Abstract
Context
Despite a lack of data, increasing numbers of patients are receiving primary androgen deprivation therapy (PADT) as an alternative to surgery, radiation or conservative management for the treatment of localized prostate cancer.
Objective
Evaluate the association between PADT and survival in elderly men with localized prostate cancer.
Main Outcome Measures
Cancer-specific and overall survival.
Design
Population-based cohort study of 19,271 men who did not receive definitive local therapy for T1-T2 prostate cancer. Instrumental variable analysis was used to address potential biases associated with unmeasured confounding variables.
Setting
Medicare patients aged ≥66 years diagnosed in 1992-2002 within predefined US geographical areas.
Results
Even though 41% of the population (median age 77) received PADT, PADT was associated with somewhat worse 10-year prostate cancer-specific survival (80.1% vs. 82.6%, hazard ratio [HR] 1.17; 95% CI 1.03–1.33) and no improvement in 10-year overall survival (30.2% vs. 30.3%,, HR 1.00; 95% CI 0.96–1.05) compared to conservative management. However, in a pre-specified subset analysis, PADT use in men with poorly-differentiated cancer was associated with marginally improved prostate cancer-specific survival (HR 0.84; 95% CI 0.70–1.00, P =0.05) but not overall survival (HR 0.92; 95% CI 0.84 – 1.01).
Conclusions
Primary androgen deprivation therapy is not associated with improved survival among the majority of elderly men with localized prostate cancer when compared with conservative management.
Keywords: prostatic neoplasm, Medicare, SEER program, antineoplastic agents, hormonal
Introduction
Among men, prostate cancer is the most common non-skin cancer and the second most common cause of cancer death.1 For the great majority of men with incident prostate cancer (~85%), disease is diagnosed at localized (T1-T2) stages,2 and standard treatment options include surgery, radiation or conservative management (ie., deferral of treatment until necessitated by disease signs or symptoms).
Although not standard or sanctioned by major groups or guidelines, a growing number of healthcare providers and patients have turned to primary androgen deprivation therapy (PADT) as an alternative to surgery, radiation or conservative management, especially among older men.3, 4 For example, in a 1999–2001 survey, PADT had become the second most common treatment approach, after surgery, for localized prostate cancer.3
Randomized clinical trials support the use of early androgen deprivation therapy as an adjunct to surgery or radiation for patients with high-risk cancer.5–10 In one study, early ADT reduced mortality by ~50% when used with radiation in high-risk disease (poorly-differentiated T1-T2, or T3-T4)5, 8 while in another study mortality was reduced by ~60% in patients with nodal disease identified at surgery.9 Consequently, many investigators have concluded that the early use of ADT is appropriate for patients with higher- or intermediate-risk disease in conjunction with local therapy, but studies that assess the use of ADT alone, as primary therapy, or in lower-risk settings are sparse.
The importance of determining the appropriate application of androgen deprivation therapy has recently increased in urgency, as a growing body of literature now demonstrates that chronic ADT use has been associated with ~10–50% increases in the risks of fracture, diabetes, coronary heart disease, myocardial infarction and sudden cardiac death, in addition to adverse effects on fat mass, cholesterol, and quality of life.11–16 There were 500% increases in the risk of gynecomastia and hot flashes in the Prostate Cancer Outcomes Study (PCOS), and a 267% increase in impotence was observed after one year of treatment.17 Finally, medical ADT is costly. The costs associated with ADT medication use in the US exceeded 1 billion dollars in 2001 and ADT drugs represented the second highest Medicare Part B drug expenditure.18
A randomized clinical trial would provide the data needed to determine the utility of PADT vs. conservative management in localized disease. Unfortunately, such a trial would take more than a decade to complete and, given current treatment practices and resources, would probably not be feasible. Observational studies are often employed to provide insight under such circumstances, although they may be more subject to biases.
Recently instrumental variable analytical (IVA) techniques have been applied successfully to observational medical studies19, 20 to minimize many of these biases so that the results of randomized clinical trials may often be mimicked with observational data.21 Instrumental variable analysis is a method of capturing the random component of patient treatment choice and using it to balance treatment groups with respect to measured and unmeasured confounders. We used this approach to assess the association between PADT and disease-specific and overall survival in men with T1-T2 prostate cancer.
Materials and Methods
Data sources
Data for this study were obtained from the population-based Surveillance, Epidemiology and End Results (SEER) program database and linked Medicare files. The SEER regions encompass approximately 14% of the US population before 2001 and 26% thereafter. The Medicare database covers approximately 97% of US persons aged ≥65 years and linkage to the SEER database was complete for approximately 93% of the patients.22 Informed consent was waived by the IRB because the data did not contain personal identifiers.
Study participants
The study cohort consisted of men (aged ≥66 years) who were SEER residents and diagnosed with T1-T2 cancer in 1992-2002 (N = 89,877). Men who died within 180 days of diagnosis were excluded (N = 1,761) (inclusion of patients dying within 180 days did not significantly alter the results). Those receiving definitive local therapy (eg., prostatectomy or radiation) within 180 days of diagnosis were also excluded (N = 31,485). To ensure that the database accurately documented the patient’s clinical course, patients without both Medicare Part A (hospitalization) and Part B (physician and outpatient) as their primary health insurance coverage during the study period were excluded (N=33,987). Those with missing data (N=2,995), unknown cancer grade (N=255) or initiation of ADT before cancer diagnosis (N=123) were also excluded.
Primary androgen deprivation therapy
Patients undergoing PADT received androgen deprivation therapy as primary cancer therapy (eg., no surgery or radiation) in the first 180 days following diagnosis. Conservative management patients were those that did not receive surgery, radiation or PADT during this time. A previous study demonstrated that patients generally start primary therapy within 6 months of diagnosis.23 Utilizing a previously described algorithm,11 Medicare physician, inpatient and outpatient claims were used to identify orchiectomy (Healthcare Common Procedure Coding System [HCPCS] codes 54520, 54521, 54522, 54530 or 54535 or ICD-9 code 624) and the use of luteinizing hormone releasing hormone (LHRH) agonists (HCPCS codes J1950, J9202, J9217, J9218, or J9219). LHRH agonists and orchiectomy were combined because previous studies have shown these treatments to be essentially equivalent.24
Study endpoints and covariates
Overall and prostate cancer-specific survival was available through December 31, 2006 and December 31, 2004, respectively. Underlying cause of death was determined from data in the SEER records. Studies have shown that cause of death in the SEER data confirm information available in medical records in 87 to 88% of cases.25, 26
Cox model covariates included age at diagnosis, race, zip code income, SEER region, urban area, marital status, cancer grade, clinical T stage, Charlson comorbidity score, and year of diagnosis. Charlson score, a powerful predictor of longevity in men with localized prostate cancer27–29 was derived from Medicare claims during the year prior to prostate cancer diagnosis using a validated algorithm.30, 31 For cancer grade, Gleason 2–4, 5–7 and 8–10 corresponded to well-differentiated, moderately-differentiated, and poorly-differentiated disease, respectively. We used clinical extension information provided by SEER to determine cancer stage (T1, T2).
