Abstract
Background
Although selenium plays an important role in muscle function, the relation between circulating selenium and muscle strength in elderly adults has not been characterized.
Objective
The objective was to examine the hypothesis that low plasma selenium is associated with poor muscle strength in older adults.
Design
We measured plasma selenium and hip, grip, and knee strength in a cross-sectional study of 891 men and women aged ≥65 y from the Invecchiare in Chianti (InCHIANTI) Study, a population-based cohort study in Tuscany (Italy). Poor muscle strength was defined as the lowest quartile of hip flexion, grip, and knee extension strength.
Results
Overall, mean (±SD) plasma selenium was 0.95 ± 0.15 μmol/L. After adjustment for age, sex, education, total energy intake, body mass index, and chronic disease, participants in the lowest versus the highest quartile of plasma selenium were at higher risk of poor hip strength [odds ratio (OR): 1.69; 95% CI: 1.02, 2.83; P = 0.04, P for linear trend = 0.04], knee strength (OR: 1.94; 95% CI: 1.18, 3.19; P = 0.009, P for linear trend = 0.01), and grip strength (OR: 1.94; 95% CI: 1.19, 3.16; P = 0.008, P for linear trend = 0.08).
Conclusions
Low plasma selenium is independently associated with poor skeletal muscle strength in community-dwelling older adults in Tuscany.
Keywords: Aging, muscle strength, sarcopenia, selenium, population-based study
INTRODUCTION
Aging is characterized by the loss of muscle strength, which in turn increases the risk of falls, hospitalization, disability, and mortality (1). The biological mechanisms involved in sarcopenia, or the loss of muscle mass and strength, are not completely understood. The accumulation of mitochondrial and nuclear DNA damage and increases in oxidative stress may lead to the loss of myocytes and, thus, may compromise the function of skeletal muscle (2). In human skeletal muscle, oxidative damage to DNA, proteins, and lipids increases with age (3–6). Selenium plays an essential role in muscle function (7), and selenoenzymes such as glutathione peroxidase and glutathione reductase protect myocytes from reactive oxygen species (8).
Human selenium deficiency is associated with skeletal myopathy and cardiomyopathy (9). In ruminant animals, selenium deficiency causes white muscle disease, a condition characterized by muscle weakness and degeneration of skeletal and cardiac muscle (10). A myopathy similar to white muscle disease has been described in human selenium deficiency (11). Although severe selenium deficiency is associated with skeletal muscle problems, including fatigue and proximal muscle weakness (12), it is not known whether a more marginal selenium status, such as that found in community-dwelling adults, is associated with muscle weakness. We hypothesized that low plasma selenium concentrations are associated with poor muscle strength in older adults. To address this hypothesis, we measured plasma selenium and upper- and lower-extremity muscle strength in older community-dwelling adults in the InCHIANTI study, a population-based study of mobility disability in the Chianti region of Tuscany, Italy.
SUBJECTS AND METHODS
Subjects
The study participants consisted of men and women aged ≥65 y who participated in the Invecchiare in Chianti (Aging in the Chianti Area) Study, otherwise known as the InCHIANTI Study, conducted in 2 small towns in Tuscany, Italy. The rationale, design, and data collection have been described elsewhere, and the main outcome of this longitudinal study is mobility disability (13). Briefly, in August 1998, 1270 people aged ≥65 y were randomly selected from the population registry of Greve in Chianti (population: 11 709) and Bagno a Ripoli (population: 4704), and of 1256 eligible subjects, 1155 (90.1%) agreed to participate. Of the 1155 participants, 1055 (91.3%) participated in the blood drawing. There were 891 (77.1%) participants who had both plasma selenium concentration and muscle strength data available for this analysis. The subjects who did not participate in the physical function evaluation were generally older and had greater comorbidity than did the subjects who participated in the evaluation, as reported elsewhere (14). Participants received an extensive description of the study and participated after providing written informed consent. The study protocol complied with the Declaration of Helsinki and was approved by the Italian National Institute of Research and Care on Aging Ethical Committee.
