated with earlier cancer death. The interpretation of this finding is questionable however for all the reasons mentioned above. In addition, the diagnosis of the causes of death is imprecise, especially in patients with a terminal disease. Henke et al reported that decreased survival was associated with the expression of epoetin receptors in head and neck tumors. This conclusion was criticized because the assay employed did not distinguish between epoetin receptors and heat shock protein 61. Whatever the substance these investigators detected, it may be a predictive factor for the risks of ESAs.
Do ESAs improve the effectiveness of radiation therapy and chemotherapy? There is no proof that this is the case, nor that ESAs may mitigate chemotherapy-induced neutropenia.
Is there a “safe” hemoglobin level attainable with ESAs? The risk of death appeared increased in most studies when hemoglobin was increased to about 12 gm/dl. Hemoglobin concentrations below 12 gm/dl appear safe.
Is there a difference between different ESAs? The answer is no. Similar effects on hemoglobin, function, and survival were reported for all three commercially available products, including epoetin α, epoetin β, and darbepoetin α.
What should be the current recommendations for the treatment of cancer patients with ESAs? Ideally, a new set of clinical trials should be conducted involving patients with the same disease, the same disease stage and receiving the same treatment, like the study already mentioned in small cell lung cancer. The criteria for administration of blood transfusions should be specified at the beginning of the study. In addition to improvement of quality of life and reduction in blood transfusion, the outcome should include some objective measures of improved energy levels, such as prevention of functional dependence in older individuals. In the absence of these studies, it appears reasonable to maintain hemoglobin levels around 12 gm/dl in patients receiving chemotherapy. Both the American Society of Clinical Oncology (ASCO) and the American Society of Hematology (ASH) concur with this recommendation 65. The NCCN guidelines for the management of older cancer patients also endorse this recommendation 66.
What does experience to date of ESAs in cancer tell us about the management of anemia of chronic inflammation/relative erythropoietin insufficiency in older patients? The most important information is that ESAs may involve serious complications and further studies are necessary to establish risk and benefits of this practice,. in the absence of more information about their therapeutic index in these situations.
Conclusions
From this review one may draw the following conclusions:
The incidence and prevalence of anemia increases with age in both men and women.
In approximately 50% of the cases, the causes of anemia are identifiable and reversible. These should be properly investigated and treated.
Inflammation causes anemia through multiple mechanisms. There is evidence that in the early phase, increasing EPO levels prevents the clinical emergence of overt anemia. However, when this compensatory mechanism fails, anemia emerges and is associated with lower than expected EPO.
In about 30% of the cases, anemia in older patients is due to absolute or relative erythropoietin deficiency. The benefits of treating these forms of anemia with ESAs have not been established and need to be studied in randomized and controlled trials.
In cancer patients, correction of anemia with ESAs has been associated with reduced number of blood transfusions and improved energy level. The treatment is associated with increased risks of thromboembolism. When hemoglobin levels were kept around 12 gm/dl there was no apparent effect of ESAs on patient survival.
