Abstract
Objectives
To evaluate the independent association between high-density lipoprotein cholesterol (HDL-C) levels and objective measures of lower extremity performance.
Design
Cross-sectional cohort study.
Setting
Community-based.
Participants
Eight hundred thirty-six nondisabled women and men aged 65 and older enrolled in the Invecchiare in Chianti study.
Mesasurements
Lower extremity performance was assessed using 4-m walking speed at fast pace, 400-m walking speed, and knee extension torque. Fasting HDL-C levels were determined using commercial enzymatic tests.
Results
The mean age of participants was 73.7 (65–92), and 55.6% were women. After adjusting for potential con-founders (sociodemographic factors, smoking, physical activity, body composition, and clinical conditions including cardiovascular and cerebrovascular disease, inflammatory markers, and serum testosterone) HDL-C levels were significantly associated with knee extension torque in men and women and with 4-m and 400-m walking speed in men. Men in the highest tertile of the HDL-C distribution (>55 mg/dL) had, on average, a three times greater probability of belonging to the best tertile of all indexes of lower extremity performance, including 4-m fast walking speed (odds ratio (OR) = 2.57, 95% = confidence interval (CI) = 1.07–6.17), 400-m walking speed (OR = 3.74, 95% CI = 1.20–11.7), and knee extension torque (OR = 3.63, 95% = CI 1.41–9.33). Path analysis suggested a direct relationship between HDL-C and knee extension torque.
Conclusion
In older nondisabled persons, HDL-C levels are highly correlated with knee extension torque and walking speed. Further research should focus on the biological mechanism of this association.
Keywords: HDL-C, cytokines, aging, disablement process, strength
A low level of high-density lipoprotein cholesterol (HDL-C) is an important risk factor for atherosclerosis and its clinical complications.1 The importance of HDL as a risk factor for cardiovascular disease and mortality has been further emphasized in the older population, where HDL-C level may have better predictive value than other lipid risk factors.2,3 More recently, the prognostic value of HDL-C levels has been expanded to other common conditions of older people, suggesting that HDL-C may have further clinical relevance in addition to the inverse relationship with atherosclerosis.4 For example, low HDL-C combined with low serum albumin identified older persons with the highest risk of all-cause and noncardiovascular mortality.5 Low HDL-C has been correlated with cognitive impairment and dementia, and at least part of the association might be independent of atherosclerotic disease.6 Furthermore, there is initial evidence that values of HDL-C are inversely correlated with physical disability and motor performance.7
The ability to remain mobile is an essential aspect of quality of life and is critical for the preservation of independence in old age. Objective measures of lower extremity function, including walking speed and muscle strength, are strong predictors of severe disability, hospitalization, institutionalization, and death.8–11 Causes of impaired mobility and lower extremity function that are potentially modifiable include a number of sociodemographic and behavioral characteristics, acute and chronic diseases,12 and several metabolic conditions, including obesity, diabetes mellitus and insulin resistance, a chronic low-grade inflammatory state,13 and a high level of oxidative stress.14 HDL-C is inversely correlated with most of the above-mentioned conditions, and recent studies have reported an antiinflammatory and antioxidant effect of Apolipoprotein A-I–containing lipoproteins,15 suggesting that, through these biological activities, HDL-C may counteract the disablement process in older persons.16
The aim of this study was therefore to evaluate the independent association between HDL-C levels and different objective measures of lower extremity performance in a sample of community-dwelling nondisabled older persons. It was hypothesized that subjects with higher HDL-C levels would have better physical performance, and the study sought to assess the direct and indirect contribution of several clinical and biochemical characteristics correlated with HDL-C levels in explaining the relationship between HDL-C and physical performance.
Methods
Sample
The InCHIANTI study is a prospective, population-based study of randomly selected older people living in two cities in the Chianti area, Tuscany, Italy. The Laboratory of Clinical Epidemiology of the Italian Research Council of Aging (Florence, Italy) designed the study to identify risk factors for late-life disability, as previously described.17 Briefly, participants were selected from the city registries of Greve in Chianti and Bagno a Ripoli using a multistage sampling method. In 1998, 1,453 persons who were randomly selected from the population agreed to participate in the project. The Italian National Research Council on Aging Ethical Committee ratified the study protocol, and participants provided written consent to participate. The present analysis was performed in 836 nondisabled persons aged 65 and older. Participants younger than 65 (n = 298), those reporting any difficulties in performing six basic activities of daily living (ADLs: eating, bathing, dressing, transferring from bed to chair, using the toilet, and walking across a small room, n = 116), and those with missing data in metabolic or physical performance measures (n = 203) were excluded.
Measures of Physical Performance
Three different and complementary objective performance-based tests were used to assess physical performance: 4-m walking speed at fast pace, 400-m walking speed, and knee extension strength. To measure walking speed on a 4-m course at fast pace, two photocells connected with a recording chronometer were placed at the beginning and end of a 4-m course. Subjects were instructed to stand with both feet touching the starting line and to walk as fast as possible, without running, immediately after a verbal command. The second objective test measured the ability to walk a standard 400-m course (20 laps of 20 m). Verbal encouragement to maintain pace was given at each lap, as was the number of laps the participant had completed. A maximum of two standing rests were permitted for less than 2 minutes each. Assistive devices (e.g., canes, walkers) were permitted. Participants were excluded from this test if they had any of the following conditions: pathological electrocardiographic abnormality, systolic blood pressure greater than 180 mmHg, diastolic blood pressure greater than 100 mmHg, resting heart rate less than 40 or greater than 135 beats per minute, myocardial infarction or episodes of angina pectoris in the past 3 months, severe dementia, or poor visual acuity. The ability to complete the 400-m walk test has demonstrated perfect 1-week test-retest reliability (κ = 1) in older adults.18 Isometric knee extension muscle strength, the third objective test, was assessed using a handheld dynamometer (Nicholas Muscle Tester; Sammon Preston, Inc., Chicago, IL) following a standard protocol that has been previously proven to be reliable (test-retest reliability 0.85, interrater reliability 0.74).19 The average results of the left and right legs were used for the analyses. Knee extension torque was calculated according to the following formula: Knee extension torque = (knee extension × 9.81) × [(calf length − 100)/100] N/dm. Data were analyzed after standardization for kilograms of weight.
