In the late 1970s, Roland Moskowitz stated, ‘‘The evolution of osteoarthritis has been characterized by a number of myths that persist even now, namely, that osteoarthritis is an inevitable disease of aging … for which little can be done therapeutically even when the diagnosis has been made’’ (1). Thirty years later, is there any hope of debunking these myths? Advanced age is the strongest risk factor for the development of osteoarthritis (OA) (2–4)—the most common form of arthritis in the United States (2), which is projected to affect some 60 million Americans by the year 2020.
Over the last 40 years, we have witnessed remarkable scientific and medical discoveries that have led to the successful treatment of many common diseases. As recently as 20 years ago, the notion that peptic ulcer disease stemmed from a bacterial infection treatable with antibiotics seemed equally as absurd as the idea of performing surgery through small incisions with no direct view of the target vessel or joint. Yet these once science fiction-like dreams have been realized and become standard medical practice. One more recent extraordinary accomplishment of medical science has been the development of targeted inhibition of inflammatory mediators. Introduction of biologic agents into the rheumatoid arthritis armamentarium has rescued many patients from total joint destruction and debilitation. In stark contrast, the OA management arsenal has been devoid of fresh recruits and new weaponry, leaving frustrated clinicians and desperate patients to resort to therapies of dubious effectiveness.
Finally, hope emerges on the horizon rising from developments in imaging, protein profiling, and molecular and integrative biology. New tools now allow the visualization of joint structure with resolution far exceeding conventional radiography. Examination of joint structures using magnetic resonance imaging (MRI) has shown that small localized defects in cartilage (5,6) is a harbinger of cartilage loss. Semiquantitative methods that assess cartilage volume, integrity, and defects in articular and periarticular structures of the knee utilized in scientific studies of the natural history of knee OA (7–10) are gaining recognition for their clinical relevance. Such studies have unveiled bony abnormalities (8,11) and meniscal damage (12,13) associated with painful symptoms and disease progression (11,14). No doubt, these highly sophisticated imaging techniques have begun to redefine the anatomic boundaries between a normally aging joint and early OA. Through administration of ‘‘challenge’’ tests of joint function, MRI spectroscopic signatures of diminished ‘‘physiologic reserve’’ can be obtained well before structural damage would otherwise be detectable (15–18). Although it would be tempting to employ these new imaging techniques and concentrate research on cartilage change and joint articulation in search of new therapeutic targets, we strongly urge consideration of all relevant components of the aging joint in synchrony. For example, age-related changes in extracellular matrix integrity that affect multiple physiological systems might also explain why older persons become more susceptible to OA.
In theory, any transition from a normal to a disease state should either emerge from or produce changes in specific biomarkers that may be detectable in the circulation (19–21). Evidence is accumulating that OA stems from the disequilibrium between degradative and repair processes that are physiologically harmonized by the cross-talk between inflammatory cytokines (22,23), growth factors (24), degradative enzymes (25,26), and chondrocyte apoptosis (27). Accordingly, inflammatory markers have been found to predict OA development and clinical progression (28–30). Functional genomics (31) and protein profiling (32), emerging technologies applicable to OA, may facilitate elucidation of the mechanisms that regulate these homeostatic pathways. Chip or mass spectroscopy-based technology allows simultaneous quantification of hundreds of proteins from small quantities of serum, plasma, or tissue. Thus, signatures of circulating proteins present in the early state or preceeding development of clinical OA may soon be available. The path from protein pattern identification to understanding pathophysiology, however, is full of potholes. First, since proteins are highly redundant in living systems, the relevance of any circulating marker can only be established in the context of carefully designed and extended longitudinal studies. Second, individual proteins may exert different actions depending on the local environment and coexpression of other proteins (e.g., IL-1 and IL-6). Finally, the possibility that over-expressed proteins could indicate activation of compensatory mechanisms should be evaluated. The coexistence of OA and other age-related conditions adds further complexity.