Instrumental variable analysis
A health service area (HSA) is defined as one or more counties that are relatively self-contained with respect to the provision of routine hospital care.32 The instrumental variable was constructed by first calculating the proportion of patients who received PADT in each HSA. Because some HSAs had small numbers of prostate cancer cases, each HSA with <50 cases was combined with the nearest (in terms of distance between geographic centers) HSA with ≥50 cases. The threshold of ≥50 cases was chosen because lower thresholds were associated with more imbalances in patient characteristics in high- and low-PADT utilization areas. The algorithm produced 66 utilization areas. High- and low-use areas corresponded to the top and bottom tertiles of PADT utilization and were used as the (binary) instrumental variable for the (binary) treatment assignment.
Previous studies have demonstrated that PADT use is highly influenced by non-medical factors,33 with tumor characteristics accounting for only 9.7% of the total variance in use.34 Our data confirmed that PADT use varied widely across HSAs, a key requirement of an instrumental variable. An instrumental variable must influence outcomes primarily through its correlation with treatment status and not through any other independent effect. We verified this assumption by comparing baseline characteristics including age at diagnosis, cancer stage and grade at diagnosis, and found them comparable between low- and high-PADT areas.
Statistical analyses
Instrumental variable analytical (IVA) methods based on the Rubin Causal Model (RCM) 21 were used to account for both measured and unmeasured (e.g., PSA, family history, diet, weight, etc.) confounders. Covariates in the IVA models included age, race, comorbidity status, cancer stage, cancer grade, income status, urban residence, marital status, and year of diagnosis. All IVA results were derived from the same models. We examined all the required assumptions to ensure the validity of our instrumental variable analysis. Traditional Cox proportional hazards model results were also reported in Table 3 for comparison to the IVA results. Analyses were conducted using SAS version 9.1 and R version 2.7.0.(R foundation for statistical Computing, Vienna, Austria). Cancer grade was a predefined measured covariate. We calculated PADT utilization for each cancer grade so that it was not necessary to assume that the patterns of PADT utilization were the same for all cancer grades within the same area. Results for well-differentiated cancer (Gleason 2–4) are not shown separately because results were unstable due to the limited sample size. Patients that differ in the likelihood of receiving PADT are compared and the treatment effect on the “marginal” population is estimated. The marginal effect (ie., “local average treatment effect”)21 of PADT was calculated as
Table 3.
Risk of mortality according to cancer grade and treatment
| Conventional Cox multivariate results† | ||||||
|---|---|---|---|---|---|---|
| PADT | Conservative management | Unadjusted HR‡ | Adjusted HR‡ (95% CI) | |||
| Events/py⊦ | Rate per 100 | Events/py⊦ | Rate per 100 | |||
| Prostate cancer-specific mortality | ||||||
| All grades | 867/32,744 | 2.6 | 693/55,424 | 1.3 | 2.12 | 1.76 (1.59–1.95)|| |
| Moderately-differentiated | 381/22,181 | 1.7 | 389/46,992 | 0.8 | 2.07 | 1.83 (1.58–2.12)|| |
| Poorly-differentiated | 483/10,196 | 4.7 | 284/6,848 | 4.1 | 1.14 | 1.12 (0.96–1.29) |
| Overall mortality | ||||||
| All grades | 4,729/39,767 | 11.9 | 6,316/66,567 | 9.5 | 1.25 | 1.17 (1.12–1.21)|| |
| Moderately-differentiated | 2,832/27,251 | 10.4 | 4,989/56,938 | 8.8 | 1.19 | 1.15 (1.10–1.21)|| |
| Poorly-differentiated | 1,849/12,115 | 15.3 | 1,145/7,903 | 14.4 | 1.05 | 1.04 (0.97–1.13) |
| IVA results† | ||||||
| High-use | Low-use | Unadjusted HR‡ | Adjusted HR‡ (95% CI) | |||
| Events/py⊦ | Rate per 100 | Events/py⊦ | Rate per 100 | |||
| Prostate cancer-specific mortality | ||||||
| All grades | 504/25,911 | 1.9 | 581/33,578 | 1.7 | 1.12 | 1.17 (1.03–1.33)|| |
| Moderately-differentiated | 245/17,568 | 1.4 | 315/29,937 | 1.1 | 1.33 | 1.43 (1.20–1.70)|| |
| Poorly-differentiated | 238/5,557 | 4.3 | 299/5,835 | 5.1 | 0.84 | 0.84 (0.70–1.00)|| |
| Overall mortality | ||||||
| All grades | 3,360/32,393 | 10.4 | 4,202/39,075 | 10.8 | 0.96 | 1.00 (0.96–1.05) |
| Moderately-differentiated | 2,098/22,332 | 9.4 | 3,425/35,397 | 9.7 | 0.97 | 1.01 (0.95–1.07) |
| Poorly-differentiated | 976/6,601 | 14.8 | 1,084/6,745 | 16.1 | 0.92 | 0.92 (0.84–1.01) |
Covariates included age, race, comorbidity status, cancer stage, cancer grade, income quartiles, urban residence, SEER region, marital status, and year of diagnosis. SEER region was not included in the instrumental variable analysis.
Hazard ratio
Person-year
P <0.05
where,
Hi = a geographic area in the upper tertile of PADT use
Lo = a geographic area in the lower tertile of PADT use
The terms are thus,
| Pr(PADT | Hi/Low) = | estimated probability of PADT use among men who had localized prostate cancer and did not have surgery or radiation as their primary cancer therapy in high/low use region. |
| Adjusted Outcomes Hi/Adjusted outcomesLo = | estimated survival probability at a particular time (eg., 5- or 10-year) among men who had localized prostate cancer and did not have surgery or radiation as their primary cancer therapy in high/low use region. |
The terms are thus,
To compute the population-adjusted survival curves, we substituted the population means (for continuous covariates) into the proportional hazards model for each combination of the categorical covariates to derive an adjusted hazard function. Then a weighted average of these adjusted hazard functions was computed with weights proportional to the numbers of subjects in each class. Finally, the population-adjusted survival curve was computed from the weighted hazard function.35 Estimates of 5- and 10-year overall and cancer-specific survival for men at average risk were derived from these adjusted curves. Confidence intervals were obtained by computing these adjusted survival curves for each of 10,000 bootstrap samples of the original data. P values and 95% confidence intervals were derived from the bootstrap estimates. Testing was 2-sided with an alpha level of 5%. Analyses were repeated for different age groups but results were similar across age groups and the interaction between age and PADT use was not significant; therefore, all age groups were combined.