Methods
Demographic information and information on smoking and medication use were collected by using standardized questionnaires. Average daily intakes of energy (kcal), carbohydrates, total protein, total lipids, etc, were estimated by using the European Prospective Investigation into Cancer and Nutrition food-frequency questionnaire, which was validated in the InCHIANTI population (15). All participants were examined by a trained geriatrician, and diseases were ascertained according to standard, preestablished criteria and algorithms based on those used in the Women’s Health and Aging Study for coronary heart disease, diabetes mellitus, chronic obstructive pulmonary disease, osteoarthritis, and cancer (16). Weight was measured with the use of a high-precision mechanical scale. Standing height was measured to the nearest 0.1 cm. Body mass index (BMI) was calculated as weight (kg)/height squared (m) Depression was defined by using the Center for Epidemiologic Studies Depression Scale (CED-S) (17).
Blood samples were collected in the morning after the subjects had fasted for 12 h. Aliquots of serum and plasma were immediately obtained and stored at −80 °C. Aliquots of plasma were shipped on dry ice to the laboratory of one of the authors (RDS) for the measurement of plasma selenium. Plasma selenium was measured by graphite furnace atomic absorption spectrometry with an AAnalyst 600 with Zeeman background correction (Perkin Elmer, Norwalk, CT). Samples were diluted 1:4 with Triton-X (Sigma Chemical, St Louis, MO) and nitric acid solution (Fisher Scientific, Pittsburgh, PA), and the matrix modifier was a palladium and magnesium nitrate solution (both from Perkin Elmer). The instrument was calibrated daily by using known plasma selenium standards (UTAK Laboratories Inc, Valencia, CA). Within-run and between-run CVs were 3.1% and 7.1%, respectively.
Serum interleukin-6 (IL-6) was measured in duplicate by high-sensitivity enzyme-linked immunsorbent assay (ELISA) (BIOSOURCE, Camarillo, CA). The lowest detectable concentration was 0.1 pg/mL, and the interassay CV was 4.5%.
Isometric muscle strength was assessed on 8 muscle groups of the lower extremity with a hand-held dynamometer according to a standard protocol (18). All measures of lower-extremity muscle strength were highly correlated (Pearson’s correlation coefficients ranging from 0.87 to 0.92). Therefore, in the analyses presented here, we used only hip flexion and knee extension to indicate lower-extremity muscle strength (19). Isometric shoulder adduction and handgrip are used as measures of upper-extremity muscle strength; we decided to use handgrip for the present analysis because the assessment of handgrip is easy, is reliable, and has been used in many studies. Furthermore, strong evidence in the literature indicates that handgrip is a strong predictor of disability and mortality (20, 21). We chose to use measures of both lower- and upper-extremity muscle strength because previous studies have suggested that the rate of age-associated decline in muscle strength is quite different in these 2 anatomical regions. In fact, the correlation between handgrip and isometric strength of the lower-extremity muscle groups was only moderately high, ranging from 0.70 to 0.72. Some participants who were unable to come to the main testing center at the 2 study sites for assessments of knee and hip strength had grip strength measured during a home visit.
Statistical analysis
Variables are reported as means (±SDs) for normally distributed variables or as percentages. Means were compared by using a t test, and percentages were compared by using chi-square tests. Because of skewed distributions, log-transformed IL-6 values were used in regression analyses. Plasma selenium was analyzed as both a continuous variable and as quartiles, where the quartiles for selenium were defined as <0.839, 0.839–0.934, 0.935–1.037, and >1.037 μmol/L. Low hip, knee, and grip strength were defined by the lowest quartile of hip, knee, and grip at enrollment at the following cutoffs: 13.0, 10.5, and 16.0 kg for women and 20.5, 16.5, and 28.0 kg for men, respectively. Logistic regression models were used to examine the relation between quartiles of plasma selenium and muscle weakness. All analyses were performed by using SAS (version 8.2; SAS Institute Inc, Cary, NC) with a statistical significance level set at P < 0.05.
RESULTS
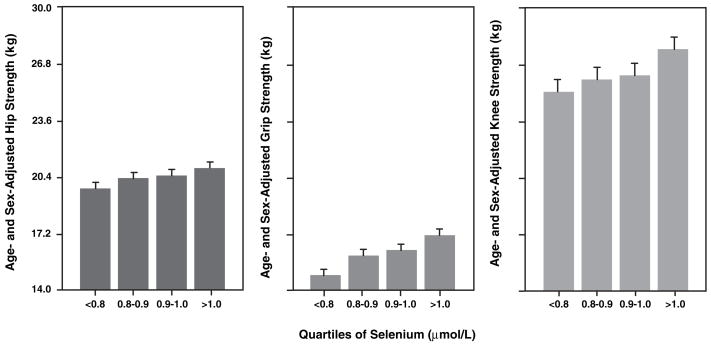
The characteristics of the study population at enrollment are shown in Table 1. Univariate logistic regression was used to examine the relation between different demographic, nutritional, and disease variables and poor muscle strength as shown in Table 2. In univariate analyses, age, education, lowest quartile of plasma selenium, total energy intake, peripheral artery disease, stroke, depression, and IL-6 (log) were significantly associated with all 3 strength measures (poor hip, knee, and grip strength). BMI and congestive heart failure were significantly associated with poor hip and knee strength but not grip strength. Age- and sex-adjusted mean hip, grip, and knee strength by quartile of plasma selenium are shown in Figure 1.