Lipid Parameters
Blood samples were obtained from participants after a 12-hour fast. Aliquots of serum were stored at −80°C and were not thawed until analyzed. HDL-C, total cholesterol, and triglycerides were determined using commercial enzymatic tests (Roche Diagnostics, Mannheim, Germany). The interassay coefficient of variation (CV) for HDL-C was less than 5.0%. Oxidized low-density lipoprotein cholesterol (LDL-C) was measured according to enzyme immunoassay (Mercodia, Uppsala, Sweden). Sex-specific HDL-C tertile cutpoints were 52 and 65 mg/dL for women and 45 and 55 mg/dL for women and men.
Covariates
Sociodemographic Characteristics
Cigarette-smoking behavior was assessed through survey questions. Physical activity during the year before the interview was assessed through an interviewer-administered questionnaire as previously described20 and coded as follows: sedentary: completely inactive or light-intensity activity less than 1 h/wk; light physical activity: light-intensity activity 2 to 4 h/wk; moderate–high physical activity: light activity at least 5 h/wk or more or moderate activity at least 1 to 2 h/wk. Daily alcohol (g/d) and total energy intake (kcal/d per kg) were estimated using the European Prospective Investigation Into Cancer and Nutrition Food Frequency Questionnaire.21 Mini-Mental State Examination score was used to evaluate cognitive function.22
Anthropometric Measures
Weight and height were measured using objective standard techniques and used to calculate body mass index (BMI, kg/m2). Waist circumference was measured at the midpoint between the lower rib margin and the iliac crest (normally umbilical level). Calf muscle cross-sectional area was evaluated using a lower leg peripheral quantitative computerized tomography (XCT 2000, Stratec, Pforzheim, Germany). Data were derived from standard 2.5-mm-thick transverse scans obtained at 66% of the tibia length, proximal to the anatomic marker, as previously described.23
Inflammatory Markers
Serum interleukin (IL)-6, IL-1β, IL-1 receptor antagonist (IL-1ra), and tumor necrosis factor alpha (TNF)-α were measured in duplicate according to high-sensitivity enzyme-linked immunoabsorbent assays (ELISA, BIOSOURCE, Camarillo, CA). The CVs were 4.5% for IL-1ra and 7% for IL-6, IL-1β, and TNF-α. Serum IL-18 was measured in duplicate using highly sensitive quantitative sandwich assays (Quantikine HS, R&D Systems, Minneapolis, MN) with a CV of 7%. Interferon gamma (IFN)-γ was determined using a multicytokine detection system (Bio-Rad, Hercules, CA), which allows detection of concentrations as low as 5 pg/mL, using a Luminex System (Austin, TX). C-reactive protein (CRP) was measured in duplicate using an ELISA and colorimetric competitive immunoassay (interassay CV 5%).
Hormone Assays
Total testosterone was assayed using commercial kits (Diagnostic Systems Laboratories, Webster, TX, CV = 9.1%). Concentration of bioavailable testosterone was calculated using the Vermeulen formula.17 Free insulin-like growth factor-1 was measured according to commercial radioimmunoassay (Diagnostic System Laboratories, CV = 7.3%).
Plasma insulin level was determined using a double-antibody, solid-phase radioimmunoassay (Sorin Biomedica, Milan, Italy). Insulin resistance (IR) was calculated according to homeostasis model assessment (HOMA): IR = Fasting 141 insulin × Fasting Glucose/22.5.24
Comorbid Diseases
The presence of specific medical conditions was established using standardized criteria that combined information from self-reported history, medical records, and a clinical medical examination. Diagnostic algorithms were modified versions of those created for the Women's Health and Aging Study.25 The following diseases were assessed: coronary artery disease (angina pectoris and acute myocardial infarction), peripheral arterial disease, stroke (and transient ischemic attack), hypertension, diabetes mellitus, congestive heart failure, and cancer. Participants were also asked to report any medication taken in the previous 2 weeks.
Statistical Analysis
Variables are reported as mean values ± standard deviations, medians and interquartile ranges (Q1–Q3), or percentages. Because of skewed distributions, log-transformed values for hormones, cytokines, and CRP concentrations were used in regression analyses. Comparisons across sex-specific HDL-C tertiles were performed using age- and sex-adjusted linear or logistic regression models. Inflammatory markers independently associated with serum HDL-C after adjustment for age and sex were used for creating an ordinal score summarizing the intensity of the proinflammatory state. The score was created as the number of inflammatory markers in the upper tertiles, among markers identified as independent correlates of HDL-C, namely, CRP (>3.9 ml/L), IL-6 (>1.75 pg/mL), IL-1ra (>163 pg/mL), IL-18 (>429 nm/mL), and TNF-α (>2.56 pg/mL). The relationship between HDL-C tertiles and 4-m walking speed, 400-m walking speed, and knee extension torque was analyzed using linear regression models. Covariates hypothesized as potential confounders of the association between HDL-C levels and physical performance were progressively added to the initial model. Sex interactions were assessed by including an interaction term in the fully adjusted model as a covariate. Multivariate polytomous logistic regression models were used to study the association between HDL-C levels and sex-specific tertile of 4-m walking speed, 400-m walking speed, and knee extension torque. Logistic regression analysis was stratified according to sex and adjusted for multiple confounders. Finally, to further examine the relationship between HDL-C and knee extension torque, structural equation modelling (path analysis) was performed using MPLUS software (Muthén & Muthén, Los Angeles, CA). This approach allows for comparing specific models that define the relationship between HDL-C and knee extension torque when considering how other factors affect HDL-C and knee extension torque. The adequacy of a model is tested by comparing the covariance matrix resulting from the model with the covariance matrix of the data. Because knee extension torque was significantly associated with HDL-C in men and women, it was decided to limit the structural equation model analysis to the relationship between HDL-C and knee extension torque.