Power calculations for determining the difference in survival between high- and low-use health service areas were carried out using simulations. Overall, the study had 80% power to detect a 7% difference in overall survival between high- and low-use PADT areas.
Results
Baseline characteristics
The total cohort consisted of 19,271 men aged ≥66 years with localized prostate cancer diagnosed in 1992-2002. By definition, none of these men received definitive local therapies (eg., radiation or surgery) in the first 180 days following diagnosis; 41% received PADT. The median age of the study cohort was 77 years and the median follow-up for overall survival was 81 months. As expected, patients receiving PADT and patients managed conservatively differed in many characteristics suggesting that there could be differences in unmeasured characteristics that might not be adjusted for by conventional statistical methods (Table 1).
Table 1.
Characteristics of the study cohort
| Characteristic | Primary androgen deprivation therapy (PADT) N=7,867 | Conservative management N=11,404 |
|---|---|---|
| Demographic characteristics | ||
| Median age (interquartile range) | 79 (74–83) | 77 (72–81) |
| African-American | 758 (9.6%) | 1,307 (11.5%) |
| Married at diagnosis | 4,911 (62.4%) | 7,302 (64.0%) |
| Urban residence | 6,299 (80.1%) | 9,411 (82.5%) |
| Median income (interquartile range) | $42,890 (33,861–57,468) | $44,022 (34,214–57,983) |
| SEER regions | ||
| Northeast | 840 (10.7%) | 964 (8.5%) |
| North-central | 1,984 (25.2%) | 3,134 (27.5%) |
| West | 4,816 (61.2%) | 6,903 (60.5%) |
| South | 227 (2.9%) | 403 (3.5%) |
| Disease characteristics | ||
| Cancer grade | ||
| Well-differentiated | 64 (0.8%) | 244 (2.1%) |
| Moderately-differentiated | 5,115 (65.0%) | 9,545 (83.7%) |
| Poorly-differentiated | 2,688 (34.2%) | 1,615 (14.2%) |
| Clinical stage at diagnosis | ||
| T1 | 3,915 (49.8%) | 7,325 (64.2%) |
| T2 | 3,952 (50.2%) | 4,079 (35.8%) |
| Comorbidity status | ||
| Charlson comorbidity score 0–1 | 7,446 (94.7%) | 10,664 (93.5%) |
| Charlson comorbidity score ≥2 | 421 (5.3%) | 740 (6.5%) |
| Year of cancer diagnosis | ||
| 1992–1997 | 2,876 (36.6%) | 5,348 (46.9%) |
| 1998–2002 | 4,991 (63.4%) | 6,056 (53.1%) |
PADT utilization (Table 2) varied widely across health service areas (31% to 53%). When we extended the window for defining PADT from 180 days to 18 months, the high/low use patterns remained the same (Table 2). Duration of use was also longer in high-PADT use areas.
Table 2.
Characteristics of men with localized prostate cancer in high-and low-use PADT health service areas
| Moderately-differentiated | Poorly-differentiated | All localized cancers | ||||
|---|---|---|---|---|---|---|
| High N=4,278 | Low N=5,898 | High N=1,433 | Low N=1,478 | High N=6,302 | Low N=6,673 | |
| PADT within 180 days | 2,058 (48.1%) | 1,502 (25.5%) | 1,058 (73.8%) | 757 (51.2%) | 3,321 (52.5%) | 2,041 (30.6%) |
| PADT within 18 months | 2,067 (48.3%) | 1,652 (28.0%) | 1,081 (75.6%) | 815 (55.1%) | 3,396 (53.9%) | 2,247 (33.7%) |
| Mean duration of PADT use (months; standard deviation) | 21 (30) | 16 (29) | 34 (34) | 26 (33) | 24 (31) | 19 (31) |
| Median age at diagnosis (years; interquartile range) | 78 (73–82) | 77 (72–81) | 79 (75–83) | 79 (74–83) | 78 (74–82) | 77 (73–81) |
| Median zip-level income (interquartile range) | $41,284 (33,816–56, 958) | $44,939 (34,490–56,912) | $42,545 (35,091–59,722) | $41,580 (31,166–52,645) | $42,408 (34,382–60,669) | $44,107 (33,176–56,041) |
| Mean Charlson score (standard deviation) | 0.22 (0.63) | 0.22 (0.64) | 0.19 (0.58) | 0.22 (0.64) | 0.21 (0.61) | 0.22 (0.64) |
| Clinical stage T1 | 2,619 (61.2%) | 3,742 (63.5%) | 698 (48.7%) | 739 (50.0%) | 3,651 (57.9%) | 3,901 (58.5%) |
Survival outcomes
There were 1,560 prostate cancer deaths and 11,045 deaths from all causes in the study cohort. Unadjusted and adjusted prostate cancer-specific and overall survival were worse for PADT treated patients when analyses were conducted using a traditional Cox multivariate model (Table 3). The Cox approach, however, is unable to adjust for unmeasured confounders and selection biases (eg., higher risk patients may be preferentially selected for PADT thus yielding apparently adverse outcomes for this group). When instrumental variable analysis was utilized (Tables 3, 4, 5 and Figure 1), PADT was still associated with increased unadjusted and adjusted cancer-specific mortality (HR 1.17, 95% CI 1.03–1.33), but there was not associated effect on unadjusted, and adjusted median overall survival (82 months vs. 82 months, HR 1.00, 95% CI 0.96–1.05). Results were similar when analyses were restricted to men with comorbidity scores of 0 or without other cancers, suggesting that the results were independent of comorbidity.
Table 4.