Table 1.
Characteristics of the study population at enrollment
| Characteristic | Value (n = 891) |
|---|---|
| Age (y) | 74.7 ± 6.71 |
| Sex (% female) | 55.7 |
| Education (y) | 5.5 ± 3.3 |
| Current smoker (%) | 13.6 |
| BMI (kg/m2) | 27.5 ± 4.0 |
| Plasma selenium (μmol/L) | 0.95 ± 0.15 |
| Plasma selenium <0.88 μmol/L (%) | 31.7 |
| Total energy intake (kcal/d) | 1934 ± 562 |
| Interleukin-6 (pg/mL) | 1.47 (0.86–2.29)2 |
| Hip strength (kg) | 20.4 ± 7.1 |
| Grip strength (kg) | 26.4 ± 11.4 |
| Knee strength (kg) | 16.1 ± 6.0 |
| Hypertension (%) | 36.1 |
| Coronary heart disease (%) | 4.8 |
| Congestive heart failure (%) | 4.0 |
| Peripheral artery disease (%) | 5.6 |
| Stroke (%) | 3.6 |
| Diabetes mellitus (%) | 10.6 |
| Chronic obstructive pulmonary disease (%) | 7.1 |
| Depression (%) | 19.6 |
| Cancer (%) | 6.1 |
| Hip osteoarthritis (%) | 5.5 |
| Knee osteoarthritis (%) | 7.2 |
x̄ ±SD (all such values).
Median; interquartile range in parentheses.
Table 2.
Odds ratios (OR) and 95% CIs for univariate relations of selenium concentration and other important confounders with hip, knee, and grip strengths1
| Low hip strength |
Low knee strength |
Low grip strength |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P |
| Age (y) | 1.14 | 1.11, 1.16 | <0.0001 | 1.13 | 1.10, 1.16 | <0.0001 | 1.11 | 1.09, 1.14 | <0.0001 |
| Sex (female) | 1.01 | 0.74, 1.37 | 0.96 | 0.99 | 0.74, 1.37 | 0.99 | 1.07 | 0.78, 1.48 | 0.68 |
| Education (y) | 0.89 | 0.84, 0.95 | 0.0001 | 0.92 | 0.87, 0.98 | 0.004 | 0.86 | 0.79, 0.92 | <0.0001 |
| Current smoker | 0.89 | 0.72, 1.10 | 0.28 | 0.99 | 0.81, 1.23 | 0.95 | 0.98 | 0.79, 1.23 | 0.87 |
| BMI (kg/m2) | 0.93 | 0.89, 0.97 | 0.0008 | 0.93 | 0.89, 0.97 | 0.0003 | 0.97 | 0.93, 1.01 | 0.14 |
| Plasma selenium (μmol/L)2 | |||||||||
| Quartile 1, <0.8 μmol/L | 2.57 | 1.64, 4.02 | <0.0001 | 2.79 | 1.80, 4.31 | <0.0001 | 2.67 | 1.66, 4.31 | <0.0001 |
| Quartile 2, 0.8–0.9 μmol/L | 2.11 | 1.34, 3.33 | 0.0001 | 1.49 | 0.95, 2.37 | 0.08 | 1.79 | 1.09, 2.93 | 0.02 |
| Quartile 3, 0.9–1.0 μmol/L | 1.31 | 0.81, 2.11 | 0.27 | 1.06 | 0.66, 1.71 | 0.81 | 1.57 | 0.95, 2.58 | 0.08 |
| Quartile 4, >1.0 μmol/L | 1.00 | — | — | 1.00 | — | — | 1.00 | — | — |
| Total energy intake (kcal/d) | 0.99 | 0.99, 1.00 | 0.0002 | 0.99 | 0.99, 1.00 | 0.0003 | 1.00 | 0.99, 1.00 | 0.02 |
| Coronary heart disease | 1.31 | 0.67, 2.56 | 0.43 | 1.67 | 0.88, 3.19 | 0.12 | 1.43 | 0.72, 2.83 | 0.31 |
| Congestive heart failure | 1.34 | 1.12, 1.60 | 0.001 | 1.45 | 1.22, 1.74 | <0.0001 | 1.10 | 0.91, 1.34 | 0.32 |
| Peripheral artery disease | 1.52 | 1.21, 1.91 | 0.0004 | 1.45 | 1.15, 1.83 | 0.002 | 1.37 | 1.07, 1.73 | 0.01 |
| Stroke | 1.55 | 1.16, 2.08 | 0.0003 | 1.50 | 1.12, 2.01 | 0.006 | 1.49 | 1.11, 2.00 | 0.008 |
| Diabetes mellitus | 1.22 | 0.84, 1.78 | 0.29 | 1.19 | 0.82, 1.75 | 0.34 | 0.98 | 0.65, 1.49 | 0.93 |
| Chronic obstructive pulmonary disease | 1.09 | 0.77, 1.53 | 0.64 | 1.93 | 0.65, 1.35 | 0.71 | 1.10 | 0.77, 1.58 | 0.59 |
| Depression | 2.14 | 1.50, 3.04 | <0.0001 | 1.99 | 1.39, 2.93 | 0.0002 | 1.55 | 1.06, 2.25 | 0.02 |
| Cancer | 0.94 | 0.49, 1.79 | 0.85 | 0.96 | 0.50, 1.83 | 0.89 | 0.92 | 0.47, 1.82 | 0.81 |
| Osteoarthritis3 | 1.25 | 0.98, 1.60 | 0.07 | 0.99 | 0.83, 1.20 | 0.99 | — | — | — |
| Log interleukin-6 | 1.60 | 1.35, 1.91 | <.0001 | 1.42 | 1.19, 1.69 | <.0001 | 1.56 | 1.30, 1.87 | <.0001 |
ORs and 95% CIs are from logistic models predicting low compared with normal strength.
P for trend [comparison of quartiles 1, 2, and 3 with quartile 4 (reference group)] was obtained by considering selenium quartile as an ordinal variable: <0.0001 for low hip, knee, and grip strength.
Hip osteoarthritis for low hip strength model; knee osteoarthritis for low knee strength model.
FIGURE 1.
Mean (±SE) hip, grip, and knee strength by selenium quartiles (n = 891). The values were adjusted by using a regression approach. Age- and sex-adjusted test for linear trend: P = 0.13 for hip strength, P = 0.04 for grip strength, and P = 0.0003 for knee strength.
Multivariate logistic regression models were used to examine the relation between quartiles of plasma selenium and poor hip, knee, and grip strength and selenium quartiles and multiple confounders (Table 3). After adjustment for age, sex, BMI, education, and total energy intake (model 1), the participants in the lowest quartile of plasma selenium had a greater risk of poor hip, knee, and grip strength than did those in the highest quartile of plasma selenium. In final models (model 2), adjustment for age, sex, BMI, education, total energy intake, congestive heart failure, peripheral artery disease, stroke, depression, and log (IL-6) in participants in the lowest quartile of plasma selenium had a greater risk of poor hip, knee, and grip strength than did those in the highest quartile of plasma selenium. In logistic regression using selenium as a continuous variable, participants with higher plasma selenium concentrations had greater hip strength (β ± SD: 1.93 ± 1.11; P = 0.05), greater knee strength (β ± SD: 4.50 ± 1.03; P < 0.0001), and greater grip strength (β ± SD: 5.26 ± 1.93; P = 0.007).
Table 3.
Odd ratios (OR) and 95% CIs for multivariate relations between selenium concentration and low hip, knee, and grip strength
| Low hip strength |
Low knee strength |
Low grip strength |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P |
| Model 11 | |||||||||
| Plasma selenium (μmol/L)2,3 | |||||||||
| Quartile 1, <0.8 μmol/L | 1.55 | 0.95, 2.54 | 0.08 | 1.82 | 1.09, 3.02 | 1.76 | 1.10, 2.82 | 0.02 | |
| Quartile 2, 0.8–0.9 μmol/L | 1.61 | 0.99, 2.64 | 0.06 | 1.40 | 0.83, 2.37 | 1.09 | 0.66, 1.78 | 0.73 | |
| Quartile 3, 0.9–1.0 μmol/L | 1.11 | 0.66, 1.85 | 0.69 | 1.39 | 0.83, 2.37 | 0.86 | 0.52, 1.43 | 0.56 | |
| Quartile 4, >1.0 μmol/L | 1.00 | — | — | 1.00 | — | — | 1.00 | — | — |
| Age (y) | 1.12 | 1.09, 1.15 | <0.0001 | 1.09 | 1.07, 1.12 | <0.0001 | 1.11 | 1.08, 1.14 | <0.0001 |
| Sex (female) | 0.63 | 0.50, 1.07 | 0.10 | 0.89 | 0.61, 1.32 | 0.59 | 0.77 | 0.53, 1.12 | 0.18 |
| Education (y) | 0.96 | 0.91, 1.02 | 0.17 | 0.93 | 0.87, 0.99 | 0.01 | 0.99 | 0.93, 1.04 | 0.61 |
| BMI (kg/m2) | 0.95 | 0.91, 0.99 | 0.03 | 0.99 | 0.95, 1.03 | 0.66 | 0.95 | 0.91, 0.99 | 0.02 |
| Total energy intake (kcal/d) | 1.00 | 0.99, 1.00 | 0.08 | 1.00 | 1.00, 1.00 | 0.74 | 1.00 | 0.99, 1.00 | 0.09 |
| Model 24 | |||||||||
| Plasma selenium (μmol/L)2,5 | |||||||||
| Quartile 1, <0.8 μmol/L | 1.69 | 1.02, 2.83 | 0.04 | 1.94 | 1.18, 3.19 | 0.009 | 1.94 | 1.19, 3.16 | |
| Quartile 2, 0.8–0.9 μmol/L | 1.78 | 1.08, 2.94 | 0.02 | 1.34 | 0.82, 2.22 | 0.24 | 1.24 | 0.76, 2.03 | |
| Quartile 3, 0.9–1.0 μmol/L | 1.36 | 0.81, 2.26 | 0.24 | 1.28 | 0.78, 2.19 | 0.35 | 0.90 | 0.54, 1.51 | |
| Quartile 4, >1.0 μmol/L | 1.00 | — | — | 1.00 | — | — | 1.00 | — | — |
| Age (y) | 1.10 | 1.07, 1.13 | <0.0001 | 1.09 | 1.06, 1.12 | <0.0001 | 1.09 | 1.07, 1.13 | <0.0001 |
| Sex (female) | 0.66 | 0.44, 0.98 | 0.04 | 1.01 | 0.68, 1.58 | 0.98 | 0.66 | 0.44, 0.97 | 0.43 |
| Education (y) | 0.99 | 0.94, 1.05 | 0.70 | 0.94 | 0.88, 1.00 | 0.05 | 0.98 | 0.92, 1.04 | 0.43 |
| BMI (kg/m2) | 0.96 | 0.91, 1.01 | 0.06 | 1.01 | 0.97, 1.05 | 0.73 | 0.95 | 0.91, 0.99 | 0.03 |
| Total energy intake (kcal/d) | 1.00 | 0.99, 1.00 | 0.04 | 1.00 | 1.00, 1.00 | 0.91 | 1.00 | 0.99, 1.00 | 0.07 |
| Congestive heart failure | 1.02 | 0.83, 1.25 | 0.87 | 0.93 | 0.75, 1.14 | 0.48 | 1.23 | 1.01, 1.50 | 0.04 |
| Peripheral artery disease | 1.31 | 1.02, 1.69 | 0.04 | 1.16 | 0.90, 1.50 | 0.25 | 1.21 | 0.93, 1.57 | 0.16 |
| Stroke | 1.26 | 0.92, 1.71 | 0.03 | 1.34 | 0.98, 1.83 | 0.06 | 1.33 | 0.98, 1.79 | 0.07 |
| Depression | 1.96 | 1.33, 2.90 | 0.007 | 137 | 0.92, 2.03 | 0.12 | 1.79 | 1.21, 2.66 | 0.004 |
| Log interleukin-6 | 1.16 | 0.95, 1.42 | 0.15 | 1.30 | 1.06, 1.60 | 0.01 | 0.99 | 0.81, 1.21 | 0.90 |
Adjusted for age and sex.
Selenium quartiles 1, 2, and 3 were compared with quartile 4 (reference group).
P for trend was obtained by considering selenium quartile as an ordinal variable: 0.04, 0.14, and 0.007 for low hip, knee, and grip strengths, respectively.
Adjusted for age, sex, education level, BMI, and total energy intake.
P for trend was obtained by considering selenium quartile as an ordinal variable: 0.04, 0.01, and 0.08 for low hip, knee, and grip strengths, respectively.
DISCUSSION
This study showed that low plasma selenium is an independent correlate of poor skeletal muscle strength in older adults living in the Tuscany community. To our knowledge, this is the first study to show an association between plasma selenium concentrations and poor muscle strength in older adults. These results suggest that there may be a continuum in the relation between plasma selenium concentrations and muscle strength, from those with severe selenium deficiency and muscle weakness to community-dwelling older adults with marginal selenium concentrations. More than 30% of older adults in the InCHIANTI Study had a plasma selenium concentration <0.88 μmol/L (<70 μg/L), the concentration below which low selenium may be limiting the synthesis of selenoproteins (22). Thus, it is possible that the activity of selenoproteins in skeletal muscle is suboptimal and may increase oxidative stress and oxidative damage to DNA, proteins, and lipid in muscle tissue.
Low selenium concentrations could also contribute to muscle weakness through the up-regulation of inflammatory cytokines that are characteristic of the age-related proinflammatory state (23). In the Women’s Health and Aging Study I, participants with low serum selenium concentrations had higher serum IL-6 concentrations (24). Selenium could also modulate arachidonic acid metabolism, a membrane lipid that regulate ion channel and therefore muscle contraction (25). In the Uppsala Longitudinal Study of Adult Men, high serum selenium concentrations were predictive of lower urinary F2-isoprostane concentrations, a biomarker of lipid peroxidation and oxidative stress (26).
A limitation of the present study was that the study design was cross-sectional, which precludes the determination of causation between observed phenomena. It may be possible that older adults with poor muscle strength have a lower dietary intake of selenium because of factors relating to their low strength, ie, being less able to shop and prepare meals higher in selenium, such as fish. In longitudinal analyses of selenium with adverse outcomes over time, there is a competing risk of mortality. Of 1389 older adults in the Etude du Vieillissement Arteriel Study in Nantes, France (27) and of 632 older women in the Women’s Health and Aging Studies I and II in Baltimore, MD (28), those with low serum and plasma selenium concentrations at enrollment had a significantly higher risk of death. In the InCHIANTI Study, the proportions of participants who died over 6 y of follow-up, from the lowest to highest quartile of plasma selenium at enrollment, were 41.3%, 27.0%, 18.1%, and 13.5%. After adjustment for age, sex, education, and chronic diseases, adults in the lowest quartile of plasma selenium at enrollment had a greater risk of mortality than did those in the highest quartile (hazard ratio: 1.60; 95% CI: 1.04, 2.47; P = 0.034) (F Lauretani, unpublished observation, 2006).
The plasma selenium concentrations in the present study are consistent with those of previous reports from Italy. In the Veneto region, mean plasma selenium concentrations were 0.82 μmol/L in adults (29) and 1.12 and 0.86 μmol/L in adults aged 65–89 and ≥90 y, respectively, from Bologna (30). Mean serum selenium concentrations in Italian adults varied between 1.09 and 1.17 μmol/L; decreasing values were observed in adults aged >60 y (31). The average daily intake of selenium in the Italian population is ≈51 μg/d, which compares with the Recommended Dietary Intake of 55 μg/d for adult men and women (22).
It is unclear whether a higher dietary intake of selenium will improve or maintain muscle strength in older community-dwelling adults with low plasma selenium concentrations. In severe selenium-deficient adults, selenium treatment was associated with an improvement in proximal muscle strength (32, 33).
In conclusion, low plasma selenium concentrations were associated with poor skeletal muscle strength in older community-dwelling adults in Tuscany, Italy. Whether selenium supplementation can slow down the age-associated decline in muscle strength and be used in selenium-deficient patients to relieve muscular complaints remains inconclusive (34) and should be tested in specifically designed clinical studies.
Footnotes
Supported in part by the Intramural Research Program, National Institute on Aging, NIH, and by National Institute on Aging contracts N01-AG-916413, N01-AG-821336, and N01-AG-5-0002 and NIA grant R01 AG027012.
The authors’ responsibilities were as follows—FL and RDS: helped design the study, analyze the data, and write the manuscript; ALR helped collect and analyze the data; JMG and SB: helped critically review the manuscript; LF: helped design the study, collect and manage the data, and critically review the manuscript. None of the authors had any financial or personal conflicts of interest.
References
- 1.Morley JE, Baumgartner RN, Roubenoff R, Mayer J, Nair KS. Sarcopenia J Lab Clin Med. 2001;137:231–43. doi: 10.1067/mlc.2001.113504. [DOI] [PubMed] [Google Scholar]
- 2.Carmeli E, Coleman R, Reznick AZ. The biochemistry of aging muscle. Exp Gerontol. 2002;37:477–89. doi: 10.1016/s0531-5565(01)00220-0. [DOI] [PubMed] [Google Scholar]
- 3.Mecocci P, Fanó G, Fulle S, et al. Age-dependent increases in oxidative damage to DNA, lipids, and proteins in human skeletal muscle. Free Radic Biol Med. 1999;26:303–8. doi: 10.1016/s0891-5849(98)00208-1. [DOI] [PubMed] [Google Scholar]
- 4.Pansarasa O, Bertorelli L, Vecchiet J, Felzani G, Marzatico F. Age-dependent changes of antioxidant activities and markers of free radical damage in human skeletal muscle. Free Radic Biol Med. 1999;27:617–22. doi: 10.1016/s0891-5849(99)00108-2. [DOI] [PubMed] [Google Scholar]
- 5.Lim PS, Cheng YM, Wei YH. Increase in oxidative damage to lipids and proteins in skeletal muscle of uremic patients. Free Radic Res. 2002;36:295–301. doi: 10.1080/10715760290019318. [DOI] [PubMed] [Google Scholar]
- 6.Gianni P, Jan KJ, Douglas MJ, Stuart PM, Tarnopolsky MA. Oxidative stress and the mitochondrial theory of aging in human skeletal muscle. Exp Gerontol. 2004;39:1391–400. doi: 10.1016/j.exger.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Rederstorff M, Krol A, Lescure A. Understanding the importance of selenium and selenoproteins in muscle function. Cell Mol Life Sci. 2006;63:52–9. doi: 10.1007/s00018-005-5313-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fulle S, Protasi F, Di Tano G, et al. The contribution of reactive oxygen species to sarcopenia and muscle ageing. Exp Gerontol. 2004;39:17–24. doi: 10.1016/j.exger.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 9.Klein EA. Selenium: epidemiology and basic science. J Urol. 2004;171(suppl):S50–3. doi: 10.1097/01.ju.0000107837.66277.e9. [DOI] [PubMed] [Google Scholar]
- 10.Lofstedt J. White muscle disease of foals. Vet Clin North Am Equine Pract. 1997;13:169–85. doi: 10.1016/s0749-0739(17)30262-6. [DOI] [PubMed] [Google Scholar]
- 11.Ishihara H, Kanda F, Matsushita T, Chihara K, Itoh K. White muscle disease in humans: myopathy caused by selenium deficiency in anorexia nervosa under long term total parenteral nutrition. J Neurol Neurosurg Psychiatry. 1999;67:829–30. doi: 10.1136/jnnp.67.6.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chariot P, Bignani O. Skeletal muscle disorders associated with selenium deficiency in humans. Muscle Nerve. 2003;27:662–8. doi: 10.1002/mus.10304. [DOI] [PubMed] [Google Scholar]
- 13.Ferrucci L, Bandinelli S, Benvenuti E, et al. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48:1618–25. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- 14.Marsh PA, Miller ME, Saikin AM, et al. Lower extremity strength and power are associated with 400-meter walk time in older adults: The InCHIANTI Study. J Gerontol A Biol Sci Med Sci. 2006;61:1186–93. doi: 10.1093/gerona/61.11.1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pisani P, Faggiano F, Krogh V, Palli D, Vineis P, Berrino F. Relative validity and reproducibility of a food frequency dietary questionnaire for use in the Italian EPIC centres. Int J Epidemiol. 1997;26(suppl 1):S152–60. doi: 10.1093/ije/26.suppl_1.s152. [DOI] [PubMed] [Google Scholar]
- 16.Guralnik JM, Fried LP, Simonsick EM, Kasper D, Lafferty ME. The Women’s Health and Aging Study: health and social characteristics of older women with disability. Bethesda, MD: National Institute on Aging; 1995. NIH publication no. 95-4009. [Google Scholar]
- 17.Radloff LS. The CED-S scale. A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 18.Bandinelli S, Benvenuti E, Del Lungo I, et al. Measuring muscular strength of the lower limbs by hand-held dynamometer: a standard protocol. Aging. 1999;11:287–93. doi: 10.1007/BF03339802. [DOI] [PubMed] [Google Scholar]
- 19.Lauretani F, Russo CR, Bandinelli S, et al. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol. 2003;95:1851–60. doi: 10.1152/japplphysiol.00246.2003. [DOI] [PubMed] [Google Scholar]
- 20.Rantanen T, Masaki K, Foley D, Izmirlian G, White L, Guralnik JM. Grip strength changes over 27 yr in Japanese-American men. J Appl Physiol. 1998;85:2047–53. doi: 10.1152/jappl.1998.85.6.2047. [DOI] [PubMed] [Google Scholar]
- 21.Rantanen T, Guralnik JM, Foley D, et al. Midlife hand grip strength as a predictor of old age disability. JAMA. 1999;281:558–60. doi: 10.1001/jama.281.6.558. [DOI] [PubMed] [Google Scholar]
- 22.Food and Nutrition Board, Institute of Medicine. Dietary reference intakes for vitamin C, vitamin E, selenium and carotenoids. Washington, DC: National Academy Press; 2000. [PubMed] [Google Scholar]
- 23.Ferrucci L, Corsi A, Lauretani F, et al. The origins of age-related proinflammatory state. Blood. 2005;105:2294–9. doi: 10.1182/blood-2004-07-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walston JD, Xue QL, Semba RD, et al. Serum antioxidants, inflammation, and mortality in older women. Am J Epidemiol. 2006;163:18–26. doi: 10.1093/aje/kwj007. [DOI] [PubMed] [Google Scholar]
- 25.Alissa EM, Bahijri Sm, Ferns GA. The controversy surrounding selenium and cardiovascular disease: a review of the evidence. Med Sci Monit. 2003;9:RA9–18. [PubMed] [Google Scholar]
- 26.Helmersson J, Árnlöv J, Vessby B, Larsson A, Alfthan G, Basu S. Serum selenium predicts levels of F2-isoprostanes and prostaglandin F2α in a 27 year follow-up study of Swedish men. Free Radic Res. 2005;39:763–70. doi: 10.1080/10715760500108513. [DOI] [PubMed] [Google Scholar]
- 27.Akbaraly NT, Arnaud J, Hininger-Favier I, Gourlet V, Roussel AM, Berr C. Selenium and mortality in the elderly: results from the EVA study. Clin Chem. 2005;51:2117–23. doi: 10.1373/clinchem.2005.055301. [DOI] [PubMed] [Google Scholar]
- 28.Ray AL, Semba RD, Walston J, et al. Low serum selenium and total carotenoids predict mortality among older women living in the community: the Women’s Health and Aging Studies. J Nutr. 2006;136:172– 6. doi: 10.1093/jn/136.1.172. [DOI] [PubMed] [Google Scholar]
- 29.Bellisola G, Perona G, Galassini S, Moschini G, Guidi GC. Plasma selenium and glutathione peroxidase activities in individuals living in the Veneto region of Italy. J Trace Elem Electrolytes Health Dis. 1993;7:242–7. [PubMed] [Google Scholar]
- 30.Ravaglia G, Forti P, Maioli F, et al. Blood micronutrient and thyroid hormone concentrations in the oldest-old. J Clin Endocrinol Metab. 2000;85:2260–5. doi: 10.1210/jcem.85.6.6627. [DOI] [PubMed] [Google Scholar]
- 31.Morisi G, Patriarca M, Marano G, Giampaoli S, Taggi F. Age and sex specific reference serum selenium levels estimated for the Italian population. Ann Ist Super Sanita. 1989;25:393–403. [PubMed] [Google Scholar]
- 32.Brown MR, Cohen HJ, Lyons JM, et al. Proximal muscle weakness and selenium deficiency associated with long term parenteral nutrition. Am J Clin Nutr. 1986;43:549–54. doi: 10.1093/ajcn/43.4.549. [DOI] [PubMed] [Google Scholar]
- 33.Yagi M, Tani T, Hashimoto T, et al. Four cases of selenium deficiency in postoperative long-term enteral nutrition. Nutrition. 1996;12:40–3. doi: 10.1016/0899-9007(95)00062-3. [DOI] [PubMed] [Google Scholar]
- 34.Robinson MF, Campbell DR, Stewart RD, et al. Effect of daily supplements of selenium on patients with muscular complaints in Otago and Canterbury. N Z Med J. 1981;93:289–92. [PubMed] [Google Scholar]