The rising threat of OA for the health of a rapidly aging population has prompted the National Institutes of Health (33) to initiate two collaborative OA research networks, the OA Biomarkers Network and the Osteoarthritis Initiative. These efforts will use state-of-the-art immunologic, biochemical, and imaging methods to identify risk factors and biomarkers associated with the development and progression of OA, with the long-term goal of identifying disease-modifying and curative therapies. Although the NIH should be applauded for these initiatives, parallel efforts to stimulate research on OA in the geriatric population are also needed. The precise mechanisms that predispose older individuals to OA have not yet been elucidated and may differ greatly from those in younger populations. There is emerging evidence that the pathophysiology of OA in older adults includes mechanisms common to many age-related chronic conditions including the accumulation of advanced glycation end products, excessive and unaltered oxidative stress, and reduced growth factor signaling (34–36). Distinguishing the boundaries that divide normal joint aging from OA-related pathology is the ultimate challenge. Due to the ubiquitous nature of OA in old age, it is exceedingly difficult to obtain tissue and cellular samples from older individuals free of objectively determined OA. This partially explains why existing in vitro studies have been conducted in a clinical vacuum—that is, without knowledge of the health status or behavioral practices (weight history and physical activity) of the patients from whom the samples were acquired. Creation of a tissue bank of specimens acquired from older persons with no apparent OA and with OA at different developmental stages complemented by extensive clinical and lifestyle information would tremendously facilitate research in this field. This resource may prove invaluable for revealing key mechanisms that protect joints from wear and tear over many years of use and misuse. Given the recent recognition of potential contributions of bone, muscle, meniscus, and tendon to OA pathogenesis, it is imperative that tissue banks expand beyond the preservation and banking of cartilage.
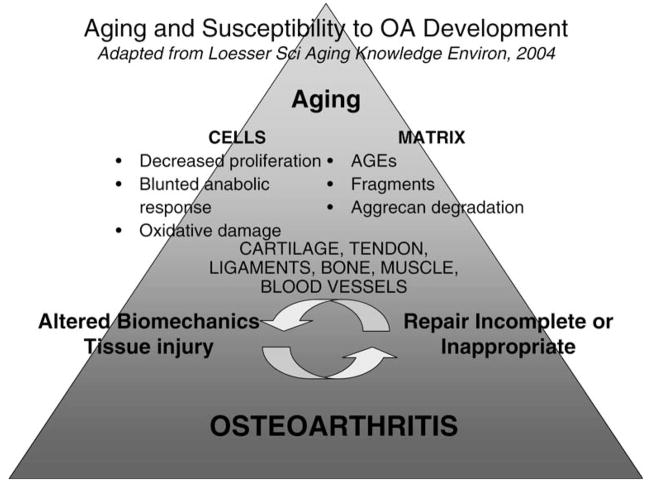
We have begun to apply extraordinarily sophisticated technology to study the interface between normal aging and OA, which has the potential to generate a massive amount of multidimensional data. Extracting the critical factors and relationships from the deafening random noise (37) will require new statistical approaches and analytic methods that can process complex relationships between and among individual biomarkers and clusters of bio-markers, and physiologic and anatomic data on multiple aging body systems. Ideally, we desire development of techniques that can be used to construct animated illustrations and quantify the transition from health to disease. In our dreams, we could imagine that we could witness the dynamic process of matrix turnover and how it is modulated by aging (Figure 1) (38). Discerning these pathways is pursuant to the development of new pharmacologic agents and restorative or regenerative interventions that can mitigate the deleterious events that transform unhealthy aging into disease.
Figure 1.
Aging and susceptibility to osteoarthritis development. [Adapted from Loeser RF, Jr. Aging cartilage and osteoarthritis—what’s the link? Sci Aging Knowledge Environ. 2004;2004(29):pe31. Used with permission.]
References
- 1.Moskowitz RW. Osteoarthritis: a new look at an old disease. Geriatrics. 1973;28:121–128. [PubMed] [Google Scholar]
- 2.Lawrence RC, Helmick CG, Arnett FC, et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum. 1998;41:778–799. doi: 10.1002/1529-0131(199805)41:5<778::AID-ART4>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 3.Leveille SG. Musculoskeletal aging. Curr Opin Rheumatol. 2004;16:114–118. doi: 10.1097/00002281-200403000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Hirsch R, Guralnik JM, Ling SM, Fried LP, Hochberg MC. The patterns and prevalence of hand osteoarthritis in a population of disabled older women: the Women’s Health and Aging Study. Osteoarthritis Cartilage. 2000;8(Suppl A):S16–S21. doi: 10.1053/joca.2000.0330. [DOI] [PubMed] [Google Scholar]