Adjusted percentage overall and prostate cancer-specific survival in high- and low-use areas with 95% bootstrapped confidence intervals†
| Characteristic | High PADT use | Low PADT use | High-Low Difference | 95% CI | ||
|---|---|---|---|---|---|---|
| Deaths/person-year | % adjusted survival | Deaths/person-year | % adjusted survival | |||
| Cancer-specific survival | ||||||
| 5-year all grades | 378/21,757 | 91.6 | 408/25,386 | 92.7 | −1.1 | −2.1 to −0.1 |
| 5-year moderately | 180/14,759 | 93.8 | 196/22,689 | 95.7 | −1.9 | −2.8 to −0.9 |
| 5-year poorly | 190/4,782 | 79.2 | 248/4,863 | 75.8 | 3.4 | 0.01 to 11.1 |
| 10-year all grades | 500/25,709 | 80.1 | 570/33,022 | 82.6 | −2.5 | −4.7 to −0.3 |
| 10-year moderately | 242/17,430 | 83.1 | 308/29,456 | 88.0 | −4.9 | −7.4 to −2.4 |
| 10-year poorly | 237/5,529 | 59.8 | 298/5,783 | 54.3 | 5.5 | 0.01 to 11.1 |
| Overall survival | ||||||
| 5-year all grades | 2,349/25,444 | 62.0 | 2,632/26,987 | 62.1 | −0.1 | −1.5 to 1.3 |
| 5-year moderately | 1,456/17,558 | 65.5 | 2,085/24,459 | 65.9 | −0.3 | −1.9 to 1.3 |
| 5-year poorly | 717/5,376 | 47.3 | 823/5,281 | 44.9 | 2.4 | −0.7 to 5.6 |
| 10-year all grades | 3,238/31,770 | 30.2 | 3,949/37,499 | 30.3 | −0.1 | −1.8 to 1.6 |
| 10-year moderately | 2,012/21,914 | 33.9 | 3,211/33,971 | 34.4 | −0.4 | −2.5 to 1.7 |
| 10-year poorly | 953/6,518 | 17.3 | 1,054/6,598 | 15.3 | 2.0 | −0.6 to 4.6 |
Covariates included age, race, comorbidity status, cancer stage, cancer grade, income quartiles, urban residence, marital status, and year of diagnosis. Because date of last follow-up differed for overall (December 31, 2006) and cancer-specific survival (December 31, 2004), the number of person-years of follow-up differs for each endpoint.
Table 5.
Marginal effect of PADT on survival: percentage difference in overall and prostate cancer-specific survival in high- and low-use areas with 95% bootstrapped confidence intervals
| 5-year survival | 10-year survival | |||||
|---|---|---|---|---|---|---|
| Risk difference† (A) | Difference in PADT use‡ (B) | Marginal effect (A/B) | Risk difference† (A) | Difference in PADT use‡ (B) | Marginal effect (A/B) | |
| Disease specific survival | ||||||
| All localized cancer | −1.1% | 21.9% | −5.0 (−9.4 to −0.6) | −2.5% | 21.9% | −11.2 (−21.2 to –1.3) |
| Moderately-differentiated | −1.9% | 22.6% | −8.3 (−12.5 to −4.1) | −4.9% | 22.6% | −21.7 (−32.8 to –10.8) |
| Poorly-differentiated cancer | 3.4% | 22.6% | 15.1 (0.0 to 30.5) | 5.5% | 22.6% | 24.5 (0.1 to 49.6) |
| Overall survival | ||||||
| All localized cancer | −0.1% | 21.9% | −0.4 (−6.7 to 6.0) | −0.1% | 21.9% | −0.5 (−8.1 to 7.3) |
| Moderately-differentiated | −0.3% | 22.6% | −1.5 (−8.5 to 5.6) | −0.4% | 22.6% | −2.0 (−11.2 to 7.4) |
| Poorly-differentiated cancer | 2.4% | 22.6% | 10.7 (−3.1 to 24.8) | 2.0% | 22.6% | 8.8 (−2.5 to 20.4) |
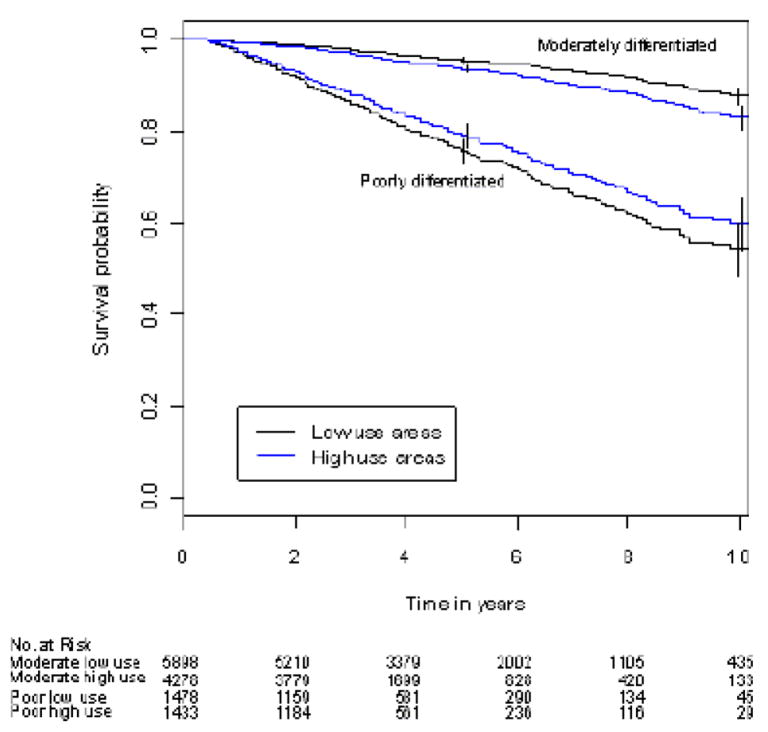
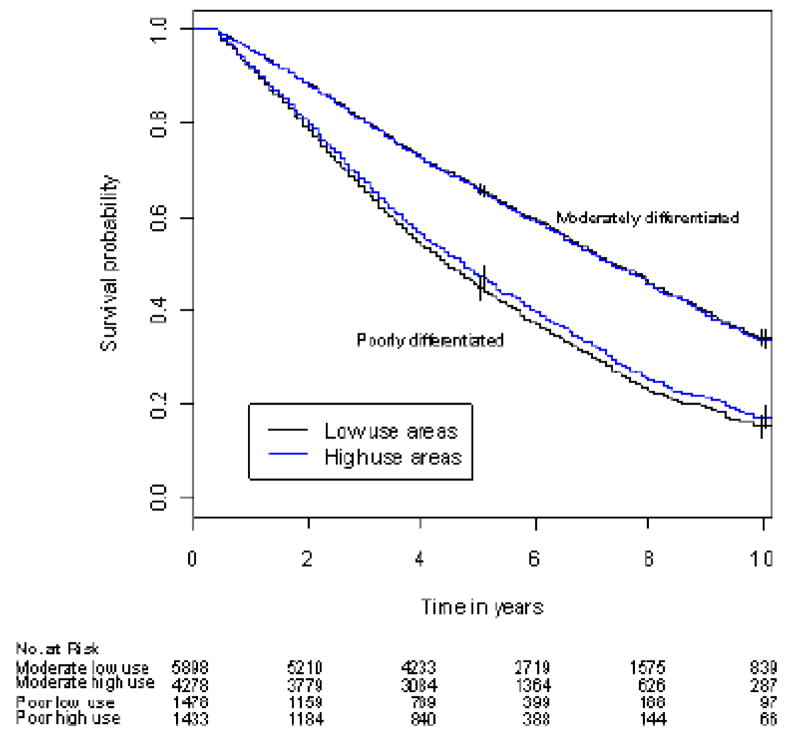
Figure 1.
