Abstract
Background
Prostate-specific antigen (PSA) level is typically used as a dichotomous test for prostate cancer, resulting in overdiagnosis for a substantial number of men. The rate at which serum PSA levels change (PSA velocity) may be an important indicator of the presence of life-threatening disease.
Methods
PSA velocity was determined in 980 men (856 without prostate cancer, 104 with prostate cancer who were alive or died of another cause, and 20 who died of prostate cancer) who were participants in the Baltimore Longitudinal Study of Aging for up to 39 years. The relative risks (RRs) of prostate cancer death and prostate cancer–specific survival stratified by PSA velocity were evaluated in the three groups of men by Cox regression and Kaplan–Meier analyses. Statistical tests were two-sided.
Results
PSA velocity measured 10–15 years before diagnosis (when most men had PSA levels below 4.0 ng/mL) was associated with cancer-specific survival 25 years later; survival was 92% (95% confidence interval [CI] = 84% to 96%) among men with PSA velocity of 0.35 ng/mL per year or less and 54% (95% CI = 15% to 82%) among men with PSA velocity above 0.35 ng/mL per year (P<.001). Furthermore, men with PSA velocity above 0.35 ng/mL per year had a higher relative risk of prostate cancer death than men with PSA velocity of 0.35 ng/mL per year or less (RR = 4.7, 95% CI = 1.3 to 16.5; P = .02); the rates per 100 000 person-years were 1240 for men with a PSA velocity above 0.35 ng/mL per year and 140 for men with a PSA velocity of 0.35 ng/mL per year or less.
Conclusions
PSA velocity may help identify men with life-threatening prostate cancer during a period when their PSA levels are associated with the presence of curable disease.
The level of prostate-specific antigen (PSA) in the blood serum is widely used to screen for prostate cancer because cancers detected by PSA screening are discovered at an earlier stage than those detected symptomatically (1). Clinicians generally agree that PSA screening can detect early-stage cancers, some of which are destined to metastasize. However, the PSA level or threshold value at which further evaluation with a prostate biopsy should be recommended remains controversial. Until recently, most clinicians had accepted a PSA threshold of 4.0 ng/mL for recommending a prostate biopsy as reasonable to balance the trade-off of missing biologically important cancers against performing unnecessary biopsies and detecting biologically unimportant cancers. Now there is growing enthusiasm among urologists to use PSA thresholds below 4.0 ng/mL for recommending prostate biopsy to increase the detection of curable disease (2). However, others have expressed concerns that lower PSA thresholds for biopsy will lead to even further increases in the overdiagnosis and overtreatment of prostate cancer (3,4). All agree that there is a need for markers that will identify those men with life-threatening prostate cancer for whom curative intervention will improve outcomes.
Rate of change in PSA level or PSA velocity has been shown to be higher among men with prostate cancer than in men without the disease in some studies (5,6). Furthermore, PSA velocity before the diagnosis of prostate cancer has been associated with surrogate biomarkers of adverse outcomes, such as pathologic tumor characteristics and time to recurrence after treatment (7,8). Recently, D’Amico et al. (9) showed that, when compared with men with a PSA velocity of 2.0 ng/mL per year or less in the year before diagnosis, men with a PSA velocity above 2.0 ng/mL per year were at an increased risk of prostate cancer death after surgical treatment. An unanswered question is whether a lower PSA velocity could identify those men with life-threatening prostate cancer during a window of curability.
If men with a relatively high PSA velocity in the year before diagnosis are more likely than men with a low PSA velocity to have advanced prostate cancer not amenable to cure with surgery, it is conceivable that these same men with life-threatening disease could have been identified earlier based on their PSA velocity at a time when the disease was still curable by local therapy (radiation or surgery). If so, then it may be appropriate to incorporate PSA velocity into decision algorithms for prostate biopsy to identify men with life-threatening cancer that will benefit from curative intervention, rather than performing biopsies on all men who reach a given threshold PSA value.
To address this issue, we used data from the Baltimore Longitudinal Study of Aging (BLSA) to compare the PSA histories of men who died of prostate cancer, men with prostate cancer who were alive or died of another cause, and men without a diagnosis of prostate cancer.
Subjects and Methods
Study Cohort
Study subjects were participants in the BLSA, a prospective cohort study of the National Institute on Aging (Bethesda, MD) (10) in which the first patient was seen on February 6, 1958. This open enrollment study includes 1806 male subjects largely from the Baltimore, MD, and Washington, DC, areas. Participants receive a comprehensive medical, physical, and neuropsychological examination at regular intervals, typically every 2 years. The Med Star Institutional Review Boards and the Institutional Review Boards of the Johns Hopkins Medical Institutions (Baltimore, MD) approved this study, and all subjects gave written informed consent for their participation in the BLSA.
Since September 9, 1991, PSA measurements and digital rectal examination were performed at each evaluation. Participants with a PSA level above 4.0 ng/mL and/or abnormality on digital rectal examination also underwent a transrectal ultrasound-directed prostate biopsy.
PSA levels were measured using frozen sera samples stored at −70 °C for visits performed before September 9, 1991. After September 9, 1991, PSA measurements were performed on sera collected prospectively at subject visits. All PSA measurements were performed using a standard monoclonal immunoradiometric assay (Tandem-R, Hybritech Inc, San Diego, CA), which has an interassay coefficient of variation of 4.9%, 3.6%, and 3.0% when the level of PSA is 3.0, 7.0, and 36.0 ng/mL, respectively (11).