Results
The mean age of participants was 73.7 (65–92), and 55.6% were women. Mean HDL-C plasma levels were 59.6 ± 15.6 mg/dL for women and 51.7 ± 12.9 mg/dL for men. Independent of age and sex, participants in the highest HDL-C tertile reported higher alcohol intake than subjects in the lowest tertile and were less likely to have hypertension, stroke, peripheral arterial disease, and diabetes mellitus (Table 1). Subjects with higher HDL-C levels had smaller calf muscle area, lower bioavailable testosterone levels, and lower levels of several markers of inflammation. Adjustment for BMI difference across tertiles of HDL-C explained the inverse relationship between HDL-C and calf muscle area. Participants with higher HDL-C level also had lower BMI, waist circumference, triglyceride levels, oxidized LDL-C levels, and HOMA-estimated insulin resistance.
Table 1. Selected General and Clinical Characteristics of the Sample According to Sex-Specific Tertiles of High-Density Lipoprotein Cholesterol (HDL-C) Levels.
| Sex-Specific HDL-C Tertiles | ||||
|---|---|---|---|---|
| Characteristic | Lowest (n = 276) | Intermediate (n = 278) | Highest (n = 280) | P-Value |
| Age, mean ± SD | 74.1 ± 6.3 | 73.3 ± 6.5 | 73.8 ± 6.6 | .67* |
| Female, % | 58.9 | 51.1 | 56.8 | .68† |
| Education, years, mean ± SD | 5.2 ± 2.9 | 5.9 ± 3.7 | 5.5 ± 3.2 | .31* |
| Current smokers, % | 15.6 | 11.2 | 16.6 | .72† |
| Alcohol intake, g/d, % | ||||
| 0.1–20 | 47.5 | 50.0 | 44.8 | |
| >20 | 18.9 | 26.3 | 33.3 | < .001† |
| Energy intake, kcal/kg per day, mean ± SD | 27.3 ± 7.7 | 28.0 ± 8.2 | 31.1 ± 9.1 | < .001 * |
| Physical activity level | ||||
| Light | 44.7 | 44.4 | 46.6 | |
| Moderate to high | 40.4 | 43.2 | 40.5 | .71† |
| Mini-Mental State Examination score, mean ± SD | 25.2 ± 3.6 | 25.3 ± 3.8 | 25.4 ± 3.4 | .72* |
| Chronic conditions, % | ||||
| Hypertension | 69.3 | 66.9 | 58.2 | .006† |
| Coronary artery disease | 11.6 | 11.5 | 9.3 | .37† |
| Congestive heart failure | 21.4 | 23.4 | 23.6 | .50† |
| Stroke or transient ischemic attack | 6.5 | 6.5 | 3.2 | .08† |
| Peripheral arterial disease | 17.4 | 14.4 | 9.3 | .006† |
| Diabetes mellitus | 13.8 | 10.8 | 8.9 | .07† |
| Cancer | 7.2 | 6.1 | 4.6 | .21† |
| Anthropometry | ||||
| Body mass index, kg/m2, mean ± SD | 28.7 ± 4.2 | 27.7 ± 3.8 | 26.2 ± 3.7 | < .001 * |
| Waist circumference, cm, mean ± SD | 95.9 ± 9.6 | 93.4 ± 10.0 | 88.8 ± 9.9 | < .001 * |
| Calf muscle area, cm2, mean ± SD | 65.0 ± 13.1 | 63.5 ± 12.1 | 60.7 ±11.4 | < .001 * |
| Lipid profile | ||||
| Total cholesterol, mg/dL, mean ± SD | 210.4 ± 38 | 220.2 ± 37 | 229.3 ± 41 | < .001 * |
| Triglycerides, mg/dL, mean ± SD | 167.1 ± 95 | 121.3 ± 49 | 95.4 ± 36.1 | < .001 * |
| LDL-C, mg/dL, mean ± SD | 134.9 ± 34 | 142.0 ± 32 | 138.0 ± 37 | .27* |
| Oxidized LDL-C, U/L, mean ± SD | 44.8 ± 14.7 | 43.5 ± 12 | 39.9 ± 12 | < .001 * |
| Lipid lowering medications, % | ||||
| Statins | 9 (3.2) | 10 (3.6) | 13 (4.6) | 0.39† |
| Fibrates | 8 (2.8) | 3 (1.1) | 1 (0.4) | 0.02† |
| Hormones, median (q1–q3 range) | ||||
| Homeostasis model assessment-estimated insulin resistance | 2.4 (3.64–1.64) | 1.99 (1.41–3.05) | 2.04 (1.37–2.86) | <.001‡ |
| Testosterone, ng/dL | 25.4 (10.2–103.4) | 21.7 (9.22–89.4) | 20.0 (7.68–90.2) | .02‡ |
| Insulin-like growth factor-1, pg/mL | 0.54 (0.40–0.68) | 0.55 (0.38–0.72) | 0.56 (0.36–0.73) | .98‡ |
| Inflammatory markers, median (q1–q3 range) | ||||
| C-reactive protein, mg/L | 3.21 (1.46–6.94) | 2.34 (1.22–4.75) | 1.83 (0.88–3.98) | <.001‡ |
| IL-6, pg/mL | 1.55 (0.87–2.80) | 1.16 (0.72–1.92) | 1.09 (0.66–1.80) | <.001‡ |
| IL-6r, ng/mL | 93.3 (69.6–119.5) | 90.9 (64.9–122.4) | 90.9 (66.6–134.2) | .83‡ |
| IL-1β, pg/mL | 0.0 (0.0–0.7) | 0.0 (0.0–0.8) | 0.0 (0.0–0.4) | .79‡ |
| IL-1 receptor antagonist, pg/mL | 166 (114–230) | 129 (98–172) | 111 (83–148) | <.001‡ |
| IL-18, ng/mL | 395 (315–512) | 376 (283–476) | 337 (260–434) | <.001‡ |
| Tumor necrosis factor-alpha, pg/mL | 2.13 (1.58–3.60) | 1.86 (1.39–2.91) | 1.83 (1.36–2.92) | .003‡ |
| Interferon gamma, pg/mL | 0.0 (0.0–0.0) | 0.0 (0.0–0.6) | 0.0 (0.0–0.0) | .89‡ |
From linear regressions, adjusted for age and sex.
