Abstract
OBJECTIVES
To determine whether lower ankle brachial index (ABI) levels are associated with lower calf skeletal muscle area and higher calf muscle percentage fat in persons with and without lower extremity peripheral arterial disease (PAD).
DESIGN
Cross-sectional.
SETTING
Three Chicago-area medical centers.
PARTICIPANTS
Four hundred thirty-nine persons with PAD (ABI<0.90) and 265 without PAD (ABI 0.90–1.30).
MEASUREMENTS
Calf muscle cross-sectional area and the percentage of fat in calf muscle were measured using computed tomography at 66.7% of the distance between the distal and proximal tibia. Physical activity was measured using an accelerometer. Functional measures included the 6-minute walk, 4-meter walking speed, and the Short Physical Performance Battery (SPPB).
RESULTS
Adjusting for age, sex, race, comorbidities, and other potential confounders, lower ABI values were associated with lower calf muscle area (ABI<0.50, 5,193 mm2; ABI 0.50–0.90, 5,536 mm2; ABI 0.91–1.30, 5,941 mm2; P for trend <.001). These significant associations remained after additional adjustment for physical activity. In participants with PAD, lower calf muscle area in the leg with higher ABI was associated with significantly poorer performance in usual- and fast-paced 4-meter walking speed and on the SPPB, adjusting for ABI, physical activity, percentage fat in calf muscle, muscle area in the leg with lower ABI, and other confounders (P<.05 for all comparisons).
CONCLUSION
These data support the hypothesis that lower extremity ischemia has a direct adverse effect on calf skeletal muscle area. This association may mediate previously established relationships between PAD and functional impairment.
Keywords: physical functioning, peripheral vascular disease, intermittent claudication, sarcopenia
Although it is known that patients with lower extremity peripheral arterial disease (PAD) have greater functional impairment and faster rates of functional decline than persons without PAD,1,2 mechanisms of functional impairment and decline in PAD are not well established. Ischemia-related pathological changes in calf skeletal muscle may contribute to functional impairment in patients with PAD, but associations between lower extremity ischemia, calf muscle characteristics, and lower extremity functioning in PAD are not well understood.
The purpose of this study was to determine whether participants with lower ankle brachial index (ABI) levels have lower calf skeletal muscle area and greater calf muscle percentage fat than participants with higher ABI levels. Associations between calf muscle area and calf muscle percentage fat and the degree of functional impairment in persons with PAD were also evaluated.
METHODS
Subjects
The institutional review board of Northwestern University Feinberg School of Medicine and Catholic Health Partners Hospitals approved the protocol. Participants gave informed consent. Participants included 325 persons attending their fourth annual follow-up visit in the Walking and Leg Circulation Study (WALCS)1,2 and 379 individuals newly identified. Participation rates and exclusion criteria for the WALCS cohort have been described.1,2 The 379 newly identified participants were recruited from among 1,804 contacted for participation. Of these 1,804, 176 (9.8%) met exclusion criteria, 131 (7.3%) refused participation, 1,021 (57%) did not respond to letters inviting their participation, and 74 (4.1%) did not attend their study appointment, leaving 402 newly identified participants. Of these, 23 did not undergo computed tomography (CT) scanning, leaving 379 newly identified participants for analyses.
All participants were aged 59 and older. Participants with PAD were identified consecutively from among patients diagnosed with PAD in three Chicago-area noninvasive vascular laboratories. Approximately half of participants without PAD were identified consecutively from patients with normal lower extremity arterial studies in the three Chicago vascular laboratories. The remainder were identified from consecutive patients in a general medicine practice at Northwestern University.
Exclusion Criteria
PAD was defined as ABI less than 0.90.1–3 Absence of PAD was defined as ABI of between 0.90 and 1.30.4 ABI less than 0.90 is 95% sensitive and 99% specific for angiographically diagnosed PAD.5 Individuals with ABI greater than 1.30 were excluded, because this indicates poorly compressible leg arteries and inability to gauge arterial perfusion accurately.4,6
The following exclusion criteria were applied at the time of original study enrollment to participants in the original WALCS cohort and those newly identified. Patients with dementia were excluded because of their inability to answer questions accurately. Nursing home residents, wheelchair-bound patients, and patients with foot or leg amputations were excluded because they have severely impaired functioning. Non-English-speaking patients were excluded because investigators were not fluent in non-English languages. Patients with recent major surgery were excluded. Finally, WALCS participants with an ABI less than 0.90 at their original visit whose ABI at the time of the computed tomography (CT) scan was 0.90 or greater were excluded, because they could not be clearly identified as having PAD.