Of the 1806 male subjects who participated in the BLSA from February 6, 1958, to May 31, 2005, 1201 had PSA measurements available. We excluded men with a diagnosis of prostate cancer who had no PSA data before their diagnosis (n = 38), men who had a simple prostatectomy for prostate enlargement and had no PSA data before their prostate surgery (n = 80), men who had taken finasteride (Proscar) at any time (n = 47), men with an unknown cause of death (n = 54), and men who had a single PSA value that was suspected to be a laboratory error, i.e., an outlier value inconsistent with other values (n = 2). After these exclusions, the study cohort included 980 men, of whom 124 had a diagnosis of prostate cancer and 856 had no known diagnosis of prostate cancer. The majority of the men were white (79%), 17% were African American, and 4% were Asian or from other ethnic groups.
Cause of death for study participants was determined from the BLSA death file, which includes information on cause of death ascertained by intermittent telephone follow-up of inactive participants, correspondence from relatives, and searches of the National Death Index. For deceased BLSA subjects, the cause of death was determined by the consensus of three physicians who reviewed all available information, including death certificates, letters from physicians and families, medical records, and autopsy reports.
As of May 31, 2005, of 124 men with prostate cancer, 66 were still alive and 58 had died. Of the deceased men, 20 had prostate cancer listed as the underlying cause of death and were considered events, whereas 38 men with prostate cancer had died of other causes. As of May 31, 2005, of 856 men without a diagnosis of prostate cancer, 684 were alive and 172 were deceased. Of these 172 men, 21 were diagnosed with prostate cancer at autopsy. Autopsy-detected prostate cancers were included among the group without prostate cancer despite the presence, in some cases, of high-grade, advanced disease. We categorized autopsy-detected cancers as noncancers because autopsies were not performed routinely on all men in this cohort and because men with autopsy-detected prostate cancer were unaware of a diagnosis of prostate cancer during their lifetime.
We divided the study cohort into three groups: 856 subjects not diagnosed with prostate cancer (no-cancer group), 104 subjects with a diagnosis of prostate cancer who were alive (n = 66) or dead of a cause other than prostate cancer (n = 38) (prostate cancer group), and 20 subjects with a diagnosis of prostate cancer who died of the disease (dead of prostate cancer). Thus, 2% of our cohort (20 of 980 subjects) died of prostate cancer (at a median of 8 years after diagnosis) at a median age of 83 years, which is similar to the approximate 2% probability of dying of prostate cancer by age 85 years for whites in the United States (12).
The median year of diagnosis was 1995 (range = 1974–2003) for the 66 men with prostate cancer who were alive, 1990 (range = 1967–1996) for the 38 men with prostate cancer who died of another cause, and 1989 (range = 1979 –1996) for the 20 men who died of prostate cancer. The median year of last PSA measurement for subjects without a diagnosis of prostate cancer was 1999 (range = 1963–2002). The stage and grade of cancers were not consistently available for subjects with a diagnosis of prostate cancer and thus were not included in analyses.
Statistical Analyses
Study group characteristics were compared using pooled t tests (in the case of equal variances) and using Cochran t tests (in the case of unequal variances). All statistical tests were two-sided; P<.05 was considered statistically significant.
All PSA measurements were censored at the time of diagnosis of prostate cancer, at the time of a prostate operation for benign disease (simple prostatectomy), or at the last participant visit. The index visit was defined as that visit within a specific time period before diagnosis at which the PSA level and PSA velocity were determined. PSA velocity in ng/mL per year was calculated for each subject (n = 788) as the running average of the rate of change over three consecutive visits (the index visit and the two preceding visits), when more than two PSA measurements were available (5), or as the simple rate of change, if only two measurements were available. The linear relationship between PSA level and PSA velocity was determined by the Pearson product–moment correlation.
We used a mixed-effects model (13) to estimate average changes in PSA levels as a function of time before diagnosis or before last evaluation for subjects without a diagnosis of prostate cancer. PSA levels were transformed as log(PSA level + 1) for the analyses. The model included time, time squared, a term for group, and interactions between time and group. Random effects were included for time and time squared within subjects. Date of diagnosis and date of last BLSA visit or evaluation were used as endpoints for subjects with a diagnosis of cancer and for subjects without prostate cancer, respectively.
We evaluated the association between PSA velocity and death from prostate cancer during the time interval of 10–15 years before diagnosis for two reasons. First, most of the men in our study who died of prostate cancer had cancer detected in the pre-PSA era, and the lead time with PSA screening is believed to be approximately 10 years (14). Because most screen-detected cancers are curable, we believe that the men in our study who died of prostate cancer may have had curable disease 10–15 years before their diagnosis date. Second, at 10–15 years before diagnosis, most men with prostate cancer in the cohort (combining those who did and did not die of disease) had PSA levels in the range (<4.0 ng/mL) at which curable disease is usually identified (15), suggesting that this time interval may have provided a window of opportunity for cure. The PSA level and PSA velocity determined at the index visit within the 10- to 15-years-before-diagnosis time frame were used in the analyses of this time period. For those subjects with more than one visit during this period, the visit closest to diagnosis was defined as the index visit. For calculation of PSA velocity at the index visit 10–15 years before diagnosis, prior PSA measurements used to determine PSA velocity could have fallen outside the 10- to 15-year time window (i.e., >15 years before diagnosis). Of 353 men with a PSA velocity determination at an index visit 10–15 years before diagnosis, 103 men had all PSA measurements taken within 10–15 years before diagnosis. The median time between the starting PSA measurement and the index visit used for calculation of PSA velocity was 5.3 years (range = 1–17.5), and the average time between measurements was 3 years.
We also evaluated the association between PSA velocity and death from prostate cancer during the time intervals 0–2, 0–5, and 5–10 years before diagnosis for comparison with the 10- to 15-years-before-diagnosis results. These analyses were performed in the same manner as the analyses of PSA velocity for the 10- to 15-years-before-diagnosis time frame. At 0–2, 0–5, and 5–10 years before diagnosis, 711, 596, and 408 men had a PSA velocity determination at the index visit, respectively.