New randomized and controlled studies are desirable in cancer patients. Such studies should involve comparable patients, i.e., patients with the same neoplasm, the same disease stage and receiving the same treatment. The outcome of these studies should include some form of objective assessment of improved energy levels, such as prevention of functional dependence in older individuals. The feasibility of these studies is doubtful however, given the limited patient availability and the concern about potential complications of ESAs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Patel KV, Guralnik JM. Epidemiology of anemia in older adults. Cambridge: Cambridge University Press; 2008. [Google Scholar]
- 2.Culleton BF, Manns BJ, Zhang J, Tonelli M, Klarenbach S, Hemmelgarn BR. Impact of anemia on hospitalization and mortality in older adults. Blood. 2006 May 15;107(10):3841–3846. doi: 10.1182/blood-2005-10-4308. [DOI] [PubMed] [Google Scholar]
- 3.Penninx BW. Anemia and functional decline. New York: Springer; 2007. [Google Scholar]
- 4.Atti AR, Palmer K, Volpato S, Zuliani G, Winblad B, Fratiglioni L. Anaemia increases the risk of dementia in cognitively intact elderly. Neurobiol Aging. 2006 Feb;27(2):278–284. doi: 10.1016/j.neurobiolaging.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 5.Penninx BW, Pluijm SM, Lips P, Woodman R, Miedema K, Guralnik JM, Deeg DJ. Late-life anemia is associated with increased risk of recurrent falls. J Am Geriatr Soc. 2005 Dec;53(12):2106–2111. doi: 10.1111/j.1532-5415.2005.00491.x. [DOI] [PubMed] [Google Scholar]
- 6.Go AS, Yang J, Ackerson LM, Lepper K, Robbins S, Massie BM, Shipak MG. Hemoglobin level, chronic kidney disease, and the risks of death and hospitalization in adults with chronic heart failure: the Anemia in Chronic Heart Failure: Outcomes and Resource Utilization (ANCHOR) Study. Circulation. 2006 Jun 13;113(23):2713–2723. doi: 10.1161/CIRCULATIONAHA.105.577577. [DOI] [PubMed] [Google Scholar]
- 7.Wu WC, Rathore SS, Wang Y, Radford MJ, Krumholz HM. Blood transfusion in elderly patients with acute myocardial infarction. N Engl J Med. 2001 Oct 25;345(17):1230–1236. doi: 10.1056/NEJMoa010615. [DOI] [PubMed] [Google Scholar]
- 8.Ania BJ, Suman VJ, Fairbanks VF, Rademacher DM, Melton LJ., 3rd Incidence of anemia in older people: an epidemiologic study in a well defined population. J Am Geriatr Soc. 1997 Jul;45(7):825–831. doi: 10.1111/j.1532-5415.1997.tb01509.x. [DOI] [PubMed] [Google Scholar]
- 9.Guralnik JM, Eisenstaedt RS, Ferrucci L, Klein HG, Woodman RC. Prevalence of anemia in persons 65 years and older in the United States: evidence for a high rate of unexplained anemia. Blood. 2004 Oct 15;104(8):2263–2268. doi: 10.1182/blood-2004-05-1812. [DOI] [PubMed] [Google Scholar]
- 10.Ferrucci L, Guralnik JM, Bandinelli S, Semba RD, Lauretani F, Corsi A, et al. Unexplained anaemia in older persons is characterised by low erythropoietin and low levels of pro-inflammatory markers. Br J Haematol. 2007 Mar;136(6):849–855. doi: 10.1111/j.1365-2141.2007.06502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrucci L, Guralnik JM, Woodman RC, Bandinelli S, Lauretani F, Corsi AM, et al. Proinflammatory state and circulating erythropoietin in persons with and without anemia. Am J Med. 2005 Nov;118(11):1288. doi: 10.1016/j.amjmed.2005.06.039. [DOI] [PubMed] [Google Scholar]
- 12.Smith RE, Jr, Aapro MS, Ludwig H, Pinter T, Smakal M, Ciuleanu TE, et al. Darbepoetin alpha for the treatment of anemia in patients with active cancer not receiving chemotherapy or radiotherapy: results of a phase III, multicenter, randomized, double-blind, placebo-controlled study. J Clin Oncol. 2008 Mar 1;26(7):1040–1050. doi: 10.1200/JCO.2007.14.2885. [DOI] [PubMed] [Google Scholar]