Figure 1A. Adjusted prostate cancer-specific survival in high- and low-use areas by cancer grade
Results were adjusted for age, race, income, marital status, urban residence, comorbidity status, year of diagnosis and cancer stage. Prostate cancer-specific survival was lower in high-PADT use areas compared with low-PADT use areas among men with moderately-differentiated cancer (P <0.001). Prostate cancer-specific survival was borderline statistically different between high- and low-PADT use areas among men with poorly-differentiated cancer (P =0.0498). Error bars represent 95% confidence intervals.
Figure 1B. Adjusted overall survival in high- and low-use areas by cancer grade
Overall survival was similar in high- and low-PADT use areas among men with moderately-differentiated cancer; median overall survival was 89 and 90 months for high- and low-use areas (P =0.671). Differences in overall survival between high- and low-PADT use areas among men with poorly-differentiated cancer did not reach statistical significance (P =0.127). Median overall survival was 57 and 54 months for high- and low-PADT use areas. The difference in median overall survival between high- and low-PADT use areas was 3 months (95% CI –1 to 7 months).
In pre-planned analyses by cancer grade, PADT was associated with either no effect, or an adverse effect on prostate cancer-specific and overall survival for poorly- or moderately-differentiated cancer, respectively, in unadjusted and adjusted Cox analyses. Evaluation by IVA, however, revealed a borderline improvement in unadjusted and adjusted median prostate cancer-specific survival in patients with poorly-differentiated cancer (Table 3, Figure 1) although the associated effect on median overall survival was not significant (57 vs. 54 months [95% C.I. 56 –61 and 53 – 57, respectively]). Similar patterns were observed for 5- and 10-year prostate cancer-specific and overall survival (Table 4). The marginal effect of PADT on cancer-specific survival was 15.1% at 5-years and 24.5% at 10 years for men with poorly-differentiated cancer (Table 5). Similar benefit, however, was not observed in men with moderately-differentiated cancer (Table 5).
Duration of treatment and survival
Most patients received PADT for extended periods. Among PADT users, only 1.1% received one month of treatment while 75% received PADT for at least 18 months and 50% received PADT for more than 30 months. Longer durations of PADT utilization were associated with lower overall and cancer-specific survival among 5,826 PADT users who survived at least 3 years (Table 6). Similar patterns were observed for all cancer grades. Sensitivity analyses restricted to patients with comorbidity scores of 0 yielded similar results.
Table 6.
Adjusted hazard ratios for overall and cancer-specific mortality among 5,826 PADT patients surviving at least 3 years
| Duration of PADT | Disease-specific mortality rate per 100 (deaths/person-year) by cancer grade | ||
|---|---|---|---|
| Moderately-differentiated | Poorly-differentiated | All localized | |
| ≤12 months | 0.39 (16/4,056) | 0.72 (7/969) | 0.45 (23/5,103) |
| 13–36 months | 1.24 (199/16,031) | 2.32 (179/7,700) | 1.58 (379/23,988) |
| Adjusted HR (95% C.I.)‡ | 2.22 (1.32, 3.74) | 2.44 (1.14, 5.23) | 2.42 (1.58, 3.72) |
| Duration of PADT | Overall mortality rate per 100 (deaths/person year) by cancer grade | ||
| Moderately-differentiated | Poorly-differentiated | All localized | |
| ≤12 months | 4.40 (242/5,498) | 5.42 (73/1,347) | 4.61 (320/6,934) |
| 13–36 months | 7.43 (1,457/19,613) | 9.70 (895/9,227) | 8.18 (2,381/29,119) |
| Adjusted HR (95% C.I.)‡ | 1.32 (1.14, 1.52) | 1.46 (1.14, 1.86) | 1.36 (1.20, 1.53) |
13–36 months vs. ≤12 months; covariates included age, race, comorbidity status, cancer stage, cancer grade, income quartiles, urban residence, SEER region, marital status, and year of diagnosis.
COMMENT
Despite the widespread use of PADT in localized (T1-T2) prostate cancer,3, 4 there is little information regarding the clinical outcomes associated with this practice. Our study was designed to evaluate the association between PADT and prostate cancer-specific and overall survival in men who did not initially receive definitive therapy (eg., surgery or radiation) for localized prostate cancer.
Utilizing instrumental variable analysis as one of the best available means of controlling for both measured and unmeasured confounding variables, we found no overall survival benefit for elderly men with localized prostate cancer receiving PADT. Results obtained with a traditional Cox model that adjusts only for measured confounding factors differed from those with the instrumental variable approach. These observations suggest that there is significant unaccounted residual bias associated with traditional analytical methods in this setting and that the instrumental variable approach may be particularly advantageous. In addition, one potential advantage of this study over clinical trials is that it includes “real-world” patients that would often be excluded from clinical trials even though these patients would receive the treatment in practice.
An interesting outcome of our study was the observation that cancer-specific survival, but not overall survival, appeared worse for men with lower risk cancer treated with PADT. This observation had also been previously documented in a randomized study of PADT in men with T0-T4 disease.36 The authors suggested several possible explanations for this finding, including competing causes of death, misclassification, and statistical variation.36 Another possibility could be that suppression of moderately- or well-differentiated cells not destined to harm a patient’s overall survival may allow for the establishment or overgrowth of more rapidly growing malignant clones (as seen in preclinical models)37–39 that increase the probability of death due to prostate cancer instead of a competing cause of death. As is evident from Figures 1A and 1B, the likelihood of death from competing causes normally exceeds the risk of death from prostate cancer in this population; this balance may be altered if PADT preferentially allows for the establishment or overgrowth of a more malignant fraction of a tumor.