Sensitivity and specificity for PSA and PSA velocity were explored using a receiver-operating curve (ROC) analysis for PSA values below 4.0 ng/mL. The area under the ROC (95% confidence interval [CI]) was calculated, and a test of the null hypothesis that the area under the curve was 50% was performed using the Wilcoxon rank sum test. The ROC analysis suggested that 0.35 ng/mL per year could be one reasonable choice—among others—to balance sensitivity and specificity for detection of life-threatening cancer, and this cut point was used in Cox proportional hazards regression analysis and Kaplan–Meier analysis.
Cox proportional hazards regression was used to evaluate associations between the covariates PSA level and PSA velocity, age at diagnosis, diagnosis date, and the main outcome—death from prostate cancer. The assumptions of proportionality have been satisfied (the hazard ratio for PSA velocity was nearly constant over time). In this analysis, time 0 was the index visit at which PSA level and PSA velocity were determined, not date of diagnosis. PSA level was treated as a binary variable using a cutoff of 2.6 ng/mL or more because in clinical practice urologists use threshold levels of PSA for recommending prostate biopsies, and a value of 2.6 ng/mL has been recommended as a biopsy threshold that will improve disease-free outcomes (16). PSA velocity was treated as a continuous variable in this analysis, and the relative risk was reported per 1 unit change (1 ng/mL per year) and per 0.1 ng/mL per year, based on units urologists use in daily practice. Age and date of diagnosis were treated as continuous variables, and relative risk was reported per unit change (i.e., 1 year). The Wald test was used to test the null hypothesis that the hazard ratio associated with a covariate was 1. Likelihood ratio tests were used to assess the fit of the model with different covariates, and all models were fit on the same cohort with no differences in missing values. The relative risk of prostate cancer death and risk per 100 000 person-years were determined from the Cox regression analysis for men with a PSA velocity above 0.35 ng/mL per year when compared with those with a PSA velocity of 0.35 ng/mL per year or less after adjustment for age and date of diagnosis.
Time to death stratified by PSA velocity (≤0.35 ng/mL per year versus >0.35 ng/mL per year) was estimated using the Kaplan–Meier method (17) with censoring of subjects who were alive as of May 31, 2005. Time 0 was the index visit 10–15 years before diagnosis at which PSA level and PSA velocity were determined. The Mantel–Haenszel log-rank test was used to test for equality of survivor functions. This analysis was done to assess the relationship between PSA velocity and prostate cancer death, not to identify a specific PSA velocity cutoff to be used in clinical practice.
Results
Cohort Demographics
The demographic characteristics of the study cohort are described in Table 1. Compared with men without a diagnosis of prostate cancer, men with a diagnosis of prostate cancer and men who died of prostate cancer were statistically significantly older at the time of the first and last PSA tests and more often had a similar number of total PSA measures (Table 1). Follow-up in years from first to last PSA test was statistically significantly longer for men with a diagnosis of prostate cancer compared with men without this diagnosis but was similar for men with prostate cancer who did and did not die of their cancer. Evaluating follow-up time from the last PSA test (for those without prostate cancer) or diagnosis (for those with prostate cancer) to censoring revealed that this period was statistically significantly longer for men with prostate cancer than for men without prostate cancer. However, on average, men who had prostate cancer but did not die of the disease were followed longer after diagnosis than men who did die of prostate cancer.
Table 1.
Description of diagnostic groups*
| Comparisons |
|||||||
|---|---|---|---|---|---|---|---|
| Characteristic | Total | No cancer | Cancer | Dead of cancer | No cancer vs. cancer | Cancer vs. dead of cancer | No cancer vs. dead of cancer |
| No. of subjects | 980 | 856 | 104 | 20 | |||
| Age at first PSA test, y | |||||||
| Median (range) | 49.8 (19.9–92.0) | 48.6 (19.9–92.0) | 55.1 (30.0–84.8) | 59.5 (38.9–86.0) | P<.001 | P = .11 | P = .002 |
| Mean (SD) | 50.4 (15.3) | 49.6 (15.4) | 55.4 (12.5) | 60.4 (12.6) | |||
| Age at last PSA test, y | |||||||
| Median (range) | 63.8 (19.9–95.3) | 62.4 (19.9–95.3) | 69.2 (47.2–88.2) | 75.0 (56.8–88.1) | P<.001 | P = .04 | P<.001 |
| Mean (SD) | 61.6 (16.4) | 60.4 (16.9) | 68.7 (9.2) | 73.4 (9.6) | |||
| Follow-up, y | |||||||
| First to last PSA test | |||||||
| Median (range) | 9.5 (0.0–38.8) | 9.0 (0.0–38.8) | 14.5 (0.0–30.6) | 13.5 (0.0–25.3) | P = .02 | P = .90 | P = .32 |
| Mean (SD) | 11.1 (9.6) | 10.8 (9.6) | 13.3 (9.7) | 13 (7.6) | |||
| Time from last PSA test or cancer iagnosis to death or censoring† | |||||||
| Median (range) | 5.5 (0.1–38.0) | 5.3 (0.1–38.0) | 11 (2.3–37.6) | 7.6 (3.0–22.1) | P<.001 | P = .06 | P = .046 |
| Mean (SD) | 7.2 (5.6) | 6.6 (5.0) | 12.3 (7.6) | 8.8 (5.8) | |||
| PSA testing | |||||||
| No. of PSA values repeated | |||||||
| Median (range) | 4.0 (1.0–15.0) | 4.0 (1.0–14.0) | 5.0 (1.0–15.0) | 5.5 (1.0–14.0) | P = .006 | P = .36 | P = .02 |
| Mean (SD) | 4.6 (3.1) | 4.5 (3.0) | 5.4 (3.3) | 6.1 (3.6) | |||
| Interval between PSA tests, y | |||||||
| Median (range) | 2.1 (0.1–21.5) | 2.1 (0.1–21.5) | 2.0 (0.1–15.6) | 2.0 (0.9–10.4) | P = .60 | P = .003 | P = .003 |
| Mean (SD) | 3.1 (2.2) | 3.1 (2.2) | 3.0 (2.4) | 2.6 (1.8) | |||
Subjects were 980 participants in the Baltimore Longitudinal Study of Aging with no diagnosis of prostate cancer, a diagnosis of prostate cancer and alive or dead of a cause other than prostate cancer, or dead of prostate cancer. Comparisons were by pooled t tests in the case of equal variances and Cochran t tests in the case of unequal variances; all statistical tests were two-sided. PSA = prostate-specific antigen; SD = standard deviation.