ABI Measurement
The ABI was measured using established methods.1–4,6 After pparticipants rested supine for 5 minutes, a handheld Doppler probe (Nicolet Vascular Pocket DopII, Golden, CO) was used to measure systolic pressures in the right brachial, dorsalis pedis, and posterior tibial arteries and in the left dorsalis pedis, posterior tibial, and brachial arteries. Pressures were measured in the order listed and repeated in reverse order. The ABI was calculated in each leg by dividing average pressures in each leg by the average of the four brachial pressures.1,2 Average brachial pressures in the arm with highest pressure were used when one brachial pressure was higher than the opposite brachial pressure in both measurement sets and the two brachial pressures differed by 10 or more mmHg in at least one measurement set, because in such cases, subclavian stenosis is possible.7 Lowest leg ABI was used in analyses.
Measuring Calf Skeletal Muscle Cross-Sectional Area
Calf muscle area was selected for study because the superficial femoral artery is the most common site of lower extremity atherosclerosis,8,9 and calf musculature receives blood supply from the superficial femoral artery. Furthermore, the calf muscles are those that classically are symptomatic in patients with PAD.10 Using a CT scanner (LightSpeed, General Electric Medical Systems, Waukesha, WI), 2.5-mm cross-sectional images of the calves were obtained at 66.7% of the distance from the distal to the proximal tibia, based on previous study.11 Cross-sectional images were analyzed using BonAlyze (BonAlyze Oy, Jyvaskyla, Finland), a software for processing CT images that identifies muscle tissue, fat, and bone.11 The muscle outline was traced manually and excluded subcutaneous fat and bone. When quantifying muscle area, the BonAlyze software quantifies voxels within a range corresponding to muscle density (9–271 mg/cm3) and excludes voxels that correspond to fat density (−270–8 mg/cm3). Intramuscular fat is quantified by summing voxels corresponding to fat within muscle tissue. Previous cadaver studies have demonstrated that these methods provide an estimate of muscle cross-sectional area that is highly correlated with direct anatomic measures.12 Because larger individuals require greater muscle mass to support their larger frame, muscle area was adjusted for the square of individual tibia length.
Functional Data
6-Minute Walk
Patients with PAD are particularly impaired in walking endurance. PAD patients experience more-significant declines in 6-minute walk performance over time than in other measures of lower extremity functioning than do persons without PAD.2 Thus, the 6-minute walk is particularly sensitive to the functional limitations experienced by persons with PAD. Following a standardized protocol,1,2,13 participants walk up and down a 100-foot hallway for 6 minutes after instructions to cover as much distance as possible.
Repeated Chair Rises
Participants sit in a straight-backed chair with arms folded across their chest and stand five times consecutively as quickly as possible. Time to complete five chair rises is measured.1,2,14,15
Standing Balance
Participants were asked to hold three increasingly difficult standing positions for 10 seconds each: standing with feet together side-by-side and parallel (side-by-side stand), standing with feet parallel with the toes of one foot adjacent to and touching the heel of the opposite foot (semi-tandem stand), and standing with one foot directly in front of the other (tandem stand).14,15
Four-Meter Walking Velocity
Walking velocity was measured using a 4-meter walk performed at usual and fastest pace. For the usual-paced walk, participants were instructed to walk at their usual pace, “as if going down the street to the store.” Each walk was performed twice. The faster walk in each pair was used in analyses.14,15
Short Physical Performance Battery
The Short Physical Performance Battery (SPPB) combines data from the usual-paced 4-meter walking velocity, time to rise from a seated position five times, and standing balance. Individuals receive a score of 0 for each task they are unable to complete. Scores of 1 to 4 are assigned for remaining tasks, based upon quartiles of performance for more than 5,000 participants in the Established Populations for the Epidemiologic Study of the Elderly.14,15 Scores are summed to obtain the SPPB score, ranging from 0 to 12.
Objective Physical Activity Measures
Physical activity levels were measured objectively over 7 days using a pedometer (Digiwalker, Yamax Inc., Tokyo, Japan) and a vertical accelerometer (Caltrac, Muscle Dynamics Fitness Network, Inc., Torrence, CA).16,17 The pedometers measured the number of steps walked.18,19 The Caltrac accelerometers are designed to calculate the number of kilocalories expended based on activity (vertical movement), age, weight, height, and sex. Data from each measure were included in the analyses. To compare activity between participants irrespective of individual variation in age, weight, height, and sex, accelerometers were programmed using identical weight, height, age, and sex for each participant.16,17 Thus, the accelerometers measured “activity units.”16,17 After wearing activity monitors continuously for 7 days, participants reported the number of activity units and steps displayed on the accelerometer and pedometer by telephone and mailed their activity monitors back to investigators.
Seventy-four percent of participants wore activity monitors. Systematic data on reasons that some participants did not wear monitors were not collected. Some participants refused to wear monitors; others wore monitors but did not return them and could not be reached at 7-day follow-up; some participants’ monitors malfunctioned; and in some instances, no monitors were available.
Leg Symptom Categories
Leg symptoms were classified into one of five groups using the San Diego Claudication Questionnaire, based on previous study.2,20 Leg symptom groups were as follows: classic intermittent claudication, leg pain on exertion and rest, atypical exertional leg pain/carry on, atypical exertional leg pain/stop, and asymptomatic (no exertional pain).