Our study had some limitations. The study was limited to men aged ≥66 years and the results could differ for younger men. The SEER-Medicare database does not capture information on antiandrogen use. Therefore, patients utilizing antiandrogens only might be misclassified into the conservative management cohort and, because a previous study40 suggested that antiandrogens may result in adverse outcomes in these patients, it is possible that the conservative management group performed unusually poorly. However, previous data from another large database (CaPSURE)41 showed that the use of antiandrogens as sole treatment for localized prostate cancer is relatively uncommon (~2%) and it is unlikely that this small subset could alter the outcomes of the conservative management group overall.
Just like the success of a randomized study is dependent on factors such as the attainment of a sufficient sample size to balance both measured and unmeasured characteristics in different treatment groups, the use of IVA to balance treatment group characteristics (eg., PSA, family history, diet, body mass, etc.) depends on finding a suitable, partly random, varying factor (instrumental variable) that can be used to balance treatment groups. Our instrumental variable (high- and low-PADT use HSAs) had excellent properties. However, as in randomized studies, it is possible that some unmeasured factors may have been somewhat imbalanced between groups. Nonetheless, sensitivity analyses, using various geographic-based instruments and removing patients with other cancers or comorbidity scores >0, yielded similar results and suggested that the analyses were robust. However, it is still possible that the use of an instrumental variable approach in this setting does not adequately control for unknown confounding variables and therefore, if possible, a randomized trial should be considered.
There are very few data comparing PADT with conservative management, or any other established treatment option (eg., surgery or radiation), in men with localized (T1-T2, NO, M0) prostate cancer even though the popularity of PADT has grown the most in this population, increasing 2-3-fold in recent years.3 Published studies have generally not provided data specific for localized (T1-T2) disease and have had limited sample sizes.42, 43 The largest published study describing PADT use among T1-T2 patients was descriptive, non-comparative, and had limited follow-up.41 The randomized Early Prostate Cancer (EPC) trial44 had a large subset of patients with T1-T2 disease, but a non-traditional form of PADT (ie., bicalutamide) was employed. Results from this trial revealed a trend toward decreased overall survival in patients treated with PADT.40 The Veterans Administration Co-operative Urological Research Group (VACURG) study45 also utilized a non-conventional form of PADT (diethylstilbestrol). Results were inconsistent, with benefit in T2 disease but harm in T1 disease. In a related randomized study (European Organization for Research and Treatment of Cancer (EORTC) Trial 30891) that included patients with both localized, and advanced disease (eg., T0-4, N0-2),36 a modest overall survival benefit was found in favor of PADT but further analyses suggested that this benefit was associated with a group of patients with high-risk disease.46 Studies by the Medical Research Council (MRC),47 the EORTC 30846,10 and the Swiss Group for Clinical Cancer Research 48 focused on patients with more advanced disease. In general, though the designs, therapies and settings vary significantly from our study, the findings of these previous studies are inferentially consistent with our documentation of a lack of overall benefit, and some suggestion of potential benefit in high-risk or advanced disease subgroups.
In summary, our analyses suggest that PADT is not associated with improved survival among the majority of elderly men with T1-T2 prostate cancer. The significant side effects and costs associated with PADT, along with our finding of a lack of overall survival benefit, suggest that healthcare providers and their patients should very carefully consider the rationale for initiating PADT in their elderly patients with T1-T2 prostate cancer.
Supplementary Material
Acknowledgments
The authors acknowledge the efforts of the Applied Research Branch, Division of Cancer Prevention and Population Science, NCI; the Office of Information Services, and the Office of Strategic Planning, HCFA; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database. The performance and design of this study was reviewed and approved by both the National Cancer Institute (NCI) and Center for Medicare and Medicaid Services (CMS). This study was supported in part by award number DAMD17-01-1-0755 from the US Army Medical Research Acquisition Activity, Fort Detrick, MD and by the Cancer Institute of New Jersey, DOD award W81XWG-05-1-0235, Ohl Foundation, and by NCI grant # R01 CA116399 and CINJ core grant NCI CA-72720-10. We thank Thanusha Puvananayagam, MPH, Cancer Institute of NJ assistant staff, for outstanding administrative and technical assistance.
Role of funding sources
The Department of Defense and National Cancer Institute provided funding for this study. The Department of Defense did not play any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. The National Cancer Institute did not play any role in the design and conduct of the study; analysis and interpretation of the data; and preparation of the manuscript, but did collect and manage the data as well as review and approve the manuscript. The study received Institutional Review Board approval from the University of Medicine and Dentistry of New Jersey, as well as the SEER program, and the Center for Medicare and Medicaid Services.
This study was supported in part by award number DAMD17-01-1-0755 from the US Army Medical Research Acquisition Activity, Fort Detrick, MD and by the Cancer Institute of New Jersey, DOD award W81XWG-05-1-0235, Ohl Foundation, and by NCI grant # R01 CA116399 and CINJ core grant NCI CA-72720-10.
Footnotes
Publisher's Disclaimer: Disclaimers: This study utilizes the Linked SEER-Medicare Database. The interpretation and reporting of these data are the sole responsibility of the authors. The content of the information does not necessarily reflect the position or the policy of the Government, and no official endorsement should be inferred.
CONFLICT OF INTEREST STATEMENT
I, Grace L. Lu-Yao, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. The authors have no conflicts of interest.