- 5.Ding C, Cicuttini F, Scott F, Cooley H, Boon C, Jones G. Natural history of knee cartilage defects and factors affecting change. Arch Intern Med. 2006;166:651–658. doi: 10.1001/archinte.166.6.651. [DOI] [PubMed] [Google Scholar]
- 6.Ding C, Cicuttini F, Scott F, Boon C, Jones G. Association of prevalent and incident knee cartilage defects with loss of tibial and patellar cartilage: a longitudinal study. Arthritis Rheum. 2005;52:3918–3927. doi: 10.1002/art.21474. [DOI] [PubMed] [Google Scholar]
- 7.Eckstein F, Cicuttini F, Raynauld JP, Waterton JC, Peterfy C. Magnetic resonance imaging (MRI) of articular cartilage in knee osteoarthritis (OA): morphological assessment. Osteoarthritis Cartilage. 2006;14(Suppl 1):46–75. doi: 10.1016/j.joca.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 8.Conaghan PG, Felson D, Gold G, Lohmander S, Totterman S, Altman R. MRI and non-cartilaginous structures in knee osteoarthritis. Osteoarthritis Cartilage. 2006;14(Suppl 1):87–94. doi: 10.1016/j.joca.2006.02.028. [DOI] [PubMed] [Google Scholar]
- 9.Hunter DJ, Conaghan PG, Peterfy CG, et al. Responsiveness, effect size, and smallest detectable difference of Magnetic Resonance Imaging in knee osteoarthritis. Osteoarthritis Cartilage. 2006;14(Suppl 1):112–115. doi: 10.1016/j.joca.2006.02.027. [DOI] [PubMed] [Google Scholar]
- 10.Peterfy C, Woodworth T, Altman R. Workshop for Consensus on Osteoarthritis Imaging: MRI of the knee. Osteoarthritis Cartilage. 2006;14(Suppl 1):44–45. [Google Scholar]
- 11.Felson DT, Chaisson CE, Hill CL, et al. The association of bone marrow lesions with pain in knee osteoarthritis. Ann Intern Med. 2001;134:541–549. doi: 10.7326/0003-4819-134-7-200104030-00007. [DOI] [PubMed] [Google Scholar]
- 12.Raynauld JP, Martel-Pelletier J, Berthiaume MJ, et al. Long term evaluation of disease progression through the quantitative magnetic resonance imaging of symptomatic knee osteoarthritis patients: correlation with clinical symptoms and radiographic changes. Arthritis Res Ther. 2006;8:R21. doi: 10.1186/ar1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hunter DJ, Zhang YQ, Niu JB, et al. The association of meniscal pathologic changes with cartilage loss in symptomatic knee osteoarthritis. Arthritis Rheum. 2006;54:795–801. doi: 10.1002/art.21724. [DOI] [PubMed] [Google Scholar]
- 14.Hunter DJ, Zhang Y, Niu J, et al. Increase in bone marrow lesions associated with cartilage loss: a longitudinal magnetic resonance imaging study of knee osteoarthritis. Arthritis Rheum. 2006;54:1529–1535. doi: 10.1002/art.21789. [DOI] [PubMed] [Google Scholar]
- 15.Gold GE, Pauly JM, Macovski A, Herfkens RJ. MR spectroscopic imaging of collagen: tendons and knee menisci. Magn Reson Med. 1995;34:647–654. doi: 10.1002/mrm.1910340502. [DOI] [PubMed] [Google Scholar]
- 16.Conley KE, Cress ME, Jubrias SA, Esselman PC, Odderson IR. From muscle properties to human performance, using magnetic resonance. J Gerontol A Biol Sci Med Sci. 1995;50(Spec Iss):35–40. doi: 10.1093/gerona/50a.special_issue.35. [DOI] [PubMed] [Google Scholar]
- 17.Crowther GJ, Jubrias SA, Gronka RK, Conley KE. A ‘‘functional biopsy’’ of muscle properties in sprinters and distance runners. Med Sci Sports Exerc. 2002;34:1719–1724. doi: 10.1097/00005768-200211000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Kemp GJ, Crowe AV, Anijeet HK, et al. Abnormal mitochondrial function and muscle wasting, but normal contractile efficiency, in haemodialysed patients studied non-invasively in vivo. Nephrol Dial Transplant. 2004;19:1520–1527. doi: 10.1093/ndt/gfh189. [DOI] [PubMed] [Google Scholar]