Time from last PSA test to censoring for those without prostate cancer or time from diagnosis of prostate cancer to death or censoring for those with prostate cancer.
Time-Dependent Evaluations of PSA and PSA Velocity
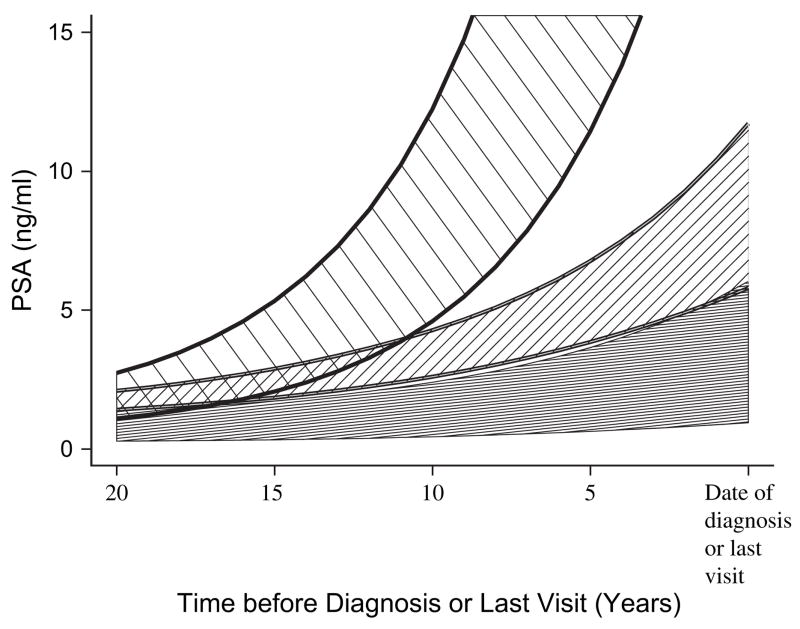
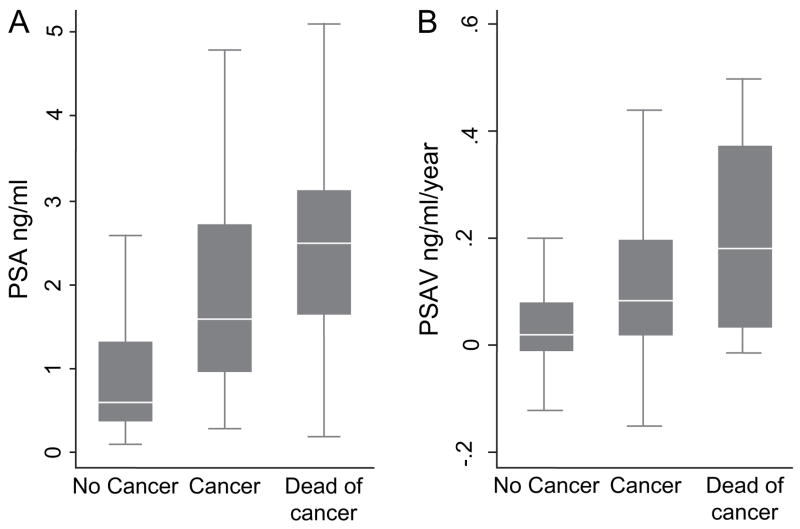
At 10–15 years before the diagnosis of prostate cancer, the PSA levels of men who died of prostate cancer were rising at an exponential rate, greater than that of men with prostate cancer who were alive or died of another cause and that of men without prostate cancer (Fig. 1). During this time, the median PSA level was 0.8 ng/mL (range = 0.1 –20.0) for all subjects and 1.9 ng/mL (range = 0.2–12.5) for subjects who died of prostate cancer, based on the mixed-effects model. We plotted the actual distributions of PSA levels and PSA velocity for the index visit at 10 –15 years before diagnosis for each study group (Fig. 2).
Fig. 1.
Average prostate-specific antigen (PSA) levels in ng/mL as a function of years before diagnosis (prostate cancer) or last visit (no prostate cancer). Subjects who died of prostate cancer (top cross-hatched area); subjects with prostate cancer who where alive or died of another cause (middle cross-hatched area); and subjects without prostate cancer (bottom solid area). Areas represent 95% confidence intervals for PSA levels based on mixed-effects models.
Fig. 2.
Distributions of prostate-specific antigen (PSA) levels and PSA velocity at 10–15 years before diagnosis for each study group. A) Distribution of PSA levels. B) Distribution of PSA velocity. Boxes represent the 25th to 75th percentiles (interquartile range = IQR); horizontal lines within boxes, the median values; and vertical lines, 1.5 times the IQR (outliers exceeding 1.5 times the IQR were removed from illustration).