Comorbidities
Algorithms developed for the Women’s Health and Aging Study and the Cardiovascular Health Study were used to document comorbidities.21 These algorithms combine data from patient report, physical examination, medical record review, medications, laboratory values, and a primary care physician questionnaire. Criteria developed by the American College of Rheumatology were used to diagnose knee and hip osteoarthritis.22,23 Comorbidities assessed were angina pectoris, diabetes mellitus, myocardial infarction, stroke, heart failure, pulmonary disease, cancer, spinal stenosis, and disk disease.
Other Measures
Height and weight were measured at the study visit. Body mass index was calculated as weight (kg)/(height (meters2)). Cigarette smoking history was based on self-report.
Statistical Analyses
Differences in continuous variables and dichotomous variables were compared between participants with and without PAD using analyses of variance and chi-square tests, respectively.
Differences in calf muscle area and percentage fat were evaluated across ABI categories using analyses of covariance, adjusting for age, race, sex, cigarette smoking, BMI, tibia length, leg symptoms, prior lower extremity revascularization, recruitment cohort (original WALCS vs newly identified), and comorbidities. In these analyses, the ABI level in the leg with lower ABI was the independent variable of interest, and the corresponding muscle characteristic in the leg with lower ABI was the dependent variable. Tibia length was a covariate, because longer tibia length is associated with greater calf muscle area.24 Analyses were repeated separately with additional adjustment for each measure of physical activity. Differences in muscle area and percentage fat were compared between legs with higher versus lower ABI levels using paired t tests in participants with PAD whose right and left leg ABIs differed by 0.20 units or more.
In participants with PAD, lower extremity performance was compared across quintiles of calf muscle area and calf muscle percentage fat using analyses of covariance, adjusting for age, sex, race, ABI, leg symptoms, BMI, tibia length, cigarette smoking, recruitment cohort, comorbidities, height, and physical activity. In these models, muscle characteristics corresponding to the leg with higher and lower ABI were separately entered into the model. These models were repeated with additional adjustment for calf muscle percentage fat and calf muscle area.
Analyses were performed using SAS Statistical Software version 9.0 (SAS Institute, Inc., Cary, NC).
RESULTS
Table 1 shows characteristics of participants according to the presence or absence of PAD. Participants with PAD were older and included higher prevalences of men, cardiovascular diseases, diabetes mellitus, and pulmonary disease than those without PAD. Participants with PAD had lower BMI and a greater pack-year history of cigarette smoking than participants without PAD. Lower extremity arthritis was more prevalent in participants without PAD.
Table 1.
Characteristics of Men and Women Aged 59 and Older with and without Lower Extremity Peripheral Arterial Disease
| All Participants (N =704) | PAD (N =439) | Non-PAD (N =265) | P-value | |
|---|---|---|---|---|
| Age (years) | 73.6 ± 8.2 | 74.9 ± 8.2 | 71.5 ± 7.7 | <0.001 |
| Male (%) | 49.2 | 53.1 | 42.6 | 0.007 |
| African-American Race (%) | 17.9 | 16.9 | 19.6 | 0.354 |
| Ankle brachial index | 0.803 ± 0.26 | 0.63 ± 0.16 | 1.088 ± 0.09 | <0.001 |
| Body mass index, (kg/m2) | 28.3 ± 5.4 | 27.8 ± 5.0 | 29.2 ± 5.8 | 0.001 |
| Cigarette smoking (pack-years) | 28.3 (33.5) | 35.5 (35.7) | 16.2 (25.1) | <0.001 |
| Angina (%) | 29.4 | 35.1 | 19.9 | <0.001 |
| Myocardial infarction (%) | 21.9 | 26.3 | 14.7 | <0.001 |
| Stroke (%) | 16.1 | 20.7 | 8.3 | <0.001 |
| Diabetes (%) | 28.6 | 31.7 | 23.4 | 0.019 |
| Lower extremity arthritis (%) | 56.4 | 52.3 | 63.4 | 0.004 |
| Pulmonary disease (%) | 41.1 | 44.2 | 35.9 | 0.029 |
| Six-minute walking distance (feet) | 1229 (433) | 1116 (397) | 1411 (429) | <0.001 |
| Four-meter normal walking velocity (meters/second) | 0.887 (0.22) | 0.854 (0.21) | 0.940 (0.21) | <0.001 |
| Four-meter fast walking velocity (meters/second) | 1.198 (0.30) | 1.151 (0.29) | 1.273 (0.28) | <0.001 |
| Summary performance score | 9.8 (2.5) | 9.5 (2.6) | 10.2 (2.4) | 0.002 |
Seventy-four percent of participants had activity monitor data. Participants with PAD who wore activity monitors had a lower prevalence of lower extremity arthritis (48.2% vs 61.5%, P =0.01) than participants without activity monitor data. Participants without PAD with activity monitor data had better SPPB scores (10.4 ± 2.1 vs 9.3 ± 3.1, P =.0.025), and included a higher prevalence of African Americans (22.0% vs 9.8%, P =0.049) and a higher prevalence of angina pectoris (22.3% vs 9.3%, P =0.045).