- 19.Poole AR. Biochemical/immunochemical biomarkers of osteoarthritis: utility for prediction of incident or progressive osteoarthritis. Rheum Dis Clin North Am. 2003;29:803–818. doi: 10.1016/s0889-857x(03)00056-5. [DOI] [PubMed] [Google Scholar]
- 20.Garnero P, Delmas PD. Biomarkers in osteoarthritis. Curr Opin Rheumatol. 2003;15:641–646. doi: 10.1097/00002281-200309000-00020. [DOI] [PubMed] [Google Scholar]
- 21.Kraus VB. Biomarkers in osteoarthritis. Curr Opin Rheumatol. 2005;17:641–646. doi: 10.1097/01.bor.0000174195.15421.17. [DOI] [PubMed] [Google Scholar]
- 22.Goldring MB. Osteoarthritis and cartilage: the role of cytokines. Curr Rheumatol Rep. 2000;2:459–465. doi: 10.1007/s11926-000-0021-y. [DOI] [PubMed] [Google Scholar]
- 23.Goldring SR, Goldring MB. The role of cytokines in cartilage matrix degeneration in osteoarthritis. Clin Orthop Relat Res. 2004;(427 Suppl):S27–S36. doi: 10.1097/01.blo.0000144854.66565.8f. [DOI] [PubMed] [Google Scholar]
- 24.Trippel SB. Growth factor inhibition: potential role in the etiopathogenesis of osteoarthritis. Clin Orthop Relat Res. 2004;(427 Suppl):S47–S52. [PubMed] [Google Scholar]
- 25.Burrage PS, Mix KS, Brinckerhoff CE. Matrix metalloproteinases: role in arthritis. Front Biosci. 2006;11:529–543. doi: 10.2741/1817. [DOI] [PubMed] [Google Scholar]
- 26.Smith GN., Jr The role of collagenolytic matrix metalloproteinases in the loss of articular cartilage in osteoarthritis. Front Biosci. 2006;11:3081–3095. doi: 10.2741/2034. [DOI] [PubMed] [Google Scholar]
- 27.Aigner T, Kim HA, Roach HI. Apoptosis in osteoarthritis. Rheum Dis Clin North Am. 2004;30:639–653. xi. doi: 10.1016/j.rdc.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 28.Sharif M, Shepstone L, Elson CJ, Dieppe PA, Kirwan JR. Increased serum C reactive protein may reflect events that precede radiographic progression in osteoarthritis of the knee. Ann Rheum Dis. 2000;59:71–74. doi: 10.1136/ard.59.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sowers M, Jannausch M, Stein E, Jamadar D, Hochberg M, Lachance L. C-reactive protein as a biomarker of emergent osteoarthritis. Osteoarthritis Cartilage. 2002;10:595–601. doi: 10.1053/joca.2002.0800. [DOI] [PubMed] [Google Scholar]
- 30.Spector TD, Hart DJ, Nandra D, et al. Low-level increases in serum C-reactive protein are present in early osteoarthritis of the knee and predict progressive disease. Arthritis Rheum. 1997;40:723–727. doi: 10.1002/art.1780400419. [DOI] [PubMed] [Google Scholar]
- 31.Aigner T, Bartnik E, Sohler F, Zimmer R. Functional genomics of osteoarthritis: on the way to evaluate disease hypotheses. Clin Orthop Relat Res. 2004;(427 Suppl):S138–S143. [PubMed] [Google Scholar]
- 32.Kingsmore SF, Patel DD. Multiplexed protein profiling on antibody-based microarrays by rolling circle amplification. Curr Opin Biotechnol. 2003;14:74–81. doi: 10.1016/s0958-1669(02)00019-8. [DOI] [PubMed] [Google Scholar]
- 33.Rooney M, Condell D, Quinlan W, et al. Analysis of the histologic variation of synovitis in rheumatoid arthritis. Arthritis Rheum. 1988;31:956–963. doi: 10.1002/art.1780310803. [DOI] [PubMed] [Google Scholar]
- 34.Loeser RF., Jr Aging cartilage and osteoarthritis—what’s the link? Sci Aging Knowledge Environ. 2004;2004(29):pe31. doi: 10.1126/sageke.2004.29.pe31. [DOI] [PubMed] [Google Scholar]
- 35.Loeser RF, Shakoor N. Aging or osteoarthritis: which is the problem? Rheum Dis Clin North Am. 2003;29:653–673. doi: 10.1016/s0889-857x(03)00062-0. [DOI] [PubMed] [Google Scholar]
- 36.Loeser RF., Jr Aging and the etiopathogenesis and treatment of osteoarthritis. Rheum Dis Clin North Am. 2000;26:547–567. doi: 10.1016/s0889-857x(05)70156-3. [DOI] [PubMed] [Google Scholar]
- 37.Kriete A. Biomarkers of aging: combinatorial or systems model? Sci Aging Knowledge Environ. 2006;2006(1):pe1. doi: 10.1126/sageke.2006.1.pe1. [DOI] [PubMed] [Google Scholar]
- 38.Loeser RF. Molecular mechanisms of cartilage destruction: mechanics, inflammatory mediators, and aging collide. Arthritis Rheum. 2006;54:1357–1360. doi: 10.1002/art.21813. [DOI] [PMC free article] [PubMed] [Google Scholar]