Delirium, defined as an acute decline of attention and cognitive function, represents a common and potentially devastating problem for hospitalized older persons. With occurrence rates from 14% to 56% and hospital mortality rates from 25% to 33% (1), delirium often initiates a cascade of events culminating in loss of independence, increased morbidity and mortality, and increased health care costs (2). We estimate, on the basis of our previous work (1,3) and extrapolations from Medicare data (4), that each year delirium complicates hospital stays for at least 20% of the 12.5 million persons aged 65 years and older who are hospitalized each year, with an increased hospital cost of $2,500 per patient attributable to delirium. This accounts for over $6.9 billion (2004 U.S. dollars) in Medicare expenditures for hospitalization attributable to delirium each year. Substantial additional direct health care costs accrue after hospital discharge because of the need for institutionalization, emergency room visits, rehospitalization, physician or clinic visits, rehabilitation services, and formal home health care, estimated at $48,785 per patient attributable to delirium, or over $100 billion per year extrapolated nationally (5).
Several recent lines of evidence have converged to highlight the interrelationship of delirium and dementia. First, delirium persists much longer than previously believed, with symptoms commonly present for months to years in many studies (6–11). The entities of persistent delirium (6–11) and reversible dementia (12) blur the distinctions between these two conditions. Second, epidemiologic studies have documented long-term cognitive decline following delirium (13). Third, dementia is the leading risk factor for delirium identified in previous studies (14,15). At least two thirds of cases of delirium occur in patients with underlying dementia or cognitive impairment, suggesting that the underlying vulnerability of the brain in dementia predisposes affected patients to the development of delirium when exposed to precipitating factors or insults such as medical illnesses, infections, medications, and medical procedures. Fourth, neuroimaging studies have documented regions of hypoperfusion in patients with delirium, suggesting that delirium may incite a derangement in brain vascular function that may lead to dementia in some cases (16,17). Fifth, dementia with Lewy bodies, which shares clinical features with delirium (e.g., visual hallucination, fluctuating symptoms) along with marked cholinergic deficiency, may reflect an overlap syndrome. Finally, previous studies have postulated shared underlying mechanisms, as both delirium and dementia have been shown to be associated with decreased cerebral oxidative metabolism, cholinergic deficiency, and inflammation (18).
To date, the pathophysiology of delirium remains poorly understood, and its underlying mechanisms are largely unknown. Understanding the pathophysiology of delirium will be critical to advancing the field and to developing optimal preventive and treatment strategies. Key issues are whether and in whom delirium leads to permanent cognitive sequelae and, if so, how does this occur? Clarification of this area may offer the unique opportunity to provide early intervention to prevent permanent neurologic damage, and to mitigate the potential downward spiral to dementia. However, studying the interface of delirium and dementia presents extreme challenges in study design and conduction, as well as in the informed consent process. To begin an exploration of this area, we convened the “Aging Brain Center Scientific Symposium: The Interface of Delirium and Dementia” on April 10, 2006. The symposium focused on studies examining the pathophysiology of delirium and the interrelationship of delirium and dementia. From the presentations at this symposium, five articles were developed as a special series for this journal. The goal of this special series of articles is to elucidate the underlying processes that lead to delirium. In addition, we generated hypotheses and new criteria that may help to identify individuals at high risk for adverse outcomes from delirium, such as those persons in whom the instability of cognitive homeostasis may have already exhausted all cognitive reserves. Finally, through this special series, we also hope to initiate a discovery process that will enable us to determine whether delirium itself leads to long-term cognitive sequelae and dementia, to explore whether delirium worsens the cognitive trajectory of an existing dementia, and to probe the pathways leading to these sequelae.