From logistic regression adjusted for age and sex.
From linear regression adjusted for age, sex, and log-transformation of the dependent variable.
SD = standard deviation; LDL-C = low-density lipoprotein cholesterol; IL = interleukin.
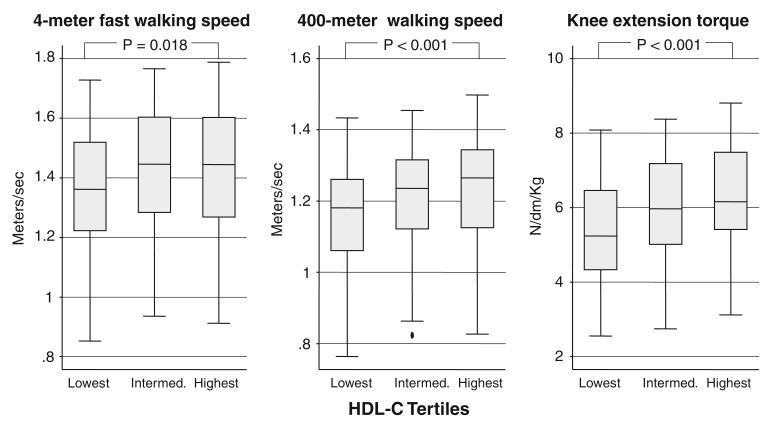
The relationships between HDL-C and 4-m fast waking speed, 400-m walking speed, and knee extension torque are shown in Figure 1. After adjustment for age and sex, there was a graded and statistically significant association between HDL-C levels and all indexes of function, with participants with the highest HDL-C levels having the best physical performance. After further adjustment for alcohol intake, physical activity, and anthropometric indicators (weight, height, waist circumference, calf muscle area), participants in the highest HDL-C tertile had faster 400-m walking speed and greater knee extension torque than subjects in the lowest HDL-C tertile, whereas difference in 4-m walking speed was no longer significant (Table 2, Model 1). Subsequently adjusting for chronic conditions associated with HDL-C levels, hormonal parameters, markers of inflammation, and oxidized LDL-C levels progressively reduced the strength of association between HDL-C and 400-m walking speed (Table 2, Models 2–4). After adjustment for the same covariates, the differences in knee extension torque across tertiles of HDL-C remained statistically significant.
Figure 1.
Box-plots showing age- and sex-adjusted performance values in 4-m fast walking speed, 400-m walking speed, and knee extension torque according to sex-specific high-density lipoprotein cholesterol (HDL-C) tertiles. The central box extends from the 25th to the 75th percentile; the line within the box represents the median value; lines projecting outside the box on either side are the “adjacent values”; points outside the lines are the “outliers.” P from age- and sex-adjusted analysis of covariance.
Table 2. Linear Regression Model Testing the Association of High-Density Lipoprotein Cholesterol (HDL-C) Levels (Sex-Specific Tertiles) and Performance Tests.
| Model 1* | Model 2† | Model 3‡ | Model 4§ | ||
|---|---|---|---|---|---|
| Performance Test | HDL-C Tertile | β‖ (Standard Error) P-Value | |||
| 4-m fast walking speed, m/s | Intermediate | 0.029 (.02) .22 | 0.028 (.02) .24 | 0.034 (.02) .16 | 0.032 (.02) .19 |
| Highest | 0.023 (.02) .37 | 0.013 (.03) .61 | 0.018 (.03) .48 | 0.019 (.03) .48 | |
| 400-m walking speed, m/s | Intermediate | 0.015 (.01) .33 | 0.017 (.02) .27 | 0.020 (.02) .20 | 0.012 (.02) .45 |
| Highest | 0.040 (.02) .02 | 0.029 (.02) .08 | 0.030 (.02) .07 | 0.019 (.02) .26 | |
| Knee extension torque, N/dm/Kg | Intermediate | 0.44 (.14) .002 | 0.42 (.14) .002 | 0.46 (.14) .001 | 0.46 (.14) .001 |
| Highest | 0.68 (.14) <.001 | 0.61 (.15) <.001 | 0.66 (.15) <.001 | 0.68 (.15) <.001 | |
Adjusted for age, sex, alcohol intake, physical activity, weight, height, waist circumference, and calf muscle area.
Adjusted for variables included in Model 1 plus additional adjustment for Mini-Mental State Examination score, hypertension, diabetes mellitus, coronary heart disease, stroke, and peripheral artery disease.
Adjusted for variables included in Model 2 plus additional adjustment for homeostasis model assessment and testosterone.
Adjusted for variables included in Model 3 plus additional adjustment for low-density lipoprotein cholesterol (LDL-C), oxidized LDL-C, and inflammation score.
β is the difference in mean adjusted value for intermediate and highest HDL-C tertiles compared with the lowest.