We first assessed risk of death from prostate cancer 10–15 years before diagnosis using proportional hazards models that included age, diagnosis date, and PSA velocity as a continuous variable. Both diagnosis date and PSA velocity were statistically significantly associated with death from prostate cancer (Table 2). For each 1 ng/mL per year increase in PSA velocity, the hazard ratio increased to 3.97 (95% CI = 1.22 to 12.9; P = .02), and for each 0.1 ng/mL per year increase in PSA velocity, the hazard ratio increased to 1.15 (95% CI = 1.02 to 1.29; P = .02). PSA level and PSA velocity were highly correlated (Pearson correlation coefficient, r = .70). The addition of PSA level as a categorical variable (cut point 2.6 ng/mL) to this model did not statistically significantly improve the fit of the model based on the likelihood ratio test (P = .65), and in a model that included age at diagnosis, diagnosis date, and PSA level (without PSA velocity), PSA level was not a statistically significant variable (P = .20).
Table 2.
Cox proportional hazards regression analysis of prostate cancer death at 10–15 years before diagnosis of prostate cancer*
| Variable | HR (95% CI) | P† |
|---|---|---|
| Age at diagnosis | 1.02 (0.95 to 1.1) | .53 |
| Date of diagnosis | 0.89 (0.81 to 0.97) | .01 |
| PSA velocity (ng/mL per year) | ||
| Per 1 unit change | 3.97 (1.22 to 12.9) | .02 |
| Per 0.1 unit change | 1.15 (1.02 to 1.29) | .02 |
Subjects were 353 participants in the Baltimore Longitudinal Study of Aging who had a PSA velocity determination at a visit 10–15 years before diagnosis of prostate cancer. Variables were continuous with hazard rate reported relative to a unit change (1 year) for age at diagnosis and date of diagnosis and 1 ng/mL or 0.1 ng/mL change for PSA velocity. Number of nonevents = 341; number of events = 12 (not all men had PSA velocity measurements at 10 –15 years before diagnosis). HR = hazard ratio; CI = confidence interval; PSA = prostate-specific antigen.
Wald test (two-sided).
As the data in Table 2 indicate, date of diagnosis may be a confounding variable in our analysis, suggesting the possibility that PSA velocity was higher among men who died of prostate cancer because they were more likely to have been diagnosed in the absence of screening with more advanced disease than men who did not die of prostate cancer. Thus, adjusting for date of diagnosis removes the confounding effect of screening or earlier diagnosis. Furthermore, adding diagnosis date to the model did not substantially improve the fit of the model, based on the likelihood ratio test (P = .30). This lack of improvement suggests that the effect of PSA velocity on the probability of prostate cancer death was not modified by date of diagnosis. We also evaluated the same model covariates 10–15 years before diagnosis but restricted the analysis to the subjects who were diagnosed before 1991 (those more likely to have been diagnosed without PSA screening; total number of subjects = 175, number of nonevents = 166, number of events = 9). In this subset, each 1 ng/mL per year increase in PSA velocity was associated with a hazard ratio increase of 3.75 (95% CI = 1.14 to 12.3; P = .03), whereas for each 0.1 ng/mL per year increase in PSA velocity, the hazard ratio increased to 1.14 (95% CI = 1.01 to 1.28; P = .03).
ROC Analysis and PSA Velocity Cut Point
No PSA level or PSA velocity cut point achieved both high sensitivity and specificity. The area under the ROC was 0.74 (95% CI = 0.54 to 0.93) (P = .001) for PSA level and 0.75 (95% CI = 0.55 to 0.95) (P = .01) for PSA velocity when PSA values were restricted to below 4.0 ng/mL. The ROC analysis suggested that 0.35 ng/mL per year could be one reasonable choice—among others—to balance sensitivity and specificity for detection of life-threatening cancer.
Relative Risk and Survival Analyses
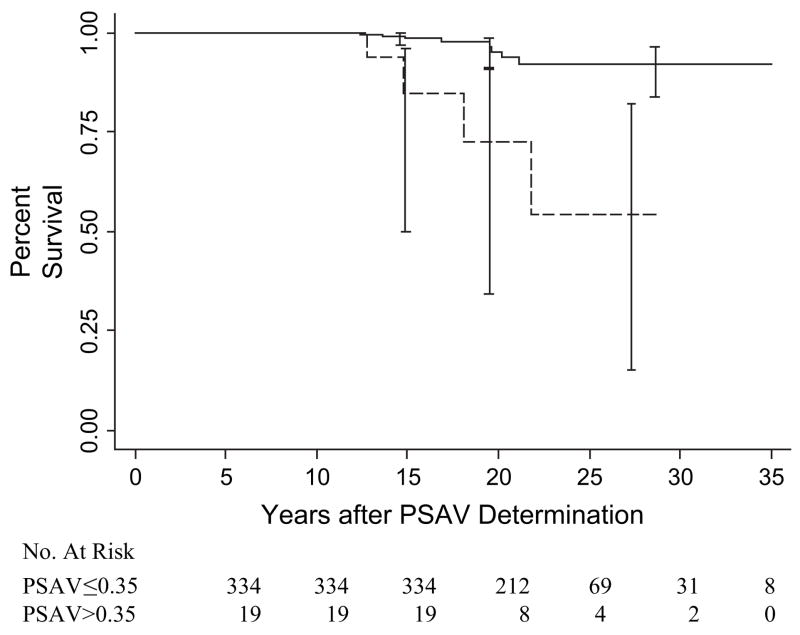
To assess the relationship between PSA velocity and survival, a PSA velocity of 0.35 ng/mL per year was used, based on the ROC analysis. When compared with subjects with a PSA velocity of 0.35 ng/mL per year or less at 10–15 years before diagnosis or last visit, subjects with a PSA velocity above 0.35 ng/mL per year had statistically significantly lower prostate cancer–specific survival at 25 years, i.e., 92% (95% CI = 84% to 96%) versus 54% (95% CI = 15% to 82%), respectively (P<.001) (Fig. 3). In the Cox regression analysis, date of diagnosis (but not age) was statistically significantly associated with prostate cancer death (Table 3). However, even after controlling for confounding by date of diagnosis, PSA velocity remained a statistically significant risk factor for death from prostate cancer. The relative risks (adjusted for age and date of diagnosis) of prostate cancer death at 5–10 and 10–15 years before diagnosis or last visit were 10.7 (95% CI = 3.3 to 34.5) (P<.001) and 4.7 (95% CI = 1.3 to 16.5) (P = .02) respectively, for men with a PSA velocity above 0.35 ng/mL per year when compared with men with a PSA velocity of 0.35 ng/mL per year or lower (Table 3). The rate per 100 000 person-years at 5–10 and 10–15 years before diagnosis was 1190 versus 96, and 1240 versus 140 when PSA velocity was above 0.35 ng/mL per year and 0.35 ng/mL per year or less, respectively.