Conceptual Framework: The Interrelationship of Delirium and Dementia
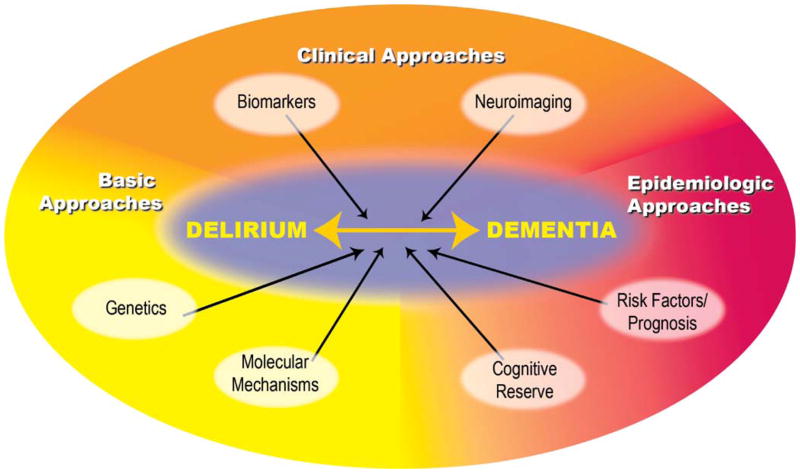
A conceptual framework for examining the interrelationship of delirium and dementia appears in Figure 1. Given the multifactorial nature of delirium, the ideal strategy will involve interdisciplinary approaches to clarify its pathophysiology and mechanisms. Thus, the framework includes overlapping clinical, basic science, and epidemiologic approaches to elucidate the fundamental pathophysiology of delirium and to provide the means to explore its long-term sequelae. The clinical approaches include determining biomarkers for delirium and applying neuroimaging techniques to the study of delirium and its sequelae. Basic science approaches include genetic and molecular approaches to clarify the underlying mechanisms of delirium. Epidemiologic approaches include prospective cohort studies to examine the risk factors for long-lasting delirium and the cognitive prognosis of delirium, and to clarify the role of cognitive reserve in determining the risk for delirium.
Figure 1.
Conceptual framework for exploring the interrelationship of delirium and dementia.
The systematic review by Marcantonio and colleagues (19) in this series suggests that elucidating serum biomarkers may considerably improve our understanding of the pathophysiology of delirium. Risk markers, present before the onset of delirium, can help to identify patients at risk of delirium or those in whom an episode of delirium may have catastrophic consequences. Disease markers, which rise and fall with delirium, are helpful to mark the onset and recovery from delirium, provide important pathophysiologic clues, and determine the effectiveness of intervention strategies. End products usually rise after the onset of delirium, and correlate with the severity of delirium or the extent of underlying damage. Ultimately, biomarkers may prove useful in confirming the diagnosis of delirium, following its time course and severity, and determining its long-term consequences.
The article by Alsop and colleagues (20) in this series reviews neuroimaging methods that may be helpful in the study of delirium. For instance, techniques of blood flow imaging can help to identify and localize early changes associated with delirium and then to follow the resolution, persistence, or evolution of these changes over time. Diffusion tensor imaging or sensitive new volumetric methods might help to identify early pathologic changes soon after a delirium episode. Using direct amyloid imaging, we may start exploring whether amyloid pathology increases the risk for delirium and whether the rate of amyloid deposition increases after delirium episodes. Conducting definitive neuroimaging studies in acutely ill older persons with delirium, however, poses substantial logistical and interpretation challenges, requiring carefully designed and conducted studies with moderate to large sample sizes. Yet, the application of these methodologies will be essential, and holds great promise for advancing our understanding of the pathophysiology of delirium and its sequelae.
In an original study reported in this series, Fong and colleagues (17) were able to obtain Single Photon Emission Computed Tomography (SPECT) scans on 22 delirious patients, including six paired scans during and after the delirium episode. This study represents the largest study of this type in the real-world hospital setting, where most delirium currently occurs, involving patients with multifactorial etiologies of delirium. The major findings of this study were hypoperfusion in the frontal, parietal, and pontine regions. Importantly, this study suggests that SPECT is a useful modality to identify and follow brain changes in delirium over time.
Genetic analysis will help to identify genetic loci associated with increased risk (such as apolipoprotein E [APOE] and others) of delirium and dementia. In addition, gene microarray analysis may help to elucidate which genes and their related products are turned on or off with delirium. To further explore the molecular mechanisms of delirium, Xie and colleagues (21) present data suggesting that the inhalational anesthetic isoflurane induces caspase-3 activation and apoptosis in a dose-dependent fashion, and Congo red—an Aβ fibrillar aggregation inhibitor—inhibits isoflurane-induced caspase-3 activation and apoptosis. The finding that anesthesia, an important risk factor for delirium, contributes to well-described mechanisms of Alzheimer’s neuro-pathogenesis provides the first demonstration of a potential pathogenic link between delirium and the more long-term sequelae of dementia.