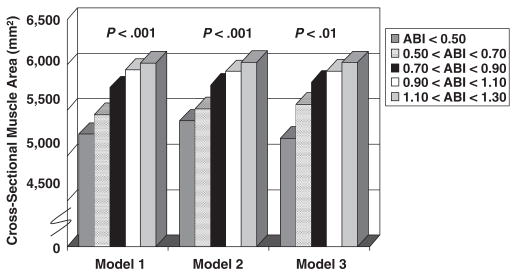
Within the entire cohort, lower ABI levels were associated with smaller calf muscle area (ABI<0.50, 5,193 mm2; ABI 0.50–0.90, 5,536 mm2; ABI 0.91–1.30, 5,941 mm2; P trend<.001), adjusting for age, sex, race, comorbidities, BMI, tibia length, recruitment cohort, lower extremity revascularization, and cigarette smoking. Lower ABI levels were associated with higher calf muscle percentage fat, adjusting for confounders (ABI<0.50, 12.8%; ABI 0.50–0.90, 11.4%; ABI 0.91–1.30, 9.2%, P trend =.02).
Figure 1 shows associations between ABI levels and adjusted muscle area in participants who wore activity monitors. These associations remained statistically significant even after additional adjustment for physical activity (P<.001) (Figure 1). Findings were not substantially changed after additional adjustment for calf muscle percentage fat (data not shown).
Figure 1.
Calf skeletal muscle area according to ankle brachial index (ABI) in men and women aged 59 and older who wore an activity monitor (n =523). Model 1 adjusts for age, sex, race, comorbidities, body mass index, tibia length, and smoking history. Model 2 adjusts for Model 1 covariates plus physical activity measured using a pedometer. Model 3 adjusts for Model 2 covariates plus physical activity measured using a Caltrac vertical accelerometer.
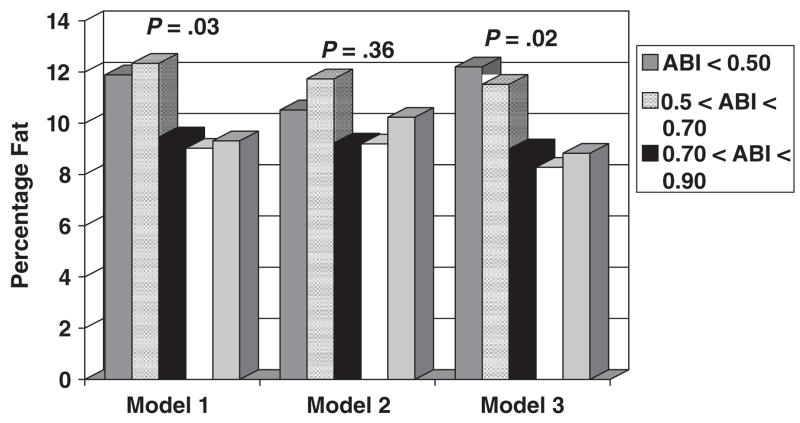
In participants who wore activity monitors, independent associations between lower ABI levels and higher calf muscle percentage fat remained statistically significant after additional adjustment for accelerometer scores (P =.02) but were not statistically significant after adjustment for pedometer-measured activity (Figure 2). When Figure 2 data were additionally adjusted for calf muscle area, associations between ABI and calf muscle percentage fat were significant for the model including adjustment for pedometer-measured physical activity (P =.043).
Figure 2.
Calf skeletal muscle percentage fat according to ankle brachial index (ABI) in men and women aged 59 and older who wore an activity monitor (n =523). Model 1 adjusts for age, sex, race, comorbidities, body mass index, and smoking history. Model 2 adjusts for Model 1 covariates plus physical activity measured using a pedometer. Model 3 adjusts for Model 2 covariates plus physical activity measured using a Caltrac vertical accelerometer.
Calf muscle area was also compared between the legs with higher and lower ABI in participants with PAD with significant discrepancies between their right and left leg ABI. In 92 participants with PAD with no history of lower extremity revascularization whose left and right leg ABI values differed by 0.20 or more, calf muscle area was lower in the leg with lower ABI than in the leg with higher ABI (5,283 ± 1,403 mm2 vs 5,511 ± 1,230 mm2, P =.001). In 198 participants with PAD with no history of lower extremity revascularization whose right and left leg ABI values differed by less than 0.20, there were no differences in calf muscle area between legs with higher and lower ABI (5,417 ± 1,452 mm2 vs 5,436 ± 1,479 mm2, respectively, P =.59).