Fig. 3.
Kaplan–Meier estimates of prostate cancer–specific survival according to prostate-specific antigen velocity (PSAV) at 10–15 years before diagnosis (prostate cancer) or last visit (no prostate cancer). Estimates of percent survival (with 95% confidence intervals) are shown for men with PSA velocity of 0.35 ng/mL per year or less (solid line) and PSA velocity above 0.35 ng/mL per year (dashed line). P<.001 (two-sided log-rank test for comparison of groups).
Table 3.
Cox regression analyses of the relative risk (RR) of prostate cancer death according to prostate-specific antigen (PSA) velocity and time before diagnosis or last visit*
| Unadjusted |
Adjusted † |
|||||
|---|---|---|---|---|---|---|
| Variable | No. of men | No. of events | RR (95% CI) | P‡ | RR (95% CI) | P‡ |
| 5–10 Years before diagnosis or before last visit | <.001 | 10.7 (3.3 to 34.5) | ||||
| PSA velocity ≤0.35 ng/mL per year | 417 | 5 | 1 (referent) | |||
| PSA velocity >0.35 ng/mL per year | 46 | 7 | 13.3 (4.16 to 42.5) | <.001 | ||
| Age at diagnosis | 1.06 (0.99 to 1.12) | .09 | ||||
| Date of diagnosis | 0.90 (0.83 to 0.98) | .009 | ||||
| 10–15 Years before diagnosis or before last visit | <.001 | 4.7 (1.33 to 16.5) | ||||
| PSA velocity ≤0.35 ng/mL per year | 334 | 8 | 1 (referent) | |||
| PSA velocity >0.35 ng/mL per year | 19 | 4 | 8.36 (2.51 to 27.9) | .02 | ||
| Age at diagnosis | 1.02 (0.95 to 1.10) | .63 | ||||
| Date of diagnosis | 0.88 (0.80 to 0.97) | .008 | ||||
Subjects were participants in the Baltimore Longitudinal Study of Aging who had a PSA velocity determination at a visit 5–10 (n = 463) or 10–15 (n = 353) years before diagnosis of prostate cancer. CI = confidence interval.
Adjusted for age at diagnosis and date of diagnosis.
Wald test (two-sided).
Discussion
We have demonstrated in an unselected cohort of men enrolled in the BLSA that PSA velocity provides information early in the disease course that is related to the chance of dying of prostate cancer. The finding that PSA velocity was associated with prostate cancer death at 10–15 years before diagnosis when PSA levels were below 4.0 ng/mL in most men (Figs. 1 and 2)—a PSA range in which most men have curable disease (15)—suggests that PSA velocity could be useful for the identification of men with potentially life-threatening disease at a time when cure is still possible with local therapy (surgery or radiation). Conversely, men with a low PSA velocity may be more appropriate candidates for observation.
An alternative interpretation of our data is that at 10 –15 years before diagnosis PSA velocity was higher among men who died of prostate cancer because they were more likely to have been diagnosed in the absence of screening with more advanced disease than men who did not die of prostate cancer. However, when we restricted our analysis to subjects who were diagnosed before 1991 and were more likely to have had a diagnosis without PSA screening, the extent of the association between PSA velocity and prostate cancer–specific death was similar to that observed when the analysis included all subjects. This similarity suggests that the observed association with PSA velocity did not differ between the pre-PSA and PSA eras. Furthermore, there was no statistically significant interaction between PSA velocity and date of diagnosis. Thus, as much as 10–15 years before diagnosis, it would appear that PSA velocity is an indicator of disease that is destined to progress and threaten life.
The PSA velocity determinations 0–5 years before diagnosis in the current study were similar to those of D’Amico et al. (9), which were made in the year before diagnosis; the 25th, 50th, and 75th percentile PSA velocity (ng/mL per year) values for all men with prostate cancer in the two studies were 0.25 and 0.5, 0.69 and 1.0, and 1.8 and 2.0, respectively. Furthermore, when compared with a PSA velocity of 2 ng/mL per year or less, a PSA velocity above 2 ng/mL per year within 2 years of diagnosis (not adjusted for tumor stage and cancer grade) was associated with a relative risk of prostate cancer death of 21.3 (95% CI = 5.6 to 81.3) in the current study. By comparison, in a univariate analysis, D’Amico et al. (9) found that a PSA velocity above 2 ng/mL per year in the year before diagnosis was associated with a relative risk of prostate cancer death of 20.4 (95% CI = 6.2 to 67.9).
