Abstract
Background
The role of nutritional status in the disablement process is still unclear. The objective of this study was to assess whether low concentrations of nutrients predict the development and course of disability.
Methods
Longitudinal study including community-dwelling women 65 years or older enrolled in the Women’s Health and Aging Study I. In total, 643 women were assessed prospectively at 6-month intervals from 1992 to 1995.
Results
Incidence rates of disability in activities of daily living (ADLs) during 3 years of follow-up. Incidence rates in the lowest quartile of each selected nutrient were compared with those in the upper quartiles. The hazard ratios were estimated from Cox models adjusted for potential confounders. Women in the lowest quartile of serum concentrations of vitamin B6 (hazard ratio [HR], 1.31; 95% confidence interval [CI], 1.03–1.67), vitamin B12 (HR, 1.40; 95% CI, 1.12–1.74), and selenium (HR, 1.38; 95% CI, 1.12–1.71) had significantly higher risk of disability in ADLs during 3 years of follow-up compared with women in the upper 3 quartiles.
Conclusions
Low serum concentrations of vitamins B6 and B12 and selenium predict subsequent disability in ADLs in older women living in the community. Nutritional status is one of the key factors to be considered in the development of strategies aimed at preventing or delaying the disablement process.
In 2003, baby boomers comprised nearly 30% of the American population. Born between 1946 and 1964, baby boomers are now aged 42 to 60 years, and in 2030 they will all be older than 65 years. Thus, an unprecedented increase of the older population is expected, and an estimated 20% of Americans will be older than 65 years.1(p12) An important implication of this demographic transition is the increased morbidity and disability.1(p1) Although reports show a decline in the disability rate,2 about 7 million persons older than 65 years in the United States are disabled2 and this has a dramatic impact on health care costs3 and the quality of life of older persons. Thus, implementation of strategies aimed at preventing or delaying the onset of disability is urgently needed.4
A crucial step to achieve this goal is the identification of those factors contributing to the disablement process. Although there is consensus that poor nutritional status is a potential factor, there is a lack of studies investigating its role in the development and course of disability.4 Our previous studies5,6 and other reports7 suggest an association of poor nutritional status with reduced physical function and disability. Most of these findings, however, are cross-sectional, and a causal role cannot be established.
The purpose of this study is to address this gap in knowledge by determining whether low serum concentrations of specific nutrients are predictors of subsequent disability in a longitudinal cohort of older women living in the community.
METHODS
The Women’s Health and Aging Study I (WHAS I) is a population-based study of risk factors contributing to the development and course of physical disability in women living in the community. The subjects in this study were recruited from an age-stratified random sample of women 65 years or older selected from Medicare enrollees residing in 12 contiguous ZIP code areas in Baltimore, Md.8 Women were screened to identify self-reported difficulties in domains of mobility, upper extremity function, higher functioning household management, and self-care. The WHAS I enrolled women 65 years or older with difficulties in 2 or more domains of function. Thus, participants had some level of difficulties in physical function but were not so disabled as to have difficulties in performing activities of daily living (ADLs).
Of the 1409 women who met study eligibility criteria, 1002 agreed to participate in the study in 1992. There were no major differences in sociodemographic or reported health characteristics between eligible participants and those who declined to participate.8
Standardized questionnaires were administered in the participant’s home by trained interviewers. Two weeks later, a trained registered nurse conducted an examination at each study participant’s home, using a standardized protocol that included physical performance measures and a directed physical examination. Of the 1002 women enrolled in the study, 734 participated in the blood draw, had serum nutrient measurements taken, and gave self-reported information on difficulty in managing ADLs at baseline and at least 1 follow-up.