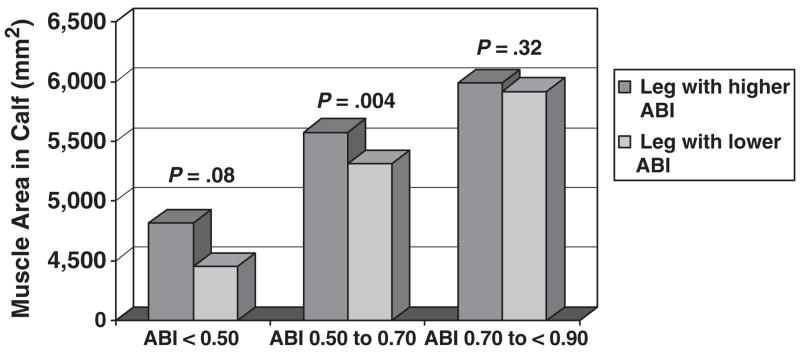
Figure 3 compares muscle area between the legs with higher and lower ABI in participants whose right and left leg ABI values differed by 0.20 or more, stratifying by the lower leg ABI. Muscle area differences between the legs with higher and lower ABI were greatest in participants with the lowest ABI levels (Figure 3), although these differences were statistically significant only for participants with lower leg ABI of 0.50 to 0.70.
Figure 3.
Calf skeletal muscle area in legs with higher versus lower ankle brachial index (ABI) values in participants with peripheral arterial disease with significant discrepancies in their left and right leg ankle brachial index values (n =92). *Data are categorized according to the leg with lower ABI. Includes only participants with peripheral arterial disease with no history of lower extremity revascularization.
Of the 92 nonrevascularized participants with PAD whose right and left leg ABIs differed by at least 0.20, calf muscle percentage fat was greater in the leg with lower ABI (11.4% ± 15.1% vs 9.47% ± 10.1%, P =.04). Differences in percentage fat between legs with lower and higher ABI values were greatest in participants with lower ABI values (data not shown). Of the 198 nonrevascularized participants with PAD whose right and left leg ABI values differed by less than 0.20, there were no differences in calf muscle percentage fat between the legs with higher and lower ABI (11.3% ± 12.4% vs 11.4% ± 12.3%, P =.69).
Table 2 shows associations between quintiles of calf muscle area and calf muscle percentage fat in the legs with higher and lower ABI and 6-minute walk performance in participants with PAD who wore pedometers. Adjusting for age, sex, race, BMI, smoking history, tibia length, ABI, leg symptoms, recruitment cohort, lower extremity revascularization, height, and activity level, calf muscle area in the leg with higher ABI was associated with significantly poorer performance on the 6-minute walk test (Table 2). After additional adjustment for calf muscle percentage fat, associations between quintiles of calf muscle area in the leg with higher ABI and lower extremity functioning were statistically significant for 6-minute walk performance (Table 2). Higher calf muscle percentage fat in the leg with higher ABI was associated with poorer performance on the 6-minute walk (Table 2). After additional adjustment for calf muscle area, associations between calf muscle percentage fat in the leg with higher ABI and 6-minute walk performance were not substantially changed (Table 2). Calf muscle characteristics in the leg with lower ABI were not associated significantly with 6-minute walk performance after adjusting for confounders (Table 2). These associations between lower calf muscle area in the leg with higher ABI and higher calf muscle percentage fat in the leg with higher ABI with poorer 6-minute walk performance were similar to those observed for other functional measures (i.e., usual- and fast-paced 4-meter walk and SPPB) (data not shown).
Table 2.