In the fully adjusted models predicting 4-m and 400-m walking speed, a statistically significant interaction was found between sex and HDL-C concentration (P-value for interaction terms = .003 and .006, respectively), suggesting that the association between walking speed and HDL-C was stronger in men than women. Accordingly, separate models were fitted in women and men, and a significant stepwise increase was found in all three performance outcomes with higher HDL-C levels in men (difference in mean adjusted value for highest HDL-C tertiles compared with the lowest tertile: 0.094 m/s, P = .04, for 4-m walking speed; 0.066 m/s, P = .03, for 400-m walking speed and 1.1 N/dm per kg, P <.001, for knee extension torque), whereas in women, the association was present only for knee extension torque (difference in mean adjusted value for highest HDL-C tertiles compared with the lowest tertile: 0.38 N/dm per kg, P = .02).
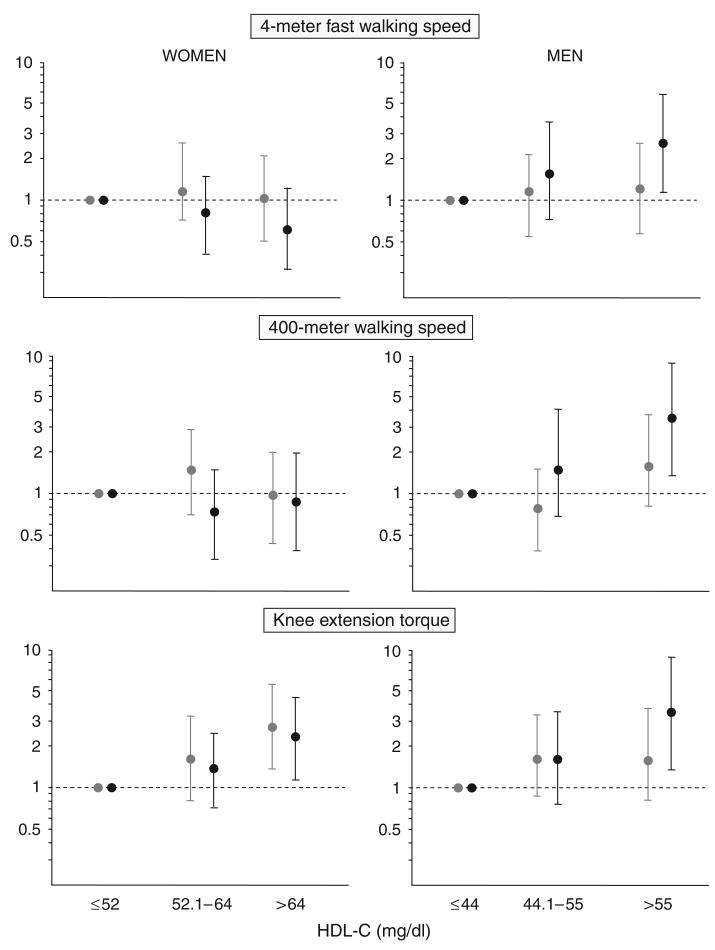
The findings reported above were confirmed according to multinomial logistic regression analysis predicting the likelihood of belonging to the highest and intermediate tertile (compared with the lowest tertile) of three performance tests according to HDL-C level and sex (Figure 2). For example, men in the highest tertile of the HDL-C distribution had, on average, a three times higher probability of belonging to the highest tertile of 4-m fast walking speed (OR = 2.57, 95% CI = 1.07–6.17), 400-m walking speed (OR = 3.74, 95% CI = 1.20–11.7), and knee extension torque (OR = 3.63, 95% CI = 1.41–9.33) than men in the lowest HDL-C tertile; women with high HDL-C levels were also more likely to be in the highest tertile of knee extension torque (OR = 2.50, 95% CI = 1.21–5.17), but no associations were found for 4-m and 400-m walking speed. The results were virtually unchanged using HDL-C as a continuous variable.
Figure 2.
Adjusted odds ratios (95% confidence intervals) comparing the likelihood of belonging to the highest (black) and intermediate (gray) tertile with that of belonging to the lowest tertile for 4-m fast walking speed, 400-m walking speed, and knee extension strength according to high-density lipoprotein cholesterol (HDL-C) levels and sex. Models are adjusted for variables included in Model 4 as described in Table 2.
Finally, to better assess the independent relationship between HDL-C and physical performance, a structural equation model (path analysis) was performed. This analytical approach allowed the direct and indirect associations between HDL-C, knee extension torque, and other parameters hypothesized to influence the association between HDL-C and performance to be simultaneously examined. In establishing the model to be tested, Table 2 was used to reduce the number of variables considered in the final model. When the variables in Model 2 (Table 2) were included, changes in the beta coefficients were observed relative to Model 1, suggesting an importance for these variables, which led to the inclusion of coronary heart disease and diabetes mellitus in the path analysis. The other variable included was inflammatory score from Model 4, because this seemed to lead to modest changes in the beta coefficients and because of interest from the underlying concept of how HDL-C might relate inflammation to change in knee torque. Figure 3 depicts the proposed model for men and women as separate groups, with each arrow representing a proposed relationship between the associated variables and the direction of the arrow implying the direction of action within the relationship. The model assumes that each variable may have a potential independent effect on each or some of the other variables in the model, including knee extension torque, and a potential indirect effect on knee extension torque through the other measures. Under this model, the main question becomes whether HDL-C has a direct effect on knee extension torque. As shown in Figure 3, HDL-C appears to have a positive and direct association with knee extension strength that was greater in men (P<.001) than in women (P = .07). The model is also consistent with the presence of an effect of inflammation, age, and diabetes mellitus on HDL-C and of inflammation, age, diabetes mellitus, and cardiovascular disease on knee extension strength. The model implies that inflammation affects HDL-C and knee extension strength but that HDL-C does not exert its influence on strength through its relationship with inflammation.
Figure 3.