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007 Jan-Feb;57(1):43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Cooperberg MR, Broering JM, Litwin MS, et al. The contemporary management of prostate cancer in the United States: lessons from the cancer of the prostate strategic urologic research endeavor (CapSURE), a national disease registry. J Urol Apr. 2004;171(4):1393–1401. doi: 10.1097/01.ju.0000107247.81471.06. [DOI] [PubMed] [Google Scholar]
- 3.Cooperberg MR, Grossfeld GD, Lubeck DP, Carroll PR. National practice patterns and time trends in androgen ablation for localized prostate cancer. J Natl Cancer Inst. 2003 Jul 2;95(13):981–989. doi: 10.1093/jnci/95.13.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akaza H. Trends in primary androgen depletion therapy for patients with localized and locally advanced prostate cancer: Japanese perspective. Cancer Sci Apr. 2006;97(4):243–247. doi: 10.1111/j.1349-7006.2006.00180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bolla M, Collette L, Blank L, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet. 2002 Jul 13;360(9327):103–106. doi: 10.1016/s0140-6736(02)09408-4. [DOI] [PubMed] [Google Scholar]
- 6.D’Amico AV, Manola J, Loffredo M, Renshaw AA, DellaCroce A, Kantoff PW. 6-Month Androgen Suppression Plus Radiation Therapy vs Radiation Therapy Alone for Patients With Clinically Localized Prostate Cancer: A Randomized Controlled Trial. JAMA. 2004 August 18, 2004;292(7):821–827. doi: 10.1001/jama.292.7.821. [DOI] [PubMed] [Google Scholar]
- 7.D’Amico AV, Loffredo M, Renshaw AA, Loffredo B, Chen MH. Six-month androgen suppression plus radiation therapy compared with radiation therapy alone for men with prostate cancer and a rapidly increasing pretreatment prostate-specific antigen level. J Clin Oncol. 2006 Sep 1;24(25):4190–4195. doi: 10.1200/JCO.2006.06.8239. [DOI] [PubMed] [Google Scholar]
- 8.Bolla M, Gonzalez D, Warde P, et al. Improved survival in patients with locally advanced prostate cancer treated with radiotherapy and goserelin. N Engl J Med. 1997 Jul 31;337(5):295–300. doi: 10.1056/NEJM199707313370502. [DOI] [PubMed] [Google Scholar]
- 9.Messing EM, Manola J, Sarosdy M, Wilding G, Crawford ED, Trump D. Immediate hormonal therapy compared with observation after radical prostatectomy and pelvic lymphadenectomy in men with node-positive prostate cancer. N Engl J Med. 1999 Dec 9;341(24):1781–1788. doi: 10.1056/NEJM199912093412401. [DOI] [PubMed] [Google Scholar]
- 10.Schroder FH, Kurth KH, Fossa SD, et al. Early versus delayed endocrine treatment of pN1-3 M0 prostate cancer without local treatment of the primary tumor: results of European Organisation for the Research and Treatment of Cancer 30846--a phase III study. J Urol Sep. 2004;172(3):923–927. doi: 10.1097/01.ju.0000135742.13171.d2. [DOI] [PubMed] [Google Scholar]
- 11.Shahinian VB, Kuo YF, Freeman JL, Goodwin JS. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med. 2005 Jan 13;352(2):154–164. doi: 10.1056/NEJMoa041943. [DOI] [PubMed] [Google Scholar]
- 12.Kapoor D, Malkin CJ, Channer KS, Jones TH. Androgens, insulin resistance and vascular disease in men. Clinical Endocrinology. 2005;63(3):239–250. doi: 10.1111/j.1365-2265.2005.02299.x. [DOI] [PubMed] [Google Scholar]
- 13.Makhsida N, Shah J, Yan G, Fisch H, Shabsigh R. Hypogonadism and metabolic syndrome: implications for testosterone therapy. J Urol Sep. 2005;174(3):827–834. doi: 10.1097/01.ju.0000169490.78443.59. [DOI] [PubMed] [Google Scholar]
- 14.Smith MR, Finkelstein JS, McGovern FJ, et al. Changes in body composition during androgen deprivation therapy for prostate cancer. J Clin Endocrinol Metab Feb. 2002;87(2):599–603. doi: 10.1210/jcem.87.2.8299. [DOI] [PubMed] [Google Scholar]
- 15.D’Amico AV, Denham JW, Crook J, et al. Influence of Androgen Suppression Therapy for Prostate Cancer on the Frequency and Timing of Fatal Myocardial Infarctions. J Clin Oncol. 2007 June 10, 2007;25(17):2420–2425. doi: 10.1200/JCO.2006.09.3369. [DOI] [PubMed] [Google Scholar]
- 16.Keating NL, O’Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006 Sep 20;24(27):4448–4456. doi: 10.1200/JCO.2006.06.2497. [DOI] [PubMed] [Google Scholar]
- 17.Potosky AL, Reeve BB, Clegg LX, et al. Quality of life following localized prostate cancer treated initially with androgen deprivation therapy or no therapy. J Natl Cancer Inst. 2002 Mar 20;94(6):430–437. doi: 10.1093/jnci/94.6.430. [DOI] [PubMed] [Google Scholar]
- 18.Part B Physician/Supplier Nat’l Data, CY 2001 Top 200 LevelII Healthcare Common Procedure Coding System (HCPCS/Alpha-Numeric) Codes.
- 19.Earle CC, Tsai JS, Gelber RD, Weinstein MC, Neumann PJ, Weeks JC. Effectiveness of chemotherapy for advanced lung cancer in the elderly: instrumental variable and propensity analysis. J Clin Oncol. 2001 Feb 15;19(4):1064–1070. doi: 10.1200/JCO.2001.19.4.1064. [DOI] [PubMed] [Google Scholar]
- 20.Stukel TA, Fisher ES, Wennberg DE, Alter DA, Gottlieb DJ, Vermeulen MJ. Analysis of Observational Studies in the Presence of Treatment Selection Bias: Effects of Invasive Cardiac Management on AMI Survival Using Propensity Score and Instrumental Variable Methods. JAMA. 2007 January 17, 2007;297(3):278–285. doi: 10.1001/jama.297.3.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Angrist J, Imbens G, Rubin D. Identification of causal effects using instrumental variables. Journal of the American Statistical Association. 1996;91(434):444–455. [Google Scholar]
- 22.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002 Aug;40(8 Suppl):IV-3–18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 23.Potosky AL, Davis WW, Hoffman RM, et al. Five-Year Outcomes After Prostatectomy or Radiotherapy for Prostate Cancer: The Prostate Cancer Outcomes Study. J Natl Cancer Inst. 2004 September 15, 2004;96(18):1358–1367. doi: 10.1093/jnci/djh259. [DOI] [PubMed] [Google Scholar]
- 24.Seidenfeld J, Samson DJ, Hasselblad V, et al. Single-therapy androgen suppression in men with advanced prostate cancer: a systematic review and meta-analysis. Ann Intern Med. 2000 Apr 4;132(7):566–577. doi: 10.7326/0003-4819-132-7-200004040-00009. [DOI] [PubMed] [Google Scholar]