Cognitive reserve, the resiliency with which an individual can cope with brain injury or stressors, affects the risk of dementia. Although a similar role has been postulated for delirium, the role of cognitive reserve in the risk of delirium has been poorly examined. In this series, Jones and colleagues (22) demonstrated that the risk of delirium was significantly higher among persons with fewer years of education in two large cohorts of hospitalized older persons, suggesting that low educational level may serve as a proxy marker of low cognitive reserve, with increased vulnerability to delirium.
Conclusions
The study of the pathophysiology of delirium may be substantially advanced by the extensive use of biomarkers, neuroimaging, and molecular approaches to elucidating delirium. Exploring the role of cognitive reserve as a marker for resiliency or low vulnerability to delirium should provide new clues in the direction of the proposed reserve–delirium–dementia pathway. Future studies to more fully address the mechanisms of delirium and its long-term sequelae will require larger, more detailed epidemiologic studies. Yet, these studies pose unique challenges in their design and conduct. Examination of the long-term prognosis of delirium and the interrelationship with dementia is best conducted in heterogeneous populations of older persons with a broad range of exposures and outcomes. However, the occurrence of delirium in the general population is relatively rare. Therefore, adequate sample sizes for these studies will be feasible and efficient only if rigorous sampling techniques are applied and appropriate high-risk populations are selected. By contrast, well-matched samples of delirious and nondelirious patients with standardized insults are preferred for elucidating specific underlying mechanisms. These studies are limited by the retrospective nature of information on the risk factor profiles of these patients prior to the event that precipitated the delirium episode. Clearly, advances in this research field require the development of new study designs that include features that minimize biases, improve efficiency, and take into account the effects of competing outcomes. Despite the barriers, meeting the challenges of these future studies will be critical to provide an innovative approach to conceptualizing reversible causes of dementia, which holds great promise for generating novel strategies to forestall or slow cognitive decline in late life.
Acknowledgments
This work was funded in part by the Education Core of the Massachusetts Alzheimer’s Disease Research Center (P50AG005134), a conference grant from the Alzheimer’s Association, grants from the National Institute on Aging (R21AG025193 and K24AG00949), the Harvard Older Americans Independence Center (P60AG00812), and the Aging Brain Center, Institute for Aging Research, Hebrew SeniorLife. Dr. Inouye holds the Milton and Shirley F. Levy Family Chair.
We thank Dr. Edward Marcantonio for helpful review of the manuscript and Jennifer Davis for graphical design assistance in creation of the figure.
References
- 1.Inouye SK, Schlesinger MJ, Lydon TJ. Delirium: a symptom of how hospital care is failing older persons and a window to improve quality of hospital care. Am J Med. 1999;106:565–573. doi: 10.1016/s0002-9343(99)00070-4. [DOI] [PubMed] [Google Scholar]
- 2.Inouye SK. Current concepts: delirium in older persons. N Engl J Med. 2006;354:1157–1165. doi: 10.1056/NEJMra052321. [DOI] [PubMed] [Google Scholar]
- 3.Inouye SK, Bogardus ST, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340:669–676. doi: 10.1056/NEJM199903043400901. [DOI] [PubMed] [Google Scholar]
- 4.U.S. Department of Health and Human Services. CMS Pub. No. 03445. Washington, DC: Centers for Medicare and Medicaid Services; 2004. 2004 CMS Statistics; p. 34. [Google Scholar]
- 5.Leslie DL, Zhang Y, Van Ness PH, Bogardus ST, Leo-Summers L, Inouye SK. One-year health care costs associated with delirium. Gerontologist. 2005;45(Spec Iss II):299. [Google Scholar]