- 13.Thomas G, Ali S, Hoebers FJ, Darcy KM, Rodgers WH, Patel M, et al. Phase III trial to evaluate the efficacy of maintaining hemoglobin levels above 12.0 g/dL with erythropoietin vs above 10.0 g/dL without erythropoietin in anemic patients receiving concurrent radiation and cisplatin for cervical cancer. Gynecol Oncol. 2008 Feb;108(2):317–325. doi: 10.1016/j.ygyno.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.FDA receives new data on risks of anemia drugs. FDA News. 2008 [Google Scholar]
- 15.Overgaard J, Hoff C, Hansen HS. Randomized study of the importance of novel erythropoiesis stimulating protein (Aranesp) for the effect of radiotherapy in patients with primary squamous cell carcinoma of the head and neck (HNSCC). The Danish Head and Neck Cancer Group DAHNCA 10 rand [abstract 6LB] Eur J Cancer Suppl. 2007;2007(5):7. [Google Scholar]
- 16.Wright JR, Ung YC, Julian JA, Pritchard KI, Whelan TJ, Smith C, et al. Randomized, double-blind, placebo-controlled trial of erythropoietin in non-small-cell lung cancer with disease-related anemia. J Clin Oncol. 2007 Mar 20;25(9):1027–1032. doi: 10.1200/JCO.2006.07.1514. [DOI] [PubMed] [Google Scholar]
- 17.Leyland-Jones B, Semiglazov V, Pawlicki M, Pienkowski T, Tjulandin S, Manikhas G, et al. Maintaining normal hemoglobin levels with epoetin alfa in mainly nonanemic patients with metastatic breast cancer receiving first-line chemotherapy: a survival study. J Clin Oncol. 2005 Sep 1;23(25):5960–5972. doi: 10.1200/JCO.2005.06.150. [DOI] [PubMed] [Google Scholar]
- 18.Hedenus M, Adriansson M, San Miguel J, Kramer MR, Juvonen E, Taylor K, et al. Darbepoetin Alfa 20000161 Study Group. Efficacy and safety of darbepoetin alfa in anaemic patients with lymphoproliferative malignancies: a randomized, double-blind, placebo-controlled study. Br J Haematol. 2003 Aug;122(3):394–403. doi: 10.1046/j.1365-2141.2003.04448.x. [DOI] [PubMed] [Google Scholar]
- 19.Henke M, Laszig R, Rube C, Schafer U, Hasse KD, Schilcher B, et al. Erythropoietin to treat head and neck cancer patients with anaemia undergoing radiotherapy: randomised, double-blind, placebo-controlled trial. Lancet. 2003 Oct 18;362(9392):1255–1260. doi: 10.1016/S0140-6736(03)14567-9. [DOI] [PubMed] [Google Scholar]
- 20.Walston J, Hadley EC, Ferrucci L, Guralnik JM, Newman AB, Studenski SA, et al. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc. 2006 Jun;54(6):991–1001. doi: 10.1111/j.1532-5415.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- 21.Ruggiero C, Ferrucci L. The endeavor of high maintenance homeostasis: resting metabolic rate and the legacy of longevity. J Gerontol A Biol Sci Med Sci. 2006 May;61(5):466–471. doi: 10.1093/gerona/61.5.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chaves PH, Semba RD, Leng SX, Woodman RC, Ferrucci L, Guralnik JM, Fried LP. Impact of anemia and cardiovascular disease on frailty status of community-dwelling older women: the Women’s Health and Aging Studies I and II. J Gerontol A Biol Sci Med Sci. 2005 Jun;60(6):729–735. doi: 10.1093/gerona/60.6.729. [DOI] [PubMed] [Google Scholar]
- 23.Woodman R, Ferrucci L, Guralnik J. Anemia in older adults. Curr Opin Hematol. 2005 Mar;12(2):123–128. doi: 10.1097/01.moh.0000154030.13020.85. [DOI] [PubMed] [Google Scholar]
- 24.Chahal HS, Drake WM. The endocrine system and ageing. J Pathol. 2007 Jan;211(2):173–180. doi: 10.1002/path.2110. [DOI] [PubMed] [Google Scholar]