Structural equation model examining the relationship between high-density lipoprotein cholesterol (HDL-C) levels and knee extension torque. Each variable has a series of arrows that reflect the proposed direction of the relationship between the variables. For continuous variables, path coefficient represents the change that occurs in the variable at the head of the arrow for each unit change in the variable at the tail of the arrow. For binary variables, path coefficient represents the log(OR) for the variable at the head of an arrow for each unit change in the variable at the tail of the arrow. Solid lines are for statistically significant coefficients (P <.1). To assess the goodness-of-fit model, the covariance matrix that resulted from the model was compared with the covariance matrix of the actual data. Because the chi-square (.947, degrees of freedom = 1) was not significant (P = .33), this model was accepted as reasonably fitting the data. CVD = cardiovascular disease.
Discussion
The findings of this study demonstrated a strong and graded relationship between plasma HDL-C levels and different objective measures of lower extremity performance in non-disabled older persons. Women and men in the highest tertile had higher knee extension torque than those in the lowest HDL-C tertile, whereas only men with higher HDL-C concentration also had faster walking speed in the 4-m and 400-m walking test. The results were based on data collected in a well-characterized random sample of older persons and were consistent using complementary analytical approaches. Multivariate adjustment for potential confounders and mediators, including several clinical and biochemical characteristics significantly associated with HDL-C level, only partially modified the findings.
The results confirm, at a population level, the findings of previous studies on HDL-C level and health status in older persons and extend the association between HDL-C and objective measure of physical limitation, a common early stage of the disablement process in older persons. In the Framingham Heart Study, healthy octogenarians were extremely unlikely to have lower levels of HDL-C than middle-aged subjects.26 Furthermore, previous studies suggested that lower HDL-C levels characterized older subjects with ADL disability and that low HDL-C predicts functional decline over time in nursing home residents.27,28 More recently, another study demonstrated a direct cross-sectional relationship between HDL-C level and objective motor performance in a population-based sample of women, independent of age, body composition, vascular and metabolic diseases, and level of physical exercise.29 In the current sample, a direct association between HDL-C and knee extension torque in both men and women was demonstrated, but no significant association was found between HDL-C and walking speed in women. Although the higher mean HDL-C level in women than in men may, at least in part, explain this finding, the reason for this sex difference in the association between HDL-C and walking speed is unclear and might deserve further investigation.
How can HDL-C level be associated with functional decline in older persons? It might be argued that low HDL-C levels, by increasing the risk of ischemic heart disease, stroke, and peripheral artery disease, could, in turn, cause decline in lower extremity performance and muscle strength.30 However, in the current study, statistically controlling for prevalent coronary artery disease, stroke, and peripheral arterial disease, objectively assessed using the ankle-brachial index, did not substantially reduce the association between HDL-C and physical performance. Moreover, restricted analyses performed in participants without prevalent cardiovascular conditions were consistent with the results obtained from the overall sample (data not shown), suggesting that the protective effect of HDL-C on the atherosclerotic process does not explain the association between HDL-C and functional impairment. Regular aerobic exercise, a powerful protective factor for maintaining mobility and physical independence in older persons,20 increases HDL-C levels in healthy sedentary persons.31 In the current study, the association between HDL-C level and physical function was independent of the amount of physical activity during the previous year. Moreover, participants who reported moderate–high physical activity performed better than sedentary participants in all three tests of lower extremity performance (data not shown), whereas no association was found between physical activity and HDL-C levels. These findings are consistent with studies suggesting that, although moderate physical activity is effective in preventing physical decline in older persons,32 there is little evidence that moderate exercise significantly increases HDL-C levels.33 Other clinical and biochemical characteristics were evaluated as potential confounders or mediators of the studied relationship, including energy and alcohol intake, cognitive decline, hypertension, obesity and body composition, diabetes mellitus,34 insulin resistance,35 inflammation,36 and oxidative damage,14 but the independent association between HDL-C and physical performance persisted even after these characteristics were taken into account in multivariable models. The path analysis (Figure 3) further supported a positive and direct relationship between HDL-C levels and knee extension torque.
Besides its classic functions in reverse cholesterol transport, removing cholesterol from peripheral tissues and delivering it to the liver and to steroidogenic organs, HDL-C has additional metabolic properties that may explain atheroprotection and may preserve quality of life and promote longevity.37 For example, HDL-C has been reported to activate endothelial nitric oxide production by stimulation of endothelial nitric oxide synthase, with resultant beneficial effects on endothelial function.38,39 In human subjects, the intravenous infusion of reconstituted HDLs containing apolipoprotein A-1 complexed with phospholipids has been found to normalize endothelial-dependent vasodilatation by increasing nitric oxide bioavailability.40 Impaired endothelium-dependent vasodilatation in arterioles of highly oxidative muscle and reduction in skeletal muscle blood flow that in turn has been linked to the age-related decline in maximal aerobic and exercise capacity characterized the aging process.41 From this point of view, it can be hypothesized that high HDL-C levels may preserve muscle and physical function, counteracting the aging-associated changes in endothelial function of skeletal muscle arteries.
In interpreting these findings, some limitations should be considered. The cross-sectional design of the study did not allow a causal relationship to be determined, and longitudinal data are needed to verify whether low HDL-C levels predict accelerated functional decline in older persons. A second limitation of the current study is the self-report and relatively simple assessment of physical activity. The combination of these two methodological caveats does not allow an effect of reverse causality (i.e., higher physical activity causing higher HDL-C) to be completely excluded, although the exclusion of people with ADL disability should have tempered this important issue.
In conclusion, this study demonstrates that, in nondisabled older persons, high levels of HDL-C are independently associated with better objective physical performance. Further studies are needed to confirm the findings, especially using prospective design, and to further elucidate the biological mechanism underlying the reported association.
Acknowledgments
This research was supported in part by the Intramural Research program of the National Institutes of Health, National Institute on Aging. The InCHIANTI study was supported as a “targeted project” (ICS 110.1\RS97.71) by the Italian Ministry of Health.
Sponsor's Role: None.