- 6.Levkoff SE, Evans DA, Litpzin B, et al. Delirium: the occurrence and persistence of symptoms among elderly hospitalized patients. Arch Intern Med. 1992;152:334–340. doi: 10.1001/archinte.152.2.334. [DOI] [PubMed] [Google Scholar]
- 7.Rockwood D. The occurrence and duration of symptoms in elderly patients with delirium. J Gerontol. 1993;48:M162–M166. doi: 10.1093/geronj/48.4.m162. [DOI] [PubMed] [Google Scholar]
- 8.Levkoff SE, Liptzin B, Evans EA, et al. Progression and resolution of delirium in elderly patients hospitalized for acute care. Am J Geriatr Psychiatry. 1994;2:230–238. doi: 10.1097/00019442-199400230-00007. [DOI] [PubMed] [Google Scholar]
- 9.Marcantonio ER, Flacker JM, Michaels M, Resnick NM. Delirium is independently associated with poor functional recovery after hip fracture. J Am Geriatr Soc. 2000;48:618–624. doi: 10.1111/j.1532-5415.2000.tb04718.x. [DOI] [PubMed] [Google Scholar]
- 10.Cole M, McCusker J, Dendukuri N, Han L. The prognostic significance of subsyndromal delirium in elderly medical inpatients. J Am Geriatr Soc. 2003;51:754–760. doi: 10.1046/j.1365-2389.2003.51255.x. [DOI] [PubMed] [Google Scholar]
- 11.McCusker J, Cole M, Dendukuri N, Han L, Belzile E. The course of delirium in older medical inpatients: a prospective study. J Gen Intern Med. 2003;18:696–704. doi: 10.1046/j.1525-1497.2003.20602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clarfield AM. The reversible dementias: do they reverse? Ann Intern Med. 1988;109:476–486. doi: 10.7326/0003-4819-109-6-476. [DOI] [PubMed] [Google Scholar]
- 13.Jackson JC, Gordon SM, Hart RP, Hopkins RO, Ely WE. The association between delirium and cognitive decline: a review of the empirical literature. Neuropsychol Rev. 2004;14:87–98. doi: 10.1023/b:nerv.0000028080.39602.17. [DOI] [PubMed] [Google Scholar]
- 14.Cole MG. Delirium in elderly patients. Am J Geriatr Psychiatry. 2004;12:7–21. [PubMed] [Google Scholar]
- 15.Inouye SK. Delirium and cognitive decline: does delirium lead to dementia? Cognitive Decline: Strategies for Prevention. In: Fillit HM, Butler RN, editors. Proceedings of a White House Conference on Aging. London: Greenwich Medical Media; 1997. pp. 85–107. [Google Scholar]
- 16.Yokota H, Ogawa S, Kurokawa A, Yamamoto Y. Regional cerebral blood flow in delirium patients. J Psychiatry Clin Neurosci. 2003;57:337–339. doi: 10.1046/j.1440-1819.2003.01126.x. [DOI] [PubMed] [Google Scholar]
- 17.Fong TG, Bogardus ST, Daftary A, et al. Interrelationship Between Delirium and Dementia. Cerebral perfusion changes in older delirious patients using 99mTc HMPAO SPECT. J Gerontol A Biol Sci Med Sci. 2006;61A:1294–1299. doi: 10.1093/gerona/61.12.1294. [DOI] [PubMed] [Google Scholar]
- 18.Eikelenboom P, Hoogendijk WJ. Do delirium and Alzheimer’s dementia share specific pathogenetic mechanisms? Dement Geriatr Cogn Disord. 1999;10:319–324. doi: 10.1159/000017162. [DOI] [PubMed] [Google Scholar]
- 19.Marcantonio ER, Rudolph JL, Culley D, Crosby G, Alsop DC, Inouye SK. Interrelationship Between Delirium and Dementia. Serum biomarkers for delirium. J Gerontol A Biol Sci Med Sci. 2006;61A:1281–1286. doi: 10.1093/gerona/61.12.1281. [DOI] [PubMed] [Google Scholar]
- 20.Alsop DC, Fearing MA, Johnson K, Sperling R, Fong TG, Inouye SK. Interrelationship Between Delirium and Dementia. The role of neuroimaging in elucidating delirium pathophysiology. J Gerontol A Biol Sci Med Sci. 2006;61A:1287–1293. doi: 10.1093/gerona/61.12.1287. [DOI] [PubMed] [Google Scholar]
- 21.Xie Z, Dong Y, Maeda U, et al. Interrelationship Between Delirium and Dementia. Isoflurane-induced apoptosis: a potential pathogenic link between delirium and dementia. J Gerontol A Biol Sci Med Sci. 2006;61A:1300–1306. doi: 10.1093/gerona/61.12.1300. [DOI] [PubMed] [Google Scholar]
- 22.Jones RN, Yang FM, Zhang Y, Kiely DK, Marcantonio ER, Inouye SK. Interrelationship Between Delirium and Dementia. Does educational attainment contribute to risk for delirium? A potential role for cognitive reserve. J Gerontol A Biol Sci Med Sci. 2006;61A:1307–1311. doi: 10.1093/gerona/61.12.1307. [DOI] [PubMed] [Google Scholar]