- 25.Ferrucci L, Maggio M, Bandinelli S, Basaria S, Lauretani F, Ble A, et al. Low testosterone levels and the risk of anemia in older men and women. Arch Intern Med. 2006 Jul 10;166(13):1380–1388. doi: 10.1001/archinte.166.13.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fein HG, Rivlin RS. Anemia in thyroid diseases. Med Clin North Am. 1975 Sep;59(5):1133–1145. doi: 10.1016/s0025-7125(16)31963-0. [DOI] [PubMed] [Google Scholar]
- 27.Ferrucci L, Corsi A, Lauretani F, Bandelli S, Bartali B, Taubb DD, et al. The origins of age-related proinflammatory state. Blood. 2005 Mar 15;105(6):2294–2299. doi: 10.1182/blood-2004-07-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yaffe K, Kanaya A, Lindquist K, Simonsick EM, Harris T, Shorr RI, et al. The metabolic syndrome, inflammation, and risk of cognitive decline. Jama. 2004 Nov 10;292(18):2237–2242. doi: 10.1001/jama.292.18.2237. [DOI] [PubMed] [Google Scholar]
- 29.Cesari M, Penninx BW, Newman AB, Kritchevsky SB, Nicklas BJ, Sutton-Tyrell K, et al. Inflammatory markers and onset of cardiovascular events: results from the Health ABC study. Circulation. 2003 Nov 11;108(19):2317–2322. doi: 10.1161/01.CIR.0000097109.90783.FC. [DOI] [PubMed] [Google Scholar]
- 30.Cesari M, Penninx BW, Pahor M. Inflammatory markers and physical performance in older persons: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2004 Mar;59(3):242–248. doi: 10.1093/gerona/59.3.m242. [DOI] [PubMed] [Google Scholar]
- 31.Alley DE, Crimmins E, Bandeen-Roche K, Guralnik J, Ferrucci L. Three-year change in inflammatory markers in elderly people and mortality: the Invecchiare in Chianti study. J Am Geriatr Soc. 2007 Nov;55(11):1801–1807. doi: 10.1111/j.1532-5415.2007.01390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferrucci L, Harris TB, Guralnik JM, Tracy RP, Corti MC, Cohen HJ, et al. Serum IL-6 level and the development of disability in older persons. J Am Geriatr Soc. 1999 Jun;47(6):639–646. doi: 10.1111/j.1532-5415.1999.tb01583.x. [DOI] [PubMed] [Google Scholar]
- 33.Lipschitz DA. The anemia of chronic disease. J Am Geriatr Soc. 1990 Nov;38(11):1258–1264. doi: 10.1111/j.1532-5415.1990.tb01509.x. [DOI] [PubMed] [Google Scholar]
- 34.van der Putten K, Braam B, Jie KE, Gaillard CA. Mechanisms of Disease: erythropoietin resistance in patients with both heart and kidney failure. Nat Clin Pract Nephrol. 2008 Jan;4(1):47–57. doi: 10.1038/ncpneph0655. [DOI] [PubMed] [Google Scholar]
- 35.Scharte M, Fink MP. Red blood cell physiology in critical illness. Crit Care Med. 2003 Dec;31(12 Suppl):S651–657. doi: 10.1097/01.CCM.0000098036.90796.ED. [DOI] [PubMed] [Google Scholar]
- 36.Ganz T. Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. Blood. 2003 Aug 1;102(3):783–788. doi: 10.1182/blood-2003-03-0672. [DOI] [PubMed] [Google Scholar]
- 37.Nemeth E, Rivera S, Gabayan V, Keller C, Pedersen BK, Ganz T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004 May;113(9):1271–1276. doi: 10.1172/JCI20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lang KS, Lang PA, Bauer C, Duranton C, Wider T, Huber SM, Lang F. Mechanisms of suicidal erythrocyte death. Cell Physiol Biochem. 2005;15(5):195–202. doi: 10.1159/000086406. [DOI] [PubMed] [Google Scholar]
- 39.Roy CN, Andrews NC. Anemia of inflammation: the hepcidin link. Curr Opin Hematol. 2005 Mar;12(2):107–111. doi: 10.1097/00062752-200503000-00001. [DOI] [PubMed] [Google Scholar]