However, our findings differ from those of D’Amico et al. (9) in two important ways. First, D’Amico et al. (9) demonstrated that a high PSA velocity in the year before diagnosis and surgery was associated with an increased risk of harboring incurable prostate cancer not amenable to cure with surgery alone. By contrast, our study suggests that values above a lower threshold of PSA velocity are associated with the presence of life-threatening disease at a time when cure may be possible because PSA levels at the time of determination of PSA velocity (i.e., 10 –15 years before diagnosis) in the current study were in the range where most men have curable disease (15). Second, the PSA velocity threshold associated with death from prostate cancer was substantially lower in the current study than in that of D’Amico et al. (0.35 versus 2) (9). We believe that this difference can be explained by the fact that PSA velocity increases directly with the PSA level (18). Because we determined PSA velocity a decade or more before diagnosis, at a time when PSA levels were below 4.0 ng/mL in most men (Fig. 1), one would expect our PSA velocity determinations to be much lower than those determined a year before diagnosis. With longer follow-up of the cohort described by D’Amico et al. (9), it is likely that velocity thresholds below 2 ng/mL per year will be associated with prostate cancer death.
Our study has several important clinical implications. First, it has been shown that there is virtually no PSA level threshold below which men can be reassured that life-threatening prostate cancer is not present (19). Lowering PSA level thresholds (<4.0 ng/mL) to achieve higher sensitivity for detection of life-threatening prostate cancer will increase the proportion of men who are diagnosed early but with the trade-off of detection of biologically irrelevant cancers that may have otherwise gone undetected (3,4). Determining PSA velocity may provide a reasonable way to balance this trade-off by initiating screening early in life, gathering a PSA testing history with which to evaluate PSA velocity, and performing prostate biopsies on those men who have a PSA velocity that suggests the presence of life-threatening cancer, even when PSA levels are lower than the previously recommended 4.0 ng/mL threshold. This would provide an alternative to using a single PSA value as an indication for biopsy of all men—an approach that risks missing important cancers if the value is too high (e.g., 4.0 ng/mL) or detecting many unimportant cancers if the value is lowered (e.g., 2.6 ng/mL) for all men.
Second, it would appear that when PSA levels are low (<4.0 ng/mL) and indicate a greater likelihood that prostate enlargement is absent (20), even small rises in PSA levels may indicate the presence of life-threatening prostate cancer. Recent data from the Prostate Cancer Prevention Trial support our findings in that men with high-grade cancers—those more likely to be life threatening — had faster rises in PSA levels (annual percent change in PSA levels) than men with lower grade cancers (21). In the Prostate Cancer Prevention Trial end of study biopsies (biopsies done not for elevated PSA or abnormal digital rectal examination), men with high-grade cancers (Gleason score ≥7) had an annual change in PSA level of 11% – 12% compared with men with low-grade cancers (Gleason score ≤6), whose annual changes were 5%–6%. For a man with a starting PSA level of 2.5 ng/mL, this would be equivalent to a PSA velocity of 0.3 ng/mL per year for high-grade cancer and 0.15 ng/mL per year for low-grade cancers.
Our data provide a further argument for PSA testing that begins relatively early in life, when PSA levels are usually lower and prostate enlargement is absent, to establish a baseline for evaluating future changes in PSA levels. We previously recommended an approach to PSA testing that begins at age 40 years and tests infrequently between ages 40 and 50 years and more frequently thereafter (22). We believe that PSA velocity may have the greatest value in predicting the presence of biologically important cancers at a curable stage in younger men (e.g., age below 60 years) without prostate enlargement and PSA levels below 4.0 ng/mL.
A number of limitations should be considered when interpreting our data. First, not all men in the BLSA had serum available for PSA testing. Second, the total number of deaths (events) from prostate cancer in this study was small. Thus, we cannot make precise estimates of the risk of death from prostate cancer based on a given PSA velocity threshold or recommend that a given PSA velocity threshold be used in clinical practice to prompt further evaluation. Third, we cannot be certain that if a life-threatening prostate cancer had been identified by PSA velocity earlier in the natural history of the disease, treatment at that point in time would have changed the outcome. Rather, PSA velocity might identify men who are destined to succumb to their disease in spite of curative intervention. Fourth, in our study, the interval between PSA level measurements was, on average, 3 years, and the same assay was used for all PSA measurements. It is possible that more frequent PSA determinations might provide a more accurate assessment of PSA velocity and an improved ability to distinguish those with life-threatening disease early. However, in clinical practice, PSA levels may be determined at irregular intervals and measured using different assays, which could result in findings that differ from the current study. Fifth, we were unable to assess the stage and grade of prostate cancer in our cohort, and this information could have provided further insight into the value of the information provided by PSA velocity. In addition, treatment given for prostate cancer subjects was not known in all cases, and treatment choice could affect outcome. Finally, our cohort was primarily white, and therefore, our findings might be less relevant for other racial/ethnic groups.
In summary, when compared with a single PSA level threshold, the rate at which PSA levels reach a threshold value (PSA velocity) may provide useful information for identifying men who need further evaluation and/or closer surveillance for the presence of life-threatening prostate cancer. When PSA levels are below 4.0 ng/mL, even small rises in PSA levels should prompt the consideration of the presence of a biologically important prostate cancer.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institutes of Health, National Institute on Aging. The study design, data analysis, and interpretation of the findings are the sole responsibility of the authors.
References
- 1.United States Department of Health and Human Services (Agency for Health-care Research and Quality) U.S. Preventive Services Task Force. [Last accessed: May 31, 2005];2002 Available at: http://www.ahcpr.gov/clinic/uspstf/uspsprca.htm#summary.