Footnotes
Conflict of Interest: The editor in chief has reviewed the conflict of interest checklist provided by the author and has determined that none of the authors have any financial or any other kind of personal conflicts with this article.
Author Contributions: Stefano Volpato: concept and design, analysis and interpretation of data, preparation of manuscript. Alessandro Ble: concept and design, interpretation of the data, preparation of manuscript. Fulvio Lauretani and Stefania Bandinelli: acquisition of subjects and data, analysis and interpretation of data. E. J. Metter: analysis and interpretation of data, preparation of manuscript. Giovanni Zuliani and Renato Fellin: concept and design, interpretation of data. Luigi Ferrucci and Jack Guralnik: concept and design, acquisition of subjects and data, interpretation of data, preparation of manuscript.
References
- 1.Assmann G, Gotto AM., Jr HDL cholesterol and protective factors in atherosclerosis. Circulation. 2004;109(Suppl 1):III8–III14. doi: 10.1161/01.CIR.0000131512.50667.46. [DOI] [PubMed] [Google Scholar]
- 2.Corti MC, Guralnik JM, Salive ME, et al. HDL cholesterol predicts coronary heart disease mortality in older persons. JAMA. 1995;274:539–544. [PubMed] [Google Scholar]
- 3.Weverling-Rijnsburger AW, Jonkers IJ, van Exel E, et al. High-density vs low-density lipoprotein cholesterol as the risk factor for coronary artery disease and stroke in old age. Arch Intern Med. 2003;163:1549–1554. doi: 10.1001/archinte.163.13.1549. [DOI] [PubMed] [Google Scholar]
- 4.Hazzard WR. Depressed albumin and high-density lipoprotein cholesterol: Signposts along the final common pathway of frailty. J Am Geriatr Soc. 2001;49:1253–1254. doi: 10.1046/j.1532-5415.2001.49245.x. [DOI] [PubMed] [Google Scholar]
- 5.Volpato S, Leveille SG, Corti MC, et al. The value of serum albumin and high-density lipoprotein cholesterol in defining mortality risk in older persons with low serum cholesterol. J Am Geriatr Soc. 2001;49:1142–1147. doi: 10.1046/j.1532-5415.2001.49229.x. [DOI] [PubMed] [Google Scholar]
- 6.van Exel E, de Craen AJ, Gussekloo J, et al. Association between high-density lipoprotein and cognitive impairment in the oldest old. Ann Neurol. 2002;51:716–721. doi: 10.1002/ana.10220. [DOI] [PubMed] [Google Scholar]
- 7.Okoro CA, Zhong Y, Ford ES, et al. Association between the metabolic syndrome and its components and gait speed among U.S. adults aged 50 years and older: a cross-sectional analysis. BMC Public Health. 2006;6:282. doi: 10.1186/1471-2458-6-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guralnik JM, Ferrucci L, Simonsick EM, et al. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Newman AB, Simonsick EM, Naydeck BL, et al. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. 2006;295:2018–2026. doi: 10.1001/jama.295.17.2018. [DOI] [PubMed] [Google Scholar]
- 10.Penninx BW, Ferrucci L, Leveille SG, et al. Lower extremity performance in nondisabled older persons as a predictor of subsequent hospitalization. J Gerontol A Biol Sci Med Sci. 2000;55A:M691–M697. doi: 10.1093/gerona/55.11.m691. [DOI] [PubMed] [Google Scholar]
- 11.Newman AB, Kupelian V, Visser M, et al. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci. 2006;61A:72–77. doi: 10.1093/gerona/61.1.72. [DOI] [PubMed] [Google Scholar]
- 12.Stuck AE, Walthert JM, Nikolaus T, et al. Risk factors for functional status decline in community-living elderly people: A systematic literature review. Soc Sci Med. 1999;48:445–469. doi: 10.1016/s0277-9536(98)00370-0. [DOI] [PubMed] [Google Scholar]
- 13.Roth SM, Metter EJ, Ling S, et al. Inflammatory factors in age-related muscle wasting. Curr Opin Rheumatol. 2006;18:625–630. doi: 10.1097/01.bor.0000245722.10136.6d. [DOI] [PubMed] [Google Scholar]
- 14.Cesari M, Kritchevsky SB, Nicklas BJ, et al. Lipoprotein peroxidation and mobility limitation: Results from the health, aging, and body composition study. Arch Intern Med. 2005;165:2148–2154. doi: 10.1001/archinte.165.18.2148. [DOI] [PubMed] [Google Scholar]
- 15.von Eckardstein A, Hersberger M, Rohrer L. Current understanding of the metabolism and biological actions of HDL. Curr Opin Clin Nutr Metab Care. 2005;8:147–152. doi: 10.1097/00075197-200503000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Nofer JR, Walter M, Assmann G. Current understanding of the role of high-density lipoproteins in atherosclerosis and senescence. Expert Rev Cardiovasc Ther. 2005;3:1071–1086. doi: 10.1586/14779072.3.6.1071. [DOI] [PubMed] [Google Scholar]
- 17.Ferrucci L, Bandinelli S, Benvenuti E, et al. Subsystems contributing to the decline in ability to walk: Bridging the gap between epidemiology and geriatric practice in the InCHIANTI Study. J Am Geriatr Soc. 2000;48:1618–1625. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- 18.Rolland YM, Cesari M, Miller ME, et al. Reliability of the 400-m usual-pace walk test as an assessment of mobility limitation in older adults. J Am Geriatr Soc. 2004;52:972–976. doi: 10.1111/j.1532-5415.2004.52267.x. [DOI] [PubMed] [Google Scholar]
- 19.Bandinelli S, Benvenuti E, Del Lungo I, et al. Measuring muscular strength of the lower limbs by hand-held dynamometer: A standard protocol. Aging (Milano) 1999;11:287–293. doi: 10.1007/BF03339802. [DOI] [PubMed] [Google Scholar]