Adjusted Associations Between Calf Muscle Characteristics and Lower Extremity Performance in Persons with Peripheral Arterial Disease (n =268)
| Lower Extremity Performance for Each Muscle Area Quintile (mm2) |
Lower Extremity Performance for Each Fat Percentage Quintile (%) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Quintiles of Calf Muscle Characteristics | Not Adjusted for Percentage Fat in Calf | P trend | Adjusted for Percentage Fat in Calf | P trend | Not Adjusted for Muscle Area | P trend | Adjusted for Muscle Area | P trend |
| Mean 6-Minute Walk Distance, Feet (95% Confidence Interval) | ||||||||
| Muscle area of leg with higher ABI | ||||||||
| 1 | 971.7 (853–1,091) | .01 | 969.7 (852–1,087) | <.01 | 1,262 (1,174–1,349) | 0.02 | 1,269 (1,180–1,357) | .02 |
| 2 | 1,167 (1,068–1,266) | 1,179 (1,079–1,278) | 1,137 (1,048–1,226) | 1,160 (1,070–1,251) | ||||
| 3 | 1,163 (1,077–1,249) | 1,166 (1,081–1,251) | 1,117 (1,046–1,189) | 1,136 (1,063–1,209) | ||||
| 4 | 1,123 (1,031–1,216) | 1,159 (1,066–1,253) | 1,148 (1,066–1,230) | 1,163 (1,080–1,246) | ||||
| 5 | 1,271 (1,161–1,381) | 1,293 (1,183–1,403) | 1,049 (948–1,151) | 1,056 (954–1,157) | ||||
| Muscle area of leg with lower ABI | ||||||||
| 1 | 1,161 (1,053–1,270) | .69 | 1,235 (1,111–1,360) | .10 | 1,081 (989–1,173) | 0.94 | 1,088 (993–1,183) | .37 |
| 2 | 1,132 (1,037–1,226) | 1,160 (1,064–1,256) | 1,206 (1,127–1,284) | 1,199 (1,119–1,280) | ||||
| 3 | 1,111 (1,023–1,198) | 1,130 (1,042–1,218) | 1,139 (1,060–1,218) | 1,147 (1,068–1,226) | ||||
| 4 | 1,164 (1,073–1,254) | 1,150 (1,055–1,244) | 1,194 (1,109–1,279) | 1,210 (1,124–1,295) | ||||
| 5 | 1,129 (1,010–1,248) | 1,091 (964–1,219) | 1,094 (998–1,190) | 1,140 (1,031–1,248) | ||||
Note: Table includes only participants who wore pedometers. Analyses adjust for age, sex, race, ankle brachial index (ABI), leg symptoms, body mass index, recruitment cohort, lower extremity revascularization history, tibia length (only for muscle area), cigarette smoking, and steps walked over 7 days, measured using pedometer+height.
Quintiles of muscle cross-sectional area: 1:<4,528 mm2; 2: 4,528 to <5,105 mm2; 3: 5,105 to<5,756 mm2; 4: 5,756 to <6,650 mm2; 5: ≥6,650 mm2.
Quintiles of calf muscle percent fat: 1:<3.9%; 2: 3.9% to<5.4%; 3: 5.4% to <8.5%; 4: 8.5% to<13.6%; 5: ≥13.6%.
DISCUSSION
Results reported here show that lower ABI levels are associated with lower adjusted calf muscle cross-sectional area and higher adjusted calf muscle percentage fat. These associations were largely independent of differences in physical activity across the ABI spectrum. Furthermore, participants with PAD whose right and left leg ABIs differed by 0.20 or more had significantly lower calf muscle area and higher calf muscle percentage fat in the leg with lowest ABI than in the leg with higher ABI. Differences in calf muscle characteristics between the right and left legs were not observed in participants with PAD whose right and left leg ABIs differed by less than 0.20. These data provide evidence for a direct detrimental effect of ischemia on calf skeletal muscle, because the potential problem of incomplete adjustment for confounding variables should be eliminated by comparing legs in the same participant.
In persons with PAD, lower calf muscle area and higher percentage calf muscle were associated with poorer lower extremity functioning, independent of ABI, physical activity, and other confounders. Muscle characteristics in the leg with higher ABI, but not those corresponding to the leg with lower ABI, were significantly associated with lower extremity performance. This latter finding, not previously studied to the authors’ knowledge, suggests that the leg with higher ABI compensates for impairments in the leg with lower ABI and ultimately determines the degree of functional impairment. Findings reported here suggest that interventions to increase calf muscle area in patients with PAD should focus particularly on the leg with higher ABI.
Although supervised treadmill walking exercise improves treadmill walking performance in patients with PAD,25 less is known about the benefits of lower extremity resistance training for persons with PAD. A previous study randomized 29 men with claudication to 12 weeks of supervised treadmill exercise, lower extremity resistance training, or no exercise. Participants in both exercise groups improved their treadmill walking performance significantly more than the control group, although the treadmill-trained group achieved greater gains in treadmill walking than the strength training group.26 A separate study of 20 patients with PAD demonstrated that lower extremity resistance training increased calf muscle fibers and calf muscle capillary density at 24-week follow-up.27 Based on these studies and data presented here, further study is needed to determine the role of strength training in patients with PAD.
The degree of calf muscle percentage fat in the leg with higher ABI was associated with lower extremity performance independent of calf muscle area. One potential mechanism for this finding is that calf muscle percentage fat influences calf muscle quality. Furthermore, as calf muscle cells atrophy or die, fat cells may proliferate to replace calf muscle. This study did not measure subcutaneous fat, because the manual outlines of muscle images deliberately excluded subcutaneous fat. Additional study is necessary to identify the mechanism of the association between higher calf muscle percentage fat and impaired leg functioning.
This study had limitations. First, the study was cross-sectional. Associations reported here cannot be construed as causal. Second, a large number of potential participants did not respond to a mailed invitation, although rates of nonparticipation were comparable with those of other large epidemiological cohort studies involving older participants.21,28 Third, activity data were not available for 26% of participants. The findings may not be generalizable to participants who did not wear activity monitors. Finally, a ceiling effect for SPPB score, which has a maximum of 12, may limit the ability to identify significant associations between calf muscle characteristics and SPPB score.