- 25.Albertsen PC, Walters S, Hanley JA. A comparison of cause of death determination in men previously diagnosed with prostate cancer who died in 1985 or 1995. J Urol. 2000;163(2):519–523. [PubMed] [Google Scholar]
- 26.Penson DF, Albertsen PC, Nelson PS, Barry M, Stanford JL. Determining cause of death in prostate cancer: are death certificates valid? J Natl Cancer Inst. 2001 Dec 5;93(23):1822–1823. doi: 10.1093/jnci/93.23.1822. [DOI] [PubMed] [Google Scholar]
- 27.Albertsen PC, Fryback DG, Storer BE, Kolon TF, Find J. Long-term survival among men with conservatively treated localized prostate cancer. JAMA. 1995;274:626–631. [PubMed] [Google Scholar]
- 28.Fowler JE, Jr, Terrell FL, Renfroe DL. Co-morbidities and survival of men with localized prostate cancer treated with surgery or radiation therapy. J Urol Nov. 1996;156(5):1714–1718. [PubMed] [Google Scholar]
- 29.D’Hoore W, Sicotte C, Tilquin C. Risk adjustment in outcome assessment: the Charlson comorbidity index. Methods Inf Med Nov. 1993;32(5):382–387. [PubMed] [Google Scholar]
- 30.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 31.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol Dec. 2000;53(12):1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 32.Makuc D, Haglund B, Ingram D, Kleinman J, Feldman J USDoHaH. Services. National center for Health Statistics; 1991. Vital and Health Statistics - Health Service Areas for the United States. [PubMed] [Google Scholar]
- 33.Shahinian VB, Kuo YF, Freeman JL, Orihuela E, Goodwin JS. Increasing use of gonadotropin-releasing hormone agonists for the treatment of localized prostate carcinoma. Cancer. 2005 Apr 15;103(8):1615–1624. doi: 10.1002/cncr.20955. [DOI] [PubMed] [Google Scholar]
- 34.Shahinian VB, Kuo Y-F, Freeman JL, Goodwin JS. Determinants of Androgen Deprivation Therapy Use for Prostate Cancer: Role of the Urologist. J Natl Cancer Inst. 2006 June 21, 2006;98(12):839–845. doi: 10.1093/jnci/djj230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Therneau TM, Grambsch PM. Modeling Survival data: extending the Cox Model. New York: Springer; 2000. [Google Scholar]
- 36.Studer UE, Whelan P, Albrecht W, et al. Immediate or deferred androgen deprivation for patients with prostate cancer not suitable for local treatment with curative intent: European Organisation for Research and Treatment of Cancer (EORTC) Trial 30891. J Clin Oncol. 2006 Apr 20;24(12):1868–1876. doi: 10.1200/JCO.2005.04.7423. [DOI] [PubMed] [Google Scholar]
- 37.Isaacs JT, Coffey DS. Adaptation versus selection as the mechanism responsible for the relapse of prostatic cancer to androgen ablation therapy as studied in the Dunning R-3327-H adenocarcinoma. Cancer Res Dec. 1981;41(12 Pt 1):5070–5075. [PubMed] [Google Scholar]
- 38.Han G, Buchanan G, Ittmann M, et al. Mutation of the androgen receptor causes oncogenic transformation of the prostate. Proc Natl Acad Sci U S A. 2005 Jan 25;102(4):1151–1156. doi: 10.1073/pnas.0408925102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Banach-Petrosky W, Jessen WJ, Ouyang X, et al. Prolonged exposure to reduced levels of androgen accelerates prostate cancer progression in Nkx3.1; Pten mutant mice. Cancer Res. 2007 Oct 1;67(19):9089–9096. doi: 10.1158/0008-5472.CAN-07-2887. [DOI] [PubMed] [Google Scholar]
- 40.Iversen P, Johansson JE, Lodding P, et al. Bicalutamide (150 mg) versus placebo as immediate therapy alone or as adjuvant to therapy with curative intent for early nonmetastatic prostate cancer: 5.3-year median followup from the Scandinavian Prostate Cancer Group Study Number 6. J Urol Nov. 2004;172(5 Pt 1):1871–1876. doi: 10.1097/01.ju.0000139719.99825.54. [DOI] [PubMed] [Google Scholar]
- 41.Kawakami J, Cowan JE, Elkin EP, Latini DM, Duchane J, Carroll PR. Androgen-deprivation therapy as primary treatment for localized prostate cancer: data from Cancer of the Prostate Strategic Urologic Research Endeavor (CaPSURE) Cancer. 2006 Apr 15;106(8):1708–1714. doi: 10.1002/cncr.21799. [DOI] [PubMed] [Google Scholar]
- 42.Akaza H, Homma Y, Usami M, et al. Efficacy of primary hormone therapy for localized or locally advanced prostate cancer: results of a 10-year follow-up. BJU Int Sep. 2006;98(3):573–579. doi: 10.1111/j.1464-410X.2006.06349.x. [DOI] [PubMed] [Google Scholar]
- 43.Labrie F, Candas B, Gomez JL, Cusan L. Can combined androgen blockade provide long-term control or possible cure of localized prostate cancer? Urology Jul. 2002;60(1):115–119. doi: 10.1016/s0090-4295(02)01639-4. [DOI] [PubMed] [Google Scholar]
- 44.McLeod DG, Iversen P, See WA, Morris T, Armstrong JON, Wirth MP. Bicalutamide 150 mg plus standard care vs standard care alone for early prostate cancer. BJU International. 2006;97(2):247–254. doi: 10.1111/j.1464-410X.2005.06051.x. [DOI] [PubMed] [Google Scholar]
- 45.The Veterans Administration Cooperative Urological Research Group. Treatment and survival of patients with cancer of the prostate. Surg Gynecol Obstet May. 1967;124(5):1011–1017. [PubMed] [Google Scholar]
- 46.Studer U, Collette L, Whelan P, et al. Patients with T0-4 N0 M0 prostate cancer not suitable for local treatment with curative intent (EORTC 30891): Which subgroup needs or does not need immediate treatment? J urol. 2006;175:1513. abstract 1592. [Google Scholar]
- 47.The Medical Research Council Prostate Cancer Working Party Investigators Group. Immediate versus deferred treatment for advanced prostatic cancer: initial results of the Medical Research Council Trial. Br J Urol Feb. 1997;79(2):235–246. doi: 10.1046/j.1464-410x.1997.d01-6840.x. [DOI] [PubMed] [Google Scholar]
- 48.Studer UE, Hauri D, Hanselmann S, et al. Immediate versus deferred hormonal treatment for patients with prostate cancer who are not suitable for curative local treatment: results of the randomized trial SAKK 08/88. J Clin Oncol. 2004 Oct 15;22(20):4109–4118. doi: 10.1200/JCO.2004.11.514. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.