- 20.Patel KV, Coppin AK, Manini TM, et al. Midlife physical activity and mobility in older age: The InCHIANTI Study. Am J Prev Med. 2006;31:217–224. doi: 10.1016/j.amepre.2006.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaaks R, Riboli E. Validation and calibration of dietary intake measurements in the EPIC project: Methodological considerations. European Prospective Investigation into Cancer and Nutrition. Int J Epidemiol. 1997;26(Suppl 1):S15–S25. doi: 10.1093/ije/26.suppl_1.s15. [DOI] [PubMed] [Google Scholar]
- 22.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 23.Russo CR, Lauretani F, Bandinelli S, et al. Aging bone in men and women: Beyond changes in bone mineral density. Osteoporos Int. 2003;14:531–538. doi: 10.1007/s00198-002-1322-y. [DOI] [PubMed] [Google Scholar]
- 24.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 25.Guralnik JM, Fried LP, Simonsick EM, et al., editors. The Women's Health and Aging Study: Health and Social Characteristics of Older Women with Disability. Bethesda: National Institute on Aging; 1995. [Google Scholar]
- 26.Schaefer EJ, Moussa PB, Wilson PW, et al. Plasma lipoproteins in healthy octogenarians: Lack of reduced high density lipoprotein cholesterol levels: Results from the Framingham Heart Study. Metabolism. 1989;38:293–296. doi: 10.1016/0026-0495(89)90113-3. [DOI] [PubMed] [Google Scholar]
- 27.Zuliani G, Palmieri E, Volpato S, et al. High-density lipoprotein cholesterol strongly discriminates between healthy free-living and disabled octo-nonagenarians: A cross sectional study. Associazione Medica Sabin. Aging (Milano) 1997;9:335–341. doi: 10.1007/BF03339612. [DOI] [PubMed] [Google Scholar]
- 28.Zuliani G, Romagnoni F, Bollini C, et al. Low levels of high-density lipoprotein cholesterol are a marker of disability in the elderly. Gerontology. 1999;45:317–322. doi: 10.1159/000022112. [DOI] [PubMed] [Google Scholar]
- 29.Guo X, Matousek M, Sundh V, et al. Motor performance in relation to age, anthropometric characteristics, and serum lipids in women. J Gerontol A Biol Sci Med Sci. 2002;57A:M37–M44. doi: 10.1093/gerona/57.1.m37. [DOI] [PubMed] [Google Scholar]
- 30.Newman AB, Arnold AM, Naydeck BL, et al. Successful aging: Effect of sub-clinical cardiovascular disease. Arch Intern Med. 2003;163:2315–2322. doi: 10.1001/archinte.163.19.2315. [DOI] [PubMed] [Google Scholar]
- 31.Giada F, Vigna GB, Vitale E, et al. Effect of age on the response of blood lipids, body composition, and aerobic power to physical conditioning and deconditioning. Metabolism. 1995;44:161–165. doi: 10.1016/0026-0495(95)90259-7. [DOI] [PubMed] [Google Scholar]
- 32.Pahor M, Blair SN, Espeland M, et al. Effects of a physical activity intervention on measures of physical performance: Results of the Lifestyle Interventions and Independence for Elders Pilot (LIFE-P) Study. J Gerontol A Biol Sci Med Sci. 2006;61A:1157–1165. doi: 10.1093/gerona/61.11.1157. [DOI] [PubMed] [Google Scholar]
- 33.Kelley GA, Kelley KS, Tran ZV. Walking, lipids, and lipoproteins: A meta-analysis of randomized controlled trials. Prev Med. 2004;38:651–661. doi: 10.1016/j.ypmed.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 34.Volpato S, Blaum C, Resnick H, et al. Comorbidities and impairments explaining the association between diabetes and lower extremity disability: The women's health and aging study. Diabetes Care. 2002;25:678–683. doi: 10.2337/diacare.25.4.678. [DOI] [PubMed] [Google Scholar]
- 35.Abbatecola AM, Ferrucci L, Ceda G, et al. Insulin resistance and muscle strength in older persons. J Gerontol A Biol Sci Med Sci. 2005;60A:1278–1282. doi: 10.1093/gerona/60.10.1278. [DOI] [PubMed] [Google Scholar]
- 36.Zuliani G, Volpato S, Ble A, et al. High interleukin-6 plasma levels are associated with low HDL-C levels in community-dwelling older adults: The In-Chianti Study. Atherosclerosis. 2007;192:384–390. doi: 10.1016/j.atherosclerosis.2006.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barter P. HDL: A recipe for longevity. Atheroscler Suppl. 2004;5:25–31. doi: 10.1016/j.atherosclerosissup.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 38.Yuhanna IS, Zhu Y, Cox BE, et al. High-density lipoprotein binding to scavenger receptor-BI activates endothelial nitric oxide synthase. Nat Med. 2001;7:853–857. doi: 10.1038/89986. [DOI] [PubMed] [Google Scholar]
- 39.Mineo C, Shaul PW. HDL stimulation of endothelial nitric oxide synthase: A novel mechanism of HDL action. Trends Cardiovasc Med. 2003;13:226–231. doi: 10.1016/s1050-1738(03)00098-7. [DOI] [PubMed] [Google Scholar]
- 40.Bisoendial RJ, Hovingh GK, Levels JH, et al. Restoration of endothelial function by increasing high-density lipoprotein in subjects with isolated low high-density lipoprotein. Circulation. 2003;107:2944–2948. doi: 10.1161/01.CIR.0000070934.69310.1A. [DOI] [PubMed] [Google Scholar]
- 41.Spier SA, Delp MD, Meininger CJ, et al. Effects of ageing and exercise training on endothelium-dependent vasodilatation and structure of rat skeletal muscle arterioles. J Physiol. 2004;556:947–958. doi: 10.1113/jphysiol.2003.060301. [DOI] [PMC free article] [PubMed] [Google Scholar]