CONCLUSION
In summary, lower ABI levels are associated with lower calf muscle cross-sectional area and higher calf muscle percentage fat. These ischemia-associated changes in calf muscle characteristics were associated with impaired leg functioning in participants with PAD. Muscle characteristics of the leg with higher ABI are more closely associated with the degree of functional impairment than muscle characteristics of the leg with lower ABI, suggesting that the leg with higher ABI compensates for the leg with lower ABI. Findings reported here support the hypothesis that direct, adverse effects of lower extremity ischemia on calf muscle area and percentage fat composition mediate PAD-related functional impairment in part.
Acknowledgments
Financial Disclosure: Supported by Grants R01-HL58099, R01-HL64739, and R01-HL71223 from the National Heart, Lung, and Blood Institute (NHLBI) and Grant RR-00048 from the National Center for Research Resources, National Institutes of Health (NIH). Supported in part by the Intramural Research Program, National Institute on Aging (NIA), NIH.
Mary McDermott receives salary support from the NHLBI and received an honorarium for educational activities from Bristol Meyers Squibb Sanofi Aventis. Frederick Hoff, Michael H. Criqui, William H. Pearce, Lu Tian, Kiang Liu, and Jin Tan receive salary support from the NHLBI. Luigi Ferrucci and Jack M. Guralnik are employed by the NIA. Leena Sharma receives salary support from the National Institute of Arthritis and Musculoskeletal Diseases.
Sponsors’ Role: The funding agency played no role in the study design, methods, subject recruitment, data collection, data analyses, or paper preparation.
Footnotes
Author Contributions: Jack M. Guralnik, Mary Mc-Dermott, Frederick Hoff, Michael H. Criqui, Luigi Ferrucci, and Kiang Liu: study conception and design. Leena Sharma, William H. Pearce, Joseph R. Schneider, Mary McDermott, Frederick Hoff, Michael H. Criqui, Luigi Ferrucci, and Kiang Liu: acquisition of subjects and data. Lu Tian, Jin Tan, William H. Pearce, Jack M. Guralnik, Joseph R. Schneider, Mary McDermott, Frederick Hoff, Michael H. Criqui, Luigi Ferrucci, and Kiang Liu: data analysis and interpretation. Lu Tian, Leena Sharma, Jin Tan, William H. Pearce, Jack M. Guralnik, Joseph R. Schneider, Mary McDermott, Frederick Hoff, Michael H. Criqui, Luigi Ferrucci, and Kiang Liu: manuscript preparation.
References
- 1.McDermott MM, Greenland P, Liu K, et al. Leg symptoms commonly reported by men and women with lower extremity peripheral arterial disease: Associated clinical characteristics and functional impairment. JAMA. 2001;286:1599–1606. doi: 10.1001/jama.286.13.1599. [DOI] [PubMed] [Google Scholar]
- 2.McDermott MM, Liu K, Greenland P, et al. Functional decline in peripheral arterial disease: Associations with the ankle brachial index and leg symptoms. JAMA. 2004;292:453–461. doi: 10.1001/jama.292.4.453. [DOI] [PubMed] [Google Scholar]
- 3.Newman AB, Siscovick DS, Manolio TA, et al. Ankle-arm index as a marker of atherosclerosis in the Cardiovascular Health Study. Circulation. 1993;88:837–845. doi: 10.1161/01.cir.88.3.837. [DOI] [PubMed] [Google Scholar]
- 4.McDermott MM, Liu K, Criqui MH, et al. Ankle-brachial index and subclinical cardiac and carotid disease. The Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol. 2005;162:33–41. doi: 10.1093/aje/kwi167. [DOI] [PubMed] [Google Scholar]
- 5.Bernstein EF, Fronek A. Current status of non-invasive tests in the diagnosis of peripheral arterial disease. Surg Clin North Am. 1982;62:473–487. doi: 10.1016/s0039-6109(16)42739-8. [DOI] [PubMed] [Google Scholar]
- 6.Hiatt WR. Medical treatment of peripheral arterial disease and claudication. N Engl J Med. 2001;344:1608–1621. doi: 10.1056/NEJM200105243442108. [DOI] [PubMed] [Google Scholar]
- 7.Shadman R, Criqui MH, Bundens WP, et al. Subclavian artery stenosis: Prevalence, risk factors, and association with cardiovascular diseases. J Am Coll Cardiol. 2004;44:618–623. doi: 10.1016/j.jacc.2004.04.044. [DOI] [PubMed] [Google Scholar]
- 8.Lindbom A. Arteriosclerosis and arterial thrombosis in the lower limb: A roentgenological study. Acta Radiol Suppl. 1950;80:1–80. [PubMed] [Google Scholar]
- 9.Hyvarinen S. Arteriographic findings of claudication patients. Ann Clin Res. 1984;16:1–45. [PubMed] [Google Scholar]