- 40.Nemeth E, Valore EV, Territo M, Schiller G, Lichtenstein A, Ganz T. Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein. Blood. 2003 Apr 1;101(7):2461–2463. doi: 10.1182/blood-2002-10-3235. [DOI] [PubMed] [Google Scholar]
- 41.Denny SD, Kuchibhatla MN, Cohen HJ. Impact of anemia on mortality, cognition, and function in community-dwelling elderly. Am J Med. 2006 Apr;119(4):327–334. doi: 10.1016/j.amjmed.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 42.Landi F, Russo A, Danese P, Liperoti R, Barillaro C, Bernabei R, Onder G. Anemia status, hemoglobin concentration, and mortality in nursing home older residents. J Am Med Dir Assoc. 2007 Jun;8(5):322–327. doi: 10.1016/j.jamda.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 43.Zakai NA, Katz R, Hirsch C, Shlipak MG, Chaves PH, Newman AB, Cushman M. A prospective study of anemia status, hemoglobin concentration, and mortality in an elderly cohort: the Cardiovascular Health Study. Arch Intern Med. 2005 Oct 24;165(19):2214–2220. doi: 10.1001/archinte.165.19.2214. [DOI] [PubMed] [Google Scholar]
- 44.Balducci L, Ershler WB. Cancer and ageing: a nexus at several levels. Nat Rev Cancer. 2005 Aug;5(8):655–662. doi: 10.1038/nrc1675. [DOI] [PubMed] [Google Scholar]
- 45.Benson K, Balducci L. Anemia and cancer. New York: Springer; 2007. [Google Scholar]
- 46.Prosnitz RG, Yao B, Farrell CL, Clough R, Brizel DM. Pretreatment anemia is correlated with the reduced effectiveness of radiation and concurrent chemotherapy in advanced head and neck cancer. Int J Radiat Oncol Biol Phys. 2005 Mar 15;61(4):1087–1095. doi: 10.1016/j.ijrobp.2004.07.710. [DOI] [PubMed] [Google Scholar]
- 47.Grogan M, Thomas GM, Melamed I, Wong FL, Pearcey RG, Joseph PK, et al. The importance of hemoglobin levels during radiotherapy for carcinoma of the cervix. Cancer. 1999 Oct 15;86(8):1528–1536. doi: 10.1002/(sici)1097-0142(19991015)86:8<1528::aid-cncr20>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 48.Extermann M, Chen H, Cantor AB, Corcoran MB, Meyer J, Grendys E, et al. Predictors of tolerance to chemotherapy in older cancer patients: a prospective pilot study. Eur J Cancer. 2002 Jul;38(11):1466–1473. doi: 10.1016/s0959-8049(02)00090-4. [DOI] [PubMed] [Google Scholar]
- 49.Schrijvers D, Highley M, De Bruyn E, Van Oosterom AT, Vermorken JB. Role of red blood cells in pharmacokinetics of chemotherapeutic agents. Anticancer Drugs. 1999 Feb;10(2):147–153. doi: 10.1097/00001813-199902000-00002. [DOI] [PubMed] [Google Scholar]
- 50.Luciani A, Balducci L, Extermann M. Fatigue may be a cause of functional dependence in older cancer patients. Am J Clin Oncol. 2008 doi: 10.1097/COC.0b013e31816d915f. In Press. [DOI] [PubMed] [Google Scholar]
- 51.Tralongo P, Respini D, Ferrau F. Fatigue and aging. Crit Rev Oncol Hematol. 2003 Oct 15;48(Suppl):S57–64. doi: 10.1016/j.critrevonc.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 52.Bennett CL, Silver SM, Djulbegovic B, Samaras AT, Blau CA, Gleason KJ, et al. Venous thromboembolism and mortality associated with recombinant erythropoietin and darbepoetin administration for the treatment of cancer-associated anemia. Jama. 2008 Feb 27;299(8):914–924. doi: 10.1001/jama.299.8.914. [DOI] [PubMed] [Google Scholar]
- 53.Curt GA, Breitbart W, Cella D, Groopman JE, Horning SJ, Itri LM, et al. Impact of cancer-related fatigue on the lives of patients: new findings from the Fatigue Coalition. Oncologist. 2000;5(5):353–360. doi: 10.1634/theoncologist.5-5-353. [DOI] [PubMed] [Google Scholar]