- 2.Zhu H, Roehl KA, Antenor JA, Catalona WJ. Biopsy of men with PSA level of 2.6 to 4.0 ng/mL associated with favorable pathologic features and PSA progression rate: a preliminary analysis. Urology. 2005;66:547–51. doi: 10.1016/j.urology.2005.03.093. [DOI] [PubMed] [Google Scholar]
- 3.Carter HB. Prostate cancers in men with low PSA levels—must we find them? N Engl J Med. 2004;350:2292–4. doi: 10.1056/NEJMe048003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Welch HG, Schwartz LM, Woloshin S. Prostate-specific antigen levels in the United States: implications of various definitions for abnormal. J Natl Cancer Inst. 2005;97:1132–7. doi: 10.1093/jnci/dji205. [DOI] [PubMed] [Google Scholar]
- 5.Carter HB, Pearson JD, Metter JE, Brant LJ, Chan DW, Andres R, et al. Longitudinal evaluation of prostate specific antigen levels in men with and without prostate disease. JAMA. 1992;267:2215. [PMC free article] [PubMed] [Google Scholar]
- 6.Smith DS, Catalona WJ. Rate of change in serum prostate specific antigen levels as a method for prostate cancer detection. J Urol. 1994;152:1163–7. doi: 10.1016/s0022-5347(17)32528-4. [DOI] [PubMed] [Google Scholar]
- 7.Goluboff ET, Heitjan DF, DeVries GM, Katz AE, Benson MC, Olsson CA. Pretreatment prostate specific antigen doubling times: use in patients before radical prostatectomy. J Urol. 1997;158:1876–8. doi: 10.1016/s0022-5347(01)64154-5. [DOI] [PubMed] [Google Scholar]
- 8.Egawa S, Arai Y, Tobisu K, Kuwao S, Kamoto T, Kakehi Y, et al. Use of pretreatment prostate specific antigen doubling time to predict outcome after radical prostatectomy. Prostate Cancer Prostatic Dis. 2000;3:269–74. doi: 10.1038/sj.pcan.4500424. [DOI] [PubMed] [Google Scholar]
- 9.D’Amico AV, Chen MH, Roehl KA, Catalona WJ. Preoperative PSA velocity and the risk of death from prostate cancer after radical prostatectomy. N Engl J Med. 2004;351:125–35. doi: 10.1056/NEJMoa032975. [DOI] [PubMed] [Google Scholar]
- 10.Shock NW, Greulich RC, Andres R, Arenberg D, Costa PT, Jr, Lakatta EG, et al. Normal human aging: the Baltimore Longitudinal Study of Aging. Washington (DC): US GPO; 1984. NIH publication no. 84–2450. [Google Scholar]
- 11.Rock RC, Chan DW, Bruzek D, Waldron C, Oesterling J, Walsh P. Evaluation of a monoclonal immunoradiometric assay for prostate-specific antigen. Clin Chem. 1987;33:2257–61. [PubMed] [Google Scholar]
- 12.SEER*Stat Database. Surveillance, Epidemiology, and End Results (SEER) Program. [Last accessed: May 31, 2005]; Available at: http://www.seer.cancer.gov.
- 13.Lindstrom MJ, Bates DM. Newton-Raphson and EM algorithms for linear mixed-effects models for repeated-measures data. J Am Stat Assoc. 1988;83:1014–22. [Google Scholar]
- 14.Draisma G, Boer R, Otto SJ, van der Cruijsen IW, Damhuis RA, Schroder FH, et al. Lead times and overdetection due to prostate-specific antigen screening: estimates from the European Randomized Study of Screening for Prostate Cancer. J Natl Cancer Inst. 2003;95:868. doi: 10.1093/jnci/95.12.868. [DOI] [PubMed] [Google Scholar]
- 15.Han M, Partin AW, Pound CR, Epstein JI, Walsh PC. Long-term biochemical disease-free and cancer-specific survival following anatomic radical retropubic prostatectomy. The 15-year Johns Hopkins experience. Urol Clin North Am. 2001;28:555–65. doi: 10.1016/s0094-0143(05)70163-4. [DOI] [PubMed] [Google Scholar]
- 16.Jang TL, Han M, Roehl KA, Hawkins SA, Catalona WJ. More favorable tumor features and progression-free survival rates in a longitudinal prostate cancer screening study: PSA era and threshold-specific effects. Urology. 2006;67:343–8. doi: 10.1016/j.urology.2005.08.048. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 18.Carter HB, Pearson JD. PSA velocity for the diagnosis of early prostate cancer: a new concept. Urol Clin North Am. 1993;20:665–70. [PubMed] [Google Scholar]
- 19.Thompson IM, Ankerst DP, Chi C, Lucia MS, Goodman PJ, Crowley JJ, et al. Operating characteristics of prostate-specific antigen in men with an initial PSA level of 3.0 ng/ml or lower. JAMA. 2005;294:66–70. doi: 10.1001/jama.294.1.66. [DOI] [PubMed] [Google Scholar]
- 20.Roehrborn CG, Boyle P, Gould AL, Waldstreicher J. Serum prostate-specific antigen as a predictor of prostate volume in men with benign prostatic hyperplasia. Urology. 1999;53:581–9. doi: 10.1016/s0090-4295(98)00655-4. [DOI] [PubMed] [Google Scholar]
- 21.Etzioni RD, Howlader N, Shaw PA, Ankerst DP, Penson DF, Goodman PJ, Thompson IM. Long-term effects of finasteride on prostate specific antigen levels: results from the prostate cancer prevention trial. J Urol. 2005;174:877–81. doi: 10.1097/01.ju.0000169255.64518.fb. [DOI] [PubMed] [Google Scholar]
- 22.Ross K, Carter HB, Pearson JD, Guess HA. Comparative efficiency of prostate specific antigen screening strategies for prostate cancer detection. JAMA. 2000;284:1399–405. doi: 10.1001/jama.284.11.1399. [DOI] [PubMed] [Google Scholar]