- 10.Rose GA. The diagnosis of ischemic heart pain and intermittent claudication in field surveys. Bull World Health Org. 1962;27:645–658. [PMC free article] [PubMed] [Google Scholar]
- 11.McDermott MM, Guralnik JM, Albay M, et al. Impairments of muscles and nerves associated with peripheral arterial disease and their relationship with lower extremity functioning: The InCHIANTI Study. J Am Geriatr Soc. 2004;52:405–410. doi: 10.1111/j.1532-5415.2004.52113.x. [DOI] [PubMed] [Google Scholar]
- 12.Mitsiopoulos N, Baumgartner RN, Heymsfield SB, et al. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol. 1999;86:1097–1098. doi: 10.1152/jappl.1998.85.1.115. [DOI] [PubMed] [Google Scholar]
- 13.Montgomery PS, Gardner AW. The clinical utility of a six-minute walk test in peripheral arterial occlusive disease patients. J Am Geriatr Soc. 1998;46:706–711. doi: 10.1111/j.1532-5415.1998.tb03804.x. [DOI] [PubMed] [Google Scholar]
- 14.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 15.Guralnik JM, Ferrucci L, Simonsick E, et al. Lower extremity function in persons over 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McDermott MM, Liu K, O’Brien E, et al. Measuring physical activity in peripheral arterial disease: A comparison of two physical activity questionnaires with an accelerometer. Angiology. 2000;51:91–100. doi: 10.1177/000331970005100201. [DOI] [PubMed] [Google Scholar]
- 17.McDermott MM, Ohlmiller SM, Liu K, et al. Gait alterations associated with walking impairment in people with peripheral arterial disease with and without intermittent claudication. J Am Geriatr Soc. 2001;49:747–754. doi: 10.1046/j.1532-5415.2001.49151.x. [DOI] [PubMed] [Google Scholar]
- 18.Crouter SE, Schneider PL, Karabulut M, et al. Validity of 10 electronic pedometers for measuring steps, distance, and energy cost. Med Sci Sports Exerc. 2003;35:1455–1460. doi: 10.1249/01.MSS.0000078932.61440.A2. [DOI] [PubMed] [Google Scholar]
- 19.Welk GJ, Differding JA, Thompson RW, et al. The utility of the Digi-Walker step counter to assess daily physical activity patterns. Med Sci Sports Exerc. 2000;32:S481–S488. doi: 10.1097/00005768-200009001-00007. [DOI] [PubMed] [Google Scholar]
- 20.Criqui MH, Denenberg JO, Bird CE, et al. The correlation between symptoms and non-invasive test results in patients referred for peripheral arterial disease testing. Vasc Med. 1996;1:65–71. doi: 10.1177/1358863X9600100112. [DOI] [PubMed] [Google Scholar]
- 21.Guralnik JM, Fried LP, Simonsick EM, et al. The Women’s Health and Aging Study: Health and Social Characteristics of Older Women with Disability (NIH Publication no. 95–4009. Bethesda, MD: National Institute on Aging; 1995. [Google Scholar]
- 22.Altman R, Alarcon G, Appelrouth D, et al. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hip. Arthritis Rheum. 1991;34:505–514. doi: 10.1002/art.1780340502. [DOI] [PubMed] [Google Scholar]
- 23.Altman R, Asch E, Bloch D, et al. Development of criteria for the classification and reporting of osteoarthritis. Arthritis Rheum. 1986;29:1039–1049. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 24.Ruff C. Allometry between length and cross-sectional dimensions of the femur and tibia in Homo Sapiens. Am J Phys Antrhopol. 1984;65:347–358. doi: 10.1002/ajpa.1330650403. [DOI] [PubMed] [Google Scholar]
- 25.Leng GC, Fowler B, Ernst E. Exercise for intermittent claudication. Cochrane Database Syst Rev. 2000;(2):CD000990. doi: 10.1002/14651858.CD000990. [DOI] [PubMed] [Google Scholar]
- 26.Hiatt WR, Wolfel EE, Meier RH, et al. Superiority of treadmill walking exercise versus strength training for patients with peripheral arterial disease. Circulation. 1994;90:1866–1874. doi: 10.1161/01.cir.90.4.1866. [DOI] [PubMed] [Google Scholar]
- 27.McGuigan MR, Bronks R, Newton RU, et al. Resistance training in patients with peripheral arterial disease: Effects on myosin isoforms, fiber type distribution, and capillary supply to skeletal muscle. J Gerontol A Biol Sci Med Sci. 2001;56A:B302–B310. doi: 10.1093/gerona/56.7.b302. [DOI] [PubMed] [Google Scholar]
- 28.Tell GS, Fried LP, Hermanson B, et al. Recruitment of adults 65 years and older as participants in the Cardiovascular Health Study. Ann Epidemiol. 1993;3:358–366. doi: 10.1016/1047-2797(93)90062-9. [DOI] [PubMed] [Google Scholar]