- 54.Aapro M, Leonard RC, Barnadas A, Marangolo M, Untch M, Malamos N, et al. Effect of once-weekly epoetin beta on survival in patients with metastatic breast cancer receiving anthracycline- and/or taxane-based chemotherapy: results of the Breast Cancer-Anemia and the Value of Erythropoietin (BRAVE) study. J Clin Oncol. 2008 Feb 1;26(4):592–598. doi: 10.1200/JCO.2007.11.5378. [DOI] [PubMed] [Google Scholar]
- 55.Littlewood TJ, Bajetta E, Nortier JW, Vercammen E, Rapoport B. Effects of epoetin alfa on hematologic parameters and quality of life in cancer patients receiving nonplatinum chemotherapy: results of a randomized, double-blind, placebo-controlled trial. J Clin Oncol. 2001 Jun 1;19(11):2865–2874. doi: 10.1200/JCO.2001.19.11.2865. [DOI] [PubMed] [Google Scholar]
- 56.Vansteenkiste J, Pirker R, Massuti B, Barata F, Font A, Fiegl M, et al. Aranesp 980297 Study Group. Double-blind, placebo-controlled, randomized phase III trial of darbepoetin alfa in lung cancer patients receiving chemotherapy. J Natl Cancer Inst. 2002 Aug 21;94(16):1211–1220. doi: 10.1093/jnci/94.16.1211. [DOI] [PubMed] [Google Scholar]
- 57.Auerbach M, Ballard H, Trout JR, McIlwain M, Ackerman A, Bahrain H, et al. Intravenous iron optimizes the response to recombinant human erythropoietin in cancer patients with chemotherapy-related anemia: a multicenter, open-label, randomized trial. J Clin Oncol. 2004 Apr 1;22(7):1301–1307. doi: 10.1200/JCO.2004.08.119. [DOI] [PubMed] [Google Scholar]
- 58.Nemeth E, Ganz T. Iron and Aging. Cambridge: Cambridge University Press; 2008. [Google Scholar]
- 59.Bohlius J, Wilson J, Seidenfeld J, Piper M, Schwarner G, Sandercock J, et al. Recombinant human erythropoietins and cancer patients: updated meta-analysis of 57 studies including 9353 patients. J Natl Cancer Inst. 2006 May 17;98(10):708–714. doi: 10.1093/jnci/djj189. [DOI] [PubMed] [Google Scholar]
- 60.Henke M, Mattern D, Pepe M, Bezay C, Weissenberger C, Werner M, Pajonk F. Do erythropoietin receptors on cancer cells explain unexpected clinical findings? J Clin Oncol. 2006 Oct 10;24(29):4708–4713. doi: 10.1200/JCO.2006.06.2737. [DOI] [PubMed] [Google Scholar]
- 61.Crawford J. Erythropoietin: high profile, high scrutiny. J Clin Oncol. 2007 Mar 20;25(9):1021–1023. doi: 10.1200/JCO.2006.08.8153. [DOI] [PubMed] [Google Scholar]
- 62.Crawford J, Henry DH, Vansteenkiste J, Ludwig H, Lillie T, Fleishman A, Glaspy J. The effect of hemoglobin (hb) level at ESA initiation on outcomes in cancer patients (pts) with chemotherapy-induced anemia (CIA): a patient-based meta-analysis from 6 randomized, placebo-controlled trials (RCTS) of darbepoetin alfa (DA) Blood. 2007 November;110:3780. [Google Scholar]
- 63.Grote T, Yeilding AL, Castillo R, Butler D, Fishkin E, Henry DH, et al. Efficacy and safety analysis of epoetin alfa in patients with small-cell lung cancer: a randomized, double-blind, placebo-controlled trial. J Clin Oncol. 2005 Dec 20;23(36):9377–9386. doi: 10.1200/JCO.2005.01.8507. [DOI] [PubMed] [Google Scholar]
- 64.Rizzo JD, Somerfield MR, Hagerty KL, Seidenfeld J, Bohlius J, Bennett CL, et al. American Society of Clinical Oncology; American Society of Hematology. Use of epoetin and darbepoetin in patients with cancer: 2007 American Society of Clinical Oncology/American Society of Hematology clinical practice guideline update. J Clin Oncol. 2008 Jan 1;26(1):132–149. doi: 10.1200/JCO.2007.14.3396. [DOI] [PubMed] [Google Scholar]
- 65.Balducci L, Cohen HJ, Engstrom PF, Ettinger DS, Halter J, Gordon LI, et al. National Comprehensive Cancer Network. Senior adult oncology clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2005 Jul;3(4):572–590. doi: 10.6004/jnccn.2005.0032. [DOI] [PubMed] [Google Scholar]