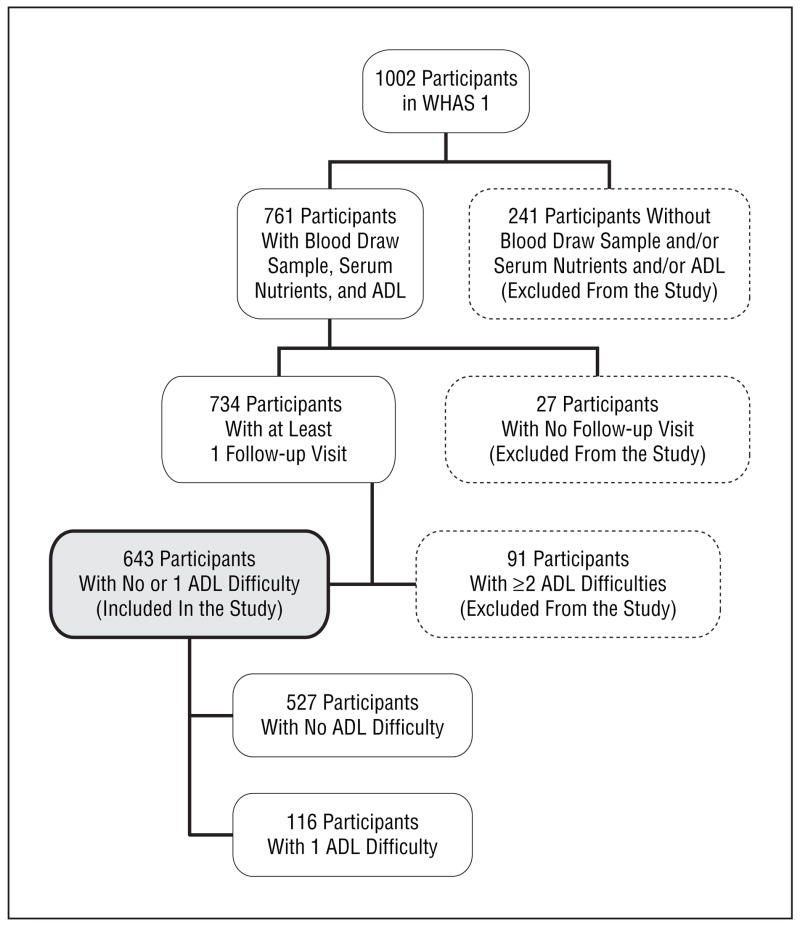
The profile of the study population is summarized in the Figure. There were no significant differences in race or body mass index (BMI) (calculated as weight in kilograms divided by height in meters squared) between those who did and did not participate in the blood draw, but women who did not participate were older (80.7 years vs 77.4 years), had less education, and had a higher prevalence of frailty compared with those who gave a blood sample.9 Further details on the methods and sampling design of the WHAS I were published elsewhere.8
Figure.
Profile of the study population. ADL denotes activity of daily living; WHAS I, Women’s Health and Aging Study I.
Standardized interviews were used to assess the perceived function in ADLs10: bathing, dressing, toileting, transferring, and eating. For the analysis presented here, disability was defined as self-reported difficulty in managing 2 or more of these ADLs.11
Weight and height were measured in a standardized position, and BMI was categorized as underweight (<18.5), reference range (18.5–24.9), overweight (≥25.0–29.9), and obese (≥30.0). Information on demographic characteristics and health status were collected in the WHAS I questionnaires. Physical activity was assessed using the Minnesota Leisure Time Activities Questionnaire.8 Chronic diseases were adjudicated by WHAS I coinvestigators based on self-report of physicians’ diagnoses, physical examination, review of medical records, and physician contact.8 Depression was assessed by using the Geriatric Depression Scale (GDS).12 For the present study we used data collected every 6 months for up to 36 months of follow-up. After excluding participants with ADL disability at baseline (n=91), the final analytical sample included 643 women.
LABORATORY ANALYSIS
Nonfasting blood samples were obtained by venipuncture between 9 AM and 2 PM. Processing, aliquoting, and freezing were performed at the Core Genetics Laboratory of The Johns Hopkins University School of Medicine, Baltimore, Md, following a standardized protocol. Blood samples were delivered to Quest Diagnostics Laboratories (Teterboro, NJ) on the day of blood draw for assays that were conducted by this commercial laboratory. For carotenoids, retinol, zinc, and selenium, serum was stored continuously at −70°C until the time of analyses. Serum carotenoids and retinol were determined by high-performance liquid chromatography.
Serum selenium and zinc were measured by graphite furnace atomic absorption spectrometry using an AAnalyst 600 spectrometer with Zeeman background correction (Perkin Elmer, Norwalk, Conn). For selenium, samples were diluted 1:4 with a triton-X (Sigma Chemical, St Louis, Mo) and nitric acid solution (Fisher Scientific, Pittsburgh, Pa), and the matrix modifier was a palladium and magnesium nitrate solution (both, Perkin Elmer). The instrument was calibrated daily using SeroNorm Trace Elements Serum, Level 1 (Accurate Chemical and Scientific Corp, Westbury, NY), with a known amount of selenium, spiked with selenium standard (Perkin Elmer). For zinc, samples were diluted 1:200 with di-water, and the matrix modifier was magnesium nitrate (Perkin Elmer). The instrument was calibrated daily using SeroNorm Trace Elements Serum Level 1 (Accurate Chemical and Scientific Corp) with a known amount of zinc. All zinc samples were run in duplicate. For both analyses, a control prepared from SeroNorm was run periodically throughout each run for quality control.
Total carotenoids were calculated as the sum of α-carotene, β-carotene, β-cryptoxanthin, lutein/zeaxanthin, and lycopene in micrograms per deciliter. Serum concentration of 25-hydroxyvitamin D was measured using a radioreceptor assay. Vitamin B6 status was assessed by pyridoxal 5-phosphate measurements using high-performance liquid chromatography. Serum vitamin B12 and folate were measured by radioimmunoassay. Within-run and between-run coefficients of variation, respectively, were 7.5% and 9.6% for 25-hydroxyvitamin D, 5.8% and 4.8% for selenium, and 2.8% and 3.9% for zinc.
STATISTICAL ANALYSIS
Descriptive analyses were performed to provide information on general characteristics of the study population. Women were assigned to quartiles based on micronutrient concentration, and a low level of each selected micronutrient was defined as the lowest quartile of the baseline distribution. We used this analytical approach because nutrient requirements for older persons are inadequately documented, and conventional cutoffs for deficiencies have been extrapolated from middle-aged populations and, consequently, may not be meaningful for older adults.
During our exploratory analyses we found that the incidence rates of ADL disability per 100 person-years were consistently higher in the first compared with the second quartile of each selected micronutrient, supporting the adequacy of using these cutoffs. The selected nutrients (and corresponding cutoff) were the following: total carotenoids (57.2 μg/dL [1.04 μmol/L]), retinol (56.4 μg/dL [1.97 μmol/L]), 25-hydroxyvitamin D (14.2 ng/mL [35.4 nmol/L]), vitamin B6 (4.4 ng/mL [17.8 nmol/L]), vitamin B12 (313.0 pg/mL [230.9 pmol/L]), folate (5.9 ng/mL [13.4 nmol/L]), selenium (105.7 μg/L [1.3 μmol/L]), and zinc (0.73 μg/dL [0.01 μmol/L]).
Because the ADL disability score was determined at 6-month intervals, grouped-time Cox proportional hazard models were used to examine the associations between low serum levels of nutrients at baseline and the risk of developing ADL disability. The hazard ratios were adjusted for age, race, education, smoking status, alcohol consumption, multivitamin use, BMI, physical activity, chronic obstructive pulmonary disease, osteoarthritis, cardiovascular diseases, and cancer. The selection of these potential confounders was based on prior evidence in the literature about their relationship with both the exposure and the outcome of interest. As shown in the Figure, women who did not have at least 1 follow-up visit because of refusal or loss to follow-up were excluded from the longitudinal analyses, as were women with ADL disability at baseline, to allow prediction of incidence of ADL disability during 3 years of follow-up.
Subjects who died, refused further participation, or were lost to follow-up after a follow-up visit were censored at that point, with disability status at their last visit considered. The hazard ratio for developing ADL disability was calculated for the lowest quartile of each nutrient’s distribution, using all other quartiles combined as the reference. We used 6 follow-up times, from baseline to 36 months, and graphically verified that the proportional hazard assumption was valid for low and not-low nutrition groups. Furthermore, we performed a log-rank test comparing censoring time for those lost to follow-up for the low and not-low micronutrient group and for low and not-low levels of each selected micronutrient. We did not find significant differences in censoring patterns. The analyses were performed using SAS statistical software (version 8.1; SAS Inc, Cary, NC).
RESULTS
The comparison of characteristics between women included and excluded from the study is reported in Table 1. In general, women who were excluded from the sample were older, and a higher proportion had BMIs lower than 18.5 and chronic diseases such as congestive heart failure (P =.004), peripheral artery disease (P =.04), stroke (P<.001), and depression (P =.005). Similar differences were observed when the comparison was restricted to women with and without ADL disability at baseline, although the difference in age between these 2 groups was not statistically significant.
Table 1.
Comparison of Characteristics Between Women Who Were Included in the Study and Those Who Were Excluded*
| Excluded From the Study |
|||||
|---|---|---|---|---|---|
| Characteristic | Included in the Study:643 With <2 ADLs† | 359 Total‡ | P Value | 91 With ≥2 ADLs§ | P Value |
| Age, mean (range), y | 77.3 (76.7–77.9) | 80.2 (79.4–81.1) | <.001 | 78.0 (76.3–79.8) | .39 |
| White race, % | 72.5 | 70.2 | .44 | 69.2 | .52 |
| Education, ≤12 y, % | 81.8 | 82.7 | .73 | 86.8 | .24 |
| Current smoking, % | 12.4 | 9.8 | .20 | 6.6 | .10 |
| Alcohol consumption, % | 17.0 | 12.5 | .06 | 12.1 | .24 |
| Multivitamin use, % | 17.9 | 15.7 | .38 | 20.0 | .63 |
| Low physical activity, % | 38.4 | 57.5 | <.001 | 72.7 | <.001 |
| BMI | |||||
| <18.5 | 8.7 | 25.3 | <.001 | 40.6 | <.001 |
| 18.5–24.9 | 24.9 | 23.1 | 13.2 | ||
| 25.0–29.9 | 32.8 | 26.5 | 20.9 | ||
| ≥30.0 | 33.6 | 25.1 | 25.3 | ||
| Chronic diseases, % | |||||
| Coronary heart disease | 23.8 | 22.0 | .70 | ||
| Congestive heart failure | 8.4 | 14.2 | .004 | 18.7 | .002 |
| Peripheral artery disease | 19.9 | 25.6 | .04 | 34.1 | .002 |
| Stroke | 3.9 | 12.5 | <.001 | 18.7 | <.001 |
| Osteoarthritis | 54.3 | 47.9 | .05 | 56.0 | .75 |
| Cancer | 11.2 | 11.4 | .91 | 11.0 | .95 |
| COPD | 16.3 | 15.6 | .76 | 14.3 | .62 |
| Diabetes mellitus | 15.1 | 17.6 | .31 | 27.5 | .003 |
| Depression|| | 28.6 | 37.2 | .005 | 51.6 | <.001 |
Abbreviations: ADL, activities of daily living; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); COPD, chronic obstructive pulmonary disease; GDS, Geriatric Depression Scale.
Data are given as percentages except where noted.
Reference group.
Excluded because subject did not have a blood draw sample or follow-up visit or had at least 2 ADLs at baseline.
Excluded because subject had at least 2 ADLs at baseline.
GDS score ≥10.
Among women who did not have ADL disability at baseline, 208 (32.3%) developed ADL disability during 3 years of follow-up, with an overall incidence rate of 13.3 per 100 person-years. The incidence rates for ADL disability among women in the lowest quartile vs those in the upper 3 quartiles of serum nutrient concentrations are shown in Table 2. The incidence rates for disability were higher in women in the lowest quartile of vitamin B6 (P =.02), vitamin B12 (P<.001), and selenium (P<.001), compared with women in the 3 upper quartiles, and was not significant for total carotenoids, retinol, 25-hydroxyvitamin D, folate, and zinc.
Table 2.
Incidence Rates of ADL Disability Over a 3-Year Interval, by Nutritional Status at Baseline
| Serum Nutrient | Participants, No. | Incidence Rate,* Lowest Quartile† | Participants, No. | Incidence Rate,* Upper 3 Quartiles | P Value |
|---|---|---|---|---|---|
| Total carotenoids, μg/dL | 150 | 14.5 | 493 | 13.0 | .58 |
| Retinol, μg/dL | 153 | 13.9 | 490 | 13.1 | .57 |
| 25-hydroxyvitamin D, ng/mL | 134 | 15.8 | 442 | 12.5 | .54 |
| Vitamin B6, ng/mL | 123 | 17.3 | 372 | 12.8 | .02 |
| Vitamin B12, pg/mL | 145 | 16.7 | 447 | 12.0 | <.001 |
| Folate, ng/mL | 148 | 14.9 | 446 | 12.5 | .61 |
| Selenium, μg/L | 145 | 21.6 | 466 | 10.8 | <.001 |
| Zinc, μg/dL | 148 | 15.4 | 486 | 12.6 | .43 |
Abbreviation: ADL, activity of daily living.
SI conversion factors: To convert 25-hydroxyvitamin D to nanomoles per liter, multiply by 2.496; carotenoids to micromoles per liter, multiply by 0.01758 for lutein and zeaxanthin, 0.01809 for β-cryptoxanthin, and 0.01863 for lycopene, α-carotene, and β-carotene (thus the conversion factor for total carotenoids is 0.01819); folate to nanomoles per liter, multiply by 2.266; retinol to micromoles per liter, multiply by 0.0349; selenium to micromoles per liter, multiply by 0.0127;vitamin B6 to nanomoles per liter, multiply by 4.046; vitamin B12 to picomoles per liter, multiply by 0.7378; and zinc to micromoles per liter, multiply by 0.0153.
Incidence rate per 100 person-years obtained from individual Cox models with the presented variables as explanatory covariates.
Lowest quartile defined among all women at baseline.
These results did not substantially change when the Cox proportional hazard models were adjusted for age, race, education, smoking status, multivitamin use, BMI, physical activity, alcohol consumption, chronic obstructive pulmonary disease, osteoarthritis, cardiovascular diseases, and cancer (Table 3).
Table 3.
Multivariate Models for Serum Nutrients at Baseline and Incidence of ADL Disability During 3 Years of Follow-up
| Serum Nutrient | HR* (95% CI) | P Value |
|---|---|---|
| Total carotenoids, μg/dL | 0.97 (0.77–1.23) | .82 |
| Retinol, μg/dL | 0.98 (0.78–1.24) | .88 |
| 25-hydroxyvitamin D, ng/mL | 0.95 (0.73–1.23) | .67 |
| Vitamin B6, ng/mL | 1.27 (0.98–1.63) | .06 |
| Vitamin B12, pg/mL | 1.36 (1.08–1.72) | .009 |
| Folate, ng/mL | 1.22 (0.95–1.57) | .12 |
| Selenium, μg/dL | 1.47 (1.19–1.83) | <.001 |
| Zinc, μg/dL | 0.99 (0.79–1.25) | .94 |
Abbreviations: ADL, activity of daily living; CI, confidence interval; HR, hazard ratio.
SI conversion factors: See SI conversion factor footnote in Table 2.
The HRs were obtained from individual Cox models adjusted for age, race, education, smoking status, alcohol consumption, multivitamin use, body mass index, physical activity, chronic obstructive pulmonary disease, osteoarthritis, cardiovascular diseases, and cancer.
COMMENT
This study sought to evaluate whether nutritional status predicts subsequent ADL disability in older women living in the community. We found that low serum concentrations of selenium and vitamins B6 and B12 are significant and independent predictors of ADL disability. These associations likely involve different causal pathways. Selenium exerts important beneficial effects.13 In particular, the selenoprotein antioxidant enzymes (eg, glutathione peroxidase) allow for the reduction of hydrogen peroxide to water, preventing lipid peroxidation and cellular damage.14 Low antioxidants may tip the balance between antioxidants and free radicals and allow increased oxidative stress. This imbalance may lead to disability through dysregulation of cellular function15 and up-regulation of proinflammatory cytokines,16 muscle and neuronal damage,17 and the exacerbation of degenerative diseases.18 The hypothesis that antioxidants play a role in the pathogenesis of disability is supported by our previous findings5,6 and other reports.7
Vitamins B6 and B12 are involved in protein and homocysteine metabolism, and their deficiencies cause hyperhomocysteinemia.19 The association of high levels of homocysteine with oxidative stress,20 endothelial dysfunction,21 occlusive vascular diseases,22 and, in particular, with decline of cognitive function23 may explain, at least in part, our findings on the association of low concentrations of B6 and B12 with disability. We evaluated this proposed pathway and found that high levels of homocysteine at baseline predicted the development of ADL disability (P<.001) over 3 years of follow-up. It is note-worthy that the relationship between vitamin B12 and disability (Table 3) was not statistically significant when homocysteine was entered in the model. This result provides empirical evidence that the relationship between vitamin B12 and disability is mediated by homocysteine. Different mechanisms may explain the “continuum” hypothesized to connect low serum levels of selenium and vitamins B6 and B12 with disability: (1) altered protein metabolism and increased levels of homocysteine, oxidative stress, and inflammatory markers resulting in protein damage and reduced muscle mass and strength (sarcopenia); (2) increased risk of developing degenerative diseases; and (3) decline of cognitive function.
To our knowledge, this is the first study to evaluate the effect of different nutritional biomarkers on subsequent ADL disability in older women living in the community.
In another study (R.D.S., R.V., B.B., et al, 2006, unpublished data), we found that low concentrations of an-tioxidants (as indicated by carotenoids), but not B complex vitamins, predicted severe walking disability as measured using walking speed. Because low concentrations of vitamins B6 and B12 contribute to cognitive decline,23 the result presented herein on the association of low concentrations of B6 and B12 with ADL disability has face validity. Although walking speed is a test of lower extremity physical function, the capacity to perform ADLs strongly depends on a complex of additional factors that are involved in the onset of disability, such as cognitive capacity. As reported by Mendes de Leon et al,24(pS356) “tests of physical function and self-reported disability represent conceptually distinct aspects of the disablement process.” The combination of these 2 studies suggests that poor nutritional status plays a role in disability, regardless of its semantic definition. The present study suggests that low levels of selenium and vitamins B6 and B12 are predictive of ADL disability among older women living in the community. Although levels of carotenoids, retinol, 25-hydroxyvitamin D, folate, and zinc could play a role in the disablement process, we did not find a significant effect of low levels of these nutrients on the development of ADL disability. Further studies are needed to confirm these results, to establish a cause-effect relationship between serum levels of nutrients and the disablement process, and to verify whether these results can be generalized to older persons with optimal physical performance.
A limitation of this study is that participants already had some level of difficulty in physical function, and this might have exacerbated the risk of undernutrition through decreased ability to shop or prepare meals. In addition, information on ADL disability was self-reported and used as a dichotomized variable. Thus, our results could be affected by misclassification bias. The probability of misclassification for our outcome, however, is independent of the exposure (nondifferential). Consequently, our findings are likely to be underestimated because they are biased toward the no-effect value. Furthermore, the effect of unmeasured confounders as explanation of these findings cannot be ruled out.
The main strength of this study is that we used measures of serum levels of nutrients rather than estimates of nutrient intake as our indicators of nutritional status. Thus, our findings are unlikely to be biased by self-report.
In conclusion, we found that low serum concentrations of selenium and vitamins B6 and B12 predict subsequent ADL disability in older women living in the community. This study addresses an important gap in knowledge by suggesting that poor nutritional status contributes to the disablement process. These results, however, do not imply a beneficial effect of nutrient supplementation on the prevention of disability but rather suggest that nutritional status is one of the key factors to be considered for the development of strategies aimed at preventing or delaying the onset of disability, and consequently for the improvement of health and the quality of life of older women living in the community.
Acknowledgments
Funding/Support: This study was funded by National Institute on Aging grants R01 AG027012, R01 AG11703-01A1, NIH-NCRR, OPD-GCRC grant RR00722, R01 AI41956; NIA contract N01-AG12112; and Cornell University Agricultural Experiment Station federal formula funds, Project No. NYC-399407.
We thank Sally Stabler, MD, for homocysteine analyses.
Footnotes
Financial Disclosure: None reported.
Author Contributions: Study concept and design: Bartali, Semba, Frongillo, Blaum, and Guralnik. Acquisition of data: Bartali, Ferrucci, and Guralnik. Analysis and interpretation of data: Bartali, Semba, Varadhan, Ricks, Blaum, Guralnik, and Fried. Drafting of the manuscript: Bartali and Semba. Critical revision of the manuscript for important intellectual content: Bartali, Frongillo, Varadhan, Ricks, Blaum, Ferrucci, Guralnik, and Fried. Statistical analysis: Bartali, Semba, Frongillo, Varadhan, Ricks, and Guralnik. Obtained funding: Guralnik. Administrative, technical, and material support: Semba. Study supervision: Bartali, Frongillo, and Fried.
References
- 1.He W, Sengupta M, Velkoff VA, DeBarros KA. US Department of Health and Human Services and Department of Commerce Web site; [Accessed September 20, 2006]. 65+in the United States: current population reports. http://www.census.gov/prod/2006pubs/p23-209.pdf. [Google Scholar]
- 2.Freedman VA, Martin LG, Schoeni RF. Recent trends in disability and functioning among older adults in the United States: a systematic review. JAMA. 2002;288:3137–3146. doi: 10.1001/jama.288.24.3137. [DOI] [PubMed] [Google Scholar]
- 3.Guralnik JM, Alecxih L, Branch LG, Wiener JM. Medical and long-term care costs when older persons become more dependent. Am J Public Health. 2002;92:1244–1245. doi: 10.2105/ajph.92.8.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferrucci L, Guralnik JM, Studenski S, Fried LP, Cutler GB, Walston JD Interventions on Frailty Working Group. Designing randomized, controlled trials aimed at preventing or delaying functional decline and disability in frail, older persons: a consensus report. J Am Geriatr Soc. 2004;52:625–634. doi: 10.1111/j.1532-5415.2004.52174.x. [DOI] [PubMed] [Google Scholar]
- 5.Bartali B, Frongillo AF, Bandinelli S, et al. Low nutrient intake is an essential component of frailty in older persons. J Gerontol A Biol Sci Med Sci. 2006;61:589–593. doi: 10.1093/gerona/61.6.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cesari M, Pahor M, Bartali B, et al. Antioxidants and physical performance in elderly persons: the Invecchiare in Chianti (InCHIANTI) study. Am J Clin Nutr. 2004;79:289–294. doi: 10.1093/ajcn/79.2.289. [DOI] [PubMed] [Google Scholar]
- 7.Snowdon DA, Gross MD, Butler SM. Antioxidants and reduced functional capacity in the elderly: findings from the Nun Study. J Gerontol A Biol Sci Med Sci. 1996;51:M10–M16. doi: 10.1093/gerona/51a.1.m10. [DOI] [PubMed] [Google Scholar]
- 8.Guralnik JM, Fried LP, Simonsick EM, Kasper D, Lafferty ME. The Women’s Health and Aging Study: Health and Social Characteristics of Older Women With Disability. Bethesda, Md: National Institute on Aging; 1995. NIH publication 95–4009. [Google Scholar]
- 9.Michelon E, Blaum C, Semba RD, Xue QL, Ricks MO, Fried LP. Vitamin and carotenoid status in older women: associations with the frailty syndrome. J Gerontol A Biol Sci Med Sci. 2006;61:600–607. doi: 10.1093/gerona/61.6.600. [DOI] [PubMed] [Google Scholar]
- 10.Katz S, Akpom CA. A measure of primary sociobiological function. Int J Health Serv. 1976;6:493–507. doi: 10.2190/UURL-2RYU-WRYD-EY3K. [DOI] [PubMed] [Google Scholar]
- 11.Guralnik JM, Fried LP, Simonsick EM, Bandeen-Roche KJ, Kasper JD. Screening the Community-Dwelling Population for Disability. [Accessed September 20, 2006]; http://www.grc.nia.nih.gov/branches/ledb/whasbook/chap1/chap1.htm.
- 12.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982–83;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 13.Rayman MP. The importance of selenium to human health. Lancet. 2000;356:233–241. doi: 10.1016/S0140-6736(00)02490-9. [DOI] [PubMed] [Google Scholar]
- 14.Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179:588–590. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- 15.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 16.Haddad JJ. Redox regulation of pro-inflammatory cytokines and IkappaB-alpha/NF-kappaB nuclear translocation and activation [published correction appears in Biochem Biophys Res Commun. 2003;301:265] Biochem Biophys Res Commun. 2002;296:847–856. doi: 10.1016/s0006-291x(02)00947-6. [DOI] [PubMed] [Google Scholar]
- 17.Mecocci P, Fano G, Fulle S. Age-dependent increases in oxidative damage to DNA, lipids, and proteins in human skeletal muscle. Free Radic Biol Med. 1999;26:303–308. doi: 10.1016/s0891-5849(98)00208-1. [DOI] [PubMed] [Google Scholar]
- 18.Heinecke JW. Oxidized amino acids: culprits in human atherosclerosis and indicators of oxidative stress. Free Radic Biol Med. 2002;32:1090–1101. doi: 10.1016/s0891-5849(02)00792-x. [DOI] [PubMed] [Google Scholar]
- 19.Selhub J. Homocysteine metabolism. Annu Rev Nutr. 1999;19:217–246. doi: 10.1146/annurev.nutr.19.1.217. [DOI] [PubMed] [Google Scholar]
- 20.Li H, Lewis A, Brodsky S, Rieger R, Iden C, Goligorsky MS. Homocysteine induces 3-hydroxy-3-methylglutaryl coenzyme a reductase in vascular endothelial cells: a mechanism for development of atherosclerosis. Circulation. 2002;105:1037–1043. doi: 10.1161/hc0902.104713. [DOI] [PubMed] [Google Scholar]
- 21.McDowell IF, Lang D. Homocysteine and endothelial dysfunction: a link with cardiovascular disease. J Nutr. 2000;130(25 suppl):369S–372S. doi: 10.1093/jn/130.2.369S. [DOI] [PubMed] [Google Scholar]
- 22.Boushey CJ, Beresford SA, Omenn GS, Motulsky AG. A quantitative assessment of plasma homocysteine as a risk factor for vascular disease: probable benefits of increasing folic acid intakes. JAMA. 1995;274:1049–1057. doi: 10.1001/jama.1995.03530130055028. [DOI] [PubMed] [Google Scholar]
- 23.Riggs KM, Spiro A, Tucker K, Rush D. Relations of vitamin B-12, vitamin B-6, folate, and homocysteine to cognitive performance in the Normative Aging Study. Am J Clin Nutr. 1996;63:306–314. doi: 10.1093/ajcn/63.3.306. [DOI] [PubMed] [Google Scholar]
- 24.Mendes de Leon CF, Guralnik JM, Bandeen-Roche K. Short-term change in physical function and disability: the Women’s Health and Aging Study. J Gerontol B Psychol Sci Soc Sci. 2002;57:S355–S365. doi: 10.1093/geronb/57.6.s355. [DOI] [PubMed] [